Abstract
The bactericidal activity of gatifloxacin, alone and in combination with isoniazid and rifampin, was studied on both exponential- and stationary-phase cultures of Mycobacterium tuberculosis strain H37Rv. On log-phase cultures, the bactericidal activity of gatifloxacin at 4 μg/ml was rapid and was very similar to that of isoniazid. At concentrations of 0.25 and 4 μg/ml, gatifloxacin enhanced the activity of isoniazid. Killing of the stationary-phase culture was biphasic. During the first 2 days, gatifloxacin at 4 μg/ml slightly increased the limited bactericidal activities of isoniazid and rifampin. However, no further additional bactericidal activity was found during further incubation with isoniazid alone or when gatifloxacin was added to either isoniazid or rifampin. This suggested that the stationary-phase culture contained a mixture of occasionally dividing bacilli that were killed during the first 2 days and true static persisters in the residual population that mimicked those in human lesions. In view of the failure of gatifloxacin to add to the sterilizing activity of isoniazid or rifampin during days 2 to 6 of exposure in the stationary-phase culture, it is unlikely to be a sterilizing drug that can be used to shorten the duration of treatment appreciably when it is added to present treatment regimens.
Tuberculosis continues to be one of the leading causes of death in the world. Although directly observed treatment, short course (DOTS), contributes to the control of the disease, noncompliance with treatment continues to be the main cause of poor results. Even in places where the DOTS strategy is reported to be a success, the main reason cited for noncompliance is the long, 6-month duration of treatment (17). Hence, shortening of the duration of treatment without compromising the cure and relapse rates still remains a major goal for control policies. Evidence that the addition of fluoroquinolones to present treatment regimens might shorten their duration has been provided by studies of experimental murine tuberculosis (14) and by a clinical trial at the Tuberculosis Research Centre, Chennai, India (16). Further clinical trials of fluoroquinolones aimed at shortening treatment, with backing in the United States (14) and from the European Commission (10), are proceeding. Among the fluoroquinolones, those with the greatest activity in tuberculosis are ofloxacin (OFX), levofloxacin, which should act in the same manner, and the newer quinolones such as 8-methoxy derivatives gatifloxacin (GAT) and moxifloxacin. In vitro studies have compared the in vitro bactericidal actions of each of these four quinolones and suggest that among the fluoroquinolones GAT and moxifloxacin have the greatest activities, even though they are still limited, against static bacterial populations of Mycobacterium tuberculosis that might resemble those in human lesions (5).
During the initial phase of chemotherapy, which lasts for about 2 days, bacilli are killed exponentially at a rapid rate, followed by a further lengthy period of much slower exponential killing (7). It is assumed that the bacilli killed in the first 2 days are actively multiplying, while those in the succeeding period are persisters killed by the slower sterilizing activities of the drugs. Drugs differ in their relative bactericidal activities during the initial 2 days, when the activity of isoniazid (INH) predominates, and in their subsequent sterilizing activity, when the activity of rifampin (RIF) predominates (2, 7). An in vitro model of drug action, a 30-day static culture, has been extensively used since 1956 and has been taken to resemble the persister population in its response to drugs (4, 11, 12). The drugs added have the same slow sterilizing action that is responsible for the prolongation of therapy. We compared the activity of GAT, with and without other antituberculosis drugs, on an actively growing log-phase, 2- to 6-day-old culture with that on a static, 30-day culture.
MATERIALS AND METHODS
Test drugs.
INH, RIF, and OFX were purchased from Sigma Chemical Co. (St. Louis, Mo.) and GAT was kindly provided by Dr. Reddy's Laboratory Ltd. (Hyderabad, India). Stock solutions of OFX and GAT were prepared in 0.1 N NaOH, stock solutions of INH were prepared in sterile distilled water, and stock solutions of RIF were prepared in dimethyl formamide. The stock solutions were sterilized by filtration through cellulose membranes with a pore size of 0.22 μm, and further dilutions were then made in sterile distilled water. INH and RIF were tested at final concentrations of 1 μg/ml, OFX was tested at a final concentration of 2 μg/ml, and GAT was tested at final concentrations of 0.25 μg/ml (GAT1) and 4 μg/ml (GAT2).
Exponential-phase and stationary-phase cultures of M. tuberculosis.
M. tuberculosis strain H37Rv was grown in 10 ml of 7H9 medium with Tween 80-albumin-dextrose for 7 days at 37°C. The total number of bacilli per milliliter in this growth medium was determined with a Thoma counting chamber. Two Erlenmeyer flasks, each of which contained 400 ml of fresh 7H9 culture medium, were stoppered with paper-wrapped bungs to reduce the level of oxygen tension but not to exclude it. The culture medium was then inoculated with an appropriate volume of the 7-day culture to give a concentration of 105 bacilli per ml. One of the flasks was incubated at 37°C for 3 days (log-phase culture), while the other flask was incubated at 37°C for 4 weeks (stationary-phase culture), during which time the flask was left undisturbed and growth continued under a 4- to 5-cm layer of medium (4).
Bactericidal actions of the drugs.
The log-phase and stationary-phase cultures of M. tuberculosis were distributed without dilution or concentration in 10-ml aliquots into 28-ml screw-cap McCartney bottles (day 0). About 1 h later, the drugs were added to duplicate aliquots of these cultures at the following final concentrations: INH, 1 μg/ml; RIF, 1 μg/ml, OFX, 2 μg/ml (4); GAT, 0.25 μg/ml (GAT1), the MIC for M. tuberculosis (15); and GAT, 4 μg/ml (GAT2), the peak concentration attainable in serum with the present dosage of 400 mg used for the treatment of human disease (3). The two GAT concentrations were tested alone and in combinations as INH-GAT, RIF-GAT, and INH-RIF-GAT. Drug-free control cultures were included. On day 3 the drugs were replenished with 50% of the original quantity for INH and RIF, 10% of the original quantity for OFX, and 25% of the original quantity for GAT to compensate for the losses during the 3 days of incubation, as estimated from standardization experiments (4). On days 0, 2, 4, and 6, serial 10-fold dilutions of these cultures were inoculated on duplicate plates of selective 7H11 agar medium (13) containing polymyxin B (200 U/ml), amphotericin B (20 μg/ml), carbenicillin (100 μg/ml), and trimethoprim (10 μg/ml) to determine CFU counts. The plates were placed in polyethylene bags and incubated at 37°C. The colonies were counted after 2 and 4 weeks of incubation (4).
Part of the experiment with log-phase cultures was repeated with CFU counts determined at 2, 6, 12, 24, 36, 48, and 72 h, since no CFU was observed after 2 days in the presence of INH alone or INH in combination with GAT.
Statistics.
The results were expressed as the mean of the duplicate experiments at each time point. Differences in the regression coefficients of the log CFU counts with different drug combinations were tested by analysis of variance by using test command in Stata software (release 8; Stata Corp., College Station, Tex.). The standard deviation of a result was obtained from the variation between the CFU counts for the duplicate cultures, estimated separately for the log-phase and the stationary-phase cultures. Counts that yielded no colonies in either of the duplicates were excluded.
Graphing.
No adequate representation on a logarithmic axis of the CFU count could be made for counts that yielded no colonies since log 0 is minus infinity. A line was therefore drawn to extrapolate the values obtained at the two previous time points, provided that it cut the x axis to the left of the time point that yielded no colonies. Otherwise, the line was drawn through log 0. In each case, the line concerned has been drawn dotted in Fig. 1 to 4 to indicate the uncertainty in its true position. Counts after the first negative count always failed to yield colonies, and their values have not been entered in the figures.
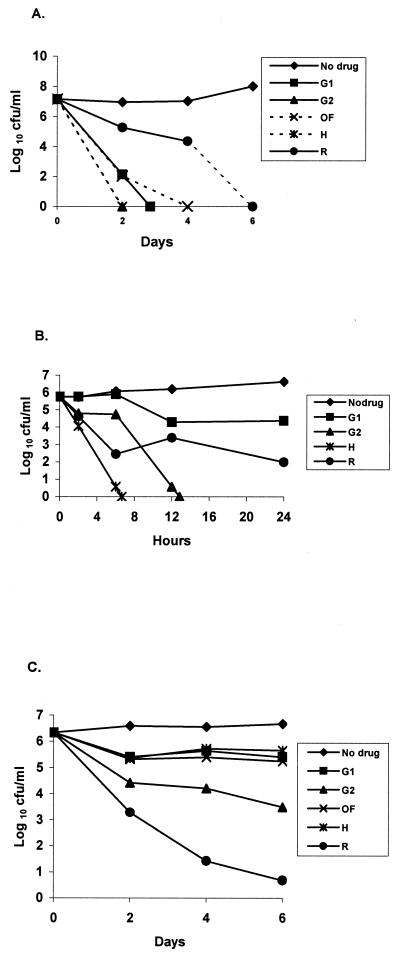
FIG. 1.
Bactericidal activities of INH at 1 μg/ml, RIF at 1 μg/ml, OFX at 2 μg/ml, GAT1 at 0.25 μg/ml, and GAT2 at 4 μg/ml (G2) against exponential-phase (A), log-phase (B), and stationary-phase (C) cultures of M. tuberculosis. H, INH; R, RIF; G1, GAT1; G2, GAT2; and OF, OFX.
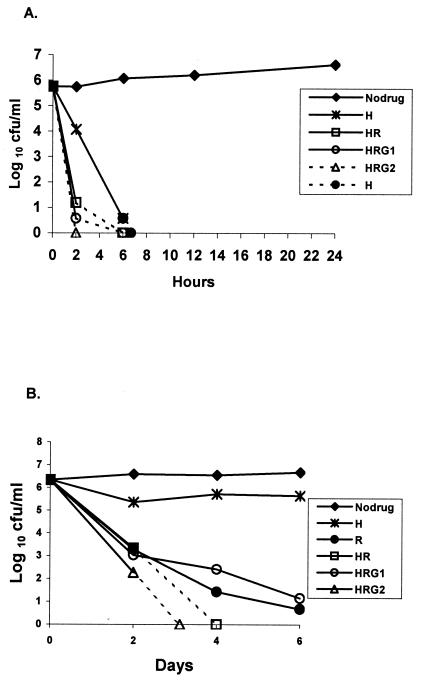
FIG. 4.
Bactericidal activities of INH, RIF, and INH-RIF with GAT1 and GAT2 against log-phase (A) and stationary-phase (B) cultures of M. tuberculosis. See Fig. 1 for drug concentrations. H, INH; R, RIF; HR, INH-RIF; HRG1, INH-RIF-GAT1; and HRG2, INH-RIF-GAT2.
RESULTS
The results of the bactericidal activities of different drugs from the two sets of experiments, one on the exponential-phase culture and the other on the stationary-phase culture, are given in successive figures, each of which describes the different comparisons of individual drugs within the same experiment. The standard deviation of a count was estimated to be 0.125 log10 (on the basis of 13 degrees of freedom) with the log-phase cultures and 0.126 log10 (on the basis of 44 degrees of freedom) with the stationary-phase cultures.
Activities of individual drugs.
The 6-day CFU counts from the log-phase culture are shown in Fig. 1A, and those from the stationary-phase cultures are shown in Fig. 1C, with the results of the repeat 24-h experiment on the log-phase culture shown in Fig. 1B. The drug-free controls showed, as expected, continuous growth in the log-phase culture but no increase in CFU in the stationary-phase culture. In the log-phase culture, INH was the most bactericidal, GAT2 was slightly less active, GAT1 and OFX were less active still, and RIF was the least active (P < 0.001). In the stationary-phase culture, RIF showed the highest activity (P < 0.001), with counts decreasing throughout the experiment. During the first 2 days, GAT2 was the most bactericidal, followed by INH, GAT1, and OFX (P < 0.001). However, none of these four drugs was bactericidal during the succeeding 4 days. This suggests that the stationary-phase culture contained two bacterial populations: a majority population (population A) of bacilli that had relatively high levels of metabolism with occasional multiplication and the remaining population (population B) of bacilli with truly static, low-level metabolism.
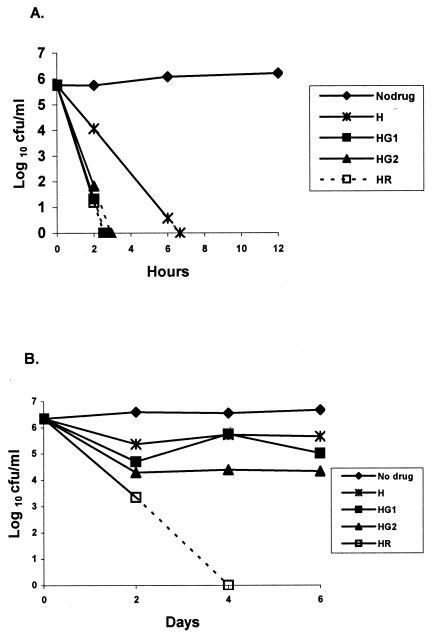
Effect of addition of GAT to INH.
The effect of combinations of GAT with INH on the log-phase culture is shown in Fig. 2A. GAT at both concentrations augmented the bactericidal activity of INH. Negative counts were obtained with GAT1-INH in just over 6 h but were obtained with GAT2-INH in less than 3 h. With the stationary-phase culture (Fig. 2B), INH-GAT1 (P < 0.05) was more active than INH alone and GAT2-INH (P < 0.001) was slightly more bactericidal. However, bactericidal activity with all three combinations occurred during the first 2 days and was not evident subsequently. The most bactericidal combination was INH-RIF, whose activity continued even after day 2.
FIG. 2.
Bactericidal activities of INH alone and INH with RIF or GAT1 and GAT2 against log-phase (A) and stationary-phase (B) cultures of M. tuberculosis. See Fig. 1 for drug concentrations. H, INH; HG1, INH-GAT1; HG2, INH-GAT2; and HR, INH-RIF.
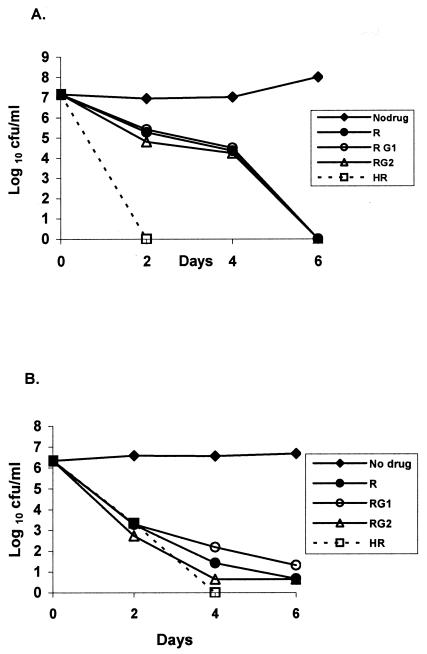
Effect of addition of GAT to RIF.
With the log-phase culture (Fig. 3A), combinations of GAT1 and GAT2 with RIF did not increase the activity of RIF alone. Indeed, RIF appeared to antagonize the bactericidal activity of GAT considerably, since the counts were 4.50 log10 CFU/ml (P < 0.001) with GAT1-RIF and 4.24 log10 CFU/ml (P < 0.001) with GAT2-RIF, while negative counts were obtained at 4 days with both GAT1 and GAT2 alone (Fig. 1A). INH-RIF was far more bactericidal. With the stationary-phase culture (Fig. 3B), there again appeared to be slight antagonism when GAT1 was added to RIF and a marginal increase in activity during the first 2 days only when GAT2 was added to RIF.
FIG. 3.
Bactericidal activities of RIF alone and RIF with INH or GAT1 and GAT2 against log-phase (A) and stationary-phase (B) cultures of M. tuberculosis. See Fig. 1 for drug concentrations. R, RIF; RG1, RIF-GAT1; RG2, RIF-GAT2; and HR, INH-RIF.
Effect of addition of GAT to INH and RIF.
There is only a suggestion (nonsignificant) that the addition of GAT1 and GAT2 to INH-RIF slightly increased the bactericidal action on the log-phase culture (Fig. 4A). With the stationary-phase culture (Fig. 4B), the addition of GAT1 to INH-RIF did not alter the activity. The addition of GAT2 increased the activity during the first 2 days (P < 0.001), but no conclusion could be drawn beyond 2 days, since negative cultures were obtained.
DISCUSSION
GAT was remarkably bactericidal against log-phase organisms, second only to INH. When GAT was added to INH it greatly increased the bactericidal activity when it was added at both the high concentration and the low concentration. However, RIF substantially inhibited the activities of GAT at both concentrations (P < 0.001). With the stationary-phase culture, the effects during the first 2 days must be distinguished from those during the next 4 days. During the first 2 days, INH was slightly bactericidal, but it had no further bactericidal activity during days 2 to 6. Since INH is a cell wall antagonist, these findings suggest that the majority bacterial population (population A) in the stationary culture was occasionally dividing and was killed during the first 2 days, leaving a remainder population (population B) insusceptible to INH because it was no longer dividing. In contrast, RIF continued to kill the bacilli during the entire 6-day period, although it did so more slowly during days 4 to 6 (Fig. 1C). This suggests that the bacilli in population A had higher metabolic rates than those in population B, since the rate of killing by RIF was slowed by factors that reduced bacillary metabolism. By considering first the effects of GAT on population A during the first 2 days, GAT increased the bactericidal activities of INH (P < 0.001) (Fig. 2B) and RIF (P < 0.001) (Fig. 3B). However, during the succeeding 4 days, GAT was not bactericidal alone on population B (Fig. 1C) and failed to demonstrate any such synergistic action. It did not significantly alter the activity of INH (Fig. 2B) or RIF (Fig. 3B), and no assessment during days 2 to 6 could be made when it was added to INH-RIF (Fig. 4B), since negative cultures were obtained after day 2.
To what extent do these findings reflect on the probable response of pulmonary tuberculosis to regimens that incorporate GAT? Are the responses to drugs of the bacilli in the log-phase and the stationary-phase cultures similar to those of bacilli in human lesions? The first study of early bactericidal activity measured the changes in CFU counts of M. tuberculosis in sputum during the treatment of patients with pulmonary tuberculosis with 22 different combinations of INH, RIF, pyrazinamide, ethambutol, and streptomycin (8). A recent reanalysis of the data showed that there was an initial 2-day period when INH was the predominant drug that killed the bacilli at the fastest rate of about log10 0.6 CFU/day and that the activity of INH was unaffected by the other drugs in the regimen (7). In contrast, regimens with RIF, but without INH, killed the bacilli at a lower rate of about log10 0.3 CFU/day (7). Actively growing organisms are thought to be killed during this initial phase. These findings are in substantial agreement with our findings on the log-phase culture, which showed that INH was the most bactericidal drug and that RIF was less so. After the first 2 days, the rate of killing in sputum slowed substantially, to about log10 0.12 CFU/day. At this time the organisms are thought to be persisters, and the predominant bactericidal drug changed to RIF, while INH no longer appeared to contribute to bactericidal activity. This again mirrors the relative activities of RIF and INH in the stationary-phase culture. Thus, the behavior of the log-phase culture reflects the activities of different drugs during the first 2 days of therapy, while the behavior of the stationary-phase culture reflects the activities of different drugs during subsequent treatment.
We must now see whether population A or population B in the stationary-phase culture most resembles the persisters found in human lesions. There is substantial evidence that INH has no bactericidal activity in regimens that start with four drugs and that continue with RIF plus INH. We have already mentioned the absence of any effect of INH in the first early bactericidal activity study. Clinical trials also provide evidence. Thus, in such regimens (conventionally referred to as 2SHRZ/RIF+INH [2 ethambutol-INH-RIF-pyrazinamide]) in eight trials, relapses occurred in 5.2% of 1,225 patients infected with initially sensitive strains and in 8.2% of 61 patients with initially infected with resistant strains, which is a nonsignificant difference (9). In a recent trial (with a 2EHRZ/RIF+INH [2 streptomycin-INH-RIF-pyrazinamide] regimen) the corresponding proportions were 3.7% of 190 patients and 4.0% of 23 patients, respectively (6). In contrast to these findings with INH, bactericidal action throughout chemotherapy is due entirely to RIF, together with pyrazinamide in the initial phase (9). This continuing action of RIF is also shown in its bactericidal action on the stationary-phase culture throughout the 6-day period (Fig. 1C). While no assessment has yet been made of the gene expression states of M. tuberculosis in sputum, the slow bactericidal activity of INH on population A and the complete absence of such activity on population B strongly suggest that it is population B that resembles the lesional persisters. If this is so, our findings show that addition of GAT does not appreciably increase the sterilizing activity of INH or RIF, although we have not been able to assess its activity against both drugs together, nor have we been able to assess the activity of GAT compared with those of regimens with pyrazinamide.
In summary, our results with GAT show that it has many of the same features as INH, particularly strong bactericidal activity against multiplying organisms but very limited sterilizing activity against persisters. This suggests that, like INH, it would be effective in retreatment regimens that do not contain RIF, since in regimens without RIF, INH is a slowly sterilizing drug (9). The concept is in agreement with assessments of retreatment regimens in murine tuberculosis (1).
Acknowledgments
We thank P. R. Narayanan, Director, and the staff members of the Bacteriology Department, Tuberculosis Research Centre, for valuable help with the conduct of this study. We gratefully acknowledge the help of F. A. Sirgel, South African National Tuberculosis Programme, Tygerberg, South Africa, and Michael H. Cynamon, Veterans Affairs Medical Centre, Syracuse, N.Y., in preparing the manuscript.
REFERENCES
- 1.Cynamon, M. H., and M. Sklaney. 2003. Gatifloxacin and ethionamide as the foundation for therapy of tuberculosis. Antimicrob. Agents Chemother. 47:2442-2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dickinson, J. M., and D. A. Mitchison. 1981. Experimental models to explain the high sterilizing activity of rifampin in the chemotherapy of tuberculosis. Am. Rev. Respir. Dis. 123:367-371. [DOI] [PubMed] [Google Scholar]
- 3.Grasela, D. M. 2000. Clinical pharmacology of gatifloxacin, a new fluoroquinolone. Clin. Infect. Dis. 31:S51-S58. [DOI] [PubMed] [Google Scholar]
- 4.Herbert, D., C. N. Paramasivan, P. Venkatesan, G. Kubendiran, R. Prabhakar, and D. A. Mitchison. 1996. Bactericidal action of ofloxacin, sulbactam-ampicillin, rifampin, and isoniazid on logarithmic- and stationary-phase cultures of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 40:2296-2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu, Y., A. R. M. Coates, and D. A. Mitchison. 2003. Sterilizing activities of fluoroquinolones against rifampin-tolerant populations of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 47:653-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jindani, A., A. J. Nunn, and D. E. Enarson. 2004. Two 8-month regimens of chemotherapy for treatment of newly diagnosed pulmonary tuberculosis: international multicentre randomized trial. Lancet 364:1244-1251. [DOI] [PubMed] [Google Scholar]
- 7.Jindani, A., C. J. Doré, and D. A. Mitchison. 2003. The bactericidal and sterilizing activities of antituberculosis drugs during the first 14 days. Am. J. Respir. Crit. Care Med. 167:1348-1354. [DOI] [PubMed] [Google Scholar]
- 8.Jindani, A., V. R. Aber, E. A. Edwards, and D. A. Mitchison. 1980. The early bactericidal activity of drugs in patients with pulmonary tuberculosis. Am. Rev. Respir. Dis. 121:939-949. [DOI] [PubMed] [Google Scholar]
- 9.Mitchison, D. A. 2000. Role of individual drugs in the chemotherapy of tuberculosis. Int. J. Tuberc. Lung Dis. 4:796-806. [PubMed] [Google Scholar]
- 10.Mitchison, D. A. 2004. Fluoroquinolones in the treatment of tuberculosis: a study in mice. Am. J. Respir. Crit. Care Med. 169:334-335. [DOI] [PubMed] [Google Scholar]
- 11.Mitchison, D. A. 1992. The Garrod Lecture. Understanding the chemotherapy of tuberculosis—current problems. J. Antimicrob. Chemother. 29:477-493. [DOI] [PubMed] [Google Scholar]
- 12.Mitchison, D. A., and J. B. Selkon. 1956. The bactericidal activities of antituberculous drugs. Am. Rev. Respir. Dis. 74S:109-116. [DOI] [PubMed] [Google Scholar]
- 13.Mitchison, D. A., B. W. Allen, L. Carrol, J. M. Dickinson, and V. R. Aber. 1971. A selective oleic acid albumin agar medium for tubercle bacilli. J. Med. Microbiol. 5:165-175. [DOI] [PubMed] [Google Scholar]
- 14.O'Brien, R. J. 2003. Development of fluoroquinolones as first-line drugs for tuberculosis—at long last! Am. J. Respir. Crit. Care Med. 168:1-2. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez, J. C., M. Ruiz, M. Lopez, and G. Royo. 2002. In vitro activity of moxifloxacin, levofloxacin, gatifloxacin and linezolid against Mycobacterium tuberculosis. Int. J. Antimicrob. Agents 20:464-467. [DOI] [PubMed] [Google Scholar]
- 16.Tuberculosis Research Centre, Indian Council of Medical Research, Chennai. 2002. Shortening short course chemotherapy. A randomised clinical trial for treatment of smear positive pulmonary tuberculosis with regimens using ofloxacin in the intensive phase. Indian J. Tuberc. 49:27-38. [Google Scholar]
- 17.World Health Organization. 2002. Global tuberculosis control: surveillance, planning and financing. World Health Organization, Geneva, Switzerland.