Abstract
We conducted a multicenter, randomized, open-label trial to compare mefloquine with a 3-day quinine plus sulphalene-pyrimethamine (SP) regimen for the treatment of imported uncomplicated malaria acquired in Africa. The end points of the study were efficacy, tolerability, and length of hospital stay. From July 1999 to February 2003, 187 patients were enrolled in five centers in Italy, of whom 93 were randomized to receive mefloquine (the M group) and 94 were randomized to receive quinine plus SP (the QSP group). Immigrants and visiting relatives and friends represented 90% of the cases and were mainly from western African countries. A slightly increased proportion of cases in the QSP group had abnormal alanine aminotransferase levels at the baseline. The early cure rate was similar in the two groups: 98.9% (confidence interval [CI] = 97 to 100%) in the M group and 96.8% (CI = 93 to 100%) in the QSP group. The extended follow-up was completed by 135 subjects (72.2%), and no case of recrudescence was detected. There were no differences in the parasite clearance time, but patients in the M group had shorter mean fever clearance time (35.9 h versus 44.4 h for the QSP group; P = 0.05) and a shorter mean hospital stay (3.9 days versus 4.6 days for the QSP group; P = 0.007). The overall proportions of reported side effects were similar in the two groups, but patients in the M group had a significantly higher rate of central nervous system disturbances (29.0% versus 9.6% for the QSP group; P < 0.001).
Malaria is the world's most important parasitic infection (5). Although it has been eradicated from temperate zones, each year an increasing number of travelers from these areas are exposed to the risk of malaria while visiting tropical countries. The risk for travelers to sub-Saharan African countries is estimated to be 1.5 to 2.4% per month of exposure in the absence of chemoprophylaxis (13). The number of malaria cases reported in European countries each year reaches 11,000, and the number of patients with falciparum malaria is estimated to be 8,000 per year; significant underreporting is assumed (6). In Italy Plasmodium falciparum malaria is the leading cause of fever imported from the tropics. The incidence of malaria in Italians who traveled to Africa is estimated to be 1.5/1,000 (9). Imported malaria in Italy is gradually becoming problematic among foreign-born persons from areas of endemicity, especially West Africa. This seems to be related to the rising flow of immigrants from Africa (3).
The regimens used in Europe for the treatment of uncomplicated falciparum malaria include quinine, either alone or in combination with tetracycline or sulfa drug combinations, and mefloquine (7, 14, 16). Newer regimens, including the fixed combinations of atovaquone-proguanil and artemether-lumefantrine, are slowly being introduced, even in the absence of efficacy data from randomized trials with nonimmune subjects. When infections are sensitive to quinine, a 3-day course of quinine and sulfadoxine-pyrimethamine can be given to overcome the problem of poor compliance with longer regimens (4, 8, 16). Sulfadoxine-pyrimethamine resistance occurs frequently in Southeast Asia and South America and occurs to such an extent that these combinations have questionable efficacies against infections acquired in these areas. Although sulfadoxine-pyrimethamine resistance is also spreading in Africa (2), the effects of resistance on the efficacy of the combination are still unclear (12).
No comparative studies of the drugs commonly used to treat imported malaria have been conducted. In a retrospective analysis of falciparum malaria cases in northern Italy, we reported (7) significantly shorter fever clearance times and hospital stays for patients treated with mefloquine compared to those for patients treated with quinine.
We now report on the results of a prospective study conducted to measure the efficacy and tolerability of mefloquine compared to those of quinine plus sulphalene-pyrimethamine (SP; Metakelfin) given for 3 days in adults with uncomplicated falciparum malaria imported from Africa.
MATERIALS AND METHODS
Study design and enrollment sites.
This was a multicenter, randomized, open-label trial. Patients were enrolled in Italy at five sites belonging to the network of the Lombardy Study Group for Research in International Health (Gruppo di Studio sulla Salute Internazionale della Regione Lombardia; SIRL). All sites are referral divisions for infectious diseases.
Patients.
Patients with a diagnosis of acute uncomplicated P. falciparum malaria were enrolled if they were over 18 years of age and had acquired P. falciparum infection in Africa.
The patients were divided into Italians and foreign-born individuals. The foreign-born individuals with malaria were further classified as immigrants who had first arrived in Italy and individuals who had been visiting relatives and friends (VRF) if they were residents of Italy and had returned back home for a short visit.
Diagnosis was always based on evidence of asexual P. falciparum parasites on Giemsa-stained thin and thick films of peripheral blood.
Patients were excluded from the study if they were diagnosed with severe or complicated malaria, defined according to the criteria set by the World Health Organization, which were revised during the conduct of the trial (15, 17). Other exclusion criteria were an inability to consume oral medications, pregnancy, the presence of a mixed parasite infection, and a history of malaria treatment (any regimen) in the 2 months preceding enrollment.
The trial was conducted in accordance with the principles of the Helsinki Declaration and its Hong Kong Amendment and according to the principles of good clinical practice, as defined by the European Community guidelines and recommendations (guideline III/3976/88-EN).
Treatment.
Patients were randomly assigned to receive either mefloquine or a combination of quinine plus SP (Metakelfin). Patients randomized to the mefloquine arm (the M group) received the drug at 25 mg/kg of body weight in three doses. The average adult dose was administered as follows: three tablets on recruitment, two tablets after 6 h, and one tablet after an additional 6 h. Patients randomized to the quinine plus SP arm (the QSP group) received 30 mg of quinine/kg in three doses a day for 3 days and a single dose of SP at 1.25/25 mg/kg, given on the first day. The drugs were readministered if vomiting occurred within 1 h of swallowing.
The protocol design allowed patients who dropped out of the study due to disease progression or vomiting to be treated with intravenous quinine.
End points.
The primary end point for efficacy was the early cure rate, defined as clinical and parasitological cure before discharge from the hospital. Patients who dropped out of the study due to adverse events were considered treatment failures. A secondary end point was the rate of recrudescence during the 28 days of follow-up. Additional treatment end points were the time required for parasite clearance, the parasite reduction ratio, the time to the clearance of fever, and the time of hospital stay.
The primary end point for tolerability was the rate of emergence of adverse events during the follow-up period during the hospital stay. Indications for early study termination were progression to severe or complicated disease; the occurrence of adverse events possibly related to study medication that, in the treating physician's opinion, warranted treatment discontinuation; and, lastly, the patient's decision. A follow-up visit was scheduled on day 28 after hospital discharge.
Measurements.
All patients were hospitalized during the acute phase of illness. A physical examination was done daily, and the temperature was recorded every 6 h. The clearance of fever was calculated as the time between admission and the first measurement below 37.0°C that was not followed by a resurgence of fever. The administration of ancillary medications (antipyretics and antiemetics) was recorded, as was the need to readminister antimalarial medications due to vomiting.
Parasitemia was quantified with a Giemsa-stained blood film (thick film) every 12 h until two consecutive smears were negative, and then parasitemia was checked at the follow-up visit. Parasitemia was expressed as the number of parasites per microliter. The time required for parasite clearance was calculated as the time between the beginning of treatment and the time when no asexual forms were found on the blood film. The parasite reduction ratio was calculated as the rate between the parasite density before treatment and that at 48 h, as described by others (14).
Inquiries on adverse events or their worsening after antimalarial treatment were made daily. A precoded list was used to register the events, but the list was not read to the patient. The treating physician established the grade of severity of the event and its relatedness to the study drug.
Hematochemical investigations were done before the beginning of treatment and at day 3. These investigations included determination of a complete blood count and measurement of creatinine, total bilirubin, alanine aminotransferase (ALT), and lactate dehydrogenase (LDH) levels. Electrocardiography was done before treatment and at day 3.
After discharge, the patients were requested to return for a follow-up visit at day 28. A clinical examination and a parasitological evaluation were done at the follow-up visit.
Statistical analysis.
Data storage and management were performed with Microsoft Access 97 software. All the data are expressed as means ± standard deviations or medians. SPSS software for Windows (version 11.0) was used for the statistical analyses. The data from the two treatment arms were compared by using the Kruskal-Wallis H test. Differences in categorical variables were assessed by the chi-square test or Fisher's exact test, as appropriate. Statistical significance was defined as a P value <0.05. Kaplan-Meier estimates were used to analyze the probabilities of detectable parasitemia between the two treatment groups; differences were evaluated by the log-rank test.
RESULTS
From July 1999 to February 2003, 187 patients were enrolled, of whom 93 were randomized to the M group and 94 were randomized to the QSP group. The most likely area of acquisition of malaria was West Africa (n = 174; 93.0%); the largest contributions came from Senegal (n = 83), Ghana (n = 36), and Nigeria (n = 24). Among the 187 patients, 20 were Italians and 167 were foreign-born individuals. Among the individuals in the latter group, information on the time of first arrival into Italy was available for 159: 128 (68.5% of all the cases) were VRF, while 31 were immigrants who had first arrived in Italy. Among the VRF group, 108 (57.7% of all the cases) had been settled in Italy for 5 years or more; the remaining 20 patients had been settled in Italy for less than 5 years.
The baseline demographic, clinical, and parasitological characteristics of the patients are summarized in Table 1 by treatment group. The two treatment groups were similar, except for significantly higher median creatinine and ALT values among the patients randomized to the QSP group. On further analysis, the proportion of patients with creatinine values above the normal range was similar in the two groups (3.3 and 6.4% in the M and QSP groups, respectively; P was not significant). On the contrary, the proportion of patients with ALT values above the normal range was higher in the QSP group than in the M group (15.2 and 28.7%, respectively; P = 0.03). However, the proportions of patients with nausea, vomiting, and liver enlargement at enrollment were similar in the two groups (data not shown).
TABLE 1.
Characteristics of patients at enrollment
| Characteristic | Q-SP group (n = 94) | M group (n = 93) | P value |
|---|---|---|---|
| No. (%) males | 80 (85.1) | 70 (75.3) | NSa |
| No. (%) foreign born | 84 (89.4) | 82 (88.1) | NS |
| Median (range) age (yr) | 34.7 (19-62) | 36.6 (20-62) | NS |
| Median (range) wt (kg) | 72 (43-162) | 69 (46-100) | NS |
| Median (range) hemoglobin concn (g/dl) | 12.5 (5.6-17.2) | 12.6 (6.5-15.9) | NS |
| Median (range) platelet count (103) | 83 (9-280) | 92 (26-344) | NS |
| Median (range) bilirubinemia (mg/dl)b | 1.2 (0.3-7) | 1.3 (0.5-7.8) | NS |
| Median (range) ALT level (U/liter)c | 37 (12-246) | 30 (11-118) | 0.02 |
| Median (range) LDH level (U/liter)d | 495 (305-1,680) | 471 (313-1328) | NS |
| Median (range) creatinine level (mg/dl)b | 1 (0.5-1.5) | 0.9 (0.5-2.3) | 0.0003 |
| Median (range) temp (°C)e | 38.5 (36-42) | 38.7 (36-40.4) | NS |
| No. (%) of patients with vomiting | 32 (34) | 29 (31.2) | NS |
| Median (range) parasitemia (%)f | 5,639 (58-195,600) | 3,701 (27-179,100) | NS |
NS, not significant.
n = 184; 93 in QSP group and 91 in M group.
n = 186; 94 in QSP group and 92 in M group.
n = 174; 86 in QSP group and 88 in M group.
n = 181; 91 in QSP group and 90 in M group.
n = 181; 92 in QSP group and 89 in M group.
The early cure rates were similar in the two groups: 98.9% (92 of 93, 95% confidence interval [CI] = 97 to 100%) for patients in the M group and 96.8% (91 of 94, 95% CI = 93 to 100%) for patients in the QSP group (Table 2). Four patients did not complete the treatment. One patient receiving mefloquine developed persistent vomiting of grade 3 severity. In three patients receiving quinine-SP, treatment was prematurely terminated due to persistent vomiting (one patient), disease progression (one patient, who developed pancreatitis and acute respiratory distress syndrome), and electrocardiographic alterations (one patient).
TABLE 2.
Cure rates and parasite and fever clearance times
| Parameter | Total | Q-SP group (n = 94) | M group (n = 93) | P value |
|---|---|---|---|---|
| No. (%) of patients: | ||||
| Completing treatment | 183 (97.9) | 91 (96.8) | 92 (98.9) | NSa |
| Cured at end of treatment | 183 (97.9) | 91 (100) | 92 (100) | NS |
| With 28-day follow-up | 135 (72.2) | 67 (71.3) | 68 (73.1) | NS |
| Cured at day 28 | 135 (72.2%) | 67 (71.3) | 68 (73.1) | NS |
| Mean parasite clearance time (h) | 48.2 | 50.6 | NS | |
| Mean fever clearance time (h) | 44.4 | 35.9 | 0.05 | |
| Mean hospital stay (days) | 4.6 | 3.9 | 0.007 |
NS, not significant.
The extended follow-up (to day 28) was completed by 135 subjects (72.2%) (Table 2). The proportions of subjects completing the extended follow-up were similar in the M and the QSP groups (73.1 and 71.3%, respectively). There were no cases of clinical or parasitological recrudescence at day 28 among the subjects being reevaluated.
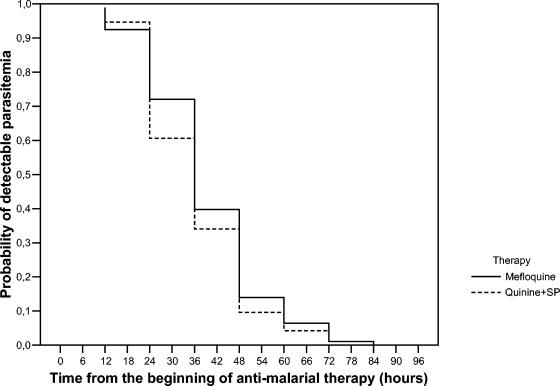
The parasite clearance times were similar in the M and the QSP groups, with mean values of 48.2 and 50.6 h, respectively (Table 2). Figure 1 shows the proportions of subjects with detectable parasitemia after treatment with the two regimens. The log-rank test showed that the rate of parasite clearance did not differ in the two arms. The mean parasite reduction ratios were 18.8 and 16.7 for the M and the QSP groups, respectively (P was not significant).
FIG. 1.
Percentage of subjects with detectable peripheral parasitemia after treatment with the two regimens. The log-rank test showed that the rate of parasite clearance did not differ in the two arms.
The mean fever clearance time was slightly but significantly shorter in patients treated with mefloquine than in patients treated with quinine-SP (35.9 and 44.4 h, respectively; P = 0.05) (Table 2).
The mean hospital stay was also significantly shorter in patients treated with mefloquine than in patients treated with quinine-SP (3.9 and 4.6 days, respectively; P = 0.007) (Table 2).
Treatment was readministered due to vomiting to eight patients (8.6%) in the M group and five patients (5.3%) in the QSP group (P was not significant). Antiemetic drugs were administered to 21 (22.6%) and 33 (35.1%) patients in the M and the QSP groups, respectively (P = 0.06). Almost all patients were administered antipyretic drugs.
Side effects (one or more) were reported by 120 (64.2%) patients (Table 3). The overall proportions of side effects were similar in the M and the QSP treatment groups (65.6 and 62.8%, respectively). The large majority of side effects were represented by gastrointestinal discomfort, which was reported by similar proportions of patients in the two groups (46.2 and 55.3%, respectively). A significantly higher proportion of patients treated with mefloquine reported central nervous system (CNS) disturbances (29.0 and 9.6%, respectively; P < 0.001) and, specifically, sleep disturbances (26.9 and 9.6%, respectively; P = 0.02). The odds ratios (ORs) of CNS and sleep disturbances for patients treated with mefloquine and patients treated with quinine-SP were 3.86 (CI = 1.59 to 9.93) and 3.47 (CI = 1.42 to 8.71), respectively. Patients treated with mefloquine were less likely to report tinnitus (2.2 and 14.9%, respectively; P = 0.02; OR = 0.13; CI = 0.02 to 0.61).
TABLE 3.
Clinical adverse events
| Adverse event | No. (%) of patients
|
P value | ||
|---|---|---|---|---|
| Total | QSP group (n = 94) | M group (n = 93) | ||
| Any side effect | 120 (64.2) | 59 (62.8) | 61 (65.6) | NSa |
| Nausea | 33 (17.6) | 20 (21.3) | 13 (14) | NS |
| Vomiting | 74 (39.6) | 42 (44.7) | 32 (34.4) | NS |
| Diarrhea | 11 (5.9) | 4 (4.3) | 7 (7.5) | NS |
| Any GIb disturbance | 95 (50.8) | 52 (55.3) | 43 (46.2) | NS |
| Sleep disturbance | 34 (18.2) | 9 (9.6) | 25 (26.9) | 0.02 (OR = 3.47; CI = 1.42-8.71) |
| Anxiety | 5 (2.7) | 1 (1) | 1 (4.3) | NS |
| Dizziness | 31 (16.6) | 13 (13.8) | 18 (19.4) | NS |
| Any CNS disturbance | 36 (19.3) | 9 (9.6) | 27 (29) | <0.001 (OR = 3.86; CI = 1.59-9.93) |
| Tinnitus | 16 (8.6) | 14 (14.9) | 2 (2.2) | 0.02 (OR = 0.13; CI = 0.02-0.61) |
NS, not significant.
GI, gastrointestinal.
DISCUSSION
We report the results of a prospective, randomized, unblinded study comparing two regimens which are commonly used in Italy and other European countries for the treatment of imported falciparum malaria. The early cure rates, the rates of recrudescence at day 28, and the parasite clearance times were similar for patients treated with mefloquine and patients treated with a short course of quinine combined with sulfa medications. The findings that all patients were cured and that all were free of recrudescence at day 28 are clinically important, although the proportion of subjects evaluated at day 28 was low. The fever clearance times and lengths of hospital stay differed between the two regimens. The mefloquine regimen achieved fever clearance faster than the short quinine course, and this probably resulted in the significantly shorter hospital stays for the patients treated with mefloquine. Although the clinical benefit of a shortening of the fever clearance time of less than 12 h is questionable, the shortening of the hospital stay by 0.7 days may be relevant in cost-effectiveness analysis.
Overall, the two groups had similar rates of side effects, and both regimens were generally well tolerated. As expected, mefloquine caused a significantly higher rate of adverse events involving the CNS. Most such events, however, were represented by sleep disturbances and were mild in grade. No severe CNS-related side effects as a result of mefloquine treatment were observed in this study; but the sample size was small, and we cannot add to the knowledge that the expected rates of severe neuropsychiatric reactions are about 0.1% among Asian patients and 0.5 to 1% among European and African patients (11). Complications like acute intravascular hemolysis are even more rare (1).
The slight increase in the proportion of patients in the QSP arm with increased AST levels at the baseline is unlikely to have affected the efficacy results. In fact, the clinical signs at the baseline, such as liver enlargement and nausea and vomiting, were similar in the two groups. The rates of gastrointestinal system-related side effects that emerged during treatment were also similar in the two groups.
The study has implications for the treatment of imported malaria in developed countries but has no relevance for the management of the disease in areas of endemicity, where other factors, including the costs of the drugs, must be taken into consideration. The results presented here mainly relate to a population of individuals with some residual immunity to malaria, as most of the enrollees were VRFs with 5 or more years of residency in areas of nonendemicity. Indeed, the present state of knowledge does not allow determination of the extent of the residual immune memory in these patients. However, these patients are similar to those with imported malaria in Europe described previously (8).
This study had several limitations. First, it was not blinded. The ability of unblinded studies to measure adverse events is weak. On the contrary, our efficacy results are unlikely to be affected by the open design of the study. Second, although no differences in the cure rates were detected, our sample size was too small to demonstrate the equivalence of the two regimens. Third, our findings apply mainly to immigrants and VRF populations and may not be applicable to all nonimmune travelers. However, immigrants and VRFs represent an increasing proportion of cases of imported malaria in Italy and Europe (9). Finally, our results are limited to patients who acquired the infection in Africa, since the short quinine-SP course that we tested might be ineffective against infections acquired in areas of widespread antifolate resistance, like Southeast Asia and South America (12). However, our results are largely relevant, at least in Italy, where more than 90% of all cases of imported P. falciparum infection are from Africa (10).
In conclusion, we have provided evidence that the cure rates for patients with imported falciparum malaria treated with mefloquine or a 3-day course of quinine-SP are similar. Mefloquine treatment resulted in a significantly shorter hospital stay and more frequently caused CNS-related side effects that were mild in grade. Clinicians may consider the use of either regimen while awaiting newer therapies, like atovaquone-proguanil (Malarone) and arthemeter-lumefantrine (Riamet). Ideally, the efficacies of these new regimens should be evaluated by use of either of the regimens that we have studied here as a comparator arm. Such studies, however, will have the same problems of sample size if the primary end point is efficacy and will have to be blinded if the primary end point is tolerability.
Acknowledgments
This study is presented for the SIRL Study Group (Gruppo di Studio sulla Salute Internazionale della Regione Lombardia), whose members include Silvio Caligaris and Cecilia Pizzocolo (Institute of Infectious and Tropical Diseases, University of Brescia), Gian Pietro Cadeo (Clinic of Infectious Diseases, Spedali Civili, Brescia), Massimo Giola (Clinic of Infectious and Tropical Diseases, University Hospital, Varese), Paolo Perini (Clinic of Infectious Diseases, A. Manzoni Hospital, Lecco), Laura Galimberti (Institute of Infectious and Tropical Diseases, University of Milan), Marco Moroni (Division of Infectious Diseases, San Anna Hospital, Como), Alessandra Donisi (Clinic of Infectious Diseases, Civil Hospital, Piacenza), Gloria Giani (I Division of Infectious Diseases, Sacco Hospital, Milan), Giorgio Perboni (Clinic of Infectious Diseases, Poma Hospital, Mantova), Alberto Volonterio (Clinic of Infectious Diseases, Ospedale Maggiore Niguarda, Milan), and Donata Galloni (Clinic of Infectious Diseases, General Hospital, Cremona).
This study received financial support from a grant from the Italian Ministry of University and Research (60%), 2002.
REFERENCES
- 1.Bisoffi, Z., S. Marocco, G. Monteiro, and M. Marsiaj. 1999. Acute intravascular haemolysis (blackwater fever) after antimalarial treatment. Trop. Med. Int. Health 4:72-73. [DOI] [PubMed] [Google Scholar]
- 2.Bousema, J. T., L. C. Gouagna, A. M. Meutstege, B. E. Okech, N. I. Akim, J. I. Githure, J. C. Beier, and R. W. Sauerwein. 2003. Treatment failure of pyrimethamine-sulphadoxine and induction of Plasmodium falciparum gametocytaemia in children in western Kenya. Trop. Med. Int. Health 8:427-430. [DOI] [PubMed] [Google Scholar]
- 3.Castelli, F., A. Matteelli, S. Caligaris, M. Gulletta, I. El-Hamad, C. Scolari, G. Chatel, and G. Carosi. 1999. Malaria in migrants. Parassitologia 41:261-265. [PubMed] [Google Scholar]
- 4.Hall, A. P., E. B. Doberstyn, V. Mettaprakong, and P. Sonkom. 1975. Falciparum malaria cured by quinine followed by sulphadoxine-pyrimethamine. Br. Med. J. 2:15-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hay, S. I., C. A. Guerra, A. J. Tatem, A. M. Noor, and R. W. Snow. 2004. The global distribution and population at risk of malaria: past, present, and future. Lancet Infect. Dis. 4:327-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jelinek, T., C. Schulte, R. Behrens, M. P. Grobusch, J. P. Coulaud, Z. Bisoffi, A. Matteelli, J. Clerinx, M. Corachan, S. Puente, I. Gjorup, G. Harms, H. Kollaritsch, A. Kotlowski, A. Bjorkmann, J. P. Delmont, J. Knobloch, L. N. Nielsen, J. Cuadros, C. Hatz, J. Beran, M. L. Schmid, M. Schulze, R. Lopez-Velez, K. Fleischer, A. Kapaun, P. McWhinney, P. Kern, J. Atougia, G. Fry, S. da Cunha, and G. Boecken. 2002. Imported falciparum malaria in Europe: sentinel surveillance data from the European Network on Surveillance of Imported Infectious Diseases. Clin. Infect. Dis. 34:572-576. [DOI] [PubMed] [Google Scholar]
- 7.Matteelli, A., P. Colombini, M. Gulletta, and F. Castelli for the SIRL Study Group. 1999. Epidemiological features and case management of imported malaria in north western Italy 1991-1995. Trop. Med. Int. Health 4:653-657. [DOI] [PubMed] [Google Scholar]
- 8.Parola, P., S. Ranque, S. Badiaga, M. Niang, O. Blin, J. J. Charbit, J. Delmont, and P. Brouqui. 2001. Controlled trial of 3-day quinine-clindamycin treatment versus 7-day quinine treatment for adult travelers with uncomplicated falciparum malaria imported from the tropics. Antimicrob. Agents Chemother. 45:932-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romi, R., G. Sabatinelli, and G. Majori. 2001. Malaria epidemiological situation in Italy and evaluation of malaria incidence in Italian travelers. J. Travel Med. 8:6-11. [DOI] [PubMed] [Google Scholar]
- 10.Romi, R., D. Boccolini, and G. Majori. 2001. Malaria incidence and mortality in Italy in 1999-2000. Euro. Surveill. 6:143-147. [DOI] [PubMed] [Google Scholar]
- 11.Ronn, A. M., J. Ronne-Rasmussen, P. C. Gotzsche, and I. C. Bygbjerg. 1998. Neuropsychiatric manifestations after mefloquine therapy for Plasmodium falciparum malaria: comparing a retrospective and a prospective study. Trop. Med. Int. Health 3:83-88. [DOI] [PubMed] [Google Scholar]
- 12.Sibley, C. H., J. E. Hyde, P. F. Sims, C. V. Plowe, J. G. Kublin, E. K. Mberu, A. F. Cowman, P. A. Winstanley, W. M. Watkins, and A. M. Nzila. 2001. Pyrimethamine-sulfadoxine resistance in Plasmodium falciparum: what next? Trends Parasitol. 17:582-588. [DOI] [PubMed] [Google Scholar]
- 13.Steffen, R. 1991. Travel medicine—prevention based on epidemiological data. Trans. R. Soc. Trop. Med. Hyg. 85:156-162. [DOI] [PubMed] [Google Scholar]
- 14.White, N. J. 1997. Assessment of the pharmacodynamic properties of antimalarial drugs in vivo. Antimicrob. Agents Chemother. 41:1413-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization. 1990. Severe falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 84(Suppl. 2):S1-S65. [PubMed] [Google Scholar]
- 16.World Health Organization. 1996. Management of uncomplicated malaria and the use of antimalarial drugs for the protection of travellers. Report WHO/MAL/96.1075. World Health Organization, Geneva, Switzerland.
- 17.World Health Organization. 2000. Severe falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 94(Suppl. 1):S1-S90. [PubMed] [Google Scholar]