Abstract
Objectives
Behavioral sleep problems (BSPs) are prevalent and consequential in young children. There is a need for screening tools that identify BSPs- which are often rooted in the parent-young child relationship- and typically respond to behavior management. Such a tool would increase capacity to identify and treat BSPs. We sought to validate a short-form version of the widely used Children's Sleep Habits Questionnaire (SF-CSHQ) that omitted items that would not be responsive to behavioral strategies.
Methods
The original 33-item CSHQ elicits parent report of “behaviorally-based” and “medically-based” sleep items (e.g., parasomnias and sleep disordered breathing). We conducted analyses to develop a SF-CSHQ that excludes its “medically-based” items, to determine a) the SF-CSHQ threshold score corresponding to the full CSHQ clinical cut-off score (≥ 41), and b) preliminary validity of this SF-CSHQ. Data were re-analyzed from the original data that established the CSHQ's psychometric properties in 4-10 year olds, and a second dataset that established its validity in 24-66 month olds.
Results
In both datasets, a threshold score of 30 had correlations of 0.90-0.94 with the original cut-off. This 23-item SF-CSHQ cut-off functioned as well as the full CSHQ cut-off in discriminating between children with vs. without a parent-reported behavioral sleep problem, and with vs. without prolonged sleep latency (per actigraphy).
Conclusion
We established preliminary validity of modified version of the widely used CSHQ. This SF-CSHQ may be useful for widening screening and first-line guidance for behavioral sleep problems in young children, among professionals who are not sleep medicine specialists.
Introduction
Insufficient and poor quality sleep in young children adversely impact behavioral and cognitive function. Preschool age (3-5 years) is a peak time for sleep disturbances, when ≈25% of children have a behavioral sleep problem (BSP).(1, 2) BSPs are problems falling or staying asleep, such as bedtime resistance, night-waking; they correspond to the “insomnia” diagnosis under the 2014 classification of sleep disorders.(3) (Previously, “sleep onset association” and “limit setting” types of behavioral insomnia were classified separately.(4)) Among children with neurodevelopmental disorders, prevalence is even higher, up to 80%. (5, 6) BSPs impede development of executive function,(7) and may increase later need for special education. However, healthy sleep practices (e.g. regular bedtime, bedtime routine), and behavioral interventions (e.g., extinction) both promote healthy sleep (8) and address sleep problems.(9) In fact, most BSPs in young children do not require specialized attention (10) Other sleep problems in young children-- sleep-disordered breathing (SDB) and parasomnias-- are not amenable to brief behavioral interventions. Parasomnias often resolve spontaneously with age, (11) while alternative gold-standard screening tools exist for SDB (12-14), which is managed by weight loss, medications, and surgery. (15)
Given the prevalence of BSPs in early childhood and their responsiveness to behavioral interventions, there is a need for criterion-referenced screening tools (10). Secondary prevention for mild BSPs could be accomplished by training a range of health professionals to deliver brief, behavioral interventions.(16) Positive effects are found when such interventions are delivered to parents by nurses and psychology trainees, (9, 17) via the internet,(18-20) and via written materials.(21) Behavioral interventions are most effective in younger (vs. older) children,(21) perhaps because they target parent-child interactions that contribute to the problem.(22)
The CSHQ is one of the most widely used multi-dimensional tools used to screen for pediatric sleep problems. It was designed to reflect common clinical symptoms presenting in school aged (4-10 year) children. Using a clinimetrics approach, i.e., one based upon symptoms or clinical presentation (23), the CSHQ includes 33 distinct items grouped into 8 subscales, based on face and content validity: bedtime resistance, sleep-onset delay, sleep duration, sleep anxiety, night-waking, parasomnias, SDB, and daytime sleepiness. Parents indicate the frequency of the sleep behavior during a typical week as ‘Usually’ [5-7 times/week], ‘Sometimes’ [2-4 times/week], or ‘Rarely’ [0-1 times/week]. Higher scores indicate worse sleep behaviors or problems. The CSHQ's validity was originally evaluated for two groups of 4-10 year olds: a community sample of elementary school children (n=469), and a sleep disorders clinic sample of 154 children, including those with a BSP (n=43), parasomnia (n=45), or SDB (polysomnography [PSG] confirmed, n=66). A total score ≥ 41 discriminated the two samples with acceptable sensitivity (0.80) and specificity (0.72).(24) Further details are presented in Methods.
The CHSQ is used in diverse community, general pediatric, and condition-specific populations. It was developed as a research tool; it has been employed in more than 300 research projects. It has been translated into at least 19 languages with adequate to good reliability and/or validity in Chinese,(25), Dutch (26), Portugese (27) and Spanish. (28) Notably, a recent paper employed the Spanish version as the gold standard for determining the validity of the shorter BEARS screener, in primary care practice.(29)
The current study aims to provide preliminary validity for a shortened version of the CSHQ (SF-CSHQ), exclusive of its parasomnia and SDB items. Such a tool would be specific to BSPs, thus identifying common sleep problems that are responsive to behavioral interventions delivered to (and administered by) parents. The 23-item SF-CSHQ evaluated in this study excludes its parasomnia (n=7) and SDB (n=3) items. A modified CSHQ excluding these items has been used by others,(30) and detected significant change after a BSP intervention.(30, 31) However, researchers who employed this modified CSHQ did not establish validity of a clinical cut-off for it. Here, we aim to determine a) the SF-CSHQ threshold score corresponding to the full CSHQ clinical cut-off score (≥ 41), and b) preliminary validity of this SF-CSHQ.
Methods
We re-analyzed datasets from two studies that had utilized the CSHQ. The first (Owens) was the original study of community and sleep disorder clinic sample data, collected in 1997-1998, that validated the CSHQ in 4-10 year olds. Analyses presented here utilize data from the sleep disorder clinic sample. (24) The second dataset collected in 2003-2005 (Goodlin-Jones)(32) included, in addition to the full CSHQ, actigraphy, sleep log, and a single-item sleep measure for younger children, aged 24-66 months. Authors of both studies agreed to share these de-identified datasets for analysis.
Description of Datasets
Owens
Total score internal consistency (α) for the community (.68) and sleep clinic (.78) samples was high using the criterion of 0.70.(33) For the bedtime resistance, sleep duration, sleep anxiety and daytime sleepiness scales, internal consistency was comparable in the community (0.63-0.70) and clinic (0.68-0.80) samples. The SDB subscale performed poorer in the community (0.51) vs. clinic (0.93) sample as did the parasomnia subscale (community=0.36, clinic= 0.56). In contrast, the night-waking subscale performed better in the community (0.54) vs. the clinic (0.44) samples. Sleep onset delay (> 20 minutes) was assessed from a single-item, thus no alpha was computed. Supporting the CSHQ's validity, the sleep clinic sample had higher (worse) scores on the total and all subscale scores, controlling for age and SES. Sensitivity and specificity were maximized using a cut-off score of ≥ 41 under the Receiver Operator Characteristic (ROC) curve.(24)
The CSHQ's development and psychometric properties have been examined by others. Of 57 pediatric sleep questionnaires analyzed, the CSHQ fulfilled 5 of 11 criteria for tool development; just two questionnaires fulfilled all steps.(12) Further, psychometric properties vary by population. In Dutch school age children, CSHQ internal consistencies ranged from 0.47-0.68, though test-retest and inter-observer reliabilities were good.(26) Among 2-5 year olds, a third of whom included early intervention or mental health program participants, subscale alphas ranged from 0.55-0.82, with, the parasomnia (0.69) and night-waking (0.68) subscales performing better than in the original community sample.(34)
Goodlin-Jones
We analyzed data from a study used to establish the full CSHQ's validity in young typically and non-typically developing children. (32) Families were recruited to a study about “sleep and waking patterns” (vs. a study of sleep problems) from community settings and a neuro-developmental disorders research registry.(35) Study participants reported on here include children who are typically developing (n= 73), with autism (n= 83), or with developmental delay without autism (n=64). The sample's full CSHQ total score had high internal consistency, 0.82. Additional sleep data included: actigraphy, a sleep log, and a single-item global assessment. Children wore actigraphs for 7 days and nights, from which the following sleep variables were derived: sleep start time, total 24 hr sleep duration, night-waking duration, and night wake-up times. Actigraphy data were averaged over one week of observations. Parents recorded sleep start and wake-up times as well as night-waking and nap durations in sleep logs on days their child wore the actigraph. Finally, parents were asked “Does your child have a sleep problem at the current time? (Yes/No).” The CSHQ significantly correlated with actigraphy-derived sleep onset (0.48), night-waking duration (0.21), total 24 h sleep (0.25), awake time (0.62), and; discriminated parents reporting their child's sleep as problematic (Yes vs. No).(32)
Statistical Analyses
To determine the SF-CSHQ threshold score that corresponds to the full CSHQ clinical cut-off score (≥ 41) we calculated linear regressions of the reduced scores on the full scores in both datasets. We calculated the threshold for the SF-CSHQ by using the regression-predicted value when the full CSHQ equals 41. We report agreement between the percentage of positive screens obtained by the full and SF-CSHQ in both datasets, using the Cohen's Kappa (standard error [SE]).
Next, we examined the SF-CSHQ's ability to discriminate children with vs. without a parent-reported sleep problem per a single-item global measure (Yes vs. No), in Goodlin-Jones' data. Such single-item measures are associated with sleep problems (i.e., onset, duration, night-waking),(36) sleep logs, the CSHQ,(37) and may correlate more highly with a child's mood and functioning than objective measures (e.g., PSG, actigraphy). (37) On a population basis, they predict quality of life, learning and behavioral outcomes at school-entry.(38)
We calculated the global measure's sensitivity and specificity to discriminate the two groups of children, using the area under the Receiver Operating Characteristics (ROC) curve. Sensitivity and specificity refers to a test's ability to detect “true positives” and “true negatives,” respectively. The area under the ROC curve describes the ability of a continuous measure to discriminate a dichotomous outcome. When the continuous measure is expected to be higher among those with the target outcome, the value ranges from 0.5 -1.0, with 0.5 indicating no systematic prediction, and 1.0 indicating the existence of a perfect cutoff, above which all subjects have the targeted outcome, and below which none do. In practice, 0.70-0.79 is considered fair discrimination, 0.80-0.89 is good, and 0.90-1.0 is excellent.(39) Measures < 0.70 are not considered useful predictors in most contexts.
Finally, we conducted additional analyses to establish preliminary validity. In the Goodlin-Jones data, we calculated Pearson correlation coefficients between the full CSHQ and SF-CSHQ scores, and actigraph measures of sleep efficiency, night-wakings, awake time, and sleep latency. We also used Pearson coefficients to determine associations between a newly derived dichotomous SF-CSHQ measure, and these actigraphy-derived measures. Using the Owens data, we sought to determine whether the SF-CSHQ could identify sleep problems independent of SDB in the subset of children (n=66) with PSG-confirmed SDB. We analyzed correlations between hourly pauses in respiration (apnea-hypopnea index [AHI]) and the SBB items of the CSHQ.
Results
Descriptions of the two samples are shown in Table 1. Both were majority male (Owens, 70.9%; Goodlin-Jones 74.8%). Mean ages in the Owens (sleep clinic) and Goodlin-Jones samples were 8.0 and 3.7 years, respectively. The percentage of children scoring above the SF-CSHQ cut-off was 81.1% and 67.2% in the Owens and Goodlin-Jones samples, respectively. Agreement between the percentage of cases falling above the cut-off, using the original and short form measures was higher in the Goodlin-Jones (0.80, SE= 0.07) compared to the Owens (0.41, SE= 0.08) sample. Given the predominance of SDB and parasomnia diagnoses in the Owens data (72%), this supports the utility of the SF-CSHQ as a measure of BSPs.
Table 1. Demographics and Short-Form CSHQ Scores.
| Owens (Clinic Sample) (n=151) | Goodlin-Jones (N=218) | |
|---|---|---|
| Female, n (%) | 44 (29.1%) | 55 (25.2%) |
| Male, n (%) | 107 (70.9%) | 163 (74.8%) |
| Age, Mean (sd) | 8.0 years (3.1) | 3.7 years (0.93)* |
| SF-CSHQ, Mean (SD) | 36.0 (6.8) | 33.7 (7.3) |
| SF-CSHQ, percentage above cut-off | 81.1% | 67.2% |
| Agreement (Cohen's Kappa), percentage above cutoff sleep using full CSHQ and SF-CSHQ versions | 0.41 (SE, 0.08) | 0.80 (SE, 0.07) |
Goodlin-Jones dataset contains date of birth, but not date of participation. Thus, age can not be calculated. Age data shown are for the n=194 children reported on in Goodlin-Jones, 2008.
In the Goodlin-Jones data, we found that E(reduced score)= -4.12 + 0.83*full score, R2 = 0.94. Accordingly, a threshold value of 41 for the full score corresponds to a reduced scale score of approximately 30. In the Owens data, we found that E(reduced score)= -6.1 + 0.80* (full score), R2 = 0.89. The somewhat lower strength of association may reflect the treatment of questionnaire item non-response. In the Owens data, missing item responses were imputed when < 20% of items were missing. In contrast, in the Goodlin-Jones data, only participants providing complete data on all items were included in the scoring.
By developmental status, the full CSHQ (cutoff= 41) and SF-CSHQ (cutoff= 30) yielded comparable rates of sleep problems in the Goodlin-Jones data. These full CSHQ vs. SF-CSHQ rates were, respectively, 70 % vs. 72% for children with autism, 74% vs. 68% for children with developmental delay, and 59% vs. 62% for typically developing children. Overall, SF-CSHQ scores were higher in the Owens sleep clinic (Mean= 36.0, SD= 6.8) versus Goodlin-Jones community and neuro-developmental sample (Mean= 33.7, SD= 7.3). There were no differences in SF-CSHQ scores by gender. [Data not shown.]
Using the Goodlin-Jones “Yes vs. No” global measure as the “gold standard” we calculated the sensitivity, specificity and area under the ROC curve for both CSHQ versions (Table 2). Using a threshold value of 41 for the full-scale score, the CSHQ has sensitivity 90.8% and specificity 45.5%. Using a threshold value of 30, the SF-CSHQ has sensitivity 89.2% and specificity 44.6%. For the full-scale score, the area under the ROC curve was 0.80 (95% CI= 0.74-0.87). For the SF-CSHQ, it was 0.79 (95% CI= 0.73-0.86) for the SF-CSHQ. Box-whisker plots of the two scale distributions in each group of children are shown in Figure 1.
Table 2. Validity Measures of Full and SF-CSHQ vs. Parental Report (Yes/No) of Sleep Problem.
| Goodlin-Jones (N=218) | |
|---|---|
| Full CSHQ | |
| Sensitivity | 90.8% |
| Specificity | 45.5% |
| Area under ROC | 0.80 (95%CI, 0.74-0.87) |
| Short Form (SF) CSHQ | |
| Sensitivity | 89.2% |
| Specificity | 44.6% |
| Area under ROC | 0.79 (95% CI, 0.73-0.86) |
Figure 1. Distributions of Full and SF-CSHQ Scores by Parental Report (Yes/No) of Sleep Problem.
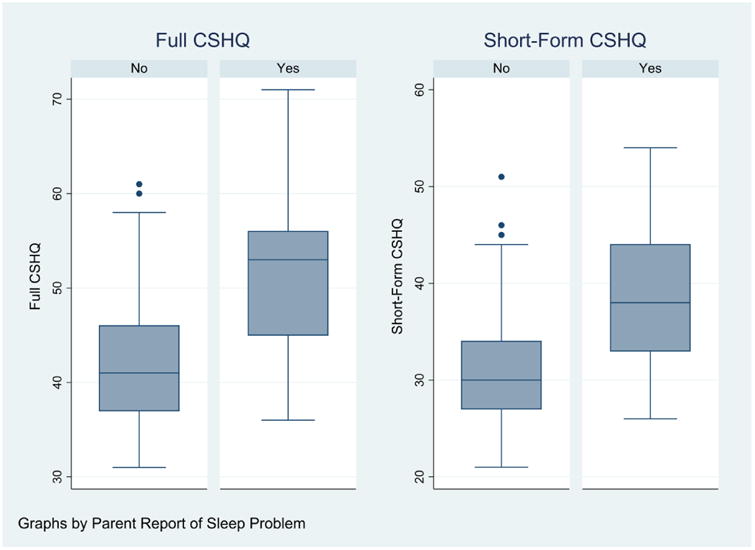
Associations between actigraphy measures (Goodline-Jones data) and the CSHQ are shown in Table 3. The correlation of the continuous SF-CSHQ score with sleep latency was statistically significant: r=0.26, p<.05. [For the continuous full-scale score, correlation to sleep latency was r=0.24 (p<.05)]. Correlations with other actigraph measures were small and did not reach significance. Sleep latency was longer among children whose SF-CSHQ score exceeded the newly derived cut-off (41 min, SD= 30), than those whose scores did not (34.8 min, SD= 18.8). As with linear measures, the dichotomous measure of the SF-CSHQ did not discriminate children by the number of night-wakings, awake time, or sleep efficiency. Use of the full-scale dichotomized CSHQ (≤ 41) produced comparable results for these actigraphy-derived measures.
Table 3. Actigraphy Associations with Full and Short Form (SF) CSHQ, by Linear and Dichotomous CSHQ Measures.
| Linear | ||||||
|---|---|---|---|---|---|---|
| Goodlin-Jones Data | Full CSHQ (linear) (Pearson r) | SF-CSHQ (linear) (Pearson r) | Full CSHQ≥41 Mean (SD) | Full-CHSQ < 41 Mean (SD) | SF-CSHQ ≥ 30 Mean (SD) | SF-CSHQ < 30 Mean (SD) |
| Sleep Efficiency: Percent of time in bed that person is awake | -0.13 | -0.12 | 89.0 (6.2) | 89.8 (6.8) | 89.2 (6.3) | 89.5 (6.7) |
| Night-Waking: Duration in minutes | -0.04 | -0.08 | 2.9 (1.8) | 3.3 (2.3) | 2.9 (1.8) | 3.4 (2.3) |
| Awake Time: Per 24 hour clock | 0.07 | 0.04 | 21.4 (8.0) | 20.2 (17.6) | 21.0 (18.0) | 20.9 (17.7) |
| Sleep Latency: Minutes to fall asleep | 0.24* | 0.26* | 41.9 (29.7) | 33.5 (18.1) | 41 min (30)* | 34.8 min (18.8)* |
p<.05
In the Owens sample, correlations between the SF-CSHQ, sleep medications, and sleep variables derived from the full CSHQ (total sleep time, sleep efficiency, and night-waking duration) were low, as shown in Table 4. Note, this sample consists primarily of children with a parasomnia or PSG-confirmed SDB (72% vs. 28% with a BSP). In the subsample of children with PSG-confirmed SDB, we confirmed that the SF-CSHQ can identify sleep problems independent of SDB, because it has very low correlations with both the SDB related subscale of the CSHQ [r=-0.01, p=0.90] and the apnea-hypopnea index [r=-0.09, p=0.19] of the PSG. By contrast the SDB items of the CSHQ omitted from the SF-CSHQ correlate moderately strongly with the AHI, r= 0.29, p=0.02 (not shown). Additionally, in this Owens subsample of children with PSG-confirmed SDB, the correlations between SF-CSHQ scores and sleep behaviors (i.e., sleep efficiency, wake duration) were low and not significant. These results favor convergent discriminant validity of the SF-CSHQ as a pure measure of sleep disturbance (not shown).
Table 4. SF-CSHQ, Sleep Medication and Sleep Measure Correlations: Owens Sleep Clinic Sample (n= 151).
| SF-CSHQ | Sleep Medications | Total Sleep Time | Sleep Efficiency | Wake Duration | |
|---|---|---|---|---|---|
| SF-CSHQ | 1.0000 | ||||
| Sleep Medications | 0.04 P = .81 N = 39 |
1.0000 | |||
| Total Sleep Time | -0.49 P = 0.70 N = 103 |
-0.03 P = 0.87 N = 40 |
1.0000 | ||
| Sleep Efficiency | 0.09 P = 0.36 N = 99 |
-0.02 P = 0.90 N = 39 |
0.47 P < 0.005 N = 144 |
1.0000 | |
| Wake Duration | -0.10 P = 0.34 N = 100 |
0.05 P < 0.76 N = 37 |
-0.33 P < 0.005 N = 143 |
-0.46 P < 0.005 N = 139 |
1.0000 |
Discussion
Criterion-referenced tools are needed to identify children with mild BSPs for whom brief interventions are often effective. The CSHQ is the most commonly used tool to screen for multi-dimensional sleep problems in children. All but its parasomnia and SDB domains reflect areas of concern that are primarily rooted in parent-child interactions, and are responsive to behavioral interventions. We re-analyzed data from two datasets used to validate the CSHQ- initially and in younger children-to determine a short form CSHQ (SF-CSHQ) cut-off score excluding parasomnia and SDB items, and to establish its preliminary validity as a screener for parent-reported BSPs. In both datasets, a score of 30 had correlations of 0.90-0.94, based on 23 of the original 33 items. The SF-CSHQ cut-off score functioned as well as the full CSHQ cut-off in discriminating between children with vs. without a parent-reported sleep problem, and between children with vs. without prolonged sleep latency as measured by actigraphy. (12, 26, 34) Furthermore, there was higher agreement between the two versions re: the percentage of positive screens in the Goodlin-Jones sample, compared with the full Owens sample which was predominately children with diagnosed SDB or parasomnia.
The SF-CSHQ shared the limited ability of the full CSHQ to predict actigraph outcomes, similar to studies comparing parent reports with objective measures.(40, 41) High correlations are found between actigraphy and sleep logs for sleep onset and wake-times (42-44); correlations for night-waking are less robust.(41, 45) Regarding PSG, studies find significant associations with the CSHQ (40) while others do not. (41) Objective measures emphasize sleep quality. Subjective reports reflect what is impactful for parents,(46) and what may respond to sleep hygiene education. CSHQ insomnia subscale scores improve after parent education, as do actigraphy measures.(31) Even for SDB, parental assessments were better predictors of SDB resolution than either PSG, clinical, or lab measures.(47) Thus, subjective assessments have a role distinct from that of objective measures.
This study has a number of strengths. We obtained and analyzed original data from two datasets that are key to validation of the CSHQ, originally and in younger children. Both included an objective assessment of sleep (actigraphy in one, PSG in the other) that we utilized in analysis. Furthermore, both datasets were utilized both to determine the SF-CSHQ threshold score, and to validate its use. The study also has limitations. Data for both were collected at least a decade earlier. Pediatric sleep medicine has seen changes in respiratory scoring of PSG,(48) and continued lack of standardized scoring for actigraphy.(49) Also, differences between the datasets in regarding measures (e.g., developmental diagnoses in one but not the other) and analysis (e.g., one suppressed an entire subscale's data if any one of its items was missing, the other did not) precluded conducting the exact same analyses in both. Conversely, such triangulation of data sources and methods, tends to strengthen the validity and applicability of findings.
One in four young children experience insomnia-related sleep difficulties. Most are transient and respond to brief educational interventions to improve sleep hygiene. Practically speaking, the SF-CSHQ is inexpensive, quick to administer, does not require computer scoring or unique credentialing and thus may be a viable screening option in wide variety of settings. Next steps might include further refinement of the SF-CSHQ such as: validation using more recent data, identification of the most discriminative items, and examination of its clinical utility. Such work would lend confidence to adding the SF-CSHQ to the toolkit of resources non-sleep specialists could use to widen secondary prevention efforts.
Even with further validation of the SF-CSHQ, there remains few evidence-based protocols (vs. information or advice) that non-sleep specialists can use to manage common sleep problems once identified. (10) A recent workshop highlighted the gap between knowledge in sleep science, and implementation of preventive interventions. One recommendation was to bolster educational interventions aimed at promoting awareness of the adverse health, functional outcomes and costs of sleep problems.(50) An ongoing study to promote sleep health literacy throughout early childhood programs seeks to bridge this gap. (51) Further work like this is needed to bridge the knowledge/action gap among school-aged children and adolescents.(52) Our findings support use of the SF-CSHQ to screen for behavioral sleep problems in settings where appropriate referral and/or delivery of evidence-based protocols by appropriately trained non-sleep specialists is available.
Acknowledgments
Funding: National Institutes of Health R01HD082129
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Karen A. Bonuck, Department of Family and Social Medicine, Albert Einstein College of Medicine, 1300 Morris Park Avenue, Bronx, NY 10461.
Beth L. Goodlin-Jones, Department of Psychiatry & Behavioral Sciences, UC Davis MIND Institute, Sacramento, CA 95817.
Clyde Schechter, Department of Family and Social Medicine, Albert Einstein College of Medicine, 1300 Morris Park Avenue, Bronx, NY 10461.
Judith Owens, Department of Neurology, Harvard Medical School, 25 Shattuck Street, Boston MA 02115.
References
- 1.Moore M, Bonuck K. Comorbid symptoms of sleep-disordered breathing and behavioral sleep problems from 18-57 months of age: a population-based study. Behav Sleep Med. 2013;11(3):222–30. doi: 10.1080/15402002.2012.666219. [DOI] [PubMed] [Google Scholar]
- 2.Owens JA, Mindell JA. Pediatric Insomnia. Pediatric clinics of North America. 2011;58(3):555–+. doi: 10.1016/j.pcl.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 3.American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 4.American Academy of Sleep Medicine, editor. International Classification of Sleep Disorders, Revised; diagnostic and coding Manual. 2nd. Illinois: 2005. [Google Scholar]
- 5.Angriman M, Caravale B, Novelli L, Ferri R, Bruni O. Sleep in children with neurodevelopmental disabilities. Neuropediatrics. 2015;46(3):199–210. doi: 10.1055/s-0035-1550151. [DOI] [PubMed] [Google Scholar]
- 6.Grigg-Damberger M, Ralls F. Treatment strategies for complex behavioral insomnia in children with neurodevelopmental disorders. Current opinion in pulmonary medicine. 2013;19(6):616–25. doi: 10.1097/MCP.0b013e328365ab89. [DOI] [PubMed] [Google Scholar]
- 7.Turnbull K, Reid GJ, Morton JB. Behavioral Sleep Problems and their Potential Impact on Developing Executive Function in Children. Sleep. 2013;36(7):1077–84. doi: 10.5665/sleep.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson KE, Miller AL, Bonuck K, Lumeng JC, Chervin RD. Evaluation of a sleep education program for low-income preschool children and their families. Sleep. 2014;37(6):1117–25. doi: 10.5665/sleep.3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hiscock H, Sciberras E, Mensah F, Gerner B, Efron D, Khano S, et al. Impact of a behavioural sleep intervention on symptoms and sleep in children with attention deficit hyperactivity disorder, and parental mental health: randomised controlled trial. BMJ (Clinical research ed) 2015;350:h68. doi: 10.1136/bmj.h68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allen SL, Howlett MD, Coulombe JA, Corkum PV. ABCs of SLEEPING: A review of the evidence behind pediatric sleep practice recommendations. Sleep medicine reviews. 2015;29:1–14. doi: 10.1016/j.smrv.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Moreno MA. Sleep Terrors and Sleepwalking: Common Parasomnias of Childhood. JAMA pediatrics. 2015;169(7):704. doi: 10.1001/jamapediatrics.2014.2140. [DOI] [PubMed] [Google Scholar]
- 12.Spruyt K, Gozal D. Pediatric sleep questionnaires as diagnostic or epidemiological tools: A review of currently available instruments. Sleep medicine reviews. 2011;15(1):19–32. doi: 10.1016/j.smrv.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Certal V, Silva H, Carvalho C, Costa-Pereira A, Azevedo I, Winck J, et al. Model for prediction of pediatric OSA: Proposal for a clinical decision rule. The Laryngoscope. 2015 doi: 10.1002/lary.25438. [DOI] [PubMed] [Google Scholar]
- 14.De Luca Canto G, Singh V, Major MP, Witmans M, El-Hakim H, Major PW, et al. Diagnostic capability of questionnaires and clinical examinations to assess sleep-disordered breathing in children: a systematic review and meta-analysis. Journal of the American Dental Association (1939) 2014;145(2):165–78. doi: 10.14219/jada.2013.26. [DOI] [PubMed] [Google Scholar]
- 15.Kaditis AG, Alonso Alvarez ML, Boudewyns A, Alexopoulos EI, Ersu R, Joosten K, et al. Obstructive sleep disordered breathing in 2-18 year-old children: diagnosis and management. The European respiratory journal. 2015 doi: 10.1183/13993003.00385-2015. [DOI] [PubMed] [Google Scholar]
- 16.Boerner KE, Coulombe JA, Corkum P. Core competencies for health professionals' training in pediatric behavioral sleep care: a Delphi study. Behav Sleep Med. 2015;13(4):265–84. doi: 10.1080/15402002.2013.874348. [DOI] [PubMed] [Google Scholar]
- 17.Quach J, Hiscock H, Ukoumunne O, Wake M. A Brief Sleep Intervention Improves Outcomes in the School Entry Year: A Randomized Controlled Trial. Pediatrics. 2011 doi: 10.1542/peds.2011-0409. [DOI] [PubMed] [Google Scholar]
- 18.Schlarb AA, Brandhorst I, H M. Mini-KiSS--a multimodal group therapy intervention for parents of young children with sleep disorders: a pilot study [article in German] Z Kinder Jugendpsychiatr Psychother. 2011;39(3):197–206. doi: 10.1024/1422-4917/a000106. [DOI] [PubMed] [Google Scholar]
- 19.Mindell JA, Du Mond CE, Sadeh A, Telofski LS, Kulkarni N, G E. Efficacy of an internet-based intervention for infant and toddler sleep disturbances. Sleep medicine reviews. 2011;34(4):451–8. doi: 10.1093/sleep/34.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonuck K, Rao T, Xu L. Pediatric Sleep Disorders and Special Educational Need at 8 Years: A Population-Based Cohort Study. Pediatrics. 2012;130(4):1439–45. doi: 10.1542/peds.2012-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meltzer LJ, Mindell JA. Systematic Review and Meta-Analysis of Behavioral Interventions for Pediatric Insomnia. Journal of pediatric psychology. 2014;39(8):932–48. doi: 10.1093/jpepsy/jsu041. [DOI] [PubMed] [Google Scholar]
- 22.Vriend J, Corkum P. Clinical management of behavioral insomnia of childhood. Psychology research and behavior management. 2011;4:69–79. doi: 10.2147/PRBM.S14057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fava GA, Tomba E, Sonino N. Clinimetrics: the science of clinical measurements. Int J Clin Pract. 2012;66(1):11–5. doi: 10.1111/j.1742-1241.2011.02825.x. [DOI] [PubMed] [Google Scholar]
- 24.Owens J, Spirito A, McGuinn M. The Children's Sleep Habits Questionnaire (CSHQ): Psychometric properties of a survey instrument for school-aged children. Sleep. 2000;23(8):1043–51. [PubMed] [Google Scholar]
- 25.Li SH, Jin XM, Shen XM, Wu SH, Jiang F, Yan CH, et al. Development and psychometric properties of the Chinese version of Children's Sleep Habits Questionnaire. Zhonghua Er Ke Za Zhi. 2007;45(3):176–80. [PubMed] [Google Scholar]
- 26.Waumans RC, Terwee CB, Van den Berg G, Knol DL, Van Litsenburg RR, Gemke RJ. Sleep and sleep disturbance in children: Reliability and validity of the Dutch version of the Child Sleep Habits Questionnaire. Sleep. 2010;33(6):841–5. doi: 10.1093/sleep/33.6.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silva FG, Silva CR, Braga LB, Neto AS. Portuguese Children's Sleep Habits Questionnaire - validation and cross-cultural comparison. J Pediatr (Rio J) 2014;90(1):78–84. doi: 10.1016/j.jped.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 28.Lucas-de la Cruz L, Martínez-Vizcaino V, Álvarez-Bueno C, Arias-Palencia N, Sánchez-López M, Notario-Pacheco B. Reliability and validity of the Spanish version of the Children's Sleep Habits Questionnaire (CSHQ-SP) in school-age children. Child: Care, Health and Development. 2016;42:675–82. doi: 10.1111/cch.12357. [DOI] [PubMed] [Google Scholar]
- 29.Bastida-Pozuelo MF, Sanchez-Ortuno MM. Preliminary analysis of the concurrent validity of the Spanish translation of the BEARS sleep screening tool for children. J Psychiatr Ment Health Nurs. 2016;23(8):513–20. doi: 10.1111/jpm.12338. [DOI] [PubMed] [Google Scholar]
- 30.Reed HE, McGrew SG, Artibee K, Surdkya K, Goldman SE, Frank K, et al. Parent-Based Sleep Education Workshops in Autism. Journal of child neurology. 2009;24(8):936–45. doi: 10.1177/0883073808331348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Veatch OJ, Reynolds A, Katz T, Weiss SK, Loh A, Wang L, et al. Sleep in Children With Autism Spectrum Disorders: How Are Measures of Parent Report and Actigraphy Related and Affected by Sleep Education? Behav Sleep Med. 2015:1–12. doi: 10.1080/15402002.2015.1065408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goodlin-Jones BL, Sitnick SL, Tang K, Liu JY, Anders TF. The children's sleep habits questionnaire in toddlers and preschool children. Journal of Developmental and Behavioral Pediatrics. 2008;29(2):82–8. doi: 10.1097/dbp.0b013e318163c39a. [DOI] [PubMed] [Google Scholar]
- 33.Nunnally J, B L. Psychometric theory. New York: McGraw-Hill Higher, INC.; 1994. [Google Scholar]
- 34.Sneddon P, Peacock GG, Crowley SL. Assessment of Sleep Problems in Preschool Aged Children: An Adaptation of the Children's Sleep Habits Questionnaire. Behav Sleep Med. 2013;11(4):283–96. doi: 10.1080/15402002.2012.707158. [DOI] [PubMed] [Google Scholar]
- 35.Goodlin-Jones BL, Tang K, Liu J, Anders TF. Sleep patterns in preschool-age children with autism, developmental delay, and typical development. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47(8):930–8. doi: 10.1097/CHI.ObO13e3181799f7c. [DOI] [PubMed] [Google Scholar]
- 36.Honomichl RD, Goodlin-Jones BL, Burnham M, Gaylor E, Anders TF. Sleep patterns of children with pervasive developmental disorders. Journal of autism and developmental disorders. 2002;32(6):553–61. doi: 10.1023/a:1021254914276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lycett K, Mensah FK, Hiscock H, Sciberras E. Comparing subjective measures of behavioral sleep problems in children with ADHD: a cross-sectional study. Sleep Med. 2015;16(11):1377–80. doi: 10.1016/j.sleep.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 38.Quach J, Hiscock H, Canterford L, Wake M. Outcomes of child sleep problems over the school-transition period: Australian population longitudinal study. Pediatrics. 2009;123(5):1287–92. doi: 10.1542/peds.2008-1860. [DOI] [PubMed] [Google Scholar]
- 39.Education. UoGCoPHIfE-BHP. Evidence-Based Practice for the Health Professions. 2016 Oct 25;Chapter 4 Section 4.6. [Google Scholar]
- 40.Malow BA, Marzec ML, McGrew SG, Wang L, Henderson LM, Stone WL. Characterizing sleep in children with autism spectrum disorders: a multidimensional approach. Sleep. 2006;29(12):1563–71. doi: 10.1093/sleep/29.12.1563. [DOI] [PubMed] [Google Scholar]
- 41.Markovich AN, Gendron MA, Corkum PV. Validating the Children's Sleep Habits Questionnaire Against Polysomnography and Actigraphy in School-Aged Children. Frontiers in psychiatry. 2014;5:188. doi: 10.3389/fpsyt.2014.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iwasaki M, Iwata S, Iemura A, Yamashita N, Tomino Y, Anme T, et al. Utility of Subjective Sleep Assessment Tools for Healthy Preschool Children: A Comparative Study Between Sleep Logs, Questionnaires, and Actigraphy. Journal of Epidemiology. 2010;20(2):143–9. doi: 10.2188/jea.JE20090054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sadeh A. A brief screening questionnaire for infant sleep problems: validation and findings for an Internet sample. Pediatrics. 2004;113(6):e570–7. doi: 10.1542/peds.113.6.e570. [DOI] [PubMed] [Google Scholar]
- 44.Sekine M, Chen X, Hamanishi S, Wang H, Yamagami T, Kagamimori S. The validity of sleeping hours of healthy young children as reported by their parents. Journal of epidemiology / Japan Epidemiological Association. 2002;12(3):237–42. doi: 10.2188/jea.12.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holley S, Hill CM, Stevenson J. A comparison of actigraphy and parental report of sleep habits in typically developing children aged 6 to 11 years. Behav Sleep Med. 2010;8(1):16–27. doi: 10.1080/15402000903425462. [DOI] [PubMed] [Google Scholar]
- 46.Dayyat EA, Spruyt K, Molfese DL, Gozal D. Sleep estimates in children: parental versus actigraphic assessments. Nature and science of sleep. 2011;3:115–23. doi: 10.2147/NSS.S25676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chervin RD, Ellenberg SS, Hou X, Marcus CL, Garetz SL, Katz ES, et al. Prognosis for Spontaneous Resolution of OSA in Children. Chest. 2015;148(5):1204–13. doi: 10.1378/chest.14-2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nixon GM, Hyde M, Biggs SN, Walter LM, Horne RS, Davey MJ. The impact of recent changes to the respiratory scoring rules in pediatrics. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2014;10(11):1217–21. doi: 10.5664/jcsm.4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meltzer LJ, Westin AM. A comparison of actigraphy scoring rules used in pediatric research. Sleep Med. 2011;12(8):793–6. doi: 10.1016/j.sleep.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parthasarathy S, Carskadon MA, Jean-Louis G, Owens J, Bramoweth A, Combs D, et al. Sleep. 2016. Implementation of Sleep and Circadian Science: Recommendations from the Sleep Research Society and National Institutes of Health Workshop. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bonuck KA, Blank A, True-Felt B, Chervin R. Promoting Sleep Health Among Families of Young Children in Head Start: Protocol for a Social-Ecological Approach. Preventing chronic disease. 2016;13:E121. doi: 10.5888/pcd13.160144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gruber R. School-based sleep education programs: A knowledge-to-action perspective regarding barriers, proposed solutions, and future directions. Sleep medicine reviews. 2016 doi: 10.1016/j.smrv.2016.10.001. [DOI] [PubMed] [Google Scholar]


