Abstract
Objective
To investigate the association between breastfeeding and endometrial cancer risk using pooled data from 17 studies participating in the Epidemiology of Endometrial Cancer Consortium.
Methods
We conducted a meta-analysis with individual-level data from three cohort and 14 case-control studies. Study-specific odds ratios (ORs) and 95% confidence intervals (CI) were estimated for the association between breastfeeding and risk of endometrial cancer using multivariable logistic regression, and pooled using random-effects meta-analysis. We investigated between-study heterogeneity with I2 and Q statistics and meta-regression.
Results
After excluding nulliparous women, the analyses included 8981 women with endometrial cancer and 17241 control women. Ever breastfeeding was associated with an 11% reduction in risk of endometrial cancer (pooled OR=0.89, 95% CI 0.81–0.98). Longer average duration of breastfeeding per child was associated with lower risk of endometrial cancer, although there appeared to be some levelling of this effect beyond 6–9 months. The association with ever breastfeeding was not explained by greater parity and did not vary notably by body mass index or histological subtype (grouped as endometrioid and mucinous versus serous and clear cell).
Conclusions
Our findings suggest that reducing endometrial cancer risk can be added to the list of maternal benefits associated with breastfeeding. Ongoing promotion, support and facilitation of this safe and beneficial behavior might therefore contribute to the prevention of this increasingly common cancer.
Introduction
Endometrial cancer is the fourth most common cancer in women in high income countries such as the USA, Canada and Australia where collectively more than 57000 women are diagnosed each year1 and incidence has been increasing over the last 20–30 years. A better understanding of its causes and ways it could be prevented is therefore required.
Exclusive breastfeeding generally suppresses ovulation and therefore maternal estrogen levels.2 Reduced estrogen levels reduce endometrial mitoses3 and might therefore reduce risk of endometrial cancer; however, clear evidence for this association is lacking. The most recent World Cancer Research Fund/American Institute for Cancer Research endometrial cancer report classified the evidence for an association with lactation as ‘limited-no conclusion’.4
Some previous studies indicate risk reduction with longer breastfeeding durations;5 some show risk reduction only for recent breastfeeding episodes;6,7 and others found no association.8,9 So, while two meta-analyses of published data suggest risk reduction with longer total durations of breastfeeding, they were unable to account for significant heterogeneity across studies.10,11 They also could not investigate factors that might modify breastfeeding associations such as body mass index, time since last pregnancy or menopausal status or how the duration of individual breastfeeding episodes might influence endometrial cancer associations. We hypothesise that, since estrogen levels during lactation are lowest while ovulation is suppressed, ongoing breastfeeding beyond this point might not confer additional benefit.
To more comprehensively assess the association between breastfeeding and endometrial cancer risk, we pooled data from 17 studies participating in the Epidemiology of Endometrial Cancer Consortium.12
Materials and Methods
Ethical approval was obtained from each study’s institutional review board. All participants provided informed consent to take part in the respective studies.
We conducted a meta-analysis with individual-participant data from 17 independent studies (14 case-control, three cohort) from the Epidemiology of Endometrial Cancer Consortium (Table 1) that provided data on breastfeeding and confounding factors. Cohort studies were analysed as nested case-control studies with four controls matched on birth year randomly selected for each case from cohort members who had not had a hysterectomy or an endometrial cancer diagnosis (or, in The Nurses’ Health Study, any other invasive cancer) by the case’s diagnosis date.
Table 1.
Characteristics of 17 E2C2 studies pooled to investigate the association between breastfeeding and endometrial cancer risk among parous women
| Study Study name | Acronym | Country | Years of recruitment/diagnosis | Cases (8981) N | Controls (17241) N | Ever breastfed N (%) | Median total breastfeeding (months)* | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case-control studies | cases | controls | cases | controls | |||||||
| Alberta Case-Control Study on Endometrial Cancer17 | ALTA | Canada | 2002–2006 | 441 | 922 | 298 (68) | 692 (75) | 6 | 9 | ||
| Australian National Endometrial Cancer Study29 | ANECS | Australia | 2005–2007 | 1119 | 659 | 913 (82) | 561 (85) | 10 | 12 | ||
| Connecticut Endometrial Cancer Study18 | CONN | USA | 2004–2009 | 505 | 558 | 218 (43) | 299 (54) | 8 | 12 | ||
| Estrogen, Diet, Genetics, and Endometrial Cancer19 | EDGE | USA | 2001–2005 | 370 | 407 | 159 (43) | 188 (46) | 6 | 9 | ||
| Fred Hutchinson Cancer Research Center25 | FHCRC | USA | 1994–2005 | 703 | 737 | 452 (64) | 514 (70) | 6 | 7 | ||
| Italian Multi Centre Study20 | IMS | Italy | 1992–2006 | 386 | 780 | 303 (79) | 592 (76) | 9 | 10 | ||
| Patient Epidemiologic Data System30 | PEDS | USA | 1982–1998 | 401 | 423 | 195 (49) | 261 (62) | 6 | 7 | ||
| Polish Endometrial Cancer Study21 | PECS | Poland | 2000–2003 | 447 | 1701 | 366 (82) | 1388 (82) | 8 | 7 | ||
| Shanghai Endometrial Cancer Study22 | SECS | China | 1997–2004 | 1089 | 1161 | 896 (83) | 1003 (86) | 14 | 13 | ||
| Turin Case Control Study | TURIN | Italy | 1998–2008 | 219 | 260 | 170 (78) | 196 (75) | 8 | 6.5 | ||
| USC LA case control24 | USC | USA | 1987–1993 | 660 | 709 | 411 (63) | 468 (66) | 3 | 5 | ||
| US Endometrial Cancer Study8 | USE | USA | 1987–1990 | 316 | 290 | 191 (60) | 181 (62) | 4 | 6 | ||
| Western New York Diet Study23 | WNYDS | USA | 1986–1991 | 185 | 532 | 91 (49) | 277 (52) | 3 | 6 | ||
| Women’s Insight and Shared Experience31 | WISE | USA | 1999–2002 | 512 | 1418 | 217 (42) | 677 (48) | 6 | 8 | ||
| Cohort studies | |||||||||||
| Black Women’s Health Study15 | BWHS | USA | 1995–2013 | 202 | 899 | 79 (39) | 388 (43) | 5 | 5 | ||
| Nurses’ Health Study16 | NHS | USA | 1986–2012 | 1197 | 4788 | 771 (64) | 3069 (64) | 5 | 5 | ||
| Swedish Women’s Lifestyle and Health Study14 | SWLHS | Sweden | 1993–2012 | 229 | 997 | 202 (88) | 921 (92) | 8 | 9 | ||
Amongst women who breastfed
The data harmonisation process for the Consortium was described elsewhere13 but, briefly, studies provided information on participants’ demographics (cases’ diagnosis age, reference date for controls, race, education) and reproductive, health and lifestyle factors (e.g., parity, oral contraceptive use (OCP), height, weight) according to specified definitions. Studies also provided information on whether women breastfed, their total duration of breastfeeding (sum of duration of all of their breastfeeding episodes) and, for some studies, breastfeeding duration for each child. For cases, studies provided tumor histology information, where available. Women with sarcomas were excluded.
For the cohort studies the data were collected in slightly different ways. For one,14 data were mostly obtained from baseline questionnaires (1991–1992), with body mass index (BMI) data updated in 2003 for cases diagnosed after this and their matched controls. For the other two15,16, most data were from questionnaires returned in the period prior to a participant becoming a case or being selected as a control, although breastfeeding data were collected only once (1986) in the Nurses’ Health Study.16
Analyses were restricted to parous women with breastfeeding information. Less than 2% of participants were missing breastfeeding/covariate data so a complete case analysis was undertaken. Study-specific odds ratios (ORs) and 95% confidence intervals (CIs) were estimated for the associations between breastfeeding variables (see below) and risk of endometrial cancer using multivariable logistic regression (conditional logistic regression for matched studies). Models were adjusted for parity (continuous), OCP use, BMI (around reference age; continuous) and education level. Study-specific ORs were pooled (pORs) using random effects models giving overall pORs and 95%CIs. Between-study heterogeneity was assessed using I2 and Q statistics.
The women were classified as having breastfed or not. Total breastfeeding duration was modelled continuously and in categories: never breastfed, ≤3 months, >3 to ≤6 months, >6 to ≤9 months, >9 to ≤12 months, and then in six-month categories up to >36 months.
Because we hypothesised that risk reduction with breastfeeding might plateau as duration of a breastfeeding episode increased (beyond return of ovulation), we examined associations with duration of individual breastfeeding episodes. First, to allow inclusion of all the studies, we divided total breastfeeding duration by the number of births for each woman to give average duration of breastfeeding/child. Then, for studies with information on individual breastfeeding episodes (8,14, 17–25 plus Turin Study), we divided total breastfeeding duration by the number of children actually breastfed. Both variables were analysed in categories (as above, but with the highest category 12–18 months) weighting the first variable by the number of births and the second by the number of children breastfed to give the risk per child for each average duration.
We assessed whether associations differed between case-control and cohort studies by stratifying the meta-analysis by study type. We also stratified analyses by number of births to assess potential for residual confounding by parity; and by BMI at reference age (<30 kg/m2 vs. ≥30 kg/m2) and early adulthood (<25 kg/m2 vs. ≥25 kg/m2, where available, as this measure was generally closer to the time of breastfeeding) to assess whether associations varied by BMI. We conducted analyses in strata of histological subtype (Type I -endometrioid and mucinous adenocarcinomas vs. Type II -serous and clear cell cancers) as etiology may vary by subtype.13 Differences between strata were assessed using random-effects meta-regression.26
We explored heterogeneity between studies by assessing whether associations varied by participant characteristics, including those already mentioned plus race (white, black, Asian); menopausal status; participant’s birth year (<1950 vs. ≥1950); and years since last pregnancy (<30 vs. ≥ 30 years). We used random-effects meta-regression26 to evaluate whether these factors explained between-study heterogeneity.
We estimated the proportion of endometrial cancers (population attributable fraction) that could be attributed to breastfeeding for ≤6 months/child using the standard formula: , where the ERR was the excess relative risk for each category of average breastfeeding/child breastfed below the >6 to 9 months category. The prevalence (px) was the proportion of women in each category. Sensitivity analyses were conducted using prevalence estimates from the studies with the lowest23 and highest prevalence22 of breastfeeding >6 months/child.
We used SAS version 9.4 (SAS Institute, Cary, NC) and STATA version 13 (StataCorp LP, College Station, TX) for statistical analyses. All statistical significance tests are two-sided.
Results
Table 1 shows details of the included studies. The analyses included 8981 parous women with endometrial cancer and 17241 parous control women. Overall, 68% of the control women had breastfed at least one child but the percentage varied considerably across studies ranging from 43%15 to 92%.14 Median total duration of breastfeeding among controls who had ever breastfed ranged across studies from five15,16,24 to 13 months.22 As expected, compared to cases, the controls had higher parity, were more likely to have used OCPs and, on average, had a lower BMI (Table 2). Control women were also somewhat more likely to have post-high school education.
Table 2.
Prevalence of covariates among parous women in the pooled analysis.
| Covariate | Cases N=8981 |
Controls N=17241 |
|||
|---|---|---|---|---|---|
|
| |||||
| Mean ±SD | % | Mean ±SD | %* | P† | |
| Age, years | 61 ±8.9 | 60 ±8.8 | <0.001 | ||
| Body mass index (kg/m2) | 29.3 ±7.0 | 25.9 ±5.0 | <0.001 | ||
| Race | <0.001 | ||||
| White | 77.4 | 73.1 | |||
| Black | 4.1 | 4.7 | |||
| Asian | 12.6 | 12.4 | |||
| Other | 3.8 | 3.0 | |||
| Missing | 2.1 | 6.8 | |||
| Education | <0.001 | ||||
| High school or less | 49.4 | 46.0 | |||
| Post high school | 49.5 | 52.5 | |||
| Missing | 1.1 | 1.5 | |||
| Parity | <0.001 | ||||
| 1 | 21.7 | 18.2 | |||
| 2 | 36.1 | 35.5 | |||
| 3 | 23.3 | 24.7 | |||
| 4 | 11.3 | 12.0 | |||
| 5 | 7.6 | 10.5 | |||
| Oral contraceptive use | <0.001 | ||||
| No | 57.5 | 50.4 | |||
| Yes | 41.9 | 49.0 | |||
| Missing | 0.6 | 0.6 | |||
SD, standard deviation
weighted by numbers of case women in each study.
differences between cases and controls were tested with t-tests for continuous variables and χ2 tests for categorical variables.
Table 3 shows the crude and adjusted associations between the various breastfeeding measures and endometrial cancer risk – for brevity we refer only to the adjusted pooled ORs (pORs) in this section. Having ever breastfed was associated with a statistically significant 11% reduced risk of endometrial cancer compared with never breastfeeding (pOR=0.89, 95%CI 0.81–0.98) (Figure 1.), although there was moderate between-study heterogeneity (I2=45%, p=0.02). For total duration of breastfeeding, estimates for durations beyond three months suggested an inverse association that became more pronounced for very long durations (pOR=0.67, 95%CI 0.53–0.83 for >36 months total duration versus never breastfeeding). However, the risk reduction associated with increasing total duration of breastfeeding was not clearly linear. Our analyses of individual episodes of breastfeeding (average breastfeeding/child, average breastfeeding/child breastfed) also showed that individual breastfeeding durations beyond three months were associated with statistically significant reductions in risk. However, beyond the >6 to 9 months category the odds ratios did not appear to decrease much further although the numbers of women who breastfed individual children for longer than this were small and therefore these estimates are less precise.
Table 3.
Associations between breastfeeding and endometrial cancer risk among parous women in the pooled analysis
| Breastfeeding variables | Cases N(%)* |
Controls N(%)* |
Crude Pooled OR (95%CI) |
Adjusted Pooled OR† (95%CI) |
I2%‡ |
|---|---|---|---|---|---|
| Ever breast fed | |||||
| No | 3049 (33.9) | 5566 (32.3) | 1.00 | 1.00 | 4 |
| Yes | 5932 (66.1) | 11675 (67.7) | 0.82 (0.75–0.90) | 0.89 (0.81–0.98) | 5 |
| Total duration of breastfeeding (months) | |||||
| Never breastfed | 3049 (34.1) | 5566 (32.3) | 1.00 | 1.00 | |
| >0 to ≤3 | 1647 (18.4) | 3059 (17.8) | 0.98 (0.88–1.09) | 1.01 (0.91–1.12) | 18 |
| >3 to ≤6 | 915 (10.2) | 2017 (11.7) | 0.83 (0.74–0.92) | 0.84 (0.73–0.98) | 39 |
| >6 to ≤9 | 710 (7.9) | 1555 (9.0) | 0.81 (0.70–0.95) | 0.90 (0.77–1.05) | 32 |
| >9 to ≤12 | 667 (7.5) | 1000 (5.8) | 0.89 (0.73–1.07) | 0.96 (0.79–1.16) | 47 |
| >12 to ≤18 | 723 (8.1) | 1413 (8.2) | 0.82 (0.73–0.93) | 0.93 (0.82–1.05) | 0 |
| >18 to ≤24 | 506 (5.7) | 998 (5.8) | 0.74 (0.64–0.85) | 0.80 (0.69–0.93) | 3 |
| >24 to ≤30 | 248 (2.8) | 598 (3.5) | 0.66 (0.51–0.84) | 0.67 (0.51–0.87) | 40 |
| >30 to ≤36 | 179 (2.0) | 319 (1.9) | 0.65 (0.53–0.81) | 0.69 (0.54–0.88) | 0 |
| >36 | 309 (3.5) | 686 (4.0) | 0.58 (0.48–0.70) | 0.67 (0.53–0.83) | 18 |
| Per 3 months of total breastfeeding | 0.97 (0.96–0.98) | 0.97 (0.96–0.98) | 26 | ||
| Average duration of breastfeeding/child (months) § | |||||
| Never breastfed | 3049 (34.3) | 5566 (32.6) | 1.00 | 1.00 | |
| >0 to ≤3 | 2777 (31.3) | 5603 (32.9) | 0.99 (0.96–1.02) | 1.01 (0.98–1.03) | 0 |
| >3 to ≤6 | 1230 (13.9) | 2618 (15.4) | 0.92 (0.89–0.97) | 0.95 (0.91–0.99) | 25 |
| >6 to ≤9 | 844 (9.5) | 1649 (9.7) | 0.92 (0.88–0.97) | 0.93 (0.88–0.98) | 31 |
| >9 to ≤12 | 752 (8.5) | 1184 (6.9) | 0.90 (0.85–0.95) | 0.92 (0.86–0.97) | 7 |
| >12 to ≤18 | 225 (2.5) | 430 (2.5) | 0.86 (0.78–0.95) | 0.88 (0.79–0.97) | 22 |
| Average duration of breastfeeding/child breastfed§‖ (months) | |||||
| Never breastfed | 1793 (32.9) | 2335 (26.4) | 1.00 | 1.00 | |
| >0 to ≤3 | 1394 (25.6) | 2324 (26.3) | 0.98 (0.92–1.04) | 0.99 (0.93–1.05) | 29 |
| >3 to ≤6 | 798 (14.6) | 1674 (18.9) | 0.92 (0.86–0.98) | 0.93 (0.88–0.98) | 15 |
| >6 to ≤9 | 616 (11.3) | 1200 (13.6) | 0.90 (0.85–0.96) | 0.89 (0.83–0.95) | 17 |
| >9 to ≤12 | 673 (12.3) | 993 (11.2) | 0.88 (0.81–0.96) | 0.87 (0.80–0.95) | 21 |
| >12 to ≤18 | 176 (3.2) | 310 (3.5) | 0.86 (0.77–0.96) | 0.86 (0.78–0.95) | 1 |
OR, odds ratio; CI, confidence interval;
May not add to total due to missing data
Adjusted for age (years), parity (continuous), oral contraceptive duration (continuous; or ever use (yes/no)17,23, BMI (around reference age, continuous), education (level achieved or number of years).
From random effects meta-analysis models. The I2% measures variation across studies that is due to heterogeneity rather than chance.
OR is for the risk change per child or per child breast fed.
Figure 1.
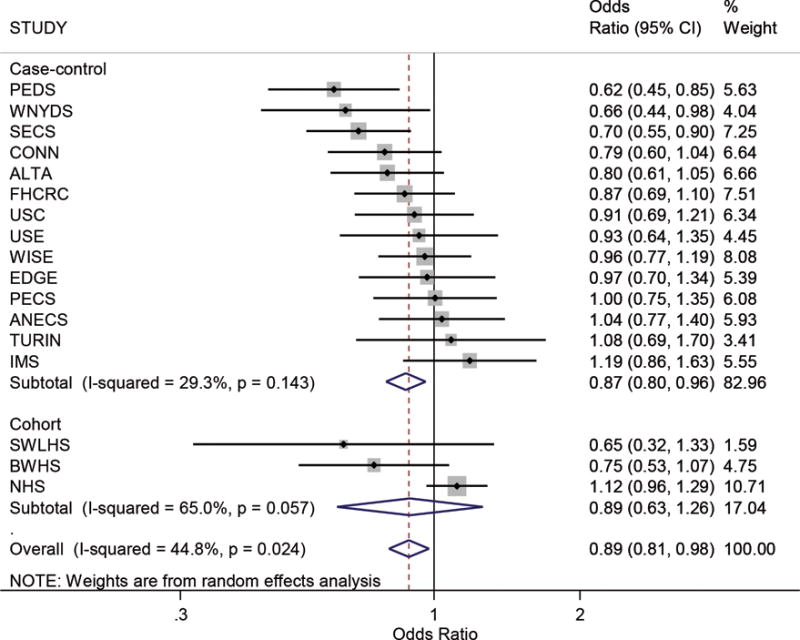
Forest plot showing study-specific estimates and 95% confidence intervals (CI) for the association between ever-breastfeeding and endometrial cancer risk amongst parous women. Estimates are stratified by study design (case-control/cohort) and ordered smallest to largest. The box size indicates the study weight, the line represents the 95% confidence interval, and the diamonds represent the pooled estimates. Higher I2 values and P values <.05 suggest statistically significant between-study heterogeneity. Study acronyms are defined in Table 1.
Figure 2 shows the results of analyses of ever-breastfeeding stratified by participant characteristics. The association did not vary substantially by tumor type (I vs. II), parity, recent BMI, or menopausal status. The magnitude of association differed somewhat by race/ethnicity, BMI in early adulthood (no association in heavier women) and time since last pregnancy but the differences were not statistically significant. For the race analyses, the number of studies included in each stratum was small; only two studies contributed to the estimate for Asian women,22,29 including one in which all women were Asian. Estimates for ever-breastfeeding differed significantly by women’s birth year (p=0.03). For women born since 1950 (likely to have breastfed after ~1970), ever breastfeeding was associated with a 28% reduction in endometrial cancer risk (OR=0.72, 95%CI 0.59–0.87) compared with never breastfeeding, whereas for women born before 1950 the risk reduction was smaller and no longer statistically significant (OR=0.93, 95%CI 0.85–1.02). It is notable that the study with the highest OR associated with ever-breastfeeding (OR=1.12, 95%CI 0.97–1.29)16 included no women born post-1950 and the participants had the highest mean age at diagnosis (65 years). Removing this study from the pooled analysis reduced the I2 from 45% to 24%.
Figure 2.
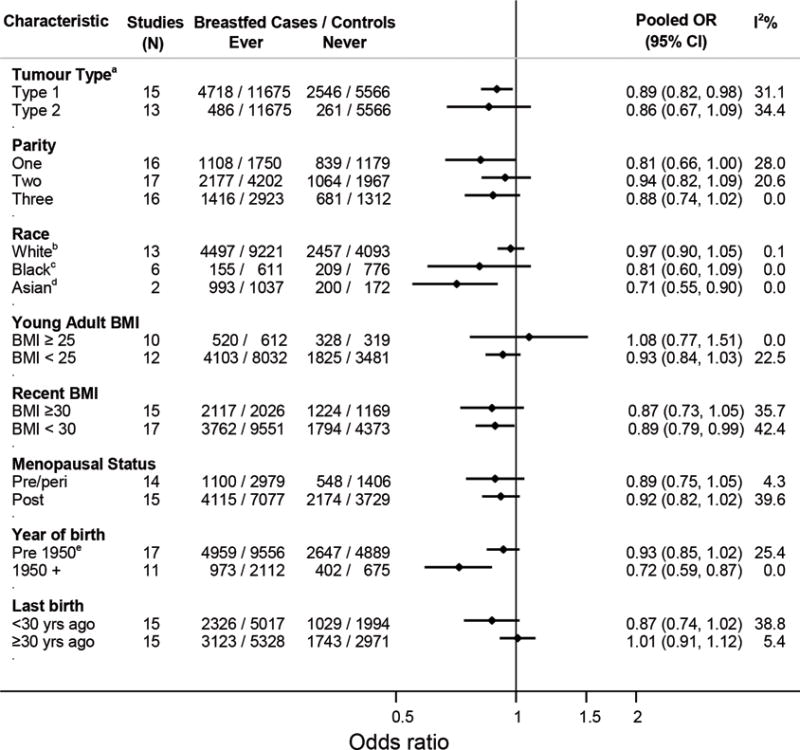
Pooled odds ratios (OR) and 95% confidence intervals (CI) for the association between ever-breastfeeding and endometrial cancer risk stratified by participant characteristics. The number of studies included in each stratum varies because some studies did not have information on the variable of interest or because there were too few women in specific strata to calculate study-specific estimates. aOnly case group was stratified. bIncludes five studies in which all women were white. cIncludes one study in which all women were black. dIncludes one study in which all women were Asian. eThe estimate for ever breastfed in women born before 1950, including only the 11 studies that also included women who were born after 1950 is 0.93 (95%CI 0.84–1.03).
Results from univariable meta-regression to evaluate potential sources of between-study heterogeneity in estimates for ever-breastfeeding are shown in Table 4. Of the factors considered, only the proportion of women whose last birth was <30 years prior to study participation (p=0.02, adjusted R2=82.3%) and the median age at diagnosis of case women in each study (p=0.03, adjusted R2=68.5%) were significantly associated with the strength of association. Although these factors were no longer statistically significant in a metaregression excluding the NHS, the adjusted R2 for each (last birth ≥30 years prior; median age at diagnosis) remained high at 41% and 68% respectively. No factors remained statistically significant when included in multivariable meta-regression, likely because of the relatively small number of studies involved.
Table 4.
Results of univariable meta-regression assessing factors associated with between-study heterogeneity in estimates for the association between ever-breastfeeding and endometrial cancer risk
| Factor | Adjusted R2%* | P-value from univariable meta-regression |
|---|---|---|
| Study design (case-control/cohort) | 9.4 | 0.48 |
| Proportion of white women | 31.0 | 0.1 |
| Median breastfeeding duration amongst women who breastfed | 4.4 | 0.5 |
| Median age at diagnosis | 68.5 | 0.03 |
| Proportion of women whose last birth was <30 years before reference date | 82.3 | 0.02 |
| Proportion of women born post-1950 | 24.9 | 0.2 |
Proportion of between-study variance explained
We estimated the proportion of endometrial cancers among parous women (population attributable fraction) that could be attributed to breastfeeding for ≤6 months per child to be 11% (range from sensitivity analyses 5–15%). The prevalence of nulliparity across the studies was 16% giving a population attributable fraction for all women of ~9% (possible range 4–13%).
Discussion
We observed a modest reduction in risk of endometrial cancer associated with breastfeeding that was not explained by greater parity and did not vary by BMI or endometrial cancer type (Type I versus Type II). The association appeared stronger with increasing duration of breastfeeding episodes up to between 6–9 months per child breastfed but thereafter the decline in risk was smaller. These results suggest that reduction in endometrial cancer risk could be added to the list of maternal benefits associated with breastfeeding for more than six months.
Strengths of our study include the large sample size, our ability to define exposure levels consistently across studies, to adjust consistently for potential confounders and the inclusion of studies from different US populations and different countries. However, some limitations should be considered. We had no information on factors that predispose women to breastfeed or data on when menstruation recommenced so could not consider these in our analyses. Most of the studies were of case-control design with potential self-selection of more health-conscious controls, perhaps more likely to have breastfed. Nevertheless, the pooled estimates for ever-breastfeeding did not vary by study design (cohort versus case-control) making selection bias less likely. Also, all studies relied on retrospective self-report of breastfeeding, which for many women occurred years prior to study participation and thus was possibly subject to recall error. This would be non-differential for the cohort studies and, again, the lack of difference in pooled estimates by study design makes substantial recall bias unlikely. Finally, despite the inclusion of >26000 women, relatively few women breastfed for long durations making estimates for these categories less precise.
An inverse association between breastfeeding and endometrial cancer risk is biologically plausible. Breastfeeding can suppress gonadotrophin-releasing hormone inhibiting ovarian follicular growth and reducing estradiol levels to within the postmenopausal range.2 At these levels, endometrial cell mitoses are virtually absent.3 Estrogen levels in breastfeeding women appear to depend on suckling stimuli, with the lowest levels found in women breastfeeding exclusively.2 Most guidelines recommend introduction of non-milk foods when infants are around six months old, so, notwithstanding considerable variation in breastfeeding practices between women, it is likely that the suckling stimulus decreases around this time and estrogen levels increase. This is consistent with our finding of levelling in risk reduction with longer durations of individual episodes of breastfeeding.
We had expected that BMI might modify the association with breastfeeding because obesity lowers sex hormone binding globulin levels, increasing bioavailable estrogen and testosterone. This, with estrone production in adipose tissue in more obese women, might negate the relative hypo-estrogenic state induced by breastfeeding. We did not observe significant effect-modification but our BMI measures may not closely reflect adiposity during breastfeeding. Most BMI data were from around diagnosis, generally years after breastfeeding. We had BMI estimates from early adulthood from 12 studies but this may have been poorly recalled since it was further in the past and may still not reflect a woman’s BMI during breastfeeding. Furthermore, few women were overweight on early adulthood measures so power may have been insufficient to detect effect-modification.
We observed moderate between-study heterogeneity in some associations, most notably for ever-breastfeeding (I2=45%). This is consistent with published meta-analyses investigating this relation,10,11 although only two studies8,20 included in those analyses were also in ours. We found the different proportions across studies of women who gave birth further in the past significantly contributed to heterogeneity. We also found the inverse association with breastfeeding was weaker in women born pre-1950. These factors may reflect attenuation of breastfeeding effects over time or differences in breastfeeding practices across birth cohorts causing different physiological effects in the endometrium. Breastfeeding rates, at least in the USA, were much lower in the 1950s and 1960s than more recently27 but whether breastfeeding practices (e.g., less demand or exclusive feeding with potentially less ovulation suppression28) also differed is not clear.
Assuming the associations we observed are causal and that prevalence of breastfeeding and nulliparity among women diagnosed with endometrial cancer in 2015 is similar to our study, it may be that, of the estimated 345,000 women diagnosed with endometrial cancer worldwide in 2015,1 around 31,000 (9%, possible range 14,500 (4%) to 43,500(13%)) might have been prevented if all parous women had been able to breastfeed their babies for more than six months each.
For health practitioners, our study suggests that promoting breastfeeding and providing support to women to breastfeed for six months and beyond might have the added benefit of contributing to the prevention of this increasingly common cancer.
Acknowledgments
Funding:
The Alberta Case-Control Study on Endometrial Cancer was funded by The Canadian Cancer Society. C. Friedenreich received career awards from the Canadian Institutes of Health Research and the Alberta Heritage Foundation for Medical Research (AHFMR) during the conduct of this study. L. Cook held a Canada Research Chair and also received career award funding from AHFMR. The Australian National Endometrial Cancer Study was funded by the National Health and Medical Research Council (NHMRC) of Australia (#339435) and Cancer Council Tasmania (#403031 and 457636). S. Jordan, A. Spurdle and P. Webb are supported by Fellowships from the NHMRC of Australia. The National Cancer Institute/the National Institutes of Health funded The Black Women’s Health Study (grant numbers: R01-CA058420, UMI-CA164974, and R03-CA169888), The Connecticut Endometrial Cancer Study (R01CA098346), The Estrogen, Diet, Genetics, and Endometrial Cancer (R01 CA83918, P30 CA008748), The Fred Hutchinson Cancer Research Center: (R35 CA39779, R01 CA47749, R01 CA75977, N01 HD 2 3166, K05 CA 92002, RO1 CA105212, and R01 CA87538). The Nurses’ Health Study: (2R01 CA082838), The Polish Endometrial Cancer Study and US Endometrial Cancer Study: (in part by intramural funds), The Shanghai Endometrial Cancer Study (R01 CA092585), The USC LA case control study (R01 CA48774 and P30 CA14089) and the Women’s Insight and Shared Experience: (P01-CA77596). The Italian Multi Centre Study was funded by The Italian Association for Cancer Research (AIRC). C.LaVecchia was supported by the Italian Foundation for Research in Cancer. TheSwedish Women’s Lifestyle and Health Study was funded by The Swedish Research Council (521-2011-2955). The Turin Case Control Study: The Italian Association for Cancer Research (AIRC) and the Ricerca Finalizzata Regione Piemonte.
References
- 1.Lyon, France: International Agency for Research on Cancer; 2013. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet] Available from: http://globocan.iarc.fr. [Google Scholar]
- 2.McNeilly AS. Lactational control of reproduction. Reprod Fertil Dev. 2001;13:583–90. doi: 10.1071/rd01056. [DOI] [PubMed] [Google Scholar]
- 3.Key TJ, Pike MC. The dose-effect relationship between ‘unopposed’ oestrogens and endometrial mitotic rate: its central role in explaining and predicting endometrial cancer risk. Br J Cancer. 1988;57:205–12. doi: 10.1038/bjc.1988.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Report. Food, nutrition, physical activity and the prevention of endometrial cancer. 2013 [Google Scholar]
- 5.Salazar-Martinez E, Lazcano-Ponce EC, Gonzalez Lira-Lira G, Escudero-De los Rios P, Salmeron-Castro J, Hernandez-Avila M. Reproductive factors of ovarian and endometrial cancer risk in a high fertility population in Mexico. Cancer Res. 1999;59:3658–62. [PubMed] [Google Scholar]
- 6.Newcomb PA, Trentham-Dietz A. Breast feeding practices in relation to endometrial cancer risk, USA. Cancer Causes Control. 2000;11:663–7. doi: 10.1023/a:1008978624266. [DOI] [PubMed] [Google Scholar]
- 7.Rosenblatt KA, Thomas DB. Prolonged lactation and endometrial cancer. WHO Collaborative Study of Neoplasia and Steroid Contraceptives. Int J Epidemiol. 1995;24:499–503. doi: 10.1093/ije/24.3.499. [DOI] [PubMed] [Google Scholar]
- 8.Brinton LA, Berman ML, Mortel R, Twiggs LB, Barrett RJ, Wilbanks GD, et al. Reproductive, menstrual, and medical risk factors for endometrial cancer: results from a case-control study. Am J Obstet Gynecol. 1992;167:1317–25. doi: 10.1016/s0002-9378(11)91709-8. [DOI] [PubMed] [Google Scholar]
- 9.Dossus L, Allen N, Kaaks R, Bakken K, Lund E, Tjonneland A, et al. Reproductive risk factors and endometrial cancer: the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2010;127:442–51. doi: 10.1002/ijc.25050. [DOI] [PubMed] [Google Scholar]
- 10.Wang L, Li J, Shi Z. Association between Breastfeeding and Endometrial Cancer Risk: Evidence from a Systematic Review and Meta-Analysis. Nutrients. 2015;7:5697–711. doi: 10.3390/nu7075248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma X, Zhao LG, Sun JW, Yang Y, Zheng JL, Gao J, et al. Association between breastfeeding and risk of endometrial cancer: a meta-analysis of epidemiological studies. Eur J Cancer Prev. 2015 doi: 10.1097/CEJ.0000000000000186. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 12.Olson SH, Chen C, De Vivo I, Doherty JA, Hartmuller V, Horn-Ross PL, et al. Maximizing resources to study an uncommon cancer: E2C2–Epidemiology of Endometrial Cancer Consortium. Cancer Causes Control. 2009;20:491–6. doi: 10.1007/s10552-008-9290-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Setiawan VW, Yang HP, Pike MC, McCann SE, Yu H, Xiang YB, et al. Type I and II endometrial cancers: have they different risk factors? J Clin Oncol. 2013;31:2607–18. doi: 10.1200/JCO.2012.48.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roswall N, Sandin S, Adami HO, Weiderpass E. Cohort Profile: The Swedish Women’s Lifestyle and Health cohort. Int J Epidemiol. 2015 doi: 10.1093/ije/dyv089. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 15.Rosenberg L, Adams-Campbell L, Palmer JR. The Black Women’s Health Study: a follow-up study for causes and preventions of illness. J Am Med Womens Assoc. 1995;50:56–8. [PubMed] [Google Scholar]
- 16.Viswanathan AN, Feskanich D, De Vivo I, Hunter DJ, Barbieri RL, Rosner B, et al. Smoking and the risk of endometrial cancer: results from the Nurses’ Health Study. Int J Cancer. 2005;114:996–1001. doi: 10.1002/ijc.20821. [DOI] [PubMed] [Google Scholar]
- 17.Friedenreich CM, Cook LS, Magliocco AM, Duggan MA, Courneya KS. Case-control study of lifetime total physical activity and endometrial cancer risk. Cancer Causes Control. 2010;21:1105–16. doi: 10.1007/s10552-010-9538-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu L, Risch H, Irwin ML, Mayne ST, Cartmel B, Schwartz P, et al. Long-term overweight and weight gain in early adulthood in association with risk of endometrial cancer. Int J Cancer. 2011;129:1237–43. doi: 10.1002/ijc.26046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fortuny J, Sima C, Bayuga S, Wilcox H, Pulick K, Faulkner S, et al. Risk of endometrial cancer in relation to medical conditions and medication use. Cancer Epidemiol Biomarkers Prev. 2009;18:1448–56. doi: 10.1158/1055-9965.EPI-08-0936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zucchetto A, Serraino D, Polesel J, Negri E, De Paoli A, Dal Maso L, et al. Hormone-related factors and gynecological conditions in relation to endometrial cancer risk. Eur J Cancer Prev. 2009;18:316–21. doi: 10.1097/cej.0b013e328329d830. [DOI] [PubMed] [Google Scholar]
- 21.Brinton LA, Sakoda LC, Lissowska J, Sherman ME, Chatterjee N, Peplonska B, et al. Reproductive risk factors for endometrial cancer among Polish women. Br J Cancer. 2007;96:1450–6. doi: 10.1038/sj.bjc.6603731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shu XO, Brinton LA, Zheng W, Gao YT, Fan J, Fraumeni JF., Jr A population-based case-control study of endometrial cancer in Shanghai, China. Int J Cancer. 1991;49:38–43. doi: 10.1002/ijc.2910490108. [DOI] [PubMed] [Google Scholar]
- 23.McCann SE, Freudenheim JL, Marshall JR, Brasure JR, Swanson MK, Graham S. Diet in the epidemiology of endometrial cancer in western New York (United States) Cancer Causes Control. 2000;11:965–74. doi: 10.1023/a:1026551309873. [DOI] [PubMed] [Google Scholar]
- 24.Pike MC, Peters RK, Cozen W, Probst-Hensch NM, Felix JC, Wan PC, et al. Estrogen-progestin replacement therapy and endometrial cancer. J Natl Cancer Inst. 1997;89:1110–6. doi: 10.1093/jnci/89.15.1110. [DOI] [PubMed] [Google Scholar]
- 25.Doherty JA, Weiss NS, Fish S, Fan W, Loomis MM, Sakoda LC, et al. Polymorphisms in nucleotide excision repair genes and endometrial cancer risk. Cancer Epidemiol Biomarkers Prev. 2011;20:1873–82. doi: 10.1158/1055-9965.EPI-11-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] The Cochrane Collaboration. 2011 Available from www.handbook.cochrane.org.2011.
- 27.Martinez GA, Nalezienski JP. The recent trend in breast-feeding. Pediatrics. 1979;64:686–92. [PubMed] [Google Scholar]
- 28.Rogers IS. Lactation and fertility. Early Hum Dev. 1997;49(Suppl):S185–90. doi: 10.1016/s0378-3782(97)00063-7. [DOI] [PubMed] [Google Scholar]
- 29.Neill AS, Nagle CM, Protani MM, Obermair A, Spurdle AB, Webb PM, et al. Aspirin, nonsteroidal anti-inflammatory drugs, paracetamol and risk of endometrial cancer: a case-control study, systematic review and meta-analysis. Int J Cancer. 2013;132:1146–55. doi: 10.1002/ijc.27717. [DOI] [PubMed] [Google Scholar]
- 30.Moysich KB, Baker JA, Rodabaugh KJ, Villella JA. Regular analgesic use and risk of endometrial cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:2923–8. doi: 10.1158/1055-9965.EPI-05-0457. [DOI] [PubMed] [Google Scholar]
- 31.Strom BL, Schinnar R, Weber AL, Bunin G, Berlin JA, Baumgarten M, et al. Case-control study of postmenopausal hormone replacement therapy and endometrial cancer. Am J Epidemiol. 2006;164:775–86. doi: 10.1093/aje/kwj316. [DOI] [PubMed] [Google Scholar]


