Highlights
-
•
Biochar increased CO2 emissions, reduced CH4 soil uptake, and reduced N2O emissions.
-
•
N2O decreased the most (42%) where all the three amendments were present.
-
•
Decreased in EI with biochar in low fertility soils is mainly through greater net primary productivity.
-
•
Biochar alone decreased SOC but increase it when applied together with urea and Tithonia.
Keywords: Biochar, Greenhouse gasses, Emission Intensity, Carbon cycling, Ultisol
Abstract
Biochar has been shown to reduce soil emissions of CO2, CH4 and N2O in short-term incubation and greenhouse experiments. Such controlled experiments failed to represent variable field conditions, and rarely included crop growth feedback. The objective of this study was to assess the effect of biochar, in comparison to green manure and mineral nitrogen, on greenhouse gas Emissions Intensity (EI = emissions in CO2 equivalents per ton of grain yield) in a low-fertility tropical Ultisol. Using a field trial in western Kenya, biochar (0 and 2.5 t ha−1; made from Eucalyptus wood) was integrated with urea (0 and 120 kg N ha−1) and green manure (Tithonia diversifolia; 0, 2.5 and 5 t ha−1) in a factorial design for four consecutive seasons from October 2012 to August 2014. Compared to the control, biochar increased soil CO2 emissions (9–33%), reduced soil CH4 uptake (7–59%) and reduced soil N2O emissions (1–42%) in each season, with no seasonal differences. N2O emissions increased following amendment with T. diversifolia (6%) and urea (13%) compared to the control. Generally, N2O emissions decreased where only biochar was applied. The greatest decrease in N2O (42%) occurred where all three amendments were applied compared to when they were added separately. EI in response to any of the amendments was lower than the control, ranging from 9 to 65% (33.0 ± 3.2 = mean ± SE). The amendments increased SOC stocks by 0.1–1.2 t ha−1 year−1 (mean ± SE of 0.8 ± 0.09 t ha−1 year−1). The results suggest decreased net EI with biochar in low fertility soils mainly through greater net primary productivity (89% of the decrease).
1. Introduction
The search for climate-smart agricultural production technologies is directing research to identify innovations that address multiple benefits such as crop productivity, carbon sequestration and mitigation of soil-atmosphere greenhouse gas (GHG) emissions. Addition of biochar (pyrogenic organic matter) to agricultural soils as a management strategy has reportedly increased crop yields in several studies but has shown variable effects on GHG fluxes (Knoblauch et al., 2011, Cayuela et al., 2014). Biochar may affect fluxes of GHGs such as CO2, CH4 and N2O by a variety of mechanisms, including: (i) the turnover rate of soil organic matter (SOM), which in turn determines the availability of C and N, the precursors for GHG production or consumption, (ii) soil physical properties (e.g. gas diffusivity, aggregation, water retention) (Quin et al., 2014); (iii) soil chemical properties (e.g. pH, Eh, availability of organic and mineral N and dissolved organic C, organo- mineral interactions); and (iv) soil biological properties (e.g. microbial community structure, microbial biomass and activity, macro-fauna activity, N cycling enzymes) (Van Zwieten et al., 2010).
Biochar may also change the effects of adding easily mineralizable organic matter as well as mineral N fertilizers on GHG emissions from soil. Additions of legume materials as a fertilizer provide both N and C that typically lead to greater GHG emissions from soils (Gentile et al., 2009). Similarly, the amount of fertilizer N additions is considered proportional to the N2O emissions (Manzoni and Porporato, 2009, Mori and Hojito, 2011). It is not clear, if simultaneous addition of biochar with either fertilizer N or legume mulch or a combination if the two may result in GHG emission reductions. Uncertainty also exists whether any emission reductions would persist over several cropping seasons.
It is not clear what role possible feedback through enhanced crop growth plays to the GHG budget. Greater crop growth and presumably greater C return to soil have been found where the pH is increased by biochar to neutral values (Jeffery et al., 2015) and this feedback would therefore be expected to be greatest in acid tropical soils. Whereas Spokas (2013) suggested that biochar has mainly shorter-term GHG mitigation effect (few days to several weeks) after application, Lentz et al. (2014) indicated that the effects may be long-lived. As such, questions remain concerning the long-term implications in cropping periods particularly for field-based biochar studies. Zimmerman et al. (2011) observed that native SOM mineralization was higher during the early incubation stage (first 90 days) and low during the later incubation stage (250–500 days). Maestrini et al. (2014) also reported pyrogenic OM (PyOM) to have promoted native OM mineralization during the first 18 days and inhibited it afterward (up to 150 days).
The objectives of this study were to determine the effect of biochar on (i) GHG fluxes (CO2, CH4 and N2O), (ii) Emissions Intensity (EI; the net CO2-equivalent for CH4 and N2O per ton of grain yield), and (iii) changes in soil organic carbon (SOC) and ecosystem carbon balance of a low-fertility tropical agricultural soil when integrated with organic and mineral N inputs. The overall hypothesis is that biochar is responsible for controlling the release of labile N and C from high N mineral and organic amendments and an accompanying reduction in CO2, CH4 and N2O emissions. Specifically, we hypothesize that compared to un-amended soil, biochar (i) reduces availability of N from both organic and mineral sources such as T. diversifolia and urea to thereby reduce N2O emission resulting from interactions of N2O with biochar; (ii) increases availability of easily mineralizable C from both soil and organic amendment to reduce the CH4 soil sink; (iii) affects emissions of CH4 and N2O early on, but not in later seasons as active surfaces get saturated with time; and (iv) increases plant growth as a result of biochar additions that are more important than changes in other soil processes affecting GHG emissions.
2. Materials and methods
2.1. Study site
The field experiment was established in September 2012 at Kapsengere on the southern Nandi hills in western Kenya (00′ 09′ 34″N and 34′ 57′ 37″E). The site receives ∼2000 mm mean annual rainfall in a bimodal distribution, with two growing seasons per year, March–July and September–January. The mean annual temperature is 26 °C. The soils are classified as Typic Kandiudults (USDA, 1999) developed on biotite-gneiss parent material. The experimental field was divided into three blocks. Soil properties before the experiment were determined by taking two samples from each block (six composite samples in total). The composite sample was obtained by mixing soil taken at four random locations. These were assumed to adequately represent the entire field where the experiment was established. The soil samples were analyzed using methods described in Fungo et al. (2014); in addition, particle size distribution was determined by the hydrometer method (Soil Texture Unit 1067; LaMotte Co., Chestertown, MD, USA) (soil properties in Table 1). The natural vegetation is composed of tropical rainforest of the Guineo-Congolian type. The experiment was conducted for four consecutive maize growing seasons: September–December 2012; March–August 2013; September–December 2013; March–August 2014. The seasons are henceforth referred to as Short Rains 2012 (SR2012); Long Rains 2013 (LR2013); Short Rains 2013 (SR2013) and Long Rains 2014 (LR2014), respectively.
Table 1.
Physical-chemical properties of the soil (0–0.2 m) and the amendments used in the field trial in western Kenya (n = 6 replicates for soil; triplicate measurements for amendments; means with standard errors in brackets).
| Property | Biochar | Soil | Green manure (T. diversifolia) |
||||
|---|---|---|---|---|---|---|---|
| Property | |||||||
| pH | 6.3 | (0.1) | 6.0 | (0.1) | N (mg kg−1) | 21.5 | (0.5) |
| C (g kg−1) | 868 | (11) | 23.3 | (0.1) | P (mg kg−1) | 2.3 | (0.1) |
| N (g kg−1) | 27.0 | (0.9) | 21.0 | (0.9) | K (mg kg−1) | 43.2 | (1.2) |
| P (mg kg−1) | 135 | (3.7) | 9.30 | (0.2) | Ca (mg kg−1) | 13.6 | (0.2) |
| K (mg kg−1) | 1490 | (14) | 223 | (10) | Na (mg kg−1) | 72.7 | (0.9) |
| Ca (mg kg−1) | 1920 | (17) | 1950 | (10) | Fe (mg kg−1) | 951 | (10) |
| Na (mg kg−1) | 180 | (7.3) | 15.9 | (0.6) | Zn (mg kg−1) | 89.7 | (1.6) |
| Mg (mg kg−1) | 150 | (4.5) | 312 | (9.4) | Mg (mg kg−1) | 2.6 | (0.0) |
| Al (mg kg−1) | 559 | (9.8) | 939 | (16) | S (mg kg−1) | 2.5 | (0.0) |
| S (mg kg−1) | 36.5 | (1.4) | 14.0 | (0.8) | Mn (mg kg−1) | 264 | (5) |
| Fe (mg kg−1) | 164 | (5.7) | 67.2 | (1.6) | Cu (mg kg−1) | 11.0 | (0.2) |
| Zn (mg kg−1) | 108 | (2.4) | 13.5 | (0.4) | B (mg kg−1) | 53.2 | (1.6) |
| Mn (mg kg−1) | 188 | (4.9) | 782 | (14) | Mo (mg kg−1) | 1.3 | (0.0) |
| Cu (mg kg−1) | 0.77 | (0.1) | 1.97 | (0.1) | |||
| B (mg kg−1) | 1.07 | (0.0) | 0.33 | (0.0) | |||
| C.E.C (meq 100 g−1) | 18.2 | (0.6) | 16.2 | (0.5) | |||
| EC (S mm−1) | 196 | (6.5) | 88.0 | (1.2) | |||
| Silt (%) | nd | 17.5 | (0.3) | ||||
| Sand (%) | nd | 10.7 | (0.4) | ||||
| Clay (%) | nd | 71.6 | (2.0) | ||||
nd = not determined.
2.2. Biochar and green manure
Biochar was produced from eucalyptus wood by chopping and grinding to pass through a 2-mm sieve. The ground material was pyrolyzed to a maximum temperature of 550 °C using a thermostat-regulated kiln with continuous agitation to provide homogeneous charring conditions and retained at this temperature for one hour before cooling to room temperature. Green manure from T. diversifolia was prepared by cutting leaves from the field and chopping them into 50-mm lengths, air-drying and grinding to pass through a 2-mm sieve before field application. The physical and chemical characteristics of the soil were analyzed following the same procedures as in Fungo et al. (2014) and are presented in Table 1.
2.3. Experimental design
The experiment was laid out in a randomized complete block design with three replicates. Treatments included the following: two levels of biochar (0 and 2.5 t ha−1); three levels of T. diversifolia green manure (0, 2.5 and 5 t ha−1); and two levels of urea application (0 and 120 kg N ha−1) in a full factorial design (Table 2). Treatments were indicative of the range of conventional management practices of many small-holder farmers in integrated soil fertility management systems. Each treatment was established in 2 × 2-m plot separated by a one meter distance within and between rows.
Table 2.
Experimental treatments for determining the effect of biochar, T. diversifolia green manure and urea on fluxes of CO2, CH4 and N2O in a maize field in western Kenya. Biochar was applied only once at the start of the experiment while urea and tithonia were applied every season for four consecutive seasons.
| Treatment | Biochar |
T. diversifolia |
Mineral N (Urea) |
|||
|---|---|---|---|---|---|---|
| Rate (t ha−1) |
Code |
Rate (t ha−1) |
Code | Rate (kg N ha−1) |
Code | |
| 1 (B0T0U0)(Control) | 0 | B0 | 0.0 | T0 | 0 | U0 |
| 2 (B0T2.5U0) | 0 | B0 | 2.5 | T2.5 | 0 | U0 |
| 3 (B0T5U0) | 0 | B0 | 5.0 | T5 | 0 | U0 |
| 4 (B0T0U120) | 0 | B0 | 0.0 | T0 | 120 | U120 |
| 5 (B0T2.5U120) | 0 | B0 | 2.5 | T2.5 | 120 | U120 |
| 6 (B0T5U120) | 0 | B0 | 5.0 | T5 | 120 | U120 |
| 7 (B2.5T0U0) | 2.5 | B2.5 | 0.0 | T0 | 0 | U0 |
| 8 (B2.5T2.5U0) | 2.5 | B2.5 | 2.5 | T2.5 | 0 | U0 |
| 9 (B2.5T5U0) | 2.5 | B2.5 | 5.0 | T5 | 0 | U0 |
| 10 (B2.5T0U120) | 2.5 | B2.5 | 0.0 | T0 | 120 | U120 |
| 11 (B2.5T2.5U120) | 2.5 | B2.5 | 2.5 | T2.5 | 120 | U120 |
| 12 (B2.5T5U120) | 2.5 | B2.5 | 5.0 | T5 | 120 | U120 |
2.4. Management of experiment
Precipitation and air temperature were monitored throughout the experiment with the help of a weather station on site. Application of biochar was done only once at the start of the first season in October 2012. The same amounts of green manure (2.5 or 5.0 t ha−1), were applied to each plot once at the start of each season (four applications in total). Mineral N (Urea; 261 kg ha−1) was applied in two splits at a total of 120 kg N ha−1 per season; 40% at planting and 60% at 30 days-after planting. Due to the inherently low fertility of the soil, 30 kg ha−1 of P as Triple Super Phosphate (TSP) (55 kg P2O5 ha−1) and 30 kg ha−1 of K as Muriate of Potash (MoP) (45 kg K2O ha−1) were applied to each plot at the start of each season. The amendments were applied by broadcasting on the soil surface by hand and immediately incorporating into the 0.1 m top soil. In plots where the combinations were applied, biochar was applied first, followed by Tithonia and then urea. Two seeds of a maize cultivar HB 513 were planted at the start of every season at a spacing of 0.25 m within and 0.5 m between rows (40 plants per plot). Weeding was done at 30 and 50 days after planting using a hand hoe. Thinning was done during the first weeding to retain one plant per planting hole.
2.5. Gas measurements
Measurements of CO2, CH4 and N2O fluxes were conducted using a static closed chamber method (Neftel et al., 2006; Morris et al., 2013). The chamber consisted of a PVC tube (diameter = 0.3 m; height = 0.15 m) transversely divided into two parts to make a base (0.05 m) and a cover (0.1 m). The base was driven into the soil to reach ∼0.02 m below the soil surface. To ensure air-tight conditions, a rubber ring was placed between the base and the cover. A photo-acoustic infrared field gas monitor (INNOVA 1402, Lumasense Technologies A/S, Ballerup, Denmark) was used to determine the gas concentrations. The accuracy and precision of standard gas concentration measurements with Photo Acoustic Spectroscopy (PAS) and Gas Chromatography (GC) have been shown to be comparable (Jung et al., 2012, Zhao et al., 2012, Iqbal et al., 2013). The gas monitor was connected to the chamber by two 0.7 m-long teflon tubes as gas inlet and outlet. Inside the cuvette, air humidity and temperature were monitored by a digital thermo-hygrometer (PCE–313 A, Paper-Consult Engineering Group, Meschede, Germany) attached to the cover from the outside while only the sensor reached inside the chamber through a rubber screw connector. Two chambers, marked “1” and “2”, were set up in each plot. All chambers marked “1” in all the treatments were sampled on day 1 and those marked “2” were sampled the following day. The values for chamber 1 and 2 for each plot were then averaged. For each gas sampling event INNOVA recorded four measurements at 2-min intervals after closing the chamber. Flux measurements were conducted weekly except during dry periods where bi-monthly measurements were taken. Fluxes are generally constant during dry, low moisture soil conditions. A total of 53 data points were obtained and used in the analysis.
2.6. Harvesting and yield determination
Above ground biomass was considered as the sum of stover, cobs and grain. Corn ears from each plot were removed from their shucks 120 days after planting, bagged, and dried for six days in a shed. Stover in each plot was cut ∼0.02 m above the soil surface, weighed and dried in a shed for six days. All above ground residues from the plot were never returned to the plot in order to determine how much of the SOC could be credited to above ground vs. below ground inputs. After drying, ears were mechanically shelled, and cob and kernel biomass was determined for each plot. Moisture content in the samples was determined by taking biomass of five fresh randomly selected plants to the oven for 48 h at 60 °C. Soil samples were taken from a 0–0.2 m depth at the beginning and end of the experimental period. For the soil samples, total organic carbon (TOC) was determined by the dry combustion method (Elementar Vario EL, Hanau, Germany) and assumed that TOC = SOC since these acid soils have negligible amounts of carbonates. The available phosphate content was determined using the Lancaster method (RDA, 1988). Exchangeable cations were estimated using Inductively Coupled Plasma Spectroscopy (OPTIMA 4300DV, Perkin Elmer, USA) after extraction with 1N ammonium acetate (pH 7.0) using a soil:water ratio of 1:20 w/v.
2.7. Data analysis
2.7.1. Data management
Cumulative gas fluxes were obtained by calculating the area under the flux-time curve and summing the results while assuming linear changes in measurements between time intervals. Global Warming Potential (GWP, the sum of cumulative gas emissions of CH4 and N2O, multiplied by the radiative forcing factor of each gas 25 and 298, respectively, for a time horizon of 100 years) (USEPA, 2007) calculated using the following equation:
| GWP = 25 × (E-CH4) + 298 × (E-N2O) |
Where, GWP is the emission in CO2-equivalents per hectare, E-CH4 and E-N2O are the emissions of CH4 and N2O per hectare during a given year, respectively. Emissions Intensity (EI) was then obtained by dividing the cumulative CO2-equivalent of the gas fluxes by the cumulative grain yield of each treatment over the experimental period.
The cumulative emission for each treatment was derived using a linear trapezoidal rule with sampling dates as the time intervals. For seasonal comparisons, the cumulative flux was restricted to the 120 days between planting and harvesting. Changes in SOC stock were calculated as the difference between values at the beginning and end of the experiment, after subtracting the C addition from biochar and T. diversifolia.
2.7.2. Statistical analysis
Differences in the net EI for each treatment were calculated as the difference between the treatment value and that of the control. Data for CO2 was normally distributed and did not require transformation but CH4 and N2O data were natural log-transformed before ANOVA. Treatment effects and their interaction were examined with repeated-measures ANOVA using comparisons with seasons. A fixed-effect model in Stata 12 (StataCorp LP, 4905 Lakeway Drive, College Station, Texas 77845 USA) was used with a nested design with biochar and Tithonia within urea. Post hoc separation of means was done using Least Significant Difference (LSD) at a 5% level of significance using the Stata 12 statistical software.
3. Results
3.1. Dynamics of GHG fluxes
3.1.1. Daily dynamics
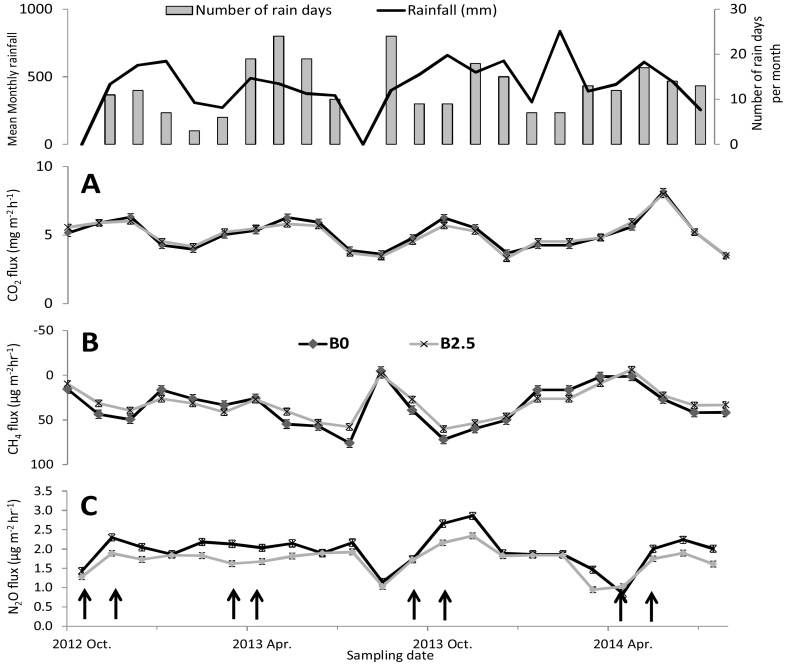
The mean daily fluxes of CO2, CH4 and N2O were 5.4 mg m−2 h−1, 39 μg m−2 h−1and 1.96 μg m−2 h−1, respectively (Fig. 1). Emissions closely followed weather patterns with higher CO2 and N2O emissions as well as lower CH4 uptake during the wet seasons. During the dry seasons, fluxes of CH4 and N2O were generally below average. Variability in daily fluxes, as expressed by the coefficient of variation (CV), was generally higher for CH4 (60%) compared to CO2 and N2O (21%). Biochar effects were not observable for daily CO2 measurements, but its effect was observed for CH4 and N2O, where it generally reduced the CH4 sink capacity of the soil and reduced N2O emission.
Fig. 1.
Weather patterns (top graph), CO2 (A), CH4 (B) and N2O (C) fluxes during four seasons of growing maize in western Kenya after amendment with mineral fertilizer (urea, 120 kg N ha−1) and green manure (T. diversifolia, 2.5 and 5.0 t ha−1) and biochar (0 or 2.5 t ha−1). Error bars are standard errors. Dates on the x-axis indicate the planting time. n = 3. Arrows at the x-axis show the dates when urea was applied.
3.1.2. Seasonal dynamics
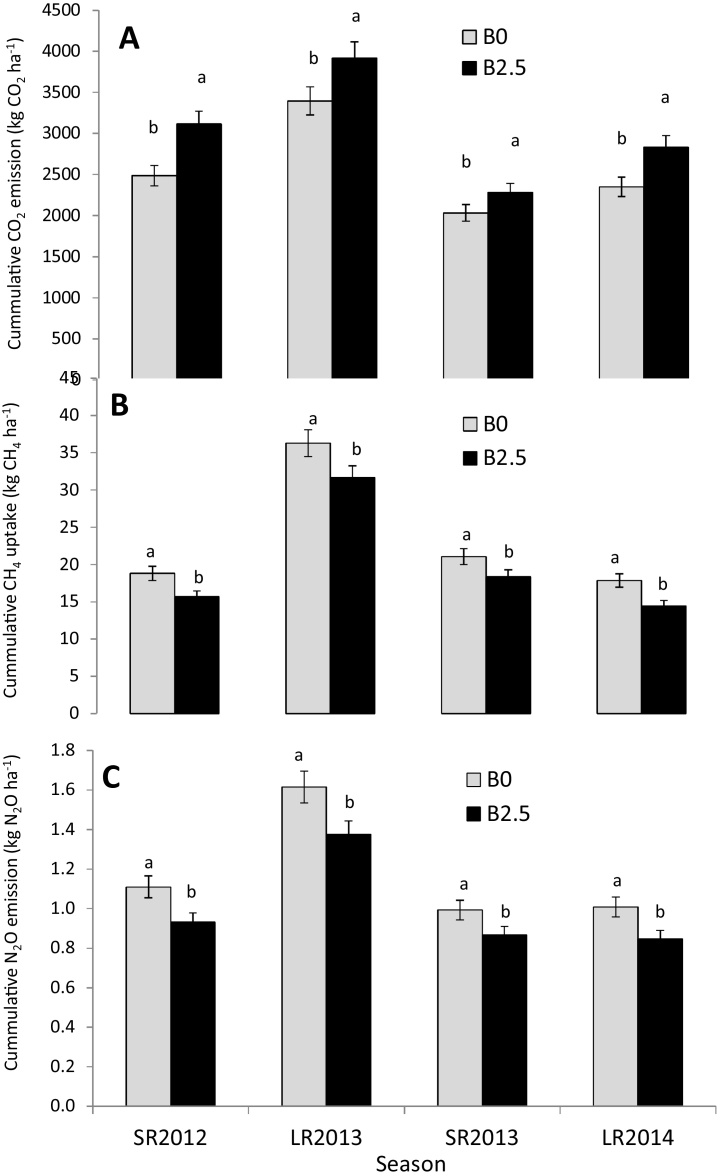
Seasonal cumulative CO2 emission increased by 17% (Fig. 2A) throughout the four seasons. CH4 uptake was reduced by 17% (Fig. 2B) in biochar-amended compared to control plots, and this reduction was observed in all the four seasons. The decrease in CH4 uptake due to biochar was maintained in all the seasons, but there was no significant difference in CH4 uptake among the seasons. Among all the seasons, LR2013 experienced the highest cumulative uptake of CH4. Consistent 15% reduction of N2O emissions were observed due to biochar additions, irrespective of season (Fig. 2C). Similar to CH4, LR2013 experienced the highest cumulative emission of N2O. Emission of N2O in this season was higher (P = 0.03) than those of all the other seasons likely because the rains were highest in this season.
Fig. 2.
Seasonal cumulative CO2, (A) emission, CH4 uptake (B), and N2O emission (C) fluxes during four-season maize trial in western Kenya after amendment with mineral fertilizer (urea) and green manure (T. diversifolia) and biochar. Within each cluster, bars with different letters are significantly different. Error bars are standard errors, n = 3.
3.1.3. Annual GHG emissions
All the three amendments, except urea (which had no effect on CH4), affected each of the GHGs (Table 3). Annual flux of CO2 was increased by all amendments (Table 3).
Table 3.
Three-way ANOVA for the effects of biochar, T. diversifolia green manure and urea on fluxes of CO2, CH4 N2O, GWP, maize grain yield and EI in maize field in western Kenya. P-values in bold show means that were significantly different p = 0.05, n = 3.
| Source of variation | CO2 |
CH4 |
N2O |
GWP |
Grain Yield |
EI |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | P | F | P | F | P | F | P | F | P | F | P | |
| Biochar | 6.11 | 0.021 | 6.29 | 0.026 | 5.65 | 0.011 | 12.5 | 0.007 | 10.5 | 0.031 | 10.6 | 0.010 |
| T. diversifolia | 12.25 | 0.000 | 3.46 | 0.047 | 5.02 | 0.015 | 10.4 | 0.025 | 1.2 | 0.96 | 12.0 | 0.017 |
| Urea | 8.62 | 0.007 | 3.01 | 0.095 | 8.43 | 0.006 | 8.0 | 0.045 | 11.4 | 0.034 | 18.0 | 0.000 |
| Biochar x T. diversifolia | 1.27 | 0.271 | 1.13 | 0.299 | 0.88 | 0.358 | 16.7 | 0.000 | 10.8 | 0.031 | 9.3 | 0.021 |
| Biochar x Urea | 1.98 | 0.160 | 1.13 | 0.339 | 4.96 | 0.037 | 11.2 | 0.016 | 10.1 | 0.038 | 15.2 | 0.000 |
| T. diversifolia x Urea | 1.67 | 0.208 | 1.04 | 0.370 | 1.27 | 0.098 | 0.0 | 0.095 | 0.0 | 0.095 | 11.2 | 0.018 |
| Biochar x T. diversifolia x Urea | 1.36 | 0.276 | 1.61 | 0.220 | 1.17 | 0.325 | 10.8 | 0.031 | 4.9 | 0.042 | 4.3 | 0.047 |
Bold numbers show significant effect at 95% level of confidence.
The increase in CO2 emissions ranged from 6 to 33%. The highest increase was observed where both T. diversifolia and urea were present (P = 0.02). On average, amendments of biochar, tithonia or urea increased CO2 emissions by 10% (P = 0.034). No interaction between either biochar and T. diversifolia or biochar and urea was observed (P > 0.05). Also, no effect of increasing T. diversifolia from 2.5 to 5.0 t ha−1 in terms of CO2 emissions was observed.
Both biochar and T. diversifolia reduced the soil uptake of CH4, while urea had no effect. Reduction in soil CH4 uptake ranged from 7 to 59% (Table 4). There was no interaction between amendments, and the level of T. diversifolia additions on CH4 uptake between the 2 and 4 t ha−1 application rates. The largest reduction in CH4 uptake was observed when biochar, tithonia and urea were added together at the highest quantities. CH4 uptake decreased progressively as more amendments were added.
Table 4.
Annual grain yield (two seasons), CH4 and N2O emissions, GWP (calculated only from CH4 and N2O) and EI of maize production over four consecutive seasons under biochar, T. diversifolia and urea amendment. Means (±S.E.) in the same column followed by different letters are significantly different at p < 0.05, n = 3.
| Treatment | CO2 (t ha−1 yr−1) |
CH4 uptake (t ha−1 yr−1) |
N2O emission (t ha−1 yr−1) |
GWP (t CO2-eq ha−1 yr−1) |
Maize Grain yield (t ha−1 yr−1) |
EI (t CO2-eq t−1 grain yr−1) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 (B0T0U0) | 3.7 | (±0.6)d | 0.061 | (±0.017)a | 0.003 | (±0.009)a | 0.67 | (±0.5)a | 14.8 | (±1.0)f | 0.05 | (±0.01)a |
| 2 (B0T2.5U0) | 4.9 | (±0.1)a | 0.057 | (±0.023)b | 0.003 | (±0.002)a | 0.57 | (±0.34)b | 15.1 | (±0.6)f | 0.04 | (±0.03)b |
| 3 (B0T5U0) | 4.8 | (±0.3)a | 0.058 | (±0.006)b | 0.003 | (±0.006)a | 0.59 | (±0.18)b | 14.9 | (±0.1)f | 0.04 | (±0.02)bc |
| 4 (B0T0U120) | 4.1 | (±0.6)ca | 0.057 | (±0.007)b | 0.003 | (±0.004)a | 0.60 | (±0.11)b | 15.9 | (±0.8)e | 0.04 | (±0.02)c |
| 5 (B0T2.5U120) | 5.0 | (±0.1)a | 0.052 | (±0.009)bc | 0.003 | (±0.007)a | 0.47 | (±0.04)c | 18.4 | (±1.4)c | 0.03 | (±0.03)c |
| 6 (B0T5U120) | 4.9 | (±0.1)c | 0.053 | (±0.010)bc | 0.002 | (±0.009)b | 0.59 | (±0.22)b | 19.1 | (±0.1)b | 0.03 | (±0.05)d |
| 7 (B2.5T0U0) | 4.0 | (±0.6)b | 0.052 | (±0.018)bc | 0.002 | (±0.005)b | 0.59 | (±0.32)b | 12.8 | (±1.8)g | 0.05 | (±0.01)a |
| 8 (B2.5T2.5U0) | 4.4 | (±0.3)a | 0.050 | (±0.006)c | 0.003 | (±0.005)a | 0.42 | (±0.06)d | 12.6 | (±1.8)g | 0.03 | (±0.06)d |
| 9 (B2.5T5U0) | 5.0 | (±0.1)b | 0.046 | (±0.029)d | 0.002 | (±0.001)b | 0.47 | (±0.18)d | 17.4 | (±1.6)d | 0.03 | (±0.01)e |
| 10 (B2.5T0U120) | 4.6 | (±0.6)b | 0.047 | (±0.014)d | 0.002 | (±0.007)b | 0.44 | (±0.36)cd | 19.8 | (±1.0)a | 0.02 | (±0.03)f |
| 11 (B2.5T2.5U120) | 4.6 | (±0.9)b | 0.055 | (±0.025)b | 0.003 | (±0.001)ab | 0.58 | (±0.34)b | 17.0 | (±3.2)d | 0.03 | (±0.01)cd |
| 12 (B2.5T5U120) | 4.4 | (±0.1)b | 0.034 | (±0.020)e | 0.002 | (±0.007)c | 0.26 | (±0.31)e | 16.9 | (±0.8)d | 0.02 | (±0.03)g |
Cumulative N2O emissions progressively decreased by up to 42% (mean ± SE of 19 ± 4.1%) where biochar was amended but did not change where T. diversifolia or urea were added on their own compared to an un-amended control (Table 4). Biochar-induced decreases in N2O emission were greatest (42%) where T. diversifolia and urea were both present compared to when they were separately applied. N2O emissions with biochar + urea additions were significantly lower than those with urea alone (Table 4). The interaction between biochar and T. diversifolia was significant only at the higher level of T. diversifolia.
3.2. Grain yield
Maize grain yields ranged from 12.6 to 19.8 t ha−1 yr−1 (mean = 16.4 t ha−1 yr−1), corresponding to a decrease of 15% and increase of 34%, respectively, compared to the control. Decreases in maize grain yield were observed where biochar was applied either alone or in combination with low amounts of T. diversifolia (B2.5T0U0, −15%, and B2.5T2.5U0, −14%, respectively). High amounts of T. diversifolia (T5) significantly increased yields by 9%. The greatest increase in maize grain yield was observed where biochar was combined with urea (B2.5T0U120, 34%). This was followed by yields where high amounts of T. diversifolia (5 t ha−1) were jointly applied with urea (treatment B0T5U120 at 29%) (Table 4). Grain yield of the combination of the three amendments was higher than either biochar or T. diversifolia applied alone (Table 4). Grain yield comprised of 35% (SD = 0.03) of total above ground biomass and this proportion was comparable for all treatments.
3.3. Global worming potential (GWP)
Biochar when applied alone caused a significantly lower GWP (8% or 6.3 ± 0.01 t CO2-eq ha−1 yr−1) compared to the control (Table 4). GWP caused by additions of T. diversifolia and urea compared to no additions (control) significantly increased by 32% and 12% corresponding to an increase by 7.7 ± 0.8 and 6.5 ± 0.6 t CO2-e ha−1 yr−1, respectively. T. diversifolia or urea additions resulted in significantly higher yield compared to the control, but were not significantly different from each other. Grain yield was significantly higher with the sole biochar addition. The interactive effect between biochar and urea increased yield significantly but no difference in yield was observed where tithonia and urea were applied together or where biochar was applied with tithonia. In a combined application of biochar, urea and T. diversifolia, doubling the amounts of T. diversifolia halved the GWP. The difference between GWP as a result of biochar + T. diversifolia and biochar + urea additions was significantly lower than the difference in the respective sole applications of T. diversifolia versus urea.
3.4. Emissions intensity (EI)
Biochar and tithonia applied alone caused significantly higher EI compared to the control, but EI as a result of urea additions was not significantly different from that of the control. There was no interactive effect between tithonia and urea application with respect to EI. Except for the interaction of 2.5 t biochar ha−1 and urea (B2.5T0U120 in Table 4), all the other treatments showed significantly higher EI than the control (p = 0.035), with increases ranging from 8 to 39%. Apart from the control, the highest EI corresponded with additions of only T. diversifolia green manure (B0T2.5U0 and B0T5U0) or urea additions (B0T0U120). The lowest EI was observed where biochar and urea were added without or with high T. diversifolia. As T. diversifolia green manure additions increased, the EI correspondingly decreased but only in the presence of biochar. The decrease in EI with greater additions of T. diversifolia corresponded to increases in CO2 emission by 14% and decreases in N2O emission by 16% (Table 4).
3.5. Changes in SOC stocks
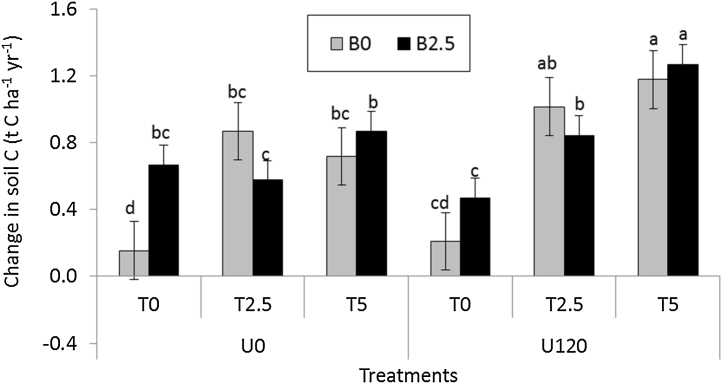
Amendments increased SOC stocks in the range of 0.7–7.1%, corresponding to 0.1–1.2 t C ha−1 year−1 (mean ± SE of 0.8 ± 0.09 t C ha−1 year−1). Except for the control (where no amendment was applied), which showed no significant difference in SOC over time, all the other treatments showed gains in SOC (Fig. 3). The smallest increment (0.2 ± 0.01 t C ha−1 yr−1) was observed without any additions while the highest increase (1.2 ± 0.04 t C ha−1 yr−1) was recorded where urea was applied with T. diversifolia irrespective of biochar additions. The increase in SOC stocks due to biochar or T. diversifolia additions was comparable (0.7 ± 0.02 t C ha−1 yr−1, or 2.8%) to the control. Soil OC stocks in response to additions of 2.5 t ha−1 of tithonia increased by 27% compared to the control, and was comparable to additions of 5 t ha−1 of tithonia. Additions of 2.5 t ha−1 of tithonia together with urea increased SOC by 39% compared to the control.
Fig. 3.
Annual changes in soil organic C stock (top 0.15 m) under biochar (B in t ha−1), T. diversifolia (T in t ha−1) and urea (U in kg N ha−1) amendments compared to the control. Means (±S.E.) followed by different letters are significantly different at p < 0.05, n = 3.
4. Discussion
4.1. Dynamics of GHGs
Stimulation of CO2 emissions following biochar addition observed in this study (Figs. 1A and 2A) concurs with what has been observed in previous studies and has been attributed to the supply of easily mineralizable C and improvement in soil physical properties for microbial activity (Lehmann et al., 2011; Sagrilo et al., 2014) and increased root respiration (Major et al., 2010). However, reduction in CO2 emission after biochar addition has also been reported in incubations without plants, and is usually associated with limited N supply due to N immobilization by amended biochar (Laird et al., 2010, Wang et al., 2015). In our case, the amount of biochar was relatively low (0.2% compared to the average 2% w/w in most studies) and could not immobilize significant amounts of soil N while crop growth significantly increased, hence the consistent increase in CO2 (that included root respiration) with all biochar plots. The lack of an interactive effect between biochar and tithonia as well as urea and tithonia with respect to CO2 emission (Table 3) was possibly due to the fact that sufficient C was supplied by biochar or T. diversifolia making further inputs temporarily unnecessary for microbial use. Contrary to our findings, Rogovska et al. (2011) found reduced CO2 emission after 500 days of incubation for a green manure-biochar mixture. The authors attributed this observation to the fact that biochar stabilized green manure C by influencing biochemical recalcitrance or physical protection of green manure C (Krull et al., 2003), or that green manure additions reduced the ability of biochar to enhance mineralization of soil organic matter. Our results did not show interactions between biochar and green manure possibly because the mixing of the two amendments was not as uniform as it was in the incubation of Rogovska et al. (2011). Whereas biochar largely remains unchanged in space, tithonia and urea may, after incorporation into the soil, leach to lower soil layers. The subsequent reactions of these amendments may thus remain independent. Some studies on placement of amendments may elucidate this notion. It is important to note that CO2 can be produced by both enhance mineralization of soil organic matter or biochar itself (Wardle et al., 2008). Our study measured only soil-atmosphere fluxes of CO2, and could not attribute the increase to a particular CO2 production process. Nonetheless, the fact that there was an increase in SOC with biochar-tithonia additions (Fig. 3) suggest an overall net positive balance towards soil C sequestration. Future studies should estimate the net effect of biochar application on soil respiration and biochar mineralization. Life cycle assessment to determine C sequestration including CO2 emitted during biochar production will also be relevant.
The upland soils are generally identified as sinks for CH4 (Chan et al., 2001) but additions of biochar and T. diversifolia in this experiment reduced the sink capacity (Figs. 1B and 2B). This can likely be attributed to the supply of easily mineralizable C that substitutes for the C oxidized by methanotrophic bacteria (Knoblauch et al., 2008; Zhang et al., 2010). The greatest reduction in CH4 uptake, coinciding with urea and high T. diversifolia input (Table 4), could be attributed to the availability of NH4+-N from urea, which is known to inhibit CH4 oxidation (Chan and Parkin, 2001, Suwanwaree and Robertson, 2005). Whereas some studies have shown increased uptake of CH4 with biochar (Liu et al., 2011, Schimmelpfennig et al., 2014), several have also shown decreases (Spokas et al., 2013; Singla and Inubushi, 2014). This suggests the complexity and variety of factors affecting CH4 fluxes in biochar-amended soils. The persistence of biochar effects on fluxes over the two years of observation (Fig. 2B) could be related to improvements in physical properties leading to better drainage and adsorption of dissolved organic carbon (Singh et al., 2012; Zhang et al., 2015) rather than N immobilization discussed above that is likely to be a transient phenomenon.
The consistent reduction in N2O with biochar amendments (Figs. 1C and 2C) is similar to previous studies (Rondon et al., 2005, Xiang et al., 2015). The reduction in N2O emissions in the present study is lower compared to other studies (such as Zhang et al., 2012a, Zhang et al., 2012b, Zhang et al., 2015) partly because the amounts of added biochar were relatively low (2.5 t ha−1 compared with 20–40 t ha−1 in all the above studies). N2O reduction could have been due to stimulation of microorganisms that can degrade more persistent SOM (Nelissen et al., 2012), resulting in more reactive surfaces. It is also plausible that adsorption of NH4+ (Taghizadeh-Toosi et al., 2012) and other potential allelochemical inhibitors of microbial metabolic pathways, such as monoterpenes and various polyphenolic compounds that are inhibitory to nitrification, could have played a role (Ball et al., 2011). That the interaction between biochar and urea (Table 3) resulted in reduced N2O emission could probably be due to a direct reaction between the biochar surfaces and N2O. Thus, the N2O emission produced from urea is countered by the biochar. Cayuela et al. (2014) have suggested the catalytic activity of biochar as capable of enhancing the reduction of N2O to N2. Direct molecular-level interaction between N2O and reducing agents has been reported (Hitoshi et al., 2002) and Fungo et al. (2014) observed that steam activation of biochar explained 56% of reduction in N2O emission from the same soil. It is plausible that such a reaction could be taking place in biochar amended soils.
The notion that a stimulatory effect of biochar on soil and/or plant respiration levels off during the first years after application (Spokas, 2013) was not seen within the two years of the experiment, as we observed consistent differences in emissions and above ground biomass production during all four cropping seasons. Several studies (e.g. Kimetu et al., 2008, Major et al., 2010, Koide et al., 2014) have shown that biochar contents remain in soil and decreased only between 0.3 and 6% of the amount applied over a period of three years. Whereas some studies (Nelissen et al., 2014) found short-lived effects of biochar (Spokas, 2013), the two-year effects observed in our study support Lentz et al. (2014), who observed a persistent effect over a 3-year period. Lentz et al. (2014) related this multi-year effect to biochar’s physical porosity and chemical binding capacity. The contrasting results in the literature indicate a need for longer-term (>5 years) studies to test this hypothesis.
Results in Fig. 2 show that biochar effects on CH4 uptake and N2O emissions can persist over two years under field conditions. This could be attributed to the fact that non-aromatic organic materials such as sugars and fats in the pores of biochar (Mukherjee et al., 2015, Koide et al., 2014) are susceptible to mineralization over time (Kim et al., 2011). According to Lentz et al. (2014), the disappearance of these organics with soil aging likely (i) increased porosity and surface area akin to the effect that activation has on charcoal (Fungo et al., 2014); and (ii) increased negatively charged sites on biochar (Cheng et al., 2008). The charged sites can bind NH4, making inorganic N only temporarily unavailable for microorganisms and leaching (Dempster et al., 2012), which can later be readily released (Wang et al., 2015). These mechanisms could have been responsible for observed persistence of biochar effect observed in this study. A change in pore size distribution in the biochar-amended soil could have influenced bacterial community composition (Lentz et al., 2014) that could conceivably also affect GHG emissions. Feng et al. (2012) reported decreased ratios of methanogenic to methanotrophic microorganisms that accounted for the decrease in CH4 emission from paddy soils. Banerjee et al. (2016) found that moisture affected microbial activity, transcription, composition and ultimately, N2O emissions. The reduced N2O mitigation effect observed by Spokas (2013) after 3 years of biochar aging could be related to the relatively large particle size (8–40 mm) of the biochar used.
4.2. Changes in soil C content
As observed in our study, a previous study carried out in the same area (Kimetu et al., 2008) found that application of biochar increased TOC contents by 6.8 times after adding biochar compared to T. diversifolia green manure. In fact, the authors observed that biochar not only remained to a greater extent unchanged, but could be protected by aggregation to a greater degree in soil in the long term. The relatively fine texture of the tested soils means that the soil may allow for more SOC protection by a combination of physical occlusion and organo-mineral interactions.
Chivenge et al. (2011a) found that in clayey soils, the addition of low quality organic resources (such as maize stover) resulted in greater stabilization of SOC and SON in the macro-aggregates, while addition of N-fertilizers enhances their decomposition and faster aggregate turnover leading to less accumulation of SOC and SON. Similar to our results, Chivenge et al. (2011b) also found no interaction between T. diversifolia with N fertilizers on similar soils with respect to changes in SOC. This suggests that microbial choice of the N source is independent of the source or that the utilization of freshly applied organic matter (C source) is consumed at the same rate as mineral N (source of N). Verchot et al. (2011) observed that most of the newly added SOC gains from green manures ended up in the coarser aggregates, and is therefore subject to turnover and loss in the event that OM inputs decline. However, using soils from the same experiment, Fungo et al. (2017) found that biochar is stored predominantly as free particulate OC in the silt and clay fraction and promoted a movement of native SOC from larger-size aggregates to the smaller-sized fraction. The increase in C stocks when T. diversifolia was added but no additive effect of T. diversifolia + biochar may be attributed to the fact that there is a balance between increased mineralization of SOM due to biochar addition on the one hand and an increase in SOC due to greater net primary productivity of root biomass on the other.
Our results (Fig. 3) show that even without returning harvested above ground biomass to the soil, any of the additions were still able to demonstrate SOC gains. It follows that returning maize stover and integrated soil fertility management with biochar can achieve even greater SOC sequestration as well as GHG emission objectives. This implies that soil amendment with organic a mineral resources increases net primary productivity and hence below ground biomass. This biomass can later build up soil organic matter stocks. In terms of climate change mitigation, this is important as it demonstrates the potential for C sequestration associated with soil management.
4.3. System C balance
In relatively low fertility soils similar to the one used in this experiment, increases in biomass production in response to biochar application have been widely reported (Major et al., 2010, Li et al., 2015). The grain and biomass yield increase may most likely be attributed in the studied acid soils to increases in direct mineral additions from biochar, improvement in soil physical properties (e.g., bulk density), greater pH and reduced Al toxicity, improved cation exchange capacity, and possibly effects on soil biota that largely remain speculative. The proportional increase in biomass is comparable to other studies on highly weathered tropical soils (Major et al., 2010, Van Zwieten et al., 2010).
The enhanced above ground biomass production that we observed (Table 4) could be due to improved soil physical and chemical properties resulting from biochar amendments. Olmo et al. (2014) found that grain production was correlated significantly and positively with soil moisture, EC, total N, Olsen P and available K, Cu and Zn, and negatively with soil compaction, consistent with the favorable changes in soil physico-chemical properties brought about by biochar additions. According to Vanlauwe et al. (2002) and Lentz et al. (2014), combining biochar with green manure more effectively utilized the two soil amendments, as it eliminates potential plant growth reductions caused by N immobilization after biochar additions and maximized green manure net N mineralization potential.
The increase in net primary productivity due to soil amendments contributes to atmospheric C capture into terrestrial C (above ground and soil organic C). However, it will be necessary for future studies to investigate other types of green manures to better understand limitations over longer periods of time. Application rates used in several other trials are unrealistic for most farming systems. Despite the relatively low application rates of both biochar and T. diversifolia used in this study compared to those of previous studies, we demonstrate that significant ecosystem C gains are practically possible in low fertility soils even with relatively moderate amounts of biochar.
The identical grain yields with additions of 2.5 t ha−1 biochar and 5 t ha−1 tithonia irrespective of urea additions (Table 4) may imply that biochar has the potential to reduce mineral N fertilizer requirements if green manure is added. In terms of GHG emissions, reduced application of mineral fertilizer is one way to reduce the impact of agriculture on climate change. This includes the avoided emissions due to fertilizer production. What remains to be determined is the trade-off between biochar production and fertilizer manufacture in terms of both cost and emissions.
Based on a yield-normalized comparison of our results (EI), biochar can potentially reduce overall GHG emission while improving crop yields and SOC. Nonetheless, the observed reduction in EI due to biochar additions needs to be further analyzed using a full Life Cycle Assessment (LCA) to account for the energy-related emissions needed to produce or transport the biochar among other emissions and emission reductions.
5. Conclusions and recommendations
We have shown that the inclusion of biochar in integrated management of low-fertility tropical agricultural soils can reduce GHG emissions and increase ecosystem C gains. The resultant C gains and GHG emission reduction benefits of biochar can be sustained for at least two consecutive years (four seasons) from the time of application under the environmental conditions studied here. This result points to the importance of plant responses for the GHG balance in biochar systems. Our results contribute to mid-term field studies but longer trials might be necessary to better understand the C balance, including different soil types and cropping systems especially where stover biomass is returned to the plot. Longer field-based studies and field studies on soil that show different plant response to organic matter additions are needed to improve understanding of linkages between nutrient use efficiency and GHG mitigation.
Acknowledgements
This study was funded by the NSF-BREAD program Grant No. IOS-09565336. Any opinions, findings, conclusions, or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the donors. Thanks to Grace Oluoch, Victor Onyango, Linda Ayieta and Benson Gudu for the support during data collection in western Kenya. The comments from the three anonymous reviewers were useful in improving this manuscript and are dully appreciated.
References
- Ball P.N., MacKenzie M.D., DeLuca T.H. Wildfire and charcoal enhance nitrification and ammonium-oxidizing bacterial abundance in dry montane forest soils. J. Environ. Qual. 2011;39:1243–1253. doi: 10.2134/jeq2009.0082. [DOI] [PubMed] [Google Scholar]
- Banerjee S., Helgason B., Wang L., Winsley T., Ferrari B.C., Siciliano S.D. Legacy effects of soil moisture on microbial community structure and N2O emissions. Soil Biol. Biochem. 2016;95:40–50. [Google Scholar]
- Cayuela M.L., van Zwieten L., Singh B.P., Jeffery S., Roig A., Sánchez-Monedero M.A. Biochar's role in mitigating soil nitrous oxide emissions: a review and meta-analysis. Agric. Ecosyst. Environ. 2014;191:5–16. [Google Scholar]
- Chan A.S.K., Parkin T.B. Methane oxidation and production activity in soils from natural and agricultural ecosystems g. J. Environ. Qual. 2001;30:1896–1903. doi: 10.2134/jeq2001.1896. [DOI] [PubMed] [Google Scholar]
- Chan A.S.K., Parkin T.B., Forest M. Methane oxidation and production activity in soils from natural and agricultural ecosystems. J. Environ. Qual. 2001:1896–1903. doi: 10.2134/jeq2001.1896. [DOI] [PubMed] [Google Scholar]
- Cheng C., Lehmann J., Engelhard M.H. Natural oxidation of black carbon in soils: changes in molecular form and surface charge along a climosequence. Geochim. Cosmochim. Acta. 2008;72:1598–1610. [Google Scholar]
- Chivenge P., Vanlauwe B., Gentile R., Six J. Comparison of organic versus mineral resource effects on short-term aggregate carbon and nitrogen dynamics in a sandy soil versus a fine textured soil. Agr. Ecosyst. Environ. 2011;140:361–371. [Google Scholar]
- Chivenge P., Vanlauwe B., Gentile R., Six J. Organic resource quality influences short-term aggregate dynamics and soil organic carbon and nitrogen accumulation. Soil Biol. Biochem. 2011;43:657–666. [Google Scholar]
- Dempster D.N., Gleeson D.B., Solaiman Z.M., Jones D.L., Murphy D.V. Decreased soil microbial biomass and nitrogen mineralization with eucalyptus biochar addition to a coarse textured soil. Plant Soil. 2012;354:311–324. [Google Scholar]
- Feng Y., Xu Y., Yu Y., Xie Z., Lin X. Soil Biology & Biochemistry Mechanisms of biochar decreasing methane emission from Chinese paddy soils. Soil Biol. Biochem. 2012;46:80–88. [Google Scholar]
- Fungo B., Guerena D., Thiongo M., Lehmann J., Neufeldt H., Kalbitz K. N2O and CH4 emission from soil amended with steam-activated biochar. J. Plant Nutr. Soil Sci. 2014;177:34–38. [Google Scholar]
- Fungo B., Lehmann J., Kalbitz K., Thiongo M., Okeyo I., Tenywa M., Neufeldt H. Aggregate size distribution in a biochar-amended tropical Ultisol under conventional hand-hoe tillage. Soil Till. Res. 2017;165:190–197. doi: 10.1016/j.still.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile R., Vanlauwe B., van Kessel C., Six J. Managing N availability and losses by combining fertilizer-N with different quality residues in Kenya. Agr. Ecosyst. Environ. 2009;131:308–314. [Google Scholar]
- Iqbal J., Castellano M.J., Parkin T.B. Evaluation of photoacoustic infrared spectroscopy for simultaneous measurement of N2O and CO2 gas concentrations and fluxes at the soil surface. Global Change Biol. 2013;19:327–336. doi: 10.1111/gcb.12021. [DOI] [PubMed] [Google Scholar]
- Jeffery S., Bezemer T.M., Cornelissen G., Kuyper T.W., Lehmann J., Mommer L., Sohi S.P., van de Voorde T.F.J., Wardle D.A., van Groenigen J.W. The way forward in biochar research: targeting trade-offs between the potential wins. Global Change Biol. 2015;7:1–13. [Google Scholar]
- Jung Y., Han B., Mostafid M.E., Chiu P., Yazdani R., Imhoff P.T. Photoacoustic infrared spectroscopy for conducting gas tracer tests and measuring water saturations in landfills. Manure Manage. 2012;32:297–304. doi: 10.1016/j.wasman.2011.09.016. [DOI] [PubMed] [Google Scholar]
- Kim E.J., Choi S.D., Chang Y.S. Levels and patterns of polycyclic aromatic hydrocarbons (PAHs) in soils after forest fires in South Korea. Environ. Sci. Pollut. Res. 2011;18:1508–1517. doi: 10.1007/s11356-011-0515-3. [DOI] [PubMed] [Google Scholar]
- Kimetu J., Lehmann J., Ngoze S., Mugendi D., Kinyangi J., Riha S., Verchot L., Recha J., Pell A. Reversibility of soil productivity decline with organic matter of differing quality along a degradation gradient. Ecosystems. 2008;11:726–739. [Google Scholar]
- Knoblauch C., Maarifat A., Pfeiffer E., Haefele S.M. Degradability of black carbon and its impact on trace gas fluxes and carbon turnover in paddy soils. Soil Biol. Biochem. 2011;43:1768–1778. [Google Scholar]
- Koide R.T., Dell C., Drohan P., Skinner H., Adler P.R. Turnover of soil carbon following addition of switchgrass-derived biochar to four soils. Soil Sci. Soc. Am. J. 2014;78:531–537. [Google Scholar]
- Laird D., Fleming P., Wang B., Horton R., Karlen D. Biochar impact on nutrient leaching from a Midwestern agricultural soil. Geoderma. 2010;158:436–442. [Google Scholar]
- Lentz R.D., Ippolito J.A., Spokas K.A. Biochar and manure effects on net nitrogen mineralization and greenhouse gas emissions from calcareous soil under corn. Soil Sci. Soc. Am. J. 2014;78:1641–1655. [Google Scholar]
- Li B., Fan C.H., Zhang H., Chen Z.Z., Sun L.Y., Xiong Z.Q. Combined effects of nitrogen fertilization and biochar on the net global warming potential, greenhouse gas intensity and net ecosystem economic budget in intensive vegetable agriculture in southeastern China. Atmos. Environ. 2015;100:10–19. [Google Scholar]
- Liu Y., Yang M., Wu Y., Wang H., Chen Y., Wu W. Reducing CH4 and CO2 emissions from waterlogged paddy soil with biochar. J. Soils Sediments. 2011;11:930–939. [Google Scholar]
- Maestrini B., Herrmann A.M., Nannipieri P., Schmidt M.W.I., Abiven S. Ryegrass-derived pyrogenic organic matter changes organic carbon and nitrogen mineralization in a temperate forest soil. Soil Biol. Biochem. 2014;69:291–301. [Google Scholar]
- Major J., Rondon M., Molina D., Riha S.J., Lehmann J. Maize yield and nutrition during 4 years after biochar application to a Colombian savanna oxisol. Plant Soil. 2010;333:117–128. [Google Scholar]
- Manzoni S., Porporato A. Soil C and nitrogen mineralization: theory and models across scales. Soil Biol. Biochem. 2009;41:1355–1379. [Google Scholar]
- Mori A., Hojito M. Effect of combined application of manure and fertilizer on N2O fluxes from a grassland soil in Nasu, Japan. Agr. Ecosyst. Environ. 2011 [Google Scholar]
- Mukherjee A., Lal R., Zimmerman A.R. Effects of biochar and other amendments on the physical properties and greenhouse gas emissions of an artificially degraded soil. Sci. Total Environ. 2015;487:26–36. doi: 10.1016/j.scitotenv.2014.03.141. [DOI] [PubMed] [Google Scholar]
- Nelissen V., Rütting T., Huygens D., Staelens J., Ruysschaert G., Boeckx P. Maize biochars accelerate short-term soil nitrogen dynamics in a loamy sand soil. Soil Biol. Biochem. 2012;55:20–27. [Google Scholar]
- Olmo M., Alburquerque J.A., Barrón V., del Campillo M.C., Gallardo A., Fuentes M., Villar R. Wheat growth and yield responses to biochar addition under Mediterranean climate conditions. Biol. Fert. Soils. 2014;50:1177–1187. [Google Scholar]
- RDA (Rural Development Administration, Korea) National Institute of Agricultural Science and Technology, RDA; Suwon: 1988. Methods of Soil Chemical Analysis. (in Korean) [Google Scholar]
- Rogovska N., Laird D., Cruse R., Fleming P., Parkin T., Meek D. Impact of biochar on manure carbon stabilization and greenhouse gas emissions. Soil Sci. Soc. Am. J. 2011;75:871. [Google Scholar]
- Rondon M.A., Ramirez J.A., Lehmann J. Greenhouse gas emissions decrease with charcoal additions to tropical soils. Proceedings of the 3rd USDA Symposium on Greenhouse Gases and Carbon Sequestration in Agriculture and Forestry. 2005:208. [Google Scholar]
- Sagrilo E., Jeffery S., Hoffland E., Kuyper T.W. Emission of CO2 from biochar-amended soils and implications for soil organic carbon. Global Change Biol. -Bioenergy. 2014;7:1294–1304. [Google Scholar]
- Schimmelpfennig S., Müller C., Grünhage L., Koch C., Kammann C. Biochar, hydrochar and uncarbonized feedstock application to permanent grassland – effects on greenhouse gas emissions and plant growth. Agr. Ecosyst. Environ. 2014;191:39–52. [Google Scholar]
- Singla A., Inubushi K. Effect of biochar on CH4 and N2O emission from soils vegetated with paddy. Paddy Water Environ. 2014;12:239–243. [Google Scholar]
- Spokas K.A. Impact of biochar field aging on laboratory greenhouse gas production potentials. Global Change Biol. Bioenergy. 2013;5:165–176. [Google Scholar]
- Suwanwaree P., Robertson G.P. Methane oxidation in forest, successional, and No-till agricultural ecosystems. Eff. Nitrogen Soil Disturb. 2005:1722–1729. [Google Scholar]
- Taghizadeh-Toosi, A., Clough, T.J., Sherlock, R.R., Condron, L.M., 2012. A wood based low-temperature biochar captures NH 3-N generated from ruminant urine-N, retaining its bioavailability, 353, 73–84.
- USEPA . USEPA; Washington, DC: 2007. Inventory of USA Greenhouse Gas Emissions and Sinks: 1990–2005. [Google Scholar]
- Van Zwieten L., Kimber S., Downie A., Morris S., Petty S., Rust J., Chan K.Y. A glasshouse study on the interaction of low mineral ash biochar with nitrogen in a sandy soil. Austr. J. Soil Res. 2010;48:569. [Google Scholar]
- Vanlauwe B., Diels J., Aihou K., Iwuafor E.N.O., Lyasse O., Sanginga N., Merckx R. Direct interactions between N fertilizer and organic matter: evidence from trials with 15N-labelled fertilizer. In: Vanlauwe B., Diels J., Sanginga N., Merckx R., editors. Integrated Plant Nutrient Management in Sub-Saharan Africa. CAB International; Wallingford, UK: 2002. pp. 173–184. [Google Scholar]
- Verchot L.V., Dutaur L., Shepherd K.D., Albrecht A. Organic matter stabilization in soil aggregates: understanding the biogeochemical mechanisms that determine the fate of carbon inputs in soils. Geoderma. 2011;161:182–193. [Google Scholar]
- Wang Z., Guo H., Shen F., Yang G., Zhang Y., Zeng Y., Wang L., Xiao H., Deng S. Biochar produced from oak sawdust by Lanthanum (La)-involved pyrolysis for adsorption of ammonium (NH4+), nitrate (NO3−), and phosphate (PO43−) Chemosphere. 2015;119:646–653. doi: 10.1016/j.chemosphere.2014.07.084. [DOI] [PubMed] [Google Scholar]
- Wardle D.A., Marie-Charlotte N., Olle Z. Fire-derived charcoal causes loss of forest humus. Science. 2008;320:2. doi: 10.1126/science.1154960. [DOI] [PubMed] [Google Scholar]
- Xiang J., Liu D., Ding W., Yuan J.J., Lin Y.X. Effects of biochar on nitrous oxide and nitric oxide emissions from paddy field during the wheat growth season. J. Cleaner Prod. 2015;104:52–58. [Google Scholar]
- Zhang D., Genxing P., Wu G.K., Wanjiru G., Li L., Zhang X., Zheng J., Zheng J., Cheng K., Joseph S., Liu X. Biochar helps enhance maize productivity and reduce greenhouse gas emissions under balanced fertilization in a rain-fed low fertility inceptisol. Chemosphere. 2015;142:106–113. doi: 10.1016/j.chemosphere.2015.04.088. [DOI] [PubMed] [Google Scholar]
- Zhang A., Bian R., Pan G., Cui L., Hussain Q., Li L., Zheng J., Zheng J., Zhang X., Han X., Yu X. Effects of biochar amendment on soil quality, crop yield and greenhouse gas emission in a Chinese rice paddy: a field study of 2 consecutive rice growing cycles. Field Crops Res. 2012;127:153–160. [Google Scholar]
- Zhang A., Liu Y., Pan G., Hussain Q., Li L., Zheng J., Zhang X. Effect of biochar amendment on maize yield and greenhouse gas emissions from a soil organic carbon poor calcareous loamy soil from Central China Plain. Plant Soil. 2012;351:263–275. [Google Scholar]
- Zhao Y., Pan Y., Rutherford J., Mitloehner F.M. Estimation of the interference in multi-gas measurements using infrared photoacoustic analyzers. Atmosphere. 2012;3:246–265. [Google Scholar]
- Zimmerman A.R., Gao B., Ahn M.Y. Positive and negative carbon mineralization priming effects among a variety of biochar-amended soils. Soil Biol. Biochem. 2011;43:1169–1179. [Google Scholar]