Abstract
AIM
To examine the incidence and trends in pediatric inflammatory bowel diseases (IBDs) over 2000-2015 and project the incidence to 2018.
METHODS
A 16-year prospective study of IBD patients < 19 years of age was conducted in the Czech Republic (the Pilsen region). All incident IBD cases within a well-defined geographical area were retrieved from a prospectively collected computerized clinical database. Historical Czech data were used for comparison (1990-2001). Our catchment population was determined from the census data. We calculated the incidence by relating the number of newly diagnosed cases to the size of the pediatric population-at-risk in each calendar year. Age/sex, disease type, place of residence, and race/ethnicity were identified.
RESULTS
In total, 170 new IBD cases [105 Crohn’s disease (CD), 48 ulcerative colitis (UC), and 17 IBD-unclassified (IBD-U)] were identified. The median age at IBD diagnosis was 14.2 years, 59.4% were males, and 97.1% were Caucasians. A male preponderance of IBD (P = 0.026) and CD (P = 0.016) was observed. With 109209 person-years in the catchment area, the average incidence of IBD per 100000 person-years was 10.0 (6.2 for CD, 2.8 for UC, and 1.0 for IBD-U) for children aged 0 to 19 years; for those aged 0 to 15 years, the incidence rate was 7.3 (4.6 for CD, 2.0 for UC, and 0.7 for IBD-U). An increase in incidence with age was observed (P = 0.0003). Over the 16-year period, the incidence increased for IBD patients (P = 0.01) and CD in particular (P < 0.0001), whereas the incidence for UC (P = 0.09) and IBD-U (P = 0.339) remained unchanged. IBD-projected data from 2016 to 2018 were 12.1, 12.3 and 12.6 per 100000 person-years, respectively.
CONCLUSION
Pediatric-onset IBD incidence is around its highest point. The increase, which is particularly pronounced for CD, may be challenging to relate to causes of pediatric disease.
Keywords: Inflammatory bowel disease, Incidence, Children, Czech Republic, Pilsen region, Projections, Crohn’s disease, Ulcerative colitis
Core tip: The incidence of inflammatory bowel diseases (IBDs) is around its highest point to date. It has been markedly rising over a 16-year period and is especially pronounced for Crohn’s disease (CD), such that CD is now more common than ulcerative colitis and IBD-unclassified. The changes in IBD incidence in developed countries cannot be explained by changes in genetic background, but the influence of environmental hazards on incidence may be involved in the pathogenesis of IBD. Analyses of time trends and the implications of environmental determinants are required to unravel concurrent factors and causal relationships with the ultimate goal of improving the current care of these patients.
INTRODUCTION
The group of life-long, chronic relapsing inflammatory bowel diseases (IBDs) of unknown origin encompasses three separate disorders: Crohn’s disease (CD), ulcerative colitis (UC), and IBD-unclassified (IBD-U). Genetic and environmental factors should be considered for IBD in childhood[1]. Approximately 25% of all diagnoses are made during childhood or adolescence[2], but a shift toward a younger age (i.e., < 6 years old) has been observed[3].
IBD is unevenly distributed throughout the world. Studies worldwide have shown a rising but variable incidence of IBD, including large increases in incidence in pediatric populations[4,5]. Pediatric IBD incidence rates are higher in North America, the United Kingdom, and northern and western Europe than in southern latitudes[6-10]. Studies that evaluated only the incidence of pediatric-onset IBD with a wide range of incidence rates have been published previously[2]. A recent analysis also identified an east-west gradient in the incidence of IBD in Europe[11].
Although no firm conclusions can be drawn with respect to addressing the rising incidence of IBD, differences in geographic distribution, and particularly in changes in incidence over time within one area, may provide new insights into concurrent etiological factors[12-15]. However, this is possible only when enough pediatric projects within one defined geographical area, in relatively homogeneous populations, provide a fundamental basis for a better understanding of the epidemiology and environmental influences in any geographically restricted pediatric population and for the assessment of IBD.
Despite a rising worldwide incidence of IBD, there is a paucity of recent information regarding the exact incidence of IBD in Czech children[16,17]. Thus, it is of great interest to explore temporal time trends with an emphasis on children in central and eastern Europe and to describe differences among geographical regions across Europe. Thus, there are conflicting data on the rate at which IBD is diagnosed and whether its incidence has declined, stabilized, or even continued to rise in Czech children. Furthermore, no studies of IBD incidence have been published using data from the past 15 years; therefore, current time trends in Czech children remain entirely unknown.
Building on our previous experience[16], the objectives of this study were: (1) to discern the current incidence of IBD using a pediatric population (< 19 years of age at diagnosis) residing in a well-defined geographical region of the Czech Republic (Figure 1); (2) to characterize differences by age and sex; and (3) to gain insights into recent trends from 2000 to 2015 and compare our results to historical incidence data from comparable Czech studies (1990-2001)[16]. A further aim was to recognize projections for future occurrence rates from the same geographical area.
Figure 1.
Map of the catchment area, the geographical area under investigation for the Czech pediatric inflammatory bowel disease study over the period of 2000-2015. Outline of the Pilsen region (red) in the Czech Republic (orange) situated in central Europe.
MATERIALS AND METHODS
Study setting
The study area covered the Pilsen region (western Bohemian region of the Czech Republic) as the catchment area (Figure 1), corresponding well to the geographical distribution of the pediatric population. The Pilsen region is one of the 14 regional administrative units in the Czech Republic (http://en.plzensky-kraj.cz/en/kategorie/pilsen-region). The child population was estimated to account for approximately 14.3% of the total population in that region (January 2015). The catchment area is the third largest region in the Czech Republic and the ninth most populous. The region area encompasses 7125 km2, having a population of approximately 577538 inhabitants (approximately 6% of the total population of the Czech Republic, 2015, Czech census data), including seven counties. The mean population density (population per square kilometer) in the entire region ranged from 52 to 1178 among the counties, with the pediatric population distributed evenly throughout all counties.
The structural settlement of the region is unbalanced. The metropolitan area of Pilsen is connected to small rural areas, whereas mid-sized towns are largely lacking. Approximately one-third of the population resides in Pilsen, and the rest is in semi-urban and rural areas. The large number of small settlements is a typical feature of the area. More than four out of five municipalities in the region have fewer than 2000 residents, and more than 30% of the region’s population resides in such small towns and villages. However, the city of Pilsen is the natural center of the region; it is currently the fourth largest city in the Czech Republic, with approximately 200000 inhabitants.
All children in our country are insured and thus have free access to health care. The Pilsen region is included in the Czech National Health System and provides universal health insurance for its residents, including free-of-charge coverage for general practitioners and hospital services. In the Czech Republic, the treatment of pediatric IBD is conducted in a tertiary hospital setting and is free for all inhabitants. Thus, the study area was well delineated as consistent with the health care system in the Czech Republic, which dictates that all children and adolescents in a specific municipality should be referred to a tertiary pediatrician gastroenterology center for the diagnosis/treatment of IBD.
Thus, as a part of a tertiary referral teaching hospital, our gastroenterology center services the whole pediatric population (< 19 years) in the catchment area. Very few, if any, children are diagnosed or treated by adult gastroenterologists in the catchment area by 19 years of age. We believe that the vast majority of pediatric IBD cases would have been referred to our IBD single center, particularly older adolescents, who were probably not diagnosed by adult gastroenterologists.
Study population and data collection
This clinical population-based prospective regional cohort consisted of children and adolescents with new onset IBD (< 19 years old at the time of diagnosis) whose residential addresses were in the Pilsen region. The study cohort included children from the same geographic region who were living in different settings (rural vs urban). Only unequivocal IBD cases were captured upon diagnosis over a 16-year period (between January 1, 2000 and December 31, 2015).
The following demographic elements were available: age, sex, race/ethnicity, place of residence, and Czech census region. Patient data were entered consistently with the first diagnosis; thus, if subjects were diagnosed initially with IBD-U and subsequently reclassified to CD and UC, the former was considered the most accurate diagnosis. Any surgical intervention needed at the time of diagnosis was also recorded. The geographical unit of a person’s residence was used to determine the place of county residence; all participating children were presumably of Czech ethnicity, which is a Caucasian population. Patients were excluded if they originated from a city outside the catchment area.
We sampled a geographically restricted pediatric population with documented IBD who were referred to a tertiary referral teaching hospital in the Czech Republic (Charles University, Faculty Hospital in Pilsen, Department of Pediatrics, Pediatric Gastroenterology Unit). This is the only tertiary pediatric center in the Pilsen region that can treat this population, and all pediatric gastroenterologists in the catchment area throughout the period were based in this hospital. Additionally, all pediatric departments within the area collaborated on the project.
Detailed information from the diagnostic work-up data for all subjects, including data from the first attendance and from the hospital discharge records, were prospectively collected from an in-house computerized clinical database (patient registries). The data on patients were collected using a data form completed by gastroenterologists. The system has been developed in cooperation with the University of West Bohemia in Pilsen, Faculty of Applied Sciences. The application, which has a common client service architecture, provides the means to collect the data both manually via structured web forms and automatically from other medical systems using the Czech data standard for the exchange of medical data (DASTA). Fully automatic data interchange between the application and hospital information system reduces the possibility of human error during data entry, thus improving the data accuracy and quality.
All data from the work-ups were present in the central dataset, forming the data network with high quality, and complete detailed information was linked at a subject level. For this reason, there was presumably no bias in the sampling or selection procedure. The size of the pediatric population (< 19 years old) and age- and sex-stratified population data were determined from census data collected between 2000 and 2015.
Diagnosis and disease subclassification
All IBD patients were diagnosed according to clinical history, physical examination, laboratory and serological testing, radiologic studies, and endoscopic appearance with stepwise biopsy for review by clinical pathologists[18]. The date of initial diagnosis was set as the day of the definitive diagnosis made during our study period, as evidenced by diagnostic evaluation or surgical findings consistent with IBD rather than symptom onset. The new IBD group was subdivided into three main clinical types: CD, UC, and IBD-U. If a distinction between UC and CD could not be determined based on these criteria, cases that were not otherwise specified in the presence of nonspecific inflammation were designated as IBD-U. The subjects without firm evidence of IBD were excluded from further analyses.
Statistical analysis
Standard descriptive statistics were used to summarize the characteristics of the entire population. The incidence rates were expressed as new cases per 100000 pediatric persons per year by dividing the observed number of ascertained new cases in a specified time period (numerator) by the size of the resident pediatric populations under effective observation from January 1, 2000 to December 31, 2015 (denominator). The data were analyzed for each time period and stratified by age, sex, and Czech census region. The IBD patients were analyzed in two separate categories with respect to age: < 10 years and 11-19 years. The age of the IBD patients was expected to have a skewed distribution because the median was used. Incidence trends and time projections of IBD and its subtypes with the number of cases as the dependent variable, and population size as the offset variable, were estimated using linear regression analyses and correlation coefficients. Student’s t-test (unpaired, 2-tailed) and stratified analysis reflecting age and sex were used to measure differences between the groups. A P-value < 0.05 and precise 95%CI were calculated to evaluate the significance and precision of the estimate.
RESULTS
Demographics and IBD incidence
The demographic characteristics are shown in Table 1. As of 2015, the data from 170 incident patients (< 19 years), including 96 subjects (< 15 years of age at diagnosis) with pediatric-onset IBD in the Pilsen region (since 2000), were entered into the electronic databases. Of the entire group, 59.4% were males. The median age in years was 14.2 (range: 1.4-18.3) for IBD, 14.1 (1.4-18.1) for CD, 14.6 (2.7-18.3) for UC, and 14.1 (2.5-17.7) for IBD-U. The median age at diagnosis did not differ between CD and UC, and there were no significant differences between the age at onset in boys and girls. The self-reported racial/ethnic distributions were 97.1% Caucasian and 2.9% other.
Table 1.
Demographics of newly diagnosed pediatric patients (n = 170) less than 19 years of age in the Pilsen area from January 1, 2000 to December 31, 2015
| CD | UC | IBD-U | IBD | |
| Total patients, n | 105 | 48 | 17 | 170 |
| Male | 68 | 24 | 9 | 101 |
| Female | 37 | 24 | 8 | 69 |
| Age in years, median (range) | 14.1 (1.4-18.1) | 14.6 (2.7-18.3) | 14.1 (2.5-17.7) | 14.2 (1.4-18.3) |
Data reported for the entire IBD population, CD, UC and IBD-U. IBD: Inflammatory bowel disease; CD: Crohn’s disease; UC: Ulcerative colitis; IBD-U: Inflammatory bowel disease-unclassified.
The number of cases diagnosed each year in the inclusion period is shown in Figure 2. Of the new cases, 105 (61.8%) were CD, 48 (28.2%) were UC and 17 (10%) were IBD-U. There was a predominance of CD (the CD to UC ratio was 2.2:1). Of the 17 subjects with IBD-U, the initial IBD-U diagnosis was changed in 13 (76.5%), with 7 (41.2%) reclassified to UC and 6 (35.3%) reclassified to CD during the follow-up. During the 16-year study period, there were 1709209 person-year observations aged < 19 years in the catchment area, summarizing the source population corresponding to 170 children who received diagnoses of IBD. Among the 0- to 15-year-olds, the overall incidence was 7.3 per 100000 person-years (95%CI: 6.1-8.5) for IBD, 4.6 (95%CI: 3.7-5.5) for CD, 2.0 (95%CI: 1.3-2.7) for UC and 0.7 (95%CI: 0.1-1.3) for IBD-U. Among 0- to 19-year-olds, the respective incidences were 10.0 (95%CI: 9.2-10.9), 6.2 (95%CI: 4.7-7.7), 2.8 (95%CI: 1.5-4.1) and 1.0 (95%CI: 0.2-1.8).
Figure 2.
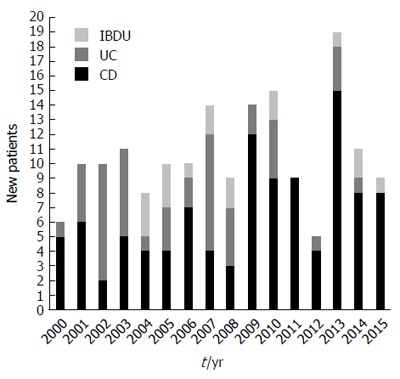
Bar graph showing the total annual numbers of newly diagnosed Crohn’s disease, ulcerative colitis and inflammatory bowel disease-unclassified individuals (< 19 yr) in the Pilsen region, 2000-2015. CD: Crohn’s disease; UC: Ulcerative colitis; IBD-U: Inflammatory bowel disease-unclassified.
IBD type by age group
Figure 3 shows the age-related incidence rates of IBD, CD, UC and IBD-U. The diagnosis was most commonly made after the age of 10 years for IBD; the age at diagnosis was less than 10 years in 30 children (17.6%). Early presentation, before the age of 6 years, was observed in 0.58%, 4.1%, 0.58% and 5.29% of children with CD, UC, IBD-U and IBD respectively. The overall incidence of IBD rose from 3.5 (95% CI: 2.5-4.3) per 100000 person-years among 0- to 10-year-olds to 16.6 (95%CI: 12.5-20.1) per 100000 person-years in 11- to 19-year-olds (P = 0.0003).
Figure 3.
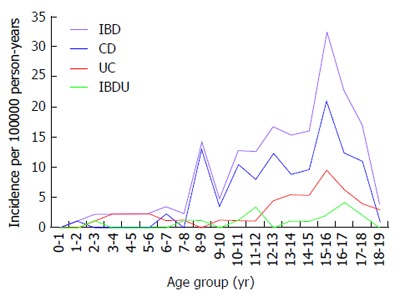
Age-specific incidence of all new-onset pediatric inflammatory bowel disease, Crohn’s disease, ulcerative colitis, and inflammatory bowel disease-unclassified per 100000 person-years (< 19 yr) in the Pilsen region, 2000-2015. The incidence of IBD significantly increased with age (P = 0.0003). IBD: Inflammatory bowel disease; CD: Crohn’s disease; UC: Ulcerative colitis; IBD-U: Inflammatory bowel disease-unclassified.
The highest age-related occurrence for IBD was observed in the 15-year-old age group. Likewise, significantly higher incidence rates were observed for CD (P = 0.0006), UC (P = 0.002) and IBD-U (P = 0.01) respectively in 11- to 19-year-olds. The age-standardized incidence was significant for both males and females in IBD (P = 0.001 and P = 0.01 respectively), CD (P = 0.006 and P = 0.01 respectively) and UC (P = 0.02 and P = 0.001 respectively), whereas it reached marginal statistical significance for IBD-U (P = 0.047 and P = 0.058 respectively). No differences were found in the age distribution of subjects among CD, UC and IBD-U in 0-to 10-year-olds, whereas the older (11 to 19 years old) group had a significantly increased incidence of CD compared with UC and IBD-U (P < 0.001).
Effect of sex
Over the 2000-2015 period, 105 patients (males/females: 68/37) were diagnosed with pediatric-onset CD, and 48 subjects with UC (males/females: 24/24) and 17 patients with IBD-U (males/females: 9/8) were diagnosed at < 19 years of age. Among all cases of IBD, 59.4% were males. The differences in sex were significant only for IBD (especially CD). More boys than girls suffered from CD. The average IBD incidence rate per 100000 person-years was 11.6 (95%CI: 9.4-14.1) for males and 8.3 (95%CI: 6.8-10.1) for females (P = 0.026). The incidence of CD was 7.8 (95%CI: 6.2-9.8) for males and 4.5 (95%CI: 2.8-4.1) for females (P = 0.016). We found an equal sex ratio in UC and IBD-U. The incidence of UC was 2.7 (95%CI: 2.1-3.1) for males and 2.9 (95%CI: 2.3-3.4) for females (P = 0.44). The incidence of IBD-U was 1.0 (95%CI: 0.8-1.5) for males and 1.0 (95%CI: 0.8-1.5) for females (P = 0.45).
IBD incidence trends between 2000 and 2015
Trends in the population under observation over the 16-year period were determined (Figure 4A-C). Incidence rates were analyzed in 1-year blocks. When analyzing the 0- to 15-year-old group, we observed no statistically significant changes in incidence for the whole group of IBD (r = 0.15, P = 0.09), IBD-U (r = 0.04, P = 0.213) or CD (r = 0.13, P = 0.109). Despite the consistent decreasing trends in incidence rates of UC, this analysis failed to reveal any statistical significance (r = - 0.01, P = 0.810). The change in incidence over the 16 years of the study period was statistically significant in the 0- to 19-year-old group in the examined region.
Figure 4.
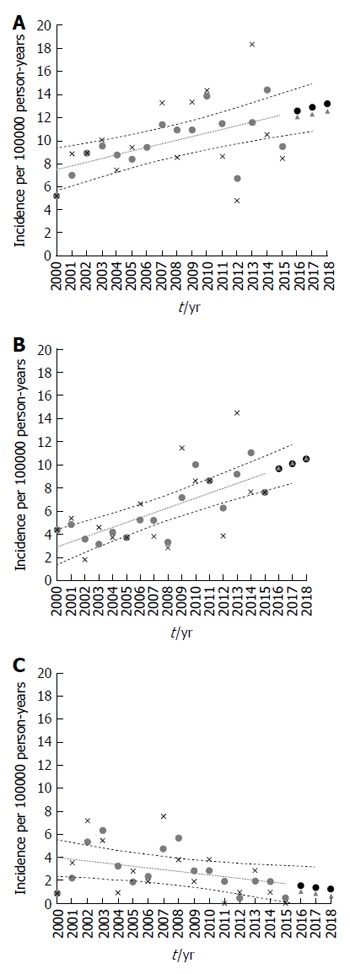
Scatter plots of the data. The average annual age-standardized incidence rate per 100000 person-years by age group (< 19 years) and the 16-year trend for inflammatory bowel disease (A), Crohn’s disease (B), and ulcerative colitis (C) in the Pilsen region over the period of 2000-2015 in the population under observation and the incidence rate projected for 2016-2018. The points are the actual data; Stars indicate the incidence of IBD (A), CD (B) and UC (C) per 100000 person-years; Blue points indicate calculating the moving average; Dotted blue line indicates a fitted linear regression model to predict trends; Triangles indicate the incidence rate projected for 2016-2018; Red points indicate calculating the moving average. There was a significant increase in incidence over the 16-year period (A: r = 0.32, P = 0.012; B: r = 0.42, P < 0.0001; C: r = - 0.15, P = 0.134).
Over the study period, the overall incidence rate of IBD and CD rose significantly (r = 0.32, P = 0.012 and r = 0.42, P < 0.0001 respectively) and showed a male preponderance. Apart from a non-significantly higher point estimate for 2000-2004, the incidence of UC decreased without reaching statistical significance (r = - 0.15, P = 0.134), and the overall rates of IBD-U remained fairly stable (r = 0.05, P = 0.341). The incidence rate of IBD increased over the study period to 5.8 (95%CI: 3.6-8.6) per 100000 person-years for girls and 11.1 (95%CI: 7.1-14.5) for boys in 2015. Similarly, the incidence rate of CD increased over the study period to 5.8 (95%CI: 3.4-9.1) per 100000 person-years for girls and 9.4 (95%CI: 7.1-13.4) for boys in 2015. In contrast, the incidence of UC decreased to 0 per 100000 person-years for both girls and boys in 2015. The most pronounced increase was observed in CD among adolescents aged 12-19 years (P = 0.001). For children less than 10 years of age, the rate remained low, limited by too few young children for the analysis in consecutive years of observations (2000-2015).
Compared with historical pediatric data in the Czech Republic from 1990 to 2001, the incidence of CD rose from 1.26 per 100000 person-years in 2001 to 5.8 per 100000 person-years in 2015 in children under 15 years of age. The incidence of UC gradually decreased from 1.84 per 100000 person-years in 1999 to 0 per 100000 person-years in 2015.
IBD projections to 2018
The IBD projections to 2018 are shown in Figure 4. The IBD incidence rates projected for 2016, 2017 and 2018 were 12.1 (95%CI: 10.6-14.6), 12.3 (10.3-15.0) and 12.6 (11.0-15.4) (Figure 4A). The incidence rates of CD projected for 2016-2018 were 9.7 (8.1-11.3), 10.2 (8.7-12.3) and 10.2 (8.7-12.3) respectively (Figure 4B). The projected UC incidence rates (Figure 4C) were 1.0 (0-3.4), 0.8 (0-3.19) and 0.6 (0- 3.2) respectively. The projected IBD-U incidence rates were 1.3 (0.5-2.2), 1.3 (0.5-2.3) and 1.4 (0.5-2.4) respectively.
DISCUSSION
Here, we report the incidence of IBD over a period of 16 years from 2000 to 2015 in Czech children (< 19 years) in a large, geographically well-defined population. To our knowledge, this is the first Czech prospective regional cohort of IBD, covering a wide area in the Pilsen region within the Czech Republic. We substantiated several important conclusions: (1) some of the highest incidence rates of pediatric IBD reported to date; (2) the higher incidence (more than twice that of UC) and male predominance of CD; (3) the significantly increased incidence of IBD (in particular CD) over time; and (4) the gradual increase in incidence rates with age. We further compared our findings with historical Czech data and showed that IBD-projected data were enhanced substantially until 2018.
We have added new insights to a global map of the incidence of IBD. We identified one of the highest incidences of IBD reported to date. The rate of IBD over the time period covered by the research was 10.0 per 100000 person-years for CD 6.2, 2.8 for UC and 1.0 for IBD-U up to 19 years of age. The respective incidences were 7.3, 4.6, 2.0 and 0.7 when considering an age of 15 years as the upper limit. We identified one of the highest incidences of childhood IBD reported to date. Our results well resemble those from areas showing similarly high IBD incidences, such as reported from studies in England[19,20], Sweden[21,22], Finland[23,24], Norway[25] and Canada[3,26]. Against this backdrop, our findings confirm high rates of IBD (primarily CD) in our country that are comparable to the rates in high-incidence Western populations[2]. IBD incidence was considerably lower in studies conducted in the United States according to data from Wisconsin[27] and northern California[28], as well as in Iceland[10], France[29], Italy[30], Scotland[9], Italy[30] and the Netherlands[15].
Our findings should be considered while bearing in mind that incidence rates are challenging due to the age limit used for pediatric patients and the heterogeneity of data collection techniques. Another important finding was a relatively low contribution of UC and IBD-U compared with the overall IBD incidence rate. In our study, the incidence of CD was more than twice that of UC. Thus, our results are similar to those of Buderus et al[31], Ashton et al[19] and the European pediatric registry EUROKIDS[32] that also showed CD accounting for most IBD cases. In contrast, Castro et al[30] showed that UC was more prevalent than CD in Italian children. An equal incidence of UC and CD occurs among adult populations[33], whereas the incidence of UC was approximately 2-fold higher than the incidence of CD in the Lazio region in Italy[34].
Next, we calculated time trends in the incidence of IBD for all three pathologies, as it is still debated whether there are temporal aspects in IBD. In a systematic pediatric review by Benchimol et al[2], only 20.1% of studies used statistical analyses to determine trends over time, while 77.8% reported a statistically significant increase in incidence of pediatric IBD. Among the studies calculating trends in the incidence of CD, 60% reported a significantly increased incidence. Among similar UC projects, 20% noted significantly increased rates[2]. Our data suggest a clear indication of the rising trend of newly diagnosed IBD, especially for CD due primarily to an increase in the incidence of CD in males. The incidence of CD more than doubled from 2000 to 2015. In contrast, the incidence of UC and IBD-U was altered very little throughout the study period. Thus, the results of our study are consistent with recent reviews[13].
Similarly, the increased incidence of IBD, with a predominance of CD over UC in recent decades, was reported in Australia[35], France[29], Sweden[22], Canada[3], Denmark[7], Scotland[36] and other countries[25,27]. In Australia, recent Victorian studies clearly showed increasing rates in children, with a greater than 10-fold increase in CD over the 30-year period to 2001. In addition, the incidence of UC also remained relatively stable in other studies, despite the increasing evidence of CD[2,29]. These figures are not fully comparable to those obtained in other pediatric studies. In northern California, Abramson et al[28] observed a several-fold rise in UC incidence, and the incidence of CD remained relatively stable over a period of 11 years, similar to the report by Orel et al[37] in Slovenia. In contrast, a Canadian study from Ontario[26], and the French EPIMAD registry between 1988 and 2007, reported a striking decrease in UC despite a persistent increase in CD[38]. An explanation for the differing trends observed in CD and UC remains elusive[39]. According to these results, we may speculate that this geographic variability is probably due to genetic and environmental implications; however, the exact triggers may not be easily identified.
We observed that IBD-U was diagnosed most frequently among the oldest group of children. The finding of 10% of subjects with IBD-U is among the lowest reported. The proportion of patients was similar to that previously reported in other pediatric observations in whom IBD-U accounts for 10% to 15% of newly diagnosed patients with IBD[8,15,20,25,30,31,36,40], but other researchers have reported a somewhat lower incidence of IBD-U[22,29,41]. Undoubtedly, our data parallel observations obtained in adults[11,34,42,43]. This is a partially unexplained phenomenon. Heyman et al[44] speculated that IBD-U might represent an evolving form of IBD that presents before a definitive diagnosis of IBD. In our series, the proportion of patients who initially presented with IBD-U decreased over time. There were still 23.5% of subjects with IBD-U, but 41.2% of cases had already evolved toward UC and 35.3% toward CD.
We observed the presenting features and compared them with the rates from the 1990s. We suggest that the incidence of pediatric IBD is increasing in the Czech Republic. These results indicate that the incidence of CD has markedly increased, while the incidence of UC has decreased substantially in the Pilsen region compared with the historical figures in the Czech Republic over the past 25 years. Indeed, an increased pediatric IBD incidence has been reported by Kolek et al[17] in a prospective population-based study from Moravia (a northern region in the Czech Republic). The incidence of CD increased from 0 to 2.7/100000 person-years between 1999 and 2001, and the incidence of UC increased from 0.68 to 1.84 per/100000 person-years between 1990 and 1999.
In the first nationwide study in Czech pediatric subjects, Pozler et al[16] published the results of a partly retrospective study exploring CD incidence in Czech children (< 15 years) between 1990 and 2001. A marked (5-fold) increase in the incidence of CD was found from 0.25/100000/year in 1990 to 1.26/100000/year in 2001[16]. The incidence of CD in children under 15 years of age increased 4.6-fold between 2001 and 2015 (1.26-5.8/100000/year), but the incidence of UC was lower than that of the hitherto published studies in the Czech Republic[16,17], remaining almost unchanged with little difference between the studies (1.84-0.9/100000/year). We also assumed that parallel increases are also occurring in adult Czech populations[11]. The reasons for these increases are largely unknown.
Although given a 25-year period, the increased risk of IBD tracks with the effects of external factors, such as environmental features that are constantly changing, rather than of shifts in the frequency of susceptibility genes alone that may change slowly[3,13]. In our population, there were important changes in higher socio-economic status and in demographic or environmental health conditions over time, which may have altered the frequency of disease. However, even in low-incidence countries, the occurrence of IBD seems to be rising. Thus, increased awareness by physicians and advancements in the diagnosis of IBD, homogeneity of registration databases, data collection methods, and possibly the completeness of case ascertainment may partially contribute to the striking increase in the incidence of IBD and geographic differences. We consider this a very plausible explanation for the precipitation of the clinical illness, although there are no clear answers from this research in terms of the relevance of the explanation in the Czech Republic. More detailed studies are warranted.
Unfortunately, data are lacking on the incidence in the pediatric population in eastern Europe because most of the published data were conducted in the United States and western Europe. However, in the past few years, recent papers have indicated a sharp increase in incidence in various parts of eastern Europe[39,45]. A recent study from western Hungary (the Veszprem province) revealed that the incidence of pediatric IBD has rapidly increased over the 35-year period. The incidence of CD and UC increased from 0 and 0.7 in 1977-1981 to 7.2 and from 5.2 in 2007-2011 per 100000 person-years[46]. By contrast, the results from our study revealed no changes in UC over time, although the incidence rates of UC in the Hungarian study increased approximately 7-fold. Very recently, the Hungarian nationwide pediatric registry data were published, with a mean incidence for the 3-year observation period from 2007 to 2009 of 7.48, 4.72, 2.32 and 0.45 for IBD, CD, UC and IBD-U respectively. The incidence of pediatric IBD in Hungary was at the higher end of the reported range[47]. Thus, the situation in our country is comparable to that in Hungary.
These findings collectively indicated that the incidence of IBD in central and eastern European countries is increasing in childhood. Consequently, these findings do not concur with the previously described west-east gradient decrease in the incidence of IBD in Europe[11], reflecting the possible geographic impact of the diagnosis of IBD. We suggest that a comparison of these studies may be indicative of the loss of this gradient in European children and exceptions to that rule. In addition, these findings reporting low incidence rates in eastern Europe contrast with the findings of a previous study in Polish children that suggested that the incidence of UC was higher than that of CD (1.3 vs 0.8/100000 person-years)[48].
In an analysis stratified by age, we uncovered a distinct contrast in the distribution of IBD with a gradual increase in older age groups, in agreement with other studies. We observed that approximately 17% of children were diagnosed with IBD by the age of 10 years, corresponding to the results reported by Buderus et al[31] in Germany and Austria. The highest rates were observed in the 11- to 19-year-old group in our study, which is consistent with previous reports[27,38,46]. In the population aged 0-10 years, the incidence of UC and CD was similar, with no differences by sex. As age increased, the incidence of IBD rose and peaked in children around the age of 15 years, with CD exhibiting a steeper increase compared with UC around puberty, although it was rare in a younger age category. The age-specific peak for diagnosis was not consistent with the findings of earlier studies by Sawczenko et al[49], van der Zaag-Loonen et al[15], Buderus et al[31] and Orel et al[37]. These discordant data are probably related to differences in the methods applied for case ascertainment, disease registries and the demographic differences of the underlying populations.
We reported a male preponderance of CD but no significant differences in sex for UC and IBD-U as previously reported[26,49] because of a doubled and significantly higher incidence of CD in males. Similar proportions regarding male susceptibility to CD have been recognized for many years[19,22,27,50], as opposed to a slight female predominance in adults[33]. Some data suggest that pediatric UC is more common in girls[29,37,51]. A systematic review by Molodecky et al[5] failed to obtain consistent results, suggesting that the disease occurred equally between the sexes in adults. Together with the different findings of the inverted male-to-female ratio in adult studies, these data suggest that biological age-related factors may contribute to predisposing people to and triggering of IBD, especially CD in Czech children. Given that our population consisted mainly of native Caucasian children, we could not estimate the specific incidence because almost all participating subjects were of similar ethnic origin. However, past studies have shown a high incidence of IBD in Caucasian children[27].
We based projections for future incidence rates on our knowledge. Our estimates for the projected rate of IBD to 2018 were alarming, which may reflect the increasing incidence of IBD over time. Our results indicate that the incidence estimates for IBD in 2018 are somewhat higher than most reported in our current study. Regarding IBD incidence rate projections, based on the most likely scenario and on the estimated trend from the linear analysis, we projected a model showing that the number of new cases of Czech children with diagnosed IBD (in particular CD) will continue to increase over an extended time period. Conversely, our projections clearly show that the incidence of UC will decrease.
To our knowledge, no study has yet focused on IBD incidence projections in children, thus making a direct comparison problematic. However, in our opinion, the fitted model actually created appraisals that seemed to be similar to those observed. Although it remains to be further confirmed, the disturbing incidence rate of 12.6 per 100000 person-years projected for 2018 appears to be realistic if current circumstances persist. The simplest explanation for this finding could be that the IBD incidence projected herein could be attributable to demographic factors and may be indicative of the persistence of previous conditions, with no major or slow changes in pediatric IBD. It will be interesting to follow these temporal trends in the coming years, as we may learn more about the role of environmental factors in the pathogenesis of IBD[12].
The strength of this study is its robust, prospective manner because the available prospective observation shows more homogeneity. In addition, we used the same defined geographic region with an ethnically homogeneous study sample, establishing well-defined selection criteria. Furthermore, we used an appropriate time period and case ascertainment identification of IBD cases, and we included patients at or shortly after diagnosis. Since newly enrolled individuals were included only once, despite meeting the inclusion criteria over multiple years, a bias may not exist for including those with more chronic or severe disease, requiring additional visits.
These strengths allowed us to provide up-to-date estimates of the incidence and trends and to depict the main differences by time period, age and sex, thus not affecting the time trend analyses. An observational survey and the appropriate type of sampling frame for study recruitment, from which subjects were selected, was critical for the sample representativeness by covering the entire population of interest with unlimited access to subspecialist care to obtain reproducible results.
The Czech Republic is a country with centralized health care for IBD, and pediatric IBD subjects are cared for only in our tertiary hospital in the catchment area. Therefore, we trust that the sample is representative due to the easily surveyable area of the Pilsen region. Due to the centralization of pediatric gastroenterology care, we believe that all pediatric patients residing in the Pilsen region were included in the database, reducing the probability of recall bias. Thus, it is highly probable that all children with IBD in the Pilsen region sought medical attention in our clinical setting. In the assessment of time trends, we reported 16 years of data with figures specifying the numeric incidence rates over a long time period. This study was also strengthened by reliable data from the files and our registry of pediatric IBD in the included area. The census data provide data sets from which we can identify a sample that is deemed to have minimal bias.
A potential weakness of our study is that our registry was based on data from one large regional referral center encompassing a specific child population, and the generalizability of the presented findings regardless of the setting may be of concern. Another possible limitation is an underestimation of rates of IBD because we were unable to exclude the possibility that the IBD subjects may have been treated in other institutions, which may have led to an underestimation of the results of the analysis. However, in the Czech pediatric population at time of this study, there was an almost universal admission policy for investigation of IBD; unlike adults, the disease is not diagnosed in outpatient settings by primary care providers or pediatricians. Our facility is the only referral center in the catchment area that provides inpatient/outpatient care, and all children with IBD are concentrated in our center for pediatric gastroenterology. Of note, in the Pilsen region, probable IBD cases in childhood are treated exclusively in our single facility. In general, adult gastroenterologists in the Czech Republic do not provide medical care for IBD-affected children until they reach 19 years of age (older teenagers with a new diagnosis of IBD are not usually referred directly to adult specialists). Therefore, we probably did not underestimate the true incidence of IBD, and the present data provide a reasonably accurate evaluation of the incidence of IBD because the major portion of the subjects would have been referred to our referral center.
Furthermore, our data did not demonstrate the apparent decline in the incidence rate after the age of 15 years, which could represent a spurious event caused by pediatric subjects being diagnosed by adult specialists and thus failing to be captured in our study. Consequently, we trust that there was no selection bias in favor of younger subjects, the capture of pediatric cases is trustworthy even in the 15- to 19-year-old age class, and the limit of 19 years better mirrors the usual situation in the pediatric population in the Czech Republic. Because our data revealed significant time trends, we regard any misclassification as stable over the studied periods and thus did not influence the time trend analyses or the actual epidemiologic characteristics.
In conclusion, the incidence of pediatric IBD, especially CD, is among the highest reported to date. This study revealed a marked increase in the incidence of CD over the 16-year period, such that CD is now much more common than UC and IBD-U in children in the Pilsen region and exhibits a persistent male preponderance. The reasons for these trends are unknown, and further studies in different regions of the country would be helpful to determine whether these trends are present in other areas of the Czech Republic. Further database projects may be attempted with software data, which should permit future research due to greater region specificity. As the changes in the past decades in industrialized countries cannot be interpreted by changes in genetic background, the influence of environmental hazards on incidence appears to be a crucial area of study. This study reaffirms the need for analyses of time trends and subsequent research to better understand the combination of genetic/family history and environmental influences, to unravel concurrent factors in the etiology of IBD and to determine causal relationships in pediatric disease.
COMMENTS
Background
Studies worldwide have shown a rising but variable incidence of inflammatory bowel disease (IBD), including in pediatric populations. Despite a rising worldwide incidence of IBD, limited data are available on the exact incidence of IBD in Czech children. The current research was designed to evaluate the incidence and overall time trends of IBD in children aged 0- to 19-years-old over the period 2000-2015 and to project incidence to 2018 in the Czech Republic.
Research frontiers
In this study, it is suggested that although no firm conclusions can be drawn to address the rising incidence of IBD, differences in the geographic distribution, and particularly changes in incidence over time within one area, may provide new insights into concurrent etiological factors. This is possible only when enough pediatric projects within one defined geographical area with a relatively homogeneous population provide a fundamental basis for a better understanding of the epidemiology and environmental influences of any geographically restricted pediatric population and for the assessment of IBD.
Innovations and breakthroughs
The findings in this and other studies suggest an increasing trend in IBD incidence. The current research adds to that literature, with the suggestion of increased rates of IBD, especially of Crohn’s disease (CD), in the time period under study from 2000 to 2015 in this pediatric population in central Europe. However, ulcerative colitis (UC) and IBD-unclassified (IBD-U) are not common forms of IBD in Czech children.
Applications
The results of this study serve as additional evidence supporting the investigation of different environmental and triggering factors in the development of IBD and its subtypes in children and adolescents.
Terminology
UC, CD and IBD-U are three primary forms of IBD. The incidence rates are expressed as new cases per 100000 pediatric persons per year.
Peer-review
The manuscript presents an interesting study, which examined the incidence in pediatric IBD during 2000-2015 in the Pilsen Region of the Czech Republic and revealed a marked increase in the incidence of CD over the 16-year period.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Czech Republic
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: This study was conducted according to good clinical practice and the Declaration of Helsinki. The research protocol was reviewed and duly approved by the relevant ethics committee.
Informed consent statement: Written informed consent was provided by the parents or caregivers of all participants prior to study inclusion in accordance with the institutional research review board requirements, and all children provided verbal consent before being included in the study.
Conflict-of-interest statement: The authors have no conflicts of interest to disclose.
Data sharing statement: There are no additional data available.
Peer-review started: December 25, 2016
First decision: January 10, 2017
Article in press: March 20, 2017
P- Reviewer: Ozen H, Zouiten-Mekki L S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Zhang FF
References
- 1.Molodecky NA, Kaplan GG. Environmental risk factors for inflammatory bowel disease. Gastroenterol Hepatol (N Y) 2010;6:339–346. [PMC free article] [PubMed] [Google Scholar]
- 2.Benchimol EI, Fortinsky KJ, Gozdyra P, Van den Heuvel M, Van Limbergen J, Griffiths AM. Epidemiology of pediatric inflammatory bowel disease: a systematic review of international trends. Inflamm Bowel Dis. 2011;17:423–439. doi: 10.1002/ibd.21349. [DOI] [PubMed] [Google Scholar]
- 3.Benchimol EI, Guttmann A, Griffiths AM, Rabeneck L, Mack DR, Brill H, Howard J, Guan J, To T. Increasing incidence of paediatric inflammatory bowel disease in Ontario, Canada: evidence from health administrative data. Gut. 2009;58:1490–1497. doi: 10.1136/gut.2009.188383. [DOI] [PubMed] [Google Scholar]
- 4.Muhvić-Urek M, Tomac-Stojmenović M, Mijandrušić-Sinčić B. Oral pathology in inflammatory bowel disease. World J Gastroenterol. 2016;22:5655–5667. doi: 10.3748/wjg.v22.i25.5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54.e42; quiz e30. doi: 10.1053/j.gastro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Urlep D, Blagus R, Orel R. Incidence Trends and Geographical Variability of Pediatric Inflammatory Bowel Disease in Slovenia: A Nationwide Study. Biomed Res Int. 2015;2015:921730. doi: 10.1155/2015/921730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larsen MD, Baldal ME, Nielsen RG, Nielsen J, Lund K, Nørgård BM. The incidence of Crohn’s disease and ulcerative colitis since 1995 in Danish children and adolescents & lt; 17 years - based on nationwide registry data. Scand J Gastroenterol. 2016;51:1100–1105. doi: 10.3109/00365521.2016.1172340. [DOI] [PubMed] [Google Scholar]
- 8.Martín-de-Carpi J, Rodríguez A, Ramos E, Jiménez S, Martínez-Gómez MJ, Medina E. Increasing incidence of pediatric inflammatory bowel disease in Spain (1996-2009): the SPIRIT Registry. Inflamm Bowel Dis. 2013;19:73–80. doi: 10.1002/ibd.22980. [DOI] [PubMed] [Google Scholar]
- 9.Henderson P, Hansen R, Cameron FL, Gerasimidis K, Rogers P, Bisset WM, Reynish EL, Drummond HE, Anderson NH, Van Limbergen J, et al. Rising incidence of pediatric inflammatory bowel disease in Scotland. Inflamm Bowel Dis. 2012;18:999–1005. doi: 10.1002/ibd.21797. [DOI] [PubMed] [Google Scholar]
- 10.Agnarsson U, Björnsson S, Jóhansson JH, Sigurdsson L. Inflammatory bowel disease in Icelandic children 1951-2010. Population-based study involving one nation over six decades. Scand J Gastroenterol. 2013;48:1399–1404. doi: 10.3109/00365521.2013.845799. [DOI] [PubMed] [Google Scholar]
- 11.Burisch J, Pedersen N, Čuković-Čavka S, Brinar M, Kaimakliotis I, Duricova D, Shonová O, Vind I, Avnstrøm S, Thorsgaard N, et al. East-West gradient in the incidence of inflammatory bowel disease in Europe: the ECCO-EpiCom inception cohort. Gut. 2014;63:588–597. doi: 10.1136/gutjnl-2013-304636. [DOI] [PubMed] [Google Scholar]
- 12.Russel MG. Changes in the incidence of inflammatory bowel disease: what does it mean? Eur J Intern Med. 2000;11:191–196. doi: 10.1016/s0953-6205(00)00090-x. [DOI] [PubMed] [Google Scholar]
- 13.Legaki E, Gazouli M. Influence of environmental factors in the development of inflammatory bowel diseases. World J Gastrointest Pharmacol Ther. 2016;7:112–125. doi: 10.4292/wjgpt.v7.i1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vind I, Riis L, Jess T, Knudsen E, Pedersen N, Elkjaer M, Bak Andersen I, Wewer V, Nørregaard P, Moesgaard F, et al. Increasing incidences of inflammatory bowel disease and decreasing surgery rates in Copenhagen City and County, 2003-2005: a population-based study from the Danish Crohn colitis database. Am J Gastroenterol. 2006;101:1274–1282. doi: 10.1111/j.1572-0241.2006.00552.x. [DOI] [PubMed] [Google Scholar]
- 15.van der Zaag-Loonen HJ, Casparie M, Taminiau JA, Escher JC, Pereira RR, Derkx HH. The incidence of pediatric inflammatory bowel disease in the Netherlands: 1999-2001. J Pediatr Gastroenterol Nutr. 2004;38:302–307. doi: 10.1097/00005176-200403000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Pozler O, Maly J, Bonova O, Dedek P, Frühauf P, Havlickova A, Janatova T, Jimramovsky F, Klimova L, Klusacek D, et al. Incidence of Crohn disease in the Czech Republic in the years 1990 to 2001 and assessment of pediatric population with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2006;42:186–189. doi: 10.1097/01.mpg.0000189328.47150.bc. [DOI] [PubMed] [Google Scholar]
- 17.Kolek A, Janout V, Tichý M, Grepl M. The incidence of inflammatory bowel disease is increasing among children 15 years old and younger in the Czech Republic. J Pediatr Gastroenterol Nutr. 2004;38:362–363. doi: 10.1097/00005176-200403000-00028. [DOI] [PubMed] [Google Scholar]
- 18.IBD Working Group of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition. Inflammatory bowel disease in children and adolescents: recommendations for diagnosis--the Porto criteria. J Pediatr Gastroenterol Nutr. 2005;41:1–7. doi: 10.1097/01.mpg.0000163736.30261.82. [DOI] [PubMed] [Google Scholar]
- 19.Ashton JJ, Wiskin AE, Ennis S, Batra A, Afzal NA, Beattie RM. Rising incidence of paediatric inflammatory bowel disease (PIBD) in Wessex, Southern England. Arch Dis Child. 2014;99:659–664. doi: 10.1136/archdischild-2013-305419. [DOI] [PubMed] [Google Scholar]
- 20.Ashton JJ, Coelho T, Ennis S, Batra A, Afzal NA, Beattie RM. Presenting phenotype of paediatric inflammatory bowel disease in Wessex, Southern England 2010-2013. Acta Paediatr. 2015;104:831–837. doi: 10.1111/apa.13017. [DOI] [PubMed] [Google Scholar]
- 21.Malmborg P, Grahnquist L, Lindholm J, Montgomery S, Hildebrand H. Increasing incidence of paediatric inflammatory bowel disease in northern Stockholm County, 2002-2007. J Pediatr Gastroenterol Nutr. 2013;57:29–34. doi: 10.1097/MPG.0b013e31828f21b4. [DOI] [PubMed] [Google Scholar]
- 22.Hildebrand H, Finkel Y, Grahnquist L, Lindholm J, Ekbom A, Askling J. Changing pattern of paediatric inflammatory bowel disease in northern Stockholm 1990-2001. Gut. 2003;52:1432–1434. doi: 10.1136/gut.52.10.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lehtinen P, Ashorn M, Iltanen S, Jauhola R, Jauhonen P, Kolho KL, Auvinen A. Incidence trends of pediatric inflammatory bowel disease in Finland, 1987-2003, a nationwide study. Inflamm Bowel Dis. 2011;17:1778–1783. doi: 10.1002/ibd.21550. [DOI] [PubMed] [Google Scholar]
- 24.Virta LJ, Saarinen MM, Kolho KL. Inflammatory Bowel Disease Incidence is on the Continuous Rise Among All Paediatric Patients Except for the Very Young: A Nationwide Registry-based Study on 28-Year Follow-up. J Crohns Colitis. 2017;11:150–156. doi: 10.1093/ecco-jcc/jjw148. [DOI] [PubMed] [Google Scholar]
- 25.Perminow G, Brackmann S, Lyckander LG, Franke A, Borthne A, Rydning A, Aamodt G, Schreiber S, Vatn MH. A characterization in childhood inflammatory bowel disease, a new population-based inception cohort from South-Eastern Norway, 2005-07, showing increased incidence in Crohn’s disease. Scand J Gastroenterol. 2009;44:446–456. doi: 10.1080/00365520802647434. [DOI] [PubMed] [Google Scholar]
- 26.Grieci T, Bütter A. The incidence of inflammatory bowel disease in the pediatric population of Southwestern Ontario. J Pediatr Surg. 2009;44:977–980. doi: 10.1016/j.jpedsurg.2009.01.038. [DOI] [PubMed] [Google Scholar]
- 27.Kugathasan S, Judd RH, Hoffmann RG, Heikenen J, Telega G, Khan F, Weisdorf-Schindele S, San Pablo W, Perrault J, Park R, et al. Epidemiologic and clinical characteristics of children with newly diagnosed inflammatory bowel disease in Wisconsin: a statewide population-based study. J Pediatr. 2003;143:525–531. doi: 10.1067/s0022-3476(03)00444-x. [DOI] [PubMed] [Google Scholar]
- 28.Abramson O, Durant M, Mow W, Finley A, Kodali P, Wong A, Tavares V, McCroskey E, Liu L, Lewis JD, et al. Incidence, prevalence, and time trends of pediatric inflammatory bowel disease in Northern California, 1996 to 2006. J Pediatr. 2010;157:233–239.e1. doi: 10.1016/j.jpeds.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 29.Auvin S, Molinié F, Gower-Rousseau C, Brazier F, Merle V, Grandbastien B, Marti R, Lerebours E, Dupas JL, Colombel JF, et al. Incidence, clinical presentation and location at diagnosis of pediatric inflammatory bowel disease: a prospective population-based study in northern France (1988-1999) J Pediatr Gastroenterol Nutr. 2005;41:49–55. doi: 10.1097/01.mpg.0000162479.74277.86. [DOI] [PubMed] [Google Scholar]
- 30.Castro M, Papadatou B, Baldassare M, Balli F, Barabino A, Barbera C, Barca S, Barera G, Bascietto F, Berni Canani R, et al. Inflammatory bowel disease in children and adolescents in Italy: data from the pediatric national IBD register (1996-2003) Inflamm Bowel Dis. 2008;14:1246–1252. doi: 10.1002/ibd.20470. [DOI] [PubMed] [Google Scholar]
- 31.Buderus S, Scholz D, Behrens R, Classen M, De Laffolie J, Keller KM, Zimmer KP, Koletzko S. Inflammatory bowel disease in pediatric patients: Characteristics of newly diagnosed patients from the CEDATA-GPGE Registry. Dtsch Arztebl Int. 2015;112:121–127. doi: 10.3238/arztebl.2015.0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Bie CI, Paerregaard A, Kolacek S, Ruemmele FM, Koletzko S, Fell JM, Escher JC. Disease phenotype at diagnosis in pediatric Crohn’s disease: 5-year analyses of the EUROKIDS Registry. Inflamm Bowel Dis. 2013;19:378–385. doi: 10.1002/ibd.23008. [DOI] [PubMed] [Google Scholar]
- 33.Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol. 2015;12:720–727. doi: 10.1038/nrgastro.2015.150. [DOI] [PubMed] [Google Scholar]
- 34.Di Domenicantonio R, Cappai G, Arcà M, Agabiti N, Kohn A, Vernia P, Biancone L, Armuzzi A, Papi C, Davoli M. Occurrence of inflammatory bowel disease in central Italy: a study based on health information systems. Dig Liver Dis. 2014;46:777–782. doi: 10.1016/j.dld.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 35.Phavichitr N, Cameron DJ, Catto-Smith AG. Increasing incidence of Crohn’s disease in Victorian children. J Gastroenterol Hepatol. 2003;18:329–332. doi: 10.1046/j.1440-1746.2003.02975.x. [DOI] [PubMed] [Google Scholar]
- 36.Henderson P, Rogers P, Gillett P, Wilson D. The epidemiology and natural history of paediatric inflammatory bowel disease in a UK region: a prospective 14-year study. Arch Dis Child. 2012;97:A53.2–A54. [Google Scholar]
- 37.Orel R, Kamhi T, Vidmar G, Mamula P. Epidemiology of pediatric chronic inflammatory bowel disease in central and western Slovenia, 1994-2005. J Pediatr Gastroenterol Nutr. 2009;48:579–586. doi: 10.1097/MPG.0b013e318164d903. [DOI] [PubMed] [Google Scholar]
- 38.Chouraki V, Savoye G, Dauchet L, Vernier-Massouille G, Dupas JL, Merle V, Laberenne JE, Salomez JL, Lerebours E, Turck D, et al. The changing pattern of Crohn’s disease incidence in northern France: a continuing increase in the 10- to 19-year-old age bracket (1988-2007) Aliment Pharmacol Ther. 2011;33:1133–1142. doi: 10.1111/j.1365-2036.2011.04628.x. [DOI] [PubMed] [Google Scholar]
- 39.Lakatos PL. Recent trends in the epidemiology of inflammatory bowel diseases: up or down? World J Gastroenterol. 2006;12:6102–6108. doi: 10.3748/wjg.v12.i38.6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turunen P, Kolho KL, Auvinen A, Iltanen S, Huhtala H, Ashorn M. Incidence of inflammatory bowel disease in Finnish children, 1987-2003. Inflamm Bowel Dis. 2006;12:677–683. doi: 10.1097/00054725-200608000-00002. [DOI] [PubMed] [Google Scholar]
- 41.Jakobsen C, Paerregaard A, Munkholm P, Wewer V. Environmental factors and risk of developing paediatric inflammatory bowel disease -- a population based study 2007-2009. J Crohns Colitis. 2013;7:79–88. doi: 10.1016/j.crohns.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 42.Sincić BM, Vucelić B, Persić M, Brncić N, Erzen DJ, Radaković B, Mićović V, Stimac D. Incidence of inflammatory bowel disease in Primorsko-goranska County, Croatia, 2000-2004: A prospective population-based study. Scand J Gastroenterol. 2006;41:437–444. doi: 10.1080/00365520500320094. [DOI] [PubMed] [Google Scholar]
- 43.Su HY, Gupta V, Day AS, Gearry RB. Rising Incidence of Inflammatory Bowel Disease in Canterbury, New Zealand. Inflamm Bowel Dis. 2016;22:2238–2244. doi: 10.1097/MIB.0000000000000829. [DOI] [PubMed] [Google Scholar]
- 44.Heyman MB, Kirschner BS, Gold BD, Ferry G, Baldassano R, Cohen SA, Winter HS, Fain P, King C, Smith T, et al. Children with early-onset inflammatory bowel disease (IBD): analysis of a pediatric IBD consortium registry. J Pediatr. 2005;146:35–40. doi: 10.1016/j.jpeds.2004.08.043. [DOI] [PubMed] [Google Scholar]
- 45.Lakatos L, Lakatos PL. Is the incidence and prevalence of inflammatory bowel diseases increasing in Eastern Europe? Postgrad Med J. 2006;82:332–337. doi: 10.1136/pgmj.2005.042416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lovasz BD, Lakatos L, Horvath A, Pandur T, Erdelyi Z, Balogh M, Szipocs I, Vegh Z, Veres G, Müller KE, et al. Incidence rates and disease course of paediatric inflammatory bowel diseases in Western Hungary between 1977 and 2011. Dig Liver Dis. 2014;46:405–411. doi: 10.1016/j.dld.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 47.Müller KE, Lakatos PL, Arató A, Kovács JB, Várkonyi Á, Szűcs D, Szakos E, Sólyom E, Kovács M, Polgár M, Nemes É, Guthy I, Tokodi I, Tóth G, Horváth Á, Tárnok A, Csoszánszki N, Balogh M, Vass N, Bódi P, Dezsőfi A, Gárdos L, Micskey E, Papp M, Cseh Á, Szabó D, Vörös P, Veres G. Incidence, Paris classification, and follow-up in a nationwide incident cohort of pediatric patients with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2013;57:576–582. doi: 10.1097/MPG.0b013e31829f7d8c. [DOI] [PubMed] [Google Scholar]
- 48.Karolewska-Bochenek K, Lazowska-Przeorek I, Albrecht P, Grzybowska K, Ryzko J, Szamotulska K, Radzikowski A, Landowski P, Krzesiek E, Ignys I, et al. Epidemiology of inflammatory bowel disease among children in Poland. A prospective, population-based, 2-year study, 2002-2004. Digestion. 2009;79:121–129. doi: 10.1159/000209382. [DOI] [PubMed] [Google Scholar]
- 49.Sawczenko A, Sandhu BK. Presenting features of inflammatory bowel disease in Great Britain and Ireland. Arch Dis Child. 2003;88:995–1000. doi: 10.1136/adc.88.11.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim BJ, Song SM, Kim KM, Lee YJ, Rhee KW, Jang JY, Park SJ, Yoon CH. Characteristics and trends in the incidence of inflammatory bowel disease in Korean children: a single-center experience. Dig Dis Sci. 2010;55:1989–1995. doi: 10.1007/s10620-009-0963-5. [DOI] [PubMed] [Google Scholar]
- 51.Wrobel I, Butzner J, Nguyen N, Withers G, Nelson K. Epidemiology of Pediatric IBD in a Population-based Cohort in Southern Alberta, Canada (1983-2005) J Pediatr Gastroenterol Nutr. 2006;43:S54–S55. [Google Scholar]


