Abstract
Furan, a possible human carcinogen, is found in heat treated foods and tobacco smoke. Previous studies have shown that humans are capable of converting furan to its reactive metabolite, cis-2-butene-1,4-dial (BDA), and therefore may be susceptible to furan toxicity. Human risk assessment of furan exposure has been stymied because of the lack of mechanism-based exposure biomarkers. Therefore, a sensitive LC-MS/MS assay for six furan metabolites was applied to measure their levels in urine from furan-exposed rodents as well as in human urine from smokers and nonsmokers. The metabolites that result from direct reaction of BDA with lysine (BDA-Nα-acetyllysine) and from cysteine-BDA-lysine cross-links (N-acetylcysteine-BDA-lysine, N-acetylcysteine-BDA-Nα-acetyllysine and their sulfoxides) were targeted in this study. Five of the six metabolites were identified in urine from rodents treated with furan by gavage. BDA-Nα-acetyllysine, N-acetylcysteine-BDA-lysine and its sulfoxide were detected in most human urine samples from three different groups. The levels of N-acetylcysteine-BDA-lysine sulfoxide were more than 10 times higher than the corresponding sulfide in many samples. The amount of this metabolite was higher in smokers relative to non-smokers and was significantly reduced following smoking cessation. Our results indicate a strong relationship between BDA-derived metabolites and smoking. Future studies will determine if levels of these biomarkers are associated with adverse health effects in humans.
Keywords: furan, biomarker, tobacco smoke, mercapturic acid, sulfoxide, lysine adduct
Graphical abstract

Introduction
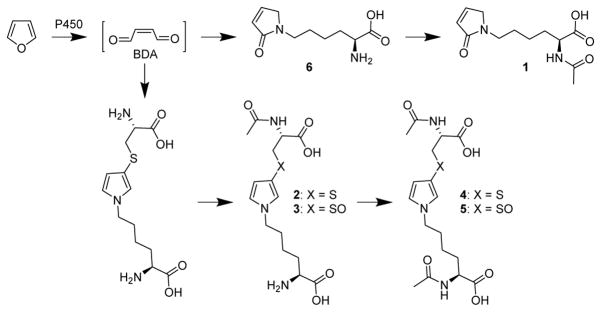
cis-2-Butene-1,4-dial (BDA) is a toxic metabolite of the liver toxicant and carcinogen, furan.1 Metabolites detected in the urine of furan-treated rats are primarily derived from the reaction of BDA with cellular nucleophiles such as lysine and cysteine (Scheme 1).2–6 Abundant metabolites include the reaction product between lysine and BDA, L-2-(acetylamino)-6-(2,5-dihydro-2-oxo-1H-pyrrol-1-yl)hexanoic acid (1), and downstream metabolites of cysteine-BDA-lysine cross-links, N-acetyl-S-[1-(5-amino-5-carboxylpentyl)-1H-pyrrol-3-yl]-L-cysteine (2) and its sulfoxide (3) as well as the N-acetyllysine derivatives of these compounds (4 and 5, respectively). These metabolites are likely degradation products of adducted proteins since approximately 13% of a 8 mg/kg dose of furan becomes covalently bound to liver proteins.7 Therefore, these urinary metabolites represent not only biomarkers of furan metabolism to BDA but also potential biomarkers of furan-derived toxicity.
Scheme 1.
Formation of urinary furan metabolites.
The human health effects of this potent liver toxicant and carcinogen are not known. Therefore, development of biomarkers of furan metabolism and toxicity for human studies is warranted. Humans are exposed to furan through processed food, pollution, car exhaust and cigarette smoke.8,9 The contribution of canned and processed foods to furan exposure is estimated to be 0.3 μg/kg/day.10,11 Smokers may be exposed to much larger amounts of furan than non-smokers since cigarette smoke contains significant levels of furan (20 – 40 μg/cigarette, depending on the method of analysis).12–14 Therefore, furan could be a significant contributor to the adverse health effects associated with tobacco smoke. To address this question, a sensitive LC-MS/MS assay was developed to quantify the levels of BDA-derived metabolites in urine. This assay was then used to determine levels of these metabolites in nonsmokers and smokers at baseline and following smoking cessation.
Materials and Methods
Chemicals and Reagents
[2H6]Acetic anhydride was purchased from Cambridge Isotope Laboratories, Inc (Andover, MA). Standards for metabolites 1, [13C615N2]1, 2, [13C615N2]2, 4 and [13C615N2]4, 5, and [13C615N2]5, were prepared as previously published.3,15,16 Concentrations of the standards in [2H6]DMSO was determined by quantitative NMR analysis as previously described.16,17 All other chemicals were purchased from Fisher Scientific (Fairlawn, NJ).
Animal Studies
All animal studies were approved by the University of Minnesota Institutional Animal Care and Use Committee. Female B6C3F1 mice (18–20 g) and male F344 rats (200–300 g) were purchased from Charles River Laboratories (Kingston, NY). F344 rats and B6C3F1 mice were selected for these studies because they have been used to assess furan toxicity in numerous short and long term studies.18–22 Male and female B6C3F1 mice have similar biochemical, proliferative and toxic responses to the hepatic effects of furan.19,21 Female mice were used because previous in vivo studies were carried out in this fashion.23 Male rats are more sensitive to the hepatocellular carcinogenic properties of furan.18 For this reason, all our metabolism studies have been performed in male F344 rats.2–5
Two groups of five mice received 0 or 8 mg/kg furan (n = 5) in 5 mL/kg corn oil by gavage. Immediately after treatment, mice were transferred to metabolism cages in groups of five. The pooled urine was collected on dry ice over 24 hours. Male F344 rats were given 0, 8 or 40 mg/kg furan in 5 mL corn oil by gavage as previously described.3 Rats were immediately transferred to metabolism cages after treatment. Urine was collected on dry ice for 24 hours.
Human urine samples
Urine from human nonsmokers (n = 15; 12 female and 3 males) was provided by the University of Minnesota Masonic Cancer Center Tobacco Biorepository. Urine from smokers at baseline (day 0) and up to 84 days after smoking cessation (n = 16; 11 females and 5 males) was collected as part of the Persistence of Biomarkers (POB) study conducted at the University of Minnesota.24 Additionally, urine samples from non-smokers and smokers were obtained from the Shanghai (all males) and Singapore (5 males and 5 females) cohort studies (five non-smokers and five smokers from each cohort).25,26 The Singapore samples were previously treated with ascorbic acid (0.4 g/20 mL urine). Pooled smoker urine provided by the laboratory of Stephen Hecht was employed for assay quality control. All urine had been stored at −20° C. Creatinine in the urine samples was measured using a colorimetric microplate assay (CRE34-K01) purchased from Eagle Bioscience (Nashua, NH). Cotinine, a measure of nicotine intake, was measured in the baseline POB study samples using an established GC-MS method.27
Urine sample preparation
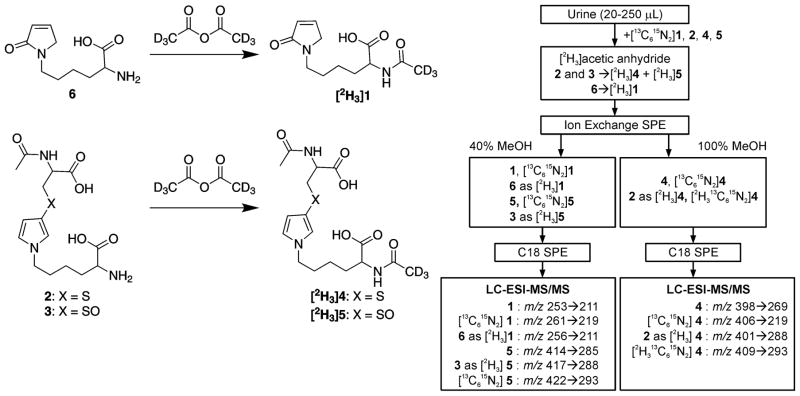
[13C615N2]Labeled internal standards (5 pmol for metabolites 1, 2, 5 and 2.5 pmol for metabolite 4) were added to 20 (rodent), 100 (Shanghai/Singapore) or 250 (POB) μL of urine in a 1.5 mL microcentrifuge tube. The urine was brought to a final volume of 1 mL by the addition of a corresponding volume of 500 mM sodium bicarbonate (POB study samples) or 750 mM sodium bicarbonate (rodent and Shanghai and Singapore cohort samples) prior to the addition of [2H6]acetic anhydride (25 μL). Tubes were briefly inverted to mix and then immediately uncapped. After 45 min at room temperature, these acetylated urine samples were applied to Oasis MAX cartridges (60 mg, Waters, Milford, MA) after the cartridges had been conditioned with 2 mL each of methanol and 2% (v/v) ammonium hydroxide. Furan metabolites were eluted from the cartridge with 1% (v/v) formic acid containing 40% (v/v) methanol (1, 6 as [2H3]1, [13C615N2]1, 5, 3 as [2H3]5, and [13C615N2]5) and 100% methanol (4, [2H3]4, [13C615N2]4 and [13C615N2]2 as [13C62H315N2]4). Fractions were collected in the presence of 15 mM ammonium acetate (200 μL) in glass vials. The organic solvent was immediately removed under reduced pressure. Following acidification with 500 μL of 1% (v/v) formic acid, the fractions were individually applied to Sep-Pak® Vac 18 cartridges (50 mg, Waters) that were preconditioned with 2 mL each methanol and water. Metabolites were eluted with 1 mL each 15% (v/v) (1, 6 as [2H3]1, [13C615N2]1, 5, 3 as [2H3]5, and [13C615N2]5) and 30% (v/v) methanol (4, 2 as [2H3]4, [13C615N2]4, and [13C615N2]2 as [13C62H315N2]4). Eluents were collected in the presence of 15 mM ammonium acetate (200 μL) in glass vials, then immediately concentrated to dryness under reduced pressure. Samples were stored at −80° C until analysis.
LC-MS/MS analysis
Samples were reconstituted in 100 μL of 15 mM ammonium acetate containing 1% (v/v) methanol for LC-MS/MS analysis. Levels of furan metabolites were measured on a Thermo Scientific TSQ Vantage mass spectrometer attached to an Eksigent nanoLC-ultra 2D pump and autosampler system using established selected reaction monitoring parameters for these metabolites.16,17 The mixtures (8 μL) were separated on a Phenomenex Synergi Hydro-RP column (250 mm × 0.5 mm, 80 Å pore size 4 μm) eluting with 15 mM ammonium acetate (solvent A) with a methanol (solvent B) gradient at a flow rate of 10 μL/min. Initial conditions were 99% A, 1% B. After 3.5 min, the gradient was linearly increased to 20% B over 17.5 min, then increased to 70% B over 5 min. After 2 min, the column was returned to initial conditions in 2 min. The electrospray ionization source was operated in negative ion mode. Source spray voltage was 3000 V. Nitrogen sheath gas pressure was 35. Q2 argon gas pressure was 1 mTorr. Declustering voltage was −5 V. Transitions monitored were as follows: 1, m/z 253 → m/z 211; 6 as [2H3]1, m/z 256 → m/z 212; [13C615N2]1, m/z 261 → m/z 219; 4, m/z 398 → m/z 269; 2 as [2H3]4, m/z 401 → m/z 272; [13C615N2]4, m/z 406 → m/z 277; [13C615N2]2 as [2H313C615N2]4, m/z 409 → m/z 280; 5, m/z 414 → m/z 285; 3 as [2H3]5, m/z 417 → 288; and [13C615N2]5, m/z 422 → m/z 293. A small subset of samples were analyzed for multiple fragments of [2H3]4 (m/z 401 → m/z 272, m/z 228 and m/z 98) and [2H313C615N2]4 (m/z 409 → m/z 280, m/z 236 and m/z 99). The scan width was 0.4 m/z and the scan time was 0.08 seconds. Q1 and Q3 resolution was 0.70 FWHM. Limits of quantitation were as follows: 1: 0.6 pmol/mL urine, 2 as [2H3]4: 0.4 pmol/mL urine; 3 as [2H3]5: 0.7 pmol/mL urine; 4: 0.2 pmol/mL urine; 5: 0.7 pmol/mL urine. Urinary metabolite levels were standardized to creatinine levels in the urine for each sample.
High resolution analysis was performed on a Thermo Scientific Orbitrap Velos using the same transitions as above with a product scan from m/z 50–300. The LC conditions were identical to the selected reaction monitoring assay.
Precision
Precision was measured by repeatedly processing pooled smoker urine at the same time as other samples and determining the standard deviation of the metabolite levels. A total of eight pooled smoker urine samples were measured on separate days. The coefficient of variation (CV) for precision of detection was 8–31% for the three analytes of interest (Table S1).
Statistical Analysis
Smoker versus non-smoker values were compared using a two tailed Student’s t-test. Cessation values were evaluated for significant changes using ANOVA followed by post-hoc t-tests. Correlations between metabolite 3 and previously measured tobacco biomarkers24 at the baseline were assessed using the Pearson’s product moment correlation coefficient, and significance was assessed for 2-sided alternatives under 5% level of significance. The tobacco constituent biomarkers were: cigarettes smoked per day, cotinine, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL), 1-hydroxypyrene (HOP), 2-hydroxyethyl mercapturic acid (HEMA), 1-(N-acetylcysteinyl)-2-hydroxy-3-butene (MHBMA), 1,2-dihydroxy-4-(N-acetylcysteinyl)butane (DHBMA), 1-(N-acetylcysteinyl)-propan-3-ol (HPMA), 2-(N-acetylcysteinyl)butan-4-ol (HMPMA), and (N-acetylcysteinyl)benzene (SPMA).
To assess whether the changes in metabolite 3 levels are significant over time, linear regression based on (normal) generalized estimating equations (GEE) was used, controlling for the repeated measures for each study subject and with an initial correlation matrix that is autoregressive with order one.28
Results
We had previously reported sensitive negative ion LC-MS/MS methods for the quantification of metabolites 1 and 4 in microsomes and hepatocytes.16,17 Metabolite 1 was detected by following the neutral loss of 42 amu (m/z 253 → m/z 211) whereas metabolite 4 was detected by monitoring the neutral loss of 129 (m/z 398 → m/z 269). Application of these methods to urine required more extensive sample clean up prior to the mass spectral analysis so we adapted a published method for the detection of urinary mercapturic acid metabolites of tobacco smoke constituents for our purposes.24 Preliminary studies indicated that there were significant contaminants that interfered with the detection of metabolite 2 when the neutral loss of 129 amu was monitored for this metabolite (m/z 356 → m/z 227). Therefore, the samples were treated with [2H6]acetic anhydride to convert metabolite 2 to [2H3]4 (Scheme 2). The selected reaction of m/z 401 to m/z 272 monitored for this derivatized metabolite yielded a chromatogram that had reduced background and fewer peaks that interfered with its detection. Conversion was considered to be close to 100% as judged by the absence of un-derivatized 2 in the urine samples treated with acetic anhydride. Reproducibility was judged by spiking pooled smokers urine with 0–24 nmol of a mixture of 2 and 4 prior to treatment with [2H6]acetic anhydride. The ratio of [2H3]4 to 4 was 1.5 ± 0.2 (n=4) in these samples after subtracting out the contribution from the pre-existing metabolites, indicating that the conversion of 2 to [2H3]4 was reproducible. The [2H3]acetylation reaction also resulted in the conversion of the BDA-lysine reaction product 6 (Scheme 1) to [2H3]1 and metabolite 3 to [2H3]5 (Scheme 2). Following acetylation, urine was subjected to a two-step solid phase extraction process using sequential mixed mode anion exchange and reverse phase cartridges (Scheme 2). Two fractions were collected from the ion exchange cartridge. The first fraction which was eluted with 40% (v/v) methanol contained 1, 6 (as [2H3]1), 3 (as [2H3]5) and 5. The second, less polar, fraction eluted with 100% methanol contained 2 (as [2H3]4) and 4. The two fractions were then concentrated and applied to C18 cartridges for an additional purification step. The fractions were dried and reconstituted in the LC mobile phase prior to LC-MS/MS analysis.
Scheme 2.
Preparation of human urine for LC-MS/MS analysis.
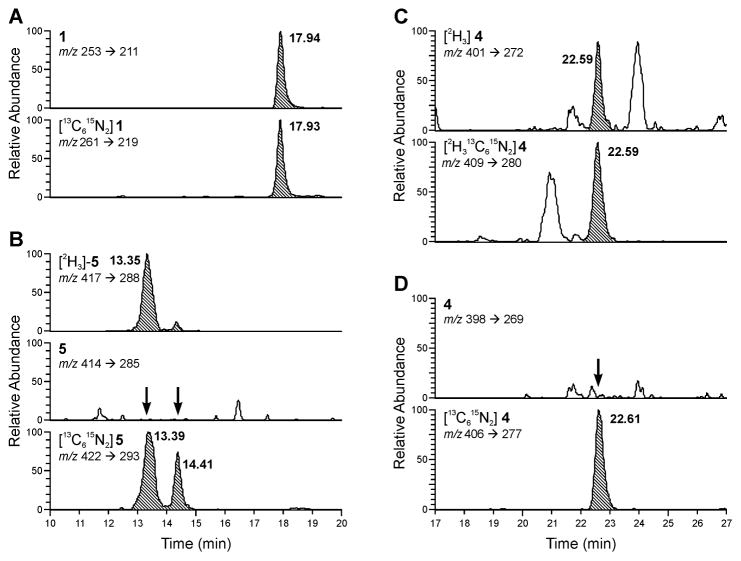
Urinary furan metabolites were initially identified by spiking the urine samples with stable isotopically labeled forms of the metabolites, [13C615N2]1, [13C615N2]2, [13C615N2]4, and [13C615N2]5 and investigating whether there were unlabeled forms of the metabolites in the urine samples that co-eluted with the internal standards. The specific mass spectral transitions monitored are outlined in Scheme 2. Representative mass chromatograms obtained for a urine sample from a human smoker are displayed in Figure 1. This sample was typical for most of the human urine samples; metabolites 1, 2 and 3 were detected whereas metabolite 4 and 5 were below the limits of detection (Table 1). Some samples contained metabolite 4 whereas metabolite 5 was at or below the limits of detection in most individuals. Metabolite 6 as [2H3]1 was identified in a subset of samples re-analyzed specifically looking for this metabolite.
Figure 1.
Representative LC-MS/MS chromatograms of a urine sample from a smoker. A) Metabolite 1; B) metabolites 3 (as [2H3]5) and 5; C) metabolite 2 (as [2H3]4); and D) metabolite 4. Peaks of interest are highlighted.
Table 1.
Furan biomarkers in various human study groups
| Urinary level of furan metabolite (pmol/mg creatinine) | ||||||
|---|---|---|---|---|---|---|
| BDA-Lysine Metabolites | N-Acetylcysteine-BDA-Lysine Metabolites | |||||
| 6 | 1 | 2 | 3 | 4 | 5 | |
| POB study | ||||||
| Non-smokers (n=15) | NDa | 420 ± 430 | 3.0 ± 4.6 | 6.5 ± 2.9 | <0.2b | <0.2b |
| Smokers (n=16) | ND | 420 ± 280 | 3.9 ± 2.7 | 69 ± 33*** | <0.2 | <0.2 |
| Shanghai cohort | ||||||
| Non-smokers (n = 5) | 13 ± 9 | 81 ± 82 | <0.8b | 1.6 ± 1.5 | <0.2 | <0.2 |
| Smokers (n = 5) | 92 ± 45** | 290 ± 130** | 7.7 ± 16* | 8.7 ± 3.5** | <0.2 | <0.2 |
| Singapore cohort | ||||||
| Non-smokers (n = 5) | 17 ± 17 | 33 ± 26 | 16 ± 14 | 1.4 ± 2.6 | <0.2 | <0.2 |
| Smokers (n = 5) | 34 ± 40 | 310 ± 220* | 14 ± 14 | 13 ± 8** | <0.2 | <0.2 |
ND = not determined.
Limits of quantitation. Significantly different from non-smokers as determined by a Student’s t-test:
p-value < 0.03;
p-value < 0.015;
p-value < 1 × 10−7.
The identities of the human metabolites 1, 2 and 3 were further confirmed with high resolution LC-MS/MS. All three metabolites had the same mass within 5 ppm as the chemically prepared standards. The mass spectrum of metabolite 1 displayed ions at m/z 82.0306, 128.0723 and 211.109 similar to synthetic 1 (Figure S1). Metabolite 2 (as [2H3]4) had fragments at m/z 98.00768 and 288.0960, the latter fragment was shifted by 1 amu due to the deuterium substitution on the acetyl group (Figure S2). The mass spectrum of metabolite 3, as [2H3]5 contained three fragments at m/z 114.00272, 244.0877 and 288.11014 (Figure S3); the latter two fragments were shifted by 1 and 3 amu, respectively, as a result of deuterium substitution on the acetyl group.
The metabolites were quantified by comparing the peak area of the analyte to that of the internal standard. Quantification of these metabolites in human urine indicated that BDA-lysine metabolite 1 was the most abundant metabolite in all samples (Table 1). In the POB study, the relative levels were: 1 > sulfoxide 3 > sulfide 2. In the Asian cohorts, the relative levels were BDA-N-acetyl-lysine metabolite 1 > sulfide 2 and sulfoxide 3. The relative levels of 2 and 3 depended on the individuals in this group with sulfide 2 was greater than sulfoxide 3 in some individuals and 3 was more abundant than 2 in others. The relative levels of these two metabolites were not dependent on smoking status.
In the Minnesota group (POB study), there was some difficulty quantifying metabolites 1 and 2. Co-elution of impurities with [13C615N2]1 and metabolite 2 affected their quantitation in numerous samples, leading to an underestimation of metabolite 1 in many smokers and an overestimation of metabolite 2 in many nonsmokers. These interfering peaks were present in only a couple of the samples from the Shanghai and Singapore cohorts.
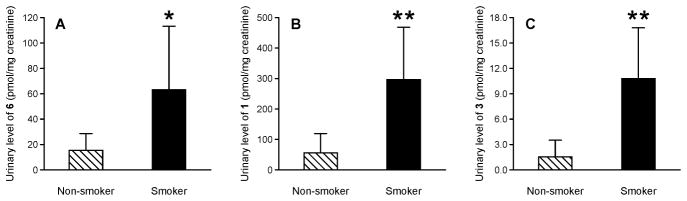
Urinary levels of sulfoxide 3 were elevated in smokers as compared to non-smokers in all three cohorts (Figures 2A and 3 and Table 1). Levels of the lysine metabolites 6 (measured as [2H3]1) and 1 were also elevated in smokers relative to non-smokers in the Singapore and Shanghai cohort samples (Figure 3 and Table 1). In the POB study, urine samples were collected at various time points following smoking cessation. Consistent with the association of sulfoxide 3 levels with smoking in this group, the levels of this metabolite decreased significantly following smoking cessation (p-value < 0.0001, Figure 2B). After three days, the levels were significantly lower when compared to baseline levels (p-value < 0.0003) with continual decrease thereafter. While the levels of metabolite 3 reached a minimum after 28 days of cessation, they were still significantly higher as compared to non-smokers even after 84 day cessation (p-value < 0.005). Unfortunately, metabolite 1 could not be followed in these cessation samples due to the presence of interfering peaks.
Figure 2.
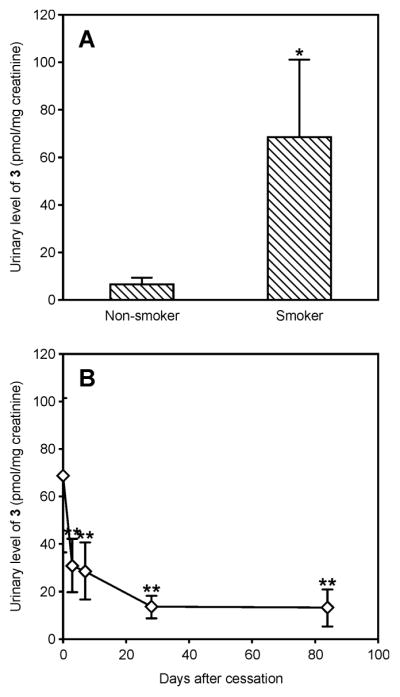
Urinary levels of metabolite 3 in A) non-smokers and smokers and B) before and after cessation. Data is presented as average urinary level normalized to creatinine ± S.D. *Metabolite 3 was significantly higher in smokers versus nonsmokers as determined by a Student t-test, p-value < 1 × 10−7. **Levels of 3 significantly dropped in smokers after cessation as determined by ANOVA followed by post-hoc Student t-test, p-value < 0.005.
Figure 3.
Urinary levels of furan metabolites in non-smokers and smokers from the Shanghai and Singapore cohorts: A) metabolite 6, B) metabolite 1 and C) metabolite 3. Data is presented as average urinary level normalized to creatinine ± S.D. These metabolites were significantly higher in smokers versus nonsmokers as determined by a Student t-test. *p-value < 0.01. ** p-value < 0.001.
To explore whether baseline levels of metabolite 3 are correlated to other biomarkers of tobacco smoke constituents, we compared the levels of this compound to those previously reported in smokers of the POB study (Figure S4).24 Levels of metabolite 3 were correlated to the number of cigarettes smoked per day (ρ = 0.464, p-value = 0.081), but the correlation coefficient was not statistically significant due to the small sample size. They were significantly correlated with several tobacco specific biomarkers such as urinary cotinine levels (ρ = 0.618, p-value = 0.014), a metabolite of nicotine and NNAL (ρ = 0.657, p-value = 0.007), a metabolite of the lung specific carcinogen, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK).29–31 HPMA and HMPMA are the biomarkers of exposure to acrolein and crotonaldehyde, respectively. These two α, β-unsaturated aldehydes have been previously quantified in cigarette smoke.32,33 Levels of metabolite 3 are significantly associated with both HPMA (ρ = 0.631, p-value = 0.011) and HMPMA (ρ = 0.615, p-value = 0.014). Borderline associations were observed for two other metabolites of tobacco smoke chemicals, pyrene and ethylene oxide (HOP:ρ = 0.513, p-value = 0.05 and HEMA: ρ = 0.48, p-value = 0.07, respectively).32,34 There was no statistically significant correlation coefficients with the mercapturic acid metabolites of 1,3-butadiene (MHBMA and DHBMA, ρ = 0.349 and 0.392, respectively) or benzene (SPMA, ρ = 0.158). Like furan, 1,3-butadiene and benzene are substrates for cytochrome P450 2E1.16,35,36
In urine from furan-treated rats and mice, metabolites 1 – 5, but not 6 (as [2H3]1), were observed (Table 2). The relative amounts of these metabolites differed between rats and mice. In rat urine, the lysine reaction product 1 was the most abundant metabolite detected by this quantitative method. The relative amounts of the metabolites derived from cysteine-BDA-lysine cross-links was sulfoxide 5 > sulfide 2 ≅ sulfoxide 4 > sulfide 3 with the sum of these four metabolites less than the levels of the BDA-lysine derived metabolite, 1. There was a roughly ten-fold increase in all the metabolites at the higher dose with no significant change in the relative distribution of each metabolite. In the mouse, the most abundant metabolite detected was the N-acetylcysteine-BDA-lysine cross-link 2. Its sulfoxide 3 was next most abundant followed by the BDA-N-acetyllysine metabolite 1. Lysine acetylation of the N-acetylcysteine-BDA-lysine cross-link 2 was a minor pathway in this species.
Table 2.
Urinary level of furan metabolite in urine from furan-treated rodents (nmol/mg creatinine)
| BDA-Lysine Metabolites | N-Acetylcysteine-BDA-Lysine Metabolites | |||||
|---|---|---|---|---|---|---|
| Species and exposure | 6 | 1 | 2 | 3 | 4 | 5 |
| Rata | ||||||
| 0 mg/kg (n = 2) | <0.0004b | 0.8,0.3 | ND,c 0.012 | <0.001, <0.001 | ND, 0.0003 | 0.001, 0.0003 |
| 8 mg/kg (n = 2) | <0.0004 | 1.2, 1.1 | 0.080, 0.066 | 0.005, 0.004 | 0.07, 0.06 | 0.21, 0.19 |
| 40 mg/kg (n = 2) | <0.0004 | 11.7, 9.3 | 1.1, 0.71 | 0.054, 0.047 | 1.2, 0.69 | 3.5, 2.2 |
| Mousea | ||||||
| 0 mg/kg (n = 1) | <0.0004 | 0.2 | 0.2 | 0.02 | <0.0002 | 0.001 |
| 8 mg/kg (n = 2) | <0.0004 | 2.2, 1.6 | 13.9, 11.5 | 2.8,5.5 | 0.001, 0.001 | 0.18, 0.33 |
Male F344 rats received 0, 8 or 40 mg/kg furan by gavage. Urine from individual animals was collected for 24 h.
Limits of quantitation
ND = not determined.
Groups of 5 female B6C3F1 mice received 0 or 8 mg/kg furan by gavage and pooled urine was collected from each group for 24 h.
Discussion
Previous studies in rats indicated that abundant urinary metabolites of furan were derived from the reaction of BDA with lysine and cysteine.3,4,6 Metabolites 1 and 6 are products of the reaction of BDA with lysine whereas metabolites 2 – 5 are derived from the crosslinking of cysteine to lysine by this reactive compound. In this report, five of these six furan metabolites were detected in urine of furan-treated rodents (metabolites 1 – 5) and four were detected in most human urine samples (metabolites 1, 2, 3 and 6).
While the cysteine-BDA-lysine derived metabolites, sulfide 4 and sulfoxide 5 were identified as major furan metabolites in rat urine using non-quantitative mass spectrometry methods,4,6 the quantitative assay presented in this report indicates that the BDA-lysine metabolite 1 is actually more abundant than both the sulfide 4 and sulfoxide 5 (Table 2). Similarly, metabolite 2 was thought to be a minor metabolite,4 but this current report demonstrates that metabolites 2 and 4 are present in comparable amounts in rat urine. The total sum of cysteine-BDA-lysine derived metabolites (2 – 5) was less than the BDA-lysine metabolite 1. This finding was somewhat surprising given that studies in rat hepatocytes indicated that the formation of glutathione-BDA-lysine metabolites occurred to a much greater extent than metabolite 1;3,17 glutathione-BDA-lysine is likely a major precursor to metabolites 2 – 5.3,17 A likely explanation for this discrepancy is that we are not accounting for all of the cysteine-BDA-lysine-derived metabolites reported in rat urine. The cysteine derivative can be metabolized by sequentially by β-lysase, S-methyltransferase and sulfur oxidases, respectively, whereas the lysine residue can undergo oxidative deamination.4 The latter pathway is reported to be significant in rats.4,6 Consequently, not all the metabolites derived from the cross-link are accounted for in this current study. Future studies will involve the development of quantitative methods for these additional metabolites.
Furan-derived metabolites have not been previously reported in mice. In this species, the cysteine-BDA-lysine derived sulfide 2 is more abundant than the BDA-lysine reaction product 1, suggesting that cysteine-BDA-lysine cross-link formation is more abundant than direct BDA-lysine adduct formation (Table 2). This is consistent with our previous observations of the relative formation of metabolite 1 and glutathione-BDA-lysine in mouse hepatocytes.17 The higher levels of metabolites 2 and 3 in the urine of mice relative to rats indicate that the cysteine and lysine residues of the cross-link are less susceptible to further metabolism in mice. Analysis by ion trap mass spectrometry indicated that the metabolic profile of furan metabolites in mice was much simpler than that observed for rats and there was little evidence of any further metabolism of the cysteine and lysine portions of this cross-link beyond acetylation of the lysine residue and S-oxidation (data not shown). The absence of these compounds will be confirmed in future studies in which quantitative methods for the other rat metabolites are employed.
Furan metabolites 1, sulfide 2 and sulfoxide 3 were detected in all but two of the human samples; significantly lower levels of sulfide 4 and sulfoxide 5 were detected in only some human samples. Unlike rodents, the non-acetylated BDA-lysine metabolite 6 was also present. The acetylated BDA-lysine compound 1 was the most abundant furan metabolite detected in the human samples, consistent with the observation that this direct reaction product was the dominant BDA-derived metabolite of furan in human hepatocytes.17 The sulfoxide metabolite 3 was the second most abundant BDA-derived reaction product detected in most of the human samples; however, sulfide 2 was more abundant than the sulfoxide 3 in some of the samples. The latter case occurred more frequently in the Asian cohort samples and was not associated with smoking status.
The observation that the sulfoxide 3 was more abundant than the corresponding mercapturic acid in all of the POB samples and more than half of the Asian co-hort samples is a novel finding. While mercapturic acid sulfoxides have been reported for a number of compounds in rodent urine,3,37,38 most human urinary biomarker studies focus on the mercapturic acid metabolites of environmental chemicals.24,39,40 The oxidation of the mercapturic acid 2 to the sulfoxide 3 likely results from an enzyme-mediated process since the ratio of the two diastereomers was ~11/1 (R/S; Figure S5). This average ratio remained constant regardless of smoking status. This contrasts with approximately equal amounts of the diastereomers formed when 2 undergoes chemical oxidation to 3.3 This evidence supports the hypothesis that the sulfoxide 3 detected in urine is formed enzymatically and not as a result of air oxidation. Cytochrome P450 and flavin-containing monooxygenase (FMO) are two enzymes capable of oxidizing mercapturic acids to their sulfoxides, with CYP3A enzymes being the most active.41–43
Levels of metabolite 3 were related to smoking in all three groups. Smoking associations were also observed for metabolites 1 and 6 in the Asian groups (Figures 2 and 3). Unfortunately, the measurement of 1 was confounded in the Minnesota study due to co-eluting contaminants with the internal standard, likely underestimating the levels of this metabolite in many smokers. Urinary levels of metabolite 3 dropped gradually following smoking cessation. This decline differed from the immediate drop observed for the mercapturic acid derivatives of other tobacco smoke chemicals such as benzene, butadiene and acrolein.24 This behavior is consistent with the expectation that 3 is derived from the degradation of BDA-modified proteins3 which would result in a gradual decline in urinary levels over time.
The abundance of furan in tobacco smoke supports furan as the precursor to metabolites 1, 3 and 6 in smoker’s urine.12–14 However, other sources cannot be excluded since there are other potential sources of BDA. First, BDA itself may be a component of tobacco smoke. This question has not been examined but other α,β-unsaturated aldehydes have been detected in tobacco smoke.32,33 Second, metabolite 3 or its precursor could be a metabolite of other tobacco constituents. Finally, BDA may be endogenously generated by radicals and reactive oxygen species-generating chemicals;44–46 both of which are abundant in cigarette smoke. Radical reactions with DNA generate the trans-isomer of BDA47 and BDA has been proposed to be a product of lipid peroxidation.48 Acrolein and crotonaldehyde are also both present in tobacco smoke and are formed as a consequence of lipid peroxidation.32,33,49 These compounds are both α,β-unsaturated aldehydes like BDA. It is interesting to note that their metabolites, HPMA and HMPMA, are correlated with metabolite 3 in the POB study samples. Further studies need to be conducted to clarify all the possible sources of BDA that result from tobacco smoking.
Tobacco smoke is not the only possible source of these metabolites. Humans are also exposed to furan through processed food, pollution, car exhaust and wood smoke.8,9 Future studies will examine the relative contribution of these exposures to the levels of these metabolites in non-smokers.
It is worth noting that the levels of metabolites 1, 2 and 3 were generally similar among the three human studies. The Shanghai and Singapore cohort specimens are more than 25 years old suggesting that the metabolites are stable with storage. In addition, the difference between smokers and nonsmokers was maintained over this period of time. This indicates that the preparatory and analysis methods described in this report could be applied to numerous existing studies to determine if there is adverse health effects associated with this exposure.
In summary, a sensitive LC-MS/MS assay was employed to detect potential furan metabolites in human urine. Several of these metabolites, 1, 3 and 6 were associated with smoking in humans, and therefore should also be explored as potential biomarkers of exposure to BDA and, possibly furan. Future studies will be performed with these biomarkers to determine if their levels are associated with adverse health effects associated with exposure to mixtures containing furan such as tobacco smoke.
Supplementary Material
Acknowledgments
Funding Sources
This research was supported by funds from the National Institute of Environmental Health Sciences [ES-10577], the Masonic Cancer Center, the Midwest Center for Occupational Health and Safety Education and Research Center (MCOHS ERC) and the University of Minnesota Grant-in-Aid of Research, Artistry and Scholarship program. MCOHS ERC is funded by the National Institute for Occupational Safety and Health (NIOSH) and the Centers for Disease Control and Prevention (CDC) [T42 OH008434]. The Masonic Cancer Center Analytical Biochemistry and Biostatistics Shared Resources are funded in part by the National Cancer Institute [P30 CA-77598]. The contents of this effort are solely the responsibility of the authors, and do not necessarily represent the official view of the MCOHS ERC, NIOSH, or the CDC or other associated entities.
The authors thank Dr. Peter Villalta and Mr. Brock Matter in Masonic Cancer Center Analytical Biochemistry Shared Resource at the University of Minnesota for assistance with mass spectrometry and Mr. Steve Carmella for helpful discussions regarding sample preparation. They also thank Dr. Stephen Hecht for the gift of urine from the Persistence of Biomarkers studies and Mr. Bob Carlson for assistance with graphic design.
Abbreviations
- ANOVA
analysis of variance
- BDA
cis-2-butene-1,4-dial
- CYP
cytochrome P450
- DHBMA
1,2-dihydroxy-4-(N-acetylcysteinyl)butane
- FMO
flavin-containing mono-oxygenase
- GC-MS
gas chromatography linked to mass spectrometry
- HMPMA
2-(N-acetylcysteinyl)butan-4-ol
- HEMA
2-hydroxyethyl mercapturic acid
- HOP
1-hydroxypyrene
- HPMA
1-(N-acetylcysteinyl)-propan-3-ol
- LC-MS/MS
liquid chromatography with tandem mass spectrometry
- MAX
mixed anionic exchange
- MHBMA
1-(N-acetylcysteinyl)-2-hydroxy-3-butene
- NNAL
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol
- NNK
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone
- POB
Persistence of Biomarkers study
- SPMA
N-acetylcysteinylbenzene
Footnotes
Supporting Information Available. Figures S1 – S3 display representative high resolution mass spectra of human urinary metabolites 1, 2 and 3. Figure S4 shows the comparison of metabolite 3 levels with previously measured tobacco smoke exposure biomarkers in the POB study. Figure S5 displays the ratio of the R to S sulfoxide diastereomers of metabolite 3 in human urine. Table S1 contains the analysis characteristics of furan metabolites in human urine. This material is available free of charge via the Internet at http://pubs.acs.org.
Reference List
- 1.Chen LJ, Hecht SS, Peterson LA. Identification of cis-2-butene-1,4-dial as a microsomal metabolite of furan. Chem Res Toxicol. 1995;8:903–906. doi: 10.1021/tx00049a001. [DOI] [PubMed] [Google Scholar]
- 2.Peterson LA, Cummings ME, Chan JY, Vu CC, Matter BA. Identification of a cis-2-butene-1,4-dial-derived glutathione conjugate in the urine of furan-treated rats. Chem Res Toxicol. 2006;19:1138–1141. doi: 10.1021/tx060111x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu D, Sullivan MM, Phillips MB, Peterson LA. Degraded protein adducts of cis-2-butene-1,4-dial are urinary and hepatocyte metabolites of furan. Chem Res Toxicol. 2009;22:997–1007. doi: 10.1021/tx800377v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu D, Peterson LA. Identification of furan metabolites derived from cysteine-cis-2-butene-1,4-dial-lysine cross-links. Chem Res Toxicol. 2010;23:142–151. doi: 10.1021/tx9003215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peterson LA, Phillips MB, Lu D, Sullivan MM. Polyamines are traps for reactive intermediates in furan metabolism. Chem Res Toxicol. 2011;24:1924–1936. doi: 10.1021/tx200273z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kellert M, Wagner S, Lutz U, Lutz WK. Biomarkers of furan exposure by metabolic profiling of rat urine with liquid chromatography-tandem mass spectrometry and principal component analysis. Chem Res Toxicol. 2008;21:761–768. doi: 10.1021/tx7004212. [DOI] [PubMed] [Google Scholar]
- 7.Burka LT, Washburn KD, Irwin RD. Disposition of [14C]furan in the male F344 rat. J Toxicol Environ Health. 1991;34:245–257. doi: 10.1080/15287399109531564. [DOI] [PubMed] [Google Scholar]
- 8.International Agency for Research on Cancer. Dry Cleaning, Some Chlorinated Solvents and Other Industrial Chemicals. IARC; Lyon, France: 1995. Furan; p. 393. [Google Scholar]
- 9.International Agency for Research on Cancer. Tobacco smoking. Vol. 38. IARC; Lyon, France: 1986. [Google Scholar]
- 10.Moro S, Chipman JK, Wegener JW, Hamberger C, Dekant W, Mally A. Furan in heat-treated foods: Formation, exposure, toxicity, and aspects of risk assessment. Mol Nutr Food Res. 2012;56:1197–1211. doi: 10.1002/mnfr.201200093. [DOI] [PubMed] [Google Scholar]
- 11.Bakhiya N, Appel KE. Toxicity and carcinogenicity of furan in human diet. Arch Toxicol. 2010;84:563–578. doi: 10.1007/s00204-010-0531-y. [DOI] [PubMed] [Google Scholar]
- 12.Hatzinikolaou DG, Lagesson V, Stavridou AJ, Pouli AE, Lagesson-Andrasko L, Stavrides JC. Analysis of the gas phase of cigarette smoke by gas chromatography coupled with UV-diode array detection. Anal Chem. 2006;78:4509–4516. doi: 10.1021/ac052004y. [DOI] [PubMed] [Google Scholar]
- 13.Pouli AE, Hatzinikolaou DG, Piperi C, Stavridou A, Psallidopoulos MC, Stavrides JC. The cytotoxic effect of volatile organic compounds of the gas phase of cigarette smoke on lung epithelial cells. Free Radic Biol Med. 2003;34:345–355. doi: 10.1016/s0891-5849(02)01289-3. [DOI] [PubMed] [Google Scholar]
- 14.Baek SO, Jenkins RA. Characterization of trace organic compounds associated with aged and diluted sidestream tobacco smoke in a controlled atmosphere-volatile organic compounds and polycyclic aromatic hydrocarbons. Atmos Environ. 2004;38:6583–6599. [Google Scholar]
- 15.Chen LJ, Hecht SS, Peterson LA. Characterization of amino acid and glutathione adducts of cis-2-butene-1,4-dial, a reactive metabolite of furan. Chem Res Toxicol. 1997;10:866–874. doi: 10.1021/tx9700174. [DOI] [PubMed] [Google Scholar]
- 16.Gates LA, Lu D, Peterson LA. Trapping of cis-2-butene-1,4-dial to measure furan metabolism in human liver microsomes by cytochrome P450 enzymes. Drug Metab Dispos. 2012;40:596–601. doi: 10.1124/dmd.111.043679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gates LA, Phillips MB, Matter BA, Peterson LA. Comparative metabolism of furan in rodent and human cryopreserved hepatocytes. Drug Metab Dispos. 2014;42:1132–1136. doi: 10.1124/dmd.114.057794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Toxicology Program. Toxicology and carcinogenesis studies of furan in F344/N rats and B6C3F1 mice vol. NTP Technical Report No. 402. US Department of Health and Human Services, Public Health Service, National Institutes of Health; Research Triangle Park, NC: 1993. [Google Scholar]
- 19.Wilson DM, Goldsworthy TL, Popp JA, Butterworth BE. Evaluation of genotoxicity, pathological lesions, and cell proliferation in livers of rats and mice treated with furan. Environ Mol Mutagen. 1992;19:209–222. doi: 10.1002/em.2850190305. [DOI] [PubMed] [Google Scholar]
- 20.Butterworth BE, Sprankle CS, Goldsworthy SM, Wilson DM, Goldsworthy TL. Expression of myc, fos, and Ha-ras in the livers of furan-treated F344 rats and B6C3F1 mice. Mol Carcinogenesis. 1994;9:24–32. doi: 10.1002/mc.2940090106. [DOI] [PubMed] [Google Scholar]
- 21.Fransson-Steen R, Goldsworthy TL, Kedderis GL, Maronpot RR. Furan-induced liver cell proliferation and apoptosis in female B6C3F1 mice. Toxicology. 1997;118:195–204. doi: 10.1016/s0300-483x(97)03618-4. [DOI] [PubMed] [Google Scholar]
- 22.Moser GJ, Foley J, Burnett M, Goldsworthy TL, Maronpot R. Furan-induced dose-response relationships for liver cytotoxicity, cell proliferation, and tumorigenicity (furan-induced liver tumorigenicity) Exp Toxicol Pathol. 2009;61:101–111. doi: 10.1016/j.etp.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Terrell AN, Huynh M, Grill AE, Kovi RC, O’Sullivan GO, Guttenplan JB, Ho YY, Peterson LA. Mutagenic activity of furan in female Big Blue B6C3F1 mice. Mutat Res. 2014;770:46–54. doi: 10.1016/j.mrgentox.2014.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carmella SG, Chen M, Han S, Briggs A, Jensen J, Hatsukami DK, Hecht SS. Effects of smoking cessation on eight urinary tobacco carcinogen and toxicant biomarkers. Chem Res Toxicol. 2009;22:734–741. doi: 10.1021/tx800479s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hecht SS, Murphy SE, Stepanov I, Nelson HH, Yuan JM. Tobacco smoke biomarkers and cancer risk among male smokers in the Shanghai cohort study. Cancer Lett. 2013;334:34–38. doi: 10.1016/j.canlet.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuan JM, Koh WP, Murphy SE, Fan Y, Wang R, Carmella SG, Han S, Wickham K, Gao YT, Yu MC, Hecht SS. Urinary levels of tobacco-specific nitrosamine metabolites in relation to lung cancer development in two prospective cohorts of cigarette smokers. Cancer Res. 2009;69:2990–2995. doi: 10.1158/0008-5472.CAN-08-4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hecht SS, Carmella SG, Chen M, Dor KJF, Miller AT, Murphy SE, Jensen JA, Zimmerman CL, Hatsukami DK. Quantitation of urinary metabolites of a tobacco-specific lung carcinogen after smoking cessation. Cancer Res. 1999;59:590–596. [PubMed] [Google Scholar]
- 28.Hardin J, Hilbe J. Generalized Estimating Equations. Chapman and Hall/CRC; London: 2003. [Google Scholar]
- 29.Murphy SE, Link CA, Jensen J, Le C, Puumala SS, Hecht SS, Carmella SG, Losey L, Hatsukami DK. A comparison of urinary biomarkers of tobacco and carcinogen exposure in smokers. Cancer Epidemiol Biomarkers Prev. 2004;13:1617–1623. [PubMed] [Google Scholar]
- 30.Hecht SS. Human urinary carcinogen metabolites: biomarkers for investigating tobacco and cancer. Carcinogenesis. 2002;23:907–922. doi: 10.1093/carcin/23.6.907. [DOI] [PubMed] [Google Scholar]
- 31.Yuan JM, Gao YT, Murphy SE, Carmella SG, Wang R, Zhong Y, Moy KA, Davis AB, Tao L, Chen M, Han S, Nelson HH, Yu MC, Hecht SS. Urinary levels of cigarette smoke constituent metabolites are prospectively associated with lung cancer development in smokers. Cancer Res. 2011;71:6749–6757. doi: 10.1158/0008-5472.CAN-11-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roemer E, Stabbert R, Rustemeier K, Veltel DJ, Meisgen TJ, Reininghaus W, Carchman RA, Gaworski CL, Podraza KF. Chemical composition, cytotoxicity and mutagenicity of smoke from US commercial and reference cigarettes smoked under two sets of machine smoking conditions. Toxicology. 2004;195:31–52. doi: 10.1016/j.tox.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 33.Counts ME, Hsu FS, Laffoon SW, Dwyer RW, Cox RH. Mainstream smoke constituent yields and predicting relationships from a worldwide market sample of cigarette brands: ISO smoking conditions. Regul Toxicol Pharmacol. 2004;39:111–134. doi: 10.1016/j.yrtph.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 34.International Agency for Research on Cancer. Tobacco smoke and involuntary smoking. 2004 monographs.iarc.fr/htdocs/monographs/vol83/
- 35.Seaton MJ, Schlosser PM, Bond JA, Medinsky MA. Benzene metabolism by human liver microsomes in relation to cytochrome P450 2E1 activity. Carcinogenesis. 1994;15:1799–1806. doi: 10.1093/carcin/15.9.1799. [DOI] [PubMed] [Google Scholar]
- 36.Duescher RJ, Elfarra AA. Human liver microsomes are efficient catalysts of 1,3-butadiene oxidation: evidence for major roles by cytochromes P450 2A6 and 2E1. Arch Biochem Biophys. 1994;311:342–349. doi: 10.1006/abbi.1994.1246. [DOI] [PubMed] [Google Scholar]
- 37.Dansette PM, Thang DC, el Amri H, Mansuy D. Evidence for thiophene-S-oxide as a primary reactive metabolite of thiophene in vivo: formation of a dihydrothiophene sulfoxide mercapturic acid. Biochem Biophys Res Commun. 1992;186:1624–1630. doi: 10.1016/s0006-291x(05)81594-3. [DOI] [PubMed] [Google Scholar]
- 38.Bakke JE, Rafter J, Larsen GL, Gustafsson JA, Gustafsson BE. Enterohepatic circulation of the mercapturic acid and cysteine conjugates of propachlor. Drug Metab Dispos. 1981;9:525–528. [PubMed] [Google Scholar]
- 39.Boettcher MI, Bolt HM, Drexler H, Angerer J. Excretion of mercapturic acids of acrylamide and glycidamide in human urine after single oral administration of deuterium-labelled acrylamide. Arch Toxicol. 2006;80:55–61. doi: 10.1007/s00204-005-0011-y. [DOI] [PubMed] [Google Scholar]
- 40.Fennell TR, Sumner SC, Snyder RW, Burgess J, Friedman MA. Kinetics of elimination of urinary metabolites of acrylamide in humans. Toxicol Sci. 2006;93:256–267. doi: 10.1093/toxsci/kfl069. [DOI] [PubMed] [Google Scholar]
- 41.Werner M, Birner G, Dekant W. Sulfoxidation of mercapturic acids derived from tri- and tetrachloroethene by cytochromes P450 3A: a bioactivation reaction in addition to deacetylation and cysteine conjugate beta-lyase mediated cleavage. Chem Res Toxicol. 1996;9:41–49. doi: 10.1021/tx950075u. [DOI] [PubMed] [Google Scholar]
- 42.Werner M, Guo Z, Birner G, Dekant W, Guengerich FP. The sulfoxidation of the hexachlorobutadiene metabolite N-acetyl-S-(1,2,3,4,4-pentachlorobutadienyl)-L-cysteine is catalyzed by human cytochrome P450 3A enzymes. Chem Res Toxicol. 1995;8:917–923. doi: 10.1021/tx00049a004. [DOI] [PubMed] [Google Scholar]
- 43.Altuntas TG, Park SB, Kharasch ED. Sulfoxidation of cysteine and mercapturic acid conjugates of the sevoflurane degradation product fluoromethyl-2,2-difluoro-1-(trifluoromethyl)vinyl ether (compound A) Chem Res Toxicol. 2004;17:435–445. doi: 10.1021/tx034254k. [DOI] [PubMed] [Google Scholar]
- 44.Frenkel K. Carcinogen-mediated oxidant formation and oxidative DNA damage. Pharmacol Ther. 1992;53:127–166. doi: 10.1016/0163-7258(92)90047-4. [DOI] [PubMed] [Google Scholar]
- 45.Ziech D, Franco R, Pappa A, Panayiotidis MI. Reactive oxygen species (ROS)--induced genetic and epigenetic alterations in human carcinogenesis. Mutat Res. 2011;711:167–173. doi: 10.1016/j.mrfmmm.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 46.Bhalla DK, Hirata F, Rishi AK, Gairola CG. Cigarette smoke, inflammation, and lung injury: a mechanistic perspective. J Toxicol Environ Health B Crit Rev. 2009;12:45–64. doi: 10.1080/10937400802545094. [DOI] [PubMed] [Google Scholar]
- 47.Chen B, Bohnert T, Zhou X, Dedon PC. 5′-(2-phosphoryl-1,4-dioxobutane) as a product of 5′-oxidation of deoxyribose in DNA: elimination as trans-1,4-dioxo-2-butene and approaches to analysis. Chem Res Toxicol. 2004;17:1406–1413. doi: 10.1021/tx049818e. [DOI] [PubMed] [Google Scholar]
- 48.Onyango AN. Small reactive carbonyl compounds as tissue lipid oxidation products; and the mechanisms of their formation thereby. Chem Phys Lipids. 2012;165:777–786. doi: 10.1016/j.chemphyslip.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 49.Voulgaridou GP, Anestopoulos I, Franco R, Panayiotidis MI, Pappa A. DNA damage induced by endogenous aldehydes: current state of knowledge. Mutat Res. 2011;711:13–27. doi: 10.1016/j.mrfmmm.2011.03.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.