Abstract
Fluoroquinolone-resistance in Pseudomonas aeruginosa may be due to efflux pump overexpression (EPO) and/or target mutations. EPO can result in multidrug resistance (MDR) due to broad substrate specificity of the pumps. MC-04,124, an efflux pump inhibitor (EPI) shown to significantly potentiate activity of levofloxacin in P. aeruginosa, was used to examine the prevalence of EPO in clinical isolates. MICs were determined for ciprofloxacin, levofloxacin, moxifloxacin, and gatifloxacin with or without EPI and for other antipseudomonal agents by using broth microdilution against P. aeruginosa isolates from adults (n = 119) and children (n = 24). The prevalence of the EPO phenotype (≥8-fold MIC decrease when tested with EPI) was compared among subgroups with different resistance profiles. The EPO phenotype was more prevalent among levofloxacin-resistant than levofloxacin-sensitive strains (61%, 48/79 versus 9%, 6/64). EPO was present in 60% of fluoroquinolone-resistant strains without cross-resistance, while it was present at variable frequencies among strains with cross-resistance to other agents: piperacillin-tazobactam (86%), ceftazidime (76%), cefepime (65%), imipenem (56%), gentamicin (55%), tobramycin (48%), and amikacin (27%). The magnitude of MIC decrease with an EPI paralleled the frequency of which the EPO phenotype was observed in different subgroups. EPI reduced the levofloxacin MIC by as much as 16-fold in eight strains for which MICs were 128 μg/ml. Efflux-mediated resistance appears to contribute significantly to fluoroquinolone resistance and MDR in P. aeruginosa. Our data support the fact that increased fluoroquinolone usage can negatively impact susceptibility of P. aeruginosa to multiple classes of antipseudomonal agents.
Pseudomonas aeruginosa is a leading pathogen that causes nosocomial pneumonia, bloodstream infections, and urinary tract infections in intensive care units (3, 37). Infections caused by P. aeruginosa are associated with significant morbidity and mortality (6, 13). The organism exhibits a high level of intrinsic resistance and only a limited number of antimicrobial agents are active against it (11). In addition, P. aeruginosa has acquired multiple mechanisms of resistance against all available antipseudomonal agents (11, 38). Specifically, target-based mutation in gyrase and/or topoisomerase and overexpression of efflux pumps (EPs) contribute to fluoroquinolone (FQ) resistance (11). Of note, in vitro data indicate that at FQ concentrations near the MIC, efflux mutants are preferentially selected before target mutations (16).
Efflux pumps present in P. aeruginosa serve physiologic functions such as the removal of intracellular toxic substances or metabolites as well as excretion of signaling molecules into the environment to facilitate cell-to-cell communication (9, 17, 28, 30, 31). Six EPs have been identified thus far: MexAB-OprM, MexCD-OprJ, MexEF-OprN, MexXY-OprM, MexJK-OprM, and MexVW-OprM (5, 20, 31). The first three have been well characterized. Each pump consists of a tripartite system: cytoplasmic membrane exporter protein in the resistance-nodulation-division family, an outer membrane protein, and a membrane fusion protein linking the exporter protein and the outer membrane protein. All of the pumps can expel a variety of compounds ranging from detergents to structurally unrelated antimicrobial agents from the cytoplasm or periplasmic space (9, 31). While each pump has a preferential set of antimicrobial agent substrates, the fluoroquinolones are universal substrates for all known Mex pumps (24, 31). Thus, FQ exposure has the potential to select for mutants with the multidrug-resistant (MDR) phenotype via efflux pump overexpression.
Large-scale surveillance studies have reported an increasing rate of FQ resistance among clinical isolates of P. aeruginosa; however, the role of efflux pump overexpression contributing to resistance is difficult to infer based on susceptibility pattern and has not been systematically examined (27). Several reports have documented the selection of the MDR phenotype by quinolones in vitro and in vivo. An early report described ciprofloxacin (CIP)-selected mutants with resistance to β-lactams, aminoglycosides, and other quinolones with several alterations in outer membrane proteins including an increase in a 54-kDa protein (18). Another report described an isolate showing the nfxC phenotype with high-level quinolone and imipenem (IPM) resistance, which was obtained from the urine of a patient treated with norfloxacin. Attributable mechanisms of resistance were alterations in DNA gyrase, loss of D2 porin, and an increase in a 50-kDa protein (33). Several others have also reported the MDR phenotype with FQ resistance and an increase of an approximately 50-kDa outer membrane protein (7, 15, 23, 32).
A series of dipeptide amide compounds have been identified as efflux pump inhibitors (EPIs) with the ability to broadly inhibit several known multidrug efflux pumps in P. aeruginosa, MexAB-OprM, MexCD-Opr, and MexEF-OprN, and possibly other efflux pumps (10, 19, 21, 22, 34-36), by competing with antibiotic substrates for binding to the pumps. Devoid of intrinsic antibacterial activity, these inhibitors have been demonstrated to (i) potentiate the activity of FQs and other antibiotics, (ii) reverse acquired FQ resistance attributable to not only efflux mutations but also to target site mutations, and (iii) markedly decrease the frequency with which highly FQ-resistant strains could be selected in vitro (22, 34). These compounds have been used to screen for efflux pump overexpression in clinical isolates of P. aeruginosa (4, 22). For this study, we employed the EPI compound, MC-04,124, to characterize the efflux pump-overexpressed phenotype in clinical isolates of P. aeruginosa (34, 40). MC-04,124 has been shown to be as potent but less toxic than earlier derivatives (MC-207,110 and MC-02,595) in this series of compounds (22, 34, 40).
We hypothesized that the widespread use of FQ agents could have a deleterious collateral effect on the susceptibility of P. aeruginosa to existing antipseudomonal agents through FQ-selected overexpression of multidrug efflux pumps. The specific objectives of our study were to use an EPI as a screening agent to evaluate (i) the prevalence of efflux pump-mediated resistance among clinical isolates of P. aeruginosa and (ii) the contribution of EP overexpression as the putative mechanism for the MDR phenotype in P. aeruginosa.
(This study was presented in part at the 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, Ill., 14 to 17 September 2003.)
MATERIALS AND METHODS
Bacterial strains.
A total of 143 strains of P. aeruginosa isolated from non-cystic fibrosis patients from August of 2000 to June of 2002 were studied (adults, n = 119 from Huntington Hospital; children, n = 24 from Childrens Hospital Los Angeles). The isolates obtained from children served as FQ-naive controls, as FQ agents are contraindicated in the pediatric population, and thus, these patients were unlikely to have been preexposed to FQs. Isolates were recovered from various sources, sputum, blood, urine, and wounds, and were stored at −70°C in Trypticase soy broth supplemented with 10% glycerol until ready for testing. Control strains included PAO1 (wild type) (12) and ATCC 27853. In addition, laboratory strains of P. aeruginosa with known genotypes overexpressing individual efflux pumps were also used (Table 1).
TABLE 1.
Synergistic in vitro activity of MC-04,124 combined with LVX against laboratory strains of P. aeruginosa
| Straina | Genotype | MIC of EPI alone (μg/ml) | LVX MIC (μg/ml) in the presence of the following concentrations of MC-04,124 (μg/ml):
|
Fold decrease in MIC at 10 μg/ml | Fold decrease in MIC at 20 μg/ml | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1.25 | 2.5 | 5 | 10 | 20 | 40 | 80 | |||||
| PAM1020 | PAO1 prototroph | >80 | 0.125 | 0.125 | 0.125 | 0.125 | 0.016 | 0.016 | 0.008 | 0.008 | 8 | 8 |
| PAM1723 | nalB ΔmexCD-oprJ::Gm ΔmexEF-oprN::ΩHg | >80 | 2 | 1 | 1 | 0.5 | 0.125 | 0.031 | 0.031 | 0.008 | 16 | 64 |
| PAM1738 | nfxB ΔmexAB-oprM::Cm ΔmexEF-oprN::ΩHg | >80 | 2 | 2 | 1 | 0.5 | 0.125 | 0.016 | 0.008 | 0.008 | 16 | 256 |
| PAM1753 | nfxC ΔmexAB-oprM::Cm ΔmexCD-oprJ::Gm | >80 | 2 | 2 | 2 | 1 | 0.125 | 0.016 | 0.008 | 0.008 | 16 | 128 |
| PAM1626 | ΔmexAB-oprM::Cm ΔmexCD-oprJ::Gm ΔmexEF-oprN::ΩHg | >80 | 0.016 | 0.016 | 0.008 | 0.008 | 0.016 | 0.008 | 0.008 | 0.008 | 1 | 2 |
Source of strains from reference 22.
Tests performed.
Checkerboard titration assay was performed to assess interaction between levofloxacin (LVX) and MC-04,124. LVX MICs were determined alone and in the presence of EPI at concentrations ranging from 1.25 to 80 μg/ml against laboratory strains of P. aeruginosa with known genotypes. A fixed concentration of 20 μg of EPI/ml was selected for testing clinical isolates in this study based on results from the checkerboard assay in order to provide clear differentiation between wild-type and pump-overexpressed strains (Table 1).
Broth microdilution tests were performed according to the National Committee for Clinical Laboratory Standards (NCCLS) recommendations to determine strain susceptibility (26). Each strain was freshly thawed and subcultured prior to testing. The following agents were tested in twofold dilutions in Mueller-Hinton broth (Becton Dickinson, Franklin Lakes, N.J.): CIP, LVX, cefepime, ceftazidime, piperacillin-tazobactam, imipenem (IPM), gentamicin, tobramycin, and amikacin. Additionally, the isolates were tested against the FQs—LVX, CIP, moxifloxacin (MOX), and gatifloxacin (GAT)—alone at concentrations ranging from 0.125 to 32 μg/ml and in the presence of 20 μg of EPI/ml. For strains with a LVX MIC >32 μg/ml and a decrease in MIC when tested in combination with EPI, additional testing with LVX concentrations up to 256 μg/ml was performed. Isolates were tested in duplicate and results were read visually and spectrophotometrically after overnight incubation at 35°C.
Definitions.
The MIC was the lowest concentration of the antimicrobial agent at which no growth was detected. Resistance was defined by MIC breakpoints according to NCCLS interpretive criteria for the respective agent. No MIC breakpoints for P. aeruginosa were provided by NCCLS for GAT and MOX, thus LVX breakpoints were used arbitrarily. An efflux pump-overexpressed phenotype was arbitrarily defined as any strain exhibiting at least an eightfold decrease in MIC to FQ when tested in the presence of an EPI (MC-04,124). This measure was based on the observation of a four- to eightfold potentiation of levofloxacin activity with this and other diamine EPI derivatives against the wild-type strain with constitutive expression of MexAB-OprM (4, 22).
Data analysis.
LVX was used as the FQ marker for comparison between MICs of FQ alone and in the presence of an EPI. The prevalence of the efflux pump-overexpressed phenotype was determined for strains overall and also for subgroups based on FQ susceptibility and pattern of cross-resistance to other antipseudomonal agents.
Subgroupings.
Isolates were subgrouped into the following categories. The levofloxacin-sensitive (LvxS) group included isolates obtained from adults and children, where isolates from children served as a FQ-naive control group. Levofloxacin-resistant (LvxR) group isolates were further divided into four groups arbitrarily based on the varied Mex pump substrate profile (1, 24, 29, 39): 1, LvxR-Only; 2, with β-lactam resistance (Lvx-BL, defined as LvxR plus resistance to all β-lactams—ceftazidime, cefepime, and piperacillin-tazobactam, excluding IPM); 3, with imipenem resistance (Lvx-IPM); and 4, with other resistance such as an aminoglycoside agent or single β-lactam agent (Lvx-Other).
The frequency of the EP-overexpressed phenotype was compared for FQ-resistant strains with and without cross-resistance to other agents. In addition, the magnitudes of activity potentiation (as measured by the magnitude of change in MICs alone versus in the presence of an EPI) were examined for each FQ agent tested and compared with each other.
Statistical analysis.
Prevalence of efflux pump overexpression, cross-resistance, and magnitude of MIC decrease with EPI were compared by chi-square or Fisher's exact test, as deemed appropriate. The significance level was 0.05. Analyses were performed using GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego, Calif.).
RESULTS
Prevalence of efflux pump-overexpressed phenotype.
Overall, the efflux pump-overexpressed phenotype was observed in a significantly greater number of LvxR than LvxS strains (61%, 48/79 versus 9%, 6/64; P < 0.0001). LvxS strains from adults were twice as likely to exhibit EP overexpression compared to strains obtained from children (12%, 5/42 versus 5%, 1/22; P > 0.33); however, LVX MICs were similar for strains obtained from adults and children. In addition, differences in the prevalence of strains showing the EP-overexpressed phenotype were noted among LvxR subgroups (Table 2). Specifically, the subgroup which demonstrated the highest prevalence of the EP-overexpressed phenotype was Lvx-BL (100%, 10/10) followed by LvxR-Only (58%, 14/24), Lvx-IPM (56%, 19/34), and Lvx-Other (45%, 5/11). The difference in prevalence of the EP-overexpressed phenotype for the above subgroups was statistically significant when compared to LvxS strains.
TABLE 2.
Prevalence of the efflux pump-overexpressed phenotypea
| Phenotype | No. of isolates | No. (%) of strains with EPO phenotype |
|---|---|---|
| LvxS (adult, children) | 64 | 6 (9) |
| 42, 22 | 5 (12), 1 (5) | |
| LvxR | 79 | 48 (61) |
| LvxR-Only | 24 | 14 (58) |
| Lvx-BL | 10 | 10 (100) |
| Lvx-IPM | 34 | 19 (56) |
| Lvx-Other | 11 | 5 (45) |
The efflux pump-overexpressed (EPO) phenotype equals at least an eightfold decrease in FQ MIC in the presence of EPI; LVX was the FQ marker. P value < 0.0001 for LvxS versus LvxR overall, LvxR-Only, Lvx-BL, or Lvx-IPM subgroups; P value = 0.002 for LvxS versus Lvx-Other.
Cross-resistance.
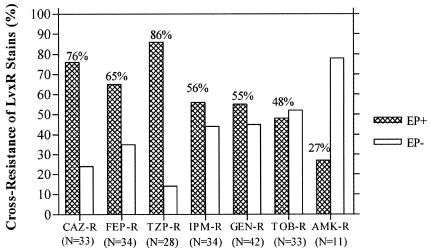
Nearly all of the LvxR isolates (≥95%) showed cross-resistance to other FQ agents: ciprofloxacin (75/79, 95%), gatifloxacin (76/79, 96%), and moxifloxacin (74/79, 95%). In addition, a substantial portion of LvxR isolates with cross-resistance to structurally unrelated antipseudomonal agents exhibited the efflux pump-overexpressed phenotype (Fig. 1). LvxR strains showing cross-resistance towards a β-lactam antipseudomonal agent have the highest prevalence of the EP-overexpressed phenotype compared to other cross-resistant subgroups. Specifically, LvxR strains which are cross-resistant to piperacillin-tazobactam have the highest prevalence of the efflux-overexpressed phenotype (85%), followed by ceftazidime (76%). The EP-overexpressed phenotype was present in about half of the strains with cross-resistance to cefepime (56%), imipenem (55%), gentamicin (54%), and tobramycin (48%).
FIG. 1.
Frequency of the efflux pump-overexpressed phenotype (EP) in LvxR strains showing cross-resistance to non-FQ antipseudomonal agents. n, number of LvxR isolates showing cross-resistance to the respective agent. CAZ, ceftazidime; FEP, cefepime; TZP, piperacillin-tazobactam; GEN, gentamicin; TOB, tobramycin; AMK, amikacin.
Magnitude of MIC decrease with EPI.
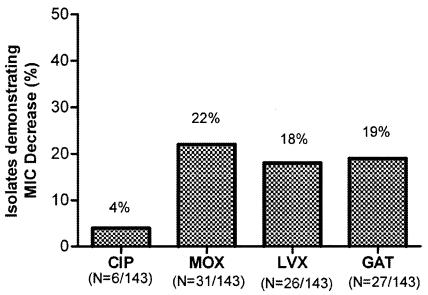
The effect of EPI on fluoroquinolone activity against P. aeruginosa was compared among the FQ agents over the concentration range of 0.5 to 8 μg/ml. The effect of EPI as measured by at least eightfold MIC decrease appeared similar on LVX, MOX, and GAT but significantly less for CIP (Fig. 2). It is notable that a substantial number of strains had MICs beyond the concentration range tested; only levofloxacin was selected for additional testing using a broader range of concentrations for those strains. An additional 28 strains demonstrating an EP-overexpressed phenotype resulted from testing of strains at a broader range of LVX concentrations (0.25 to 256 μg/ml); of those, 13 strains exhibited a 32-fold LVX MIC decrease while one strain showed as much as 64-fold MIC decrease in the presence of EPI.
FIG. 2.
Effect of EPI on fluoroquinolone MICs. All strains were tested over the same range of concentrations (0.5 to 8 μg/ml) for all four FQ agents. n, number of strains showing at least an 8-fold decrease in MIC with an EPI; an additional 28 isolates showed a ≥8-fold decrease in the LVX MIC when tested at a broader concentration range (0.25 to 256 μg/ml). P was ≤0.0002 for CIP versus MOX, LVX, or GAT. P was >0.05 for comparison amongst MOX, LVX, and GAT.
The MICs at which 50 and 90% of the isolates tested were inhibited (MIC50s and MIC90s) of LVX alone and in the presence of an EPI are shown for each subgroup in Table 3. MIC50 decreased by one twofold dilution from 0.5 to 0.25 μg/ml for LVX-susceptible strains. In contrast, MIC50 dropped by three twofold dilutions from 64 to 8 μg/ml in LVX-resistant strains. Reversal of FQ resistance was observed in over one-third (37%, 29/79) of LvxR strains based on decreases in LVX MICs to susceptible breakpoint concentration following the addition of an EPI. For the eight strains with an LVX MIC as high as 128 μg/ml, EPI potentiated the activity of LVX to an MIC of 8 μg/ml against 75% (6/8) of strains and to an MIC as low as 2 μg/ml in one strain. Of note, two subgroups with the highest MIC90s (LvxR-Only and Lvx-BL) also had the greatest decrease in MIC90 in the presence of an EPI from 128 to 16 and 8 μg/ml, respectively.
TABLE 3.
Levofloxacin MIC alone and in the presence of an EPI
| Parameter | Result (μg/ml) for:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| LvxS
|
LvxR
|
|||||||
| All (n = 64) | Children (n = 22) | Adults (n = 42) | All (n = 79) | Lvx-Only (n = 24) | Lvx-BL (n = 10) | Lvx-IPM (n = 34) | Lvx-Other (n = 11) | |
| LVX MIC50 | ||||||||
| Alone | 0.5 | 0.5 | 0.5 | 64 | 32 | 64 | 64 | 64 |
| +EPI | 0.25 | 0.25 | 0.25 | 8 | 8 | 8 | 8 | 8 |
| Lvx MIC90 | ||||||||
| Alone | 2 | 2 | 2 | 128 | 128 | 128 | 64 | >32 |
| +EPI | 0.5 | 0.5 | 0.5 | >32 | 16 | 8 | >32 | >32 |
| Lvx MIC range | ||||||||
| Alone | 0.25-2 | 0.25-2 | 0.25-2 | 8-128a | 8-128b | 8-128c | 8-64d | 8->32e |
| +EPI | 0.25-0.5 | 0.25-0.5 | 0.25-0.5 | 0.5->32 | 0.25->32 | 0.5-8 | 0.5->32 | 0.25->32 |
All LvxR strains: MIC for eight strains = 128 μg/ml (for six of the eight strains, MIC with an EPI = 8 μg/ml); MIC for 25 strains = 64 μg/ml (for 14 of the 25 strains, MIC with an EPI = 8 μg/ml; for 8 of the 25 strains, MIC with an EPI = 2 μg/ml).
LvxR-Only subgroup: MIC for three strains = 128 μg/ml (for one of the three strains, MIC with an EPI = 2 μg/ml); MIC for five strains = 64 μg/ml (for two of the five strains, MIC with an EPI = 2 μg/ml).
Lvx-BL subgroup: MIC for four strains = 128 μg/ml (for all four strains, MIC with an EPI = 8 μg/ml); MIC for five strains = 64 μg/ml (for two of the five strains, MIC with an EPI = 8 μg/ml; for three of the five strains, MIC with an EPI = 2 μg/ml).
Lvx-IPM subgroup: MIC for 14 strains = 64 μg/ml (for 9 of the 14 strains, MIC with an EPI = 8 μg/ml).
Lvx-Other subgroup: MIC for one strain = 128 μg/ml (with an EPI, MIC = 8 μg/ml); MIC for one strain = 64 μg/ml (with an EPI, MIC = 8 μg/ml).
DISCUSSION
Data from the Centers for Disease Control and Prevention-National Nosocomial Infections Surveillance indicate a 50% increase in the incidence of levofloxacin-resistant P. aeruginosa strains associated with nosocomial infections in intensive care units between 1994 to 1999 (3). Parallel to the rise in FQ resistance is a 78% increase in FQ prescribing between 1992 and 2000 in the United States based on ambulatory care prescription data (25). It is notable that many of the FQ-resistant strains are associated with cross-resistance to structurally unrelated antimicrobial agents. Thus, a link between the increased use of FQs and collateral deleterious effect on P. aeruginosa susceptibility has been suggested based on data from epidemiologic studies (2, 27).
Large-scale surveillance studies thus far have tracked the change in P. aeruginosa susceptibilities over time but have not evaluated the underlying mechanisms responsible for the increase in P. aeruginosa resistance (2, 8, 14, 27). To date, only one epidemiologic study has investigated the contribution of EP overexpression to FQ resistance among clinical isolates of P. aeruginosa obtained from 12 countries using EP-specific and broad-spectrum inhibitors (4). Pump profiles among studied strains varied widely by country of origin. Results specific to strains from the United States (n = 258) indicate that 50% of the clinical isolates overexpressed the MexAB-OprM pump, with one-third of the isolates (32%) expressing a combination of efflux pumps and/or target mutations. However, this study did not explore the role of EP overexpression in strains with the MDR phenotype.
In our study, we determined the prevalence of the efflux pump-overexpressed phenotype and compared FQ-susceptible and FQ-resistant clinical isolates of P. aeruginosa obtained from non-cystic fibrosis adult and pediatric patients. We included susceptible strains from patients (i.e., pediatrics) who were unlikely to have been preexposed to the FQ agents as controls. We screened for the presence of the EP-overexpressed phenotype using a broad-spectrum EPI (MC-04,124) based on its ability to potentiate levofloxacin activity (10, 22, 34, 40). The efflux pump-overexpressed phenotype was present in the majority (61%) of FQ-resistant strains compared to only 9% of FQ-susceptible strains.
In addition, we observed that strains expressing multidrug resistance were significantly associated with the efflux pump-overexpressed phenotype. The presence of the EP-overexpressed phenotype in all FQ-resistant strains with cross-resistance to non-imipenem β-lactams is remarkable. By analyzing the individual β-lactam agent, we found that the EP-overexpressed phenotype is most frequently encountered in the presence of coresistance to piperacillin-tazobactam (85%) followed by ceftazidime (76%).
EPI appears to have the strongest effect on potentiating LVX activity in strains showing the greatest degree of resistance to LVX. Notably, MIC90s for the subgroup of strains resistant to all β-lactam agents (excluding imipenem) decreased by 16-fold (128 to 8 μg/ml) in the presence of an EPI compared to fourfold (2 to 0.5 μg/ml) for levofloxacin-susceptible clinical strains and twofold (0.5 to 0.25 μg/ml) for the PAO1 wild-type strain. The effect of the EPI on FQ MICs appears to be similar for LVX, MOX, and GAT but lower for CIP when tested over the same MIC range. An accurate assessment and comparison of the potentiating effect of EPI on FQ agents other than LVX may be limited by the substantial number of strains with MICs beyond the upper limit of concentration range tested. However, it is possible that the differential effects observed may be a function of differential binding affinity of the EPI or the Mex systems expressed towards each FQ agent. Kohler et al. demonstrated that the quinolones differed in their ability to select for a particular efflux system depending on its C-7 and C-6 constituents (16). Ciprofloxacin demonstrated preferential selection for the MexCD-OprJ system (16). Levofloxacin, however, had an eightfold decrease in MIC (in the presence of an EPI) for all strains expressing the mexAB-oprM (22).
Interestingly, use of an EPI with broad activity against known Mex pumps (MC-04,124) did not potentiate LVX activity at all in 18% (14/79) of our FQ-resistant strains while the LVX MIC was decreased fourfold in 19% and twofold in 3% of the remaining FQ-resistant strains, suggesting that other mechanisms such as target mutations may contribute significantly to resistance in these strains. It is possible that the prevalence of the EP-overexpressed phenotype may be greater if testing to determine actual MICs was performed at the higher range of concentrations for other FQ agents besides LVX. In addition, our arbitrary measure of the efflux-overexpressed phenotype based on an eightfold MIC decrease may have underestimated the contribution of efflux pumps to FQ resistance.
Our demonstration of the widespread presence of the EP-overexpressed phenotype in FQ-resistant and MDR strains requires genotypic confirmation. Further studies are planned to explore other mechanisms that may have contributed to FQ resistance (i.e., target mutations) and MDR in our strains (i.e., target mutations, β-lactamase production, and loss of outer membrane porins).
Efflux pump-mediated resistance is an underrecognized mechanism contributing to resistance in clinical isolates of P. aeruginosa. We have demonstrated that the efflux pump-overexpressed phenotype appears to be widespread among clinical strains which have developed resistance to the fluoroquinolone class of agents. Broader testing of P. aeruginosa isolates in large-scale surveillance studies is warranted to establish the true prevalence of the efflux-overexpressed phenotype. More importantly, we have provided support for the link between FQ resistance and multidrug resistance through phenotypic confirmation of EP overexpression. Strains which overexpress efflux pumps are frequently resistant to the fluoroquinolones and the β-lactams simultaneously, leaving few available treatment options. Considering that the FQs appear to be universal substrates for the Mex pumps and that these pumps have broad substrate specificity for structurally unrelated compounds, increased exposure through widespread prescribing of the fluoroquinolones will undoubtedly promote the selection for strains overexpressing efflux pumps. The continued emergence and increasing prevalence of FQ-resistant and MDR P. aeruginosa is of grave concern and urgently calls for clinicians to spare the use of fluoroquinolones as first-line therapy in order to preserve the utility of the existing antipseudomonal armamentarium.
Acknowledgments
This study was supported in part by an unrestricted educational grant from Wyeth Pharmaceuticals.
We are thankful for the assistance of Jasmine Razeghi, Megan Nguyen, Clark Inderlied, and Misa Nakayama and the Microbiology Laboratories at Huntington Hospital and Childrens Hospital Los Angeles in saving bacterial isolates.
We thank Kevin Ward and Janet Hindler at the Clinical Microbiology Laboratory at UCLA Medical Center for preparing the microtiter trays for susceptibility testing and Lyn Cote from Mpex Pharmaceuticals for performing checkerboard titration assays.
REFERENCES
- 1.Aeschlimann, J. R. 2003. The role of multidrug efflux pumps in the antibiotic resistance of Pseudomonas aeruginosa and other gram-negative bacteria. Pharmacotherapy 23:916-924. [DOI] [PubMed] [Google Scholar]
- 2.Bhavnani, S., W. Callen, A. Forrest, K. Glilliland, D. Collins, J. Paladino, and J. Schentag. 2003. Effect of fluoroquinolone expenditures on susceptibility of Pseudomonas aeruginosa to ciprofloxacin in U.S. hospitals. Am. J. Health Syst. Pharm. 60:1962-1970. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention-National Nosocomial Infections Surveillance. 1999. Hospital infection program. NNIS antimicrobial resistance ICU surveillance report, 1999. Centers for Disease Control and Prevention, Atlanta, Ga.
- 4.Cho, D., D. Lofland, J. Blais, K. Tangen, D. Cotter, O. Lomovskaya, S. Chamberland, and M. Dudley. 1999. Prevalence of efflux pumps among clinical isolates of fluoroquinolone-resistant Pseudomonas aeruginosa, abstract 1267. Program Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., San Francisco, Calif.
- 5.Chuanchuen, R., C. T. Narasaki, and H. P. Shweizer. 2002. The MexJK efflux pump of Pseudomonas aeruginosa requires OprM for antibiotic efflux but not for efflux of triclosan. J. Bacteriol. 184:5036-5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fagon, J.-Y., J. Chastre, M. Wolff, C. Gervais, S. Parer-Aubas, F. Stephan, T. Similowski, A. Mercat, J.-L. Diehl, J.-P. Sollet, and A. Tenaillon. 2000. Invasive and non-invasive strategies for management of suspected ventilator-associated pneumonia. Ann. Intern. Med. 132:621-630. [DOI] [PubMed] [Google Scholar]
- 7.Fukuda, H., M. Hosaka, K. Hirai, and S. Iyobe. 1990. New norfloxacin resistance gene in Pseudomonas aeruginosa PAO. Antimicrob. Agents Chemother. 34:1757-1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gales, A., R. Jones, J. Turnidge, R. Rennie, and R. Ramphal. 2001. Characterization of Pseudomonas aeruginosa isolates: occurrence rates, antimicrobial susceptibility patterns, and molecular typing in the global SENTRY antimicrobial surveillance program, 1997-1999. Clin. Infect. Dis. 32(Suppl. 2):S146-S155. [DOI] [PubMed] [Google Scholar]
- 9.Gotoh, N., H. Tsujimoto, M. Tsuda, K. Okamoto, A. Nomura, T. Wada, M. Nakahashi, and T. Nishino. 1998. Characterization of the MexC-MexD-OprJ multidrug efflux system in ΔmexA-mexB-oprM mutants of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 42:1938-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Griffith, D., O. Lomovskaya, V. J. Lee, and M. Dudley. 2000. Potentiation of levofloxacin (Levo) by MC-02,595, a broad-spectrum efflux pump inhibitor (EPI) in mouse models of infection due to Pseudomonas aeruginosa (PA) with combinations of different Mex pump expression and a gyrA mutation, abstr. 1496. Program Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., Toronto, Canada.
- 11.Hancock, R. E. W. 1998. Resistance mechanisms in Pseudomonas aeruginosa and other nonfermentative gram-negative bacteria. Clin. Infect. Dis. 27(Suppl. 1):S93-S99. [DOI] [PubMed] [Google Scholar]
- 12.Hancock, R. E. W., and H. Nikaido. 1978. Outer membranes of gram-negative bacteria XIX. Isolation from Pseudomonas aeruginosa PAO1 and use in reconstitution and definition of the permeability barrier. J. Bacteriol. 136:381-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris, A., C. Torres-Viera, L. Vekataraman, P. DeGirolami, M. Samore, and Y. Carmeli. 1999. Epidemiology and clinical outcomes of patients with multiresistant Pseudomonas aeruginosa. Clin. Infect. Dis. 28:1128-1133. [DOI] [PubMed] [Google Scholar]
- 14.Hill, H. A., M. J. Habe, J. E. McGowen, S. K. Fridkin, J. R. Edwards, F. C. Tenover, and R. P. Gaynes. 2001. A link between quinolone use and resistance in P. aeruginosa. Preliminary data from Project ICARE. Abstracts of the 39th Annual Infectious Diseases Society Meeting. Clin. Infect. Dis. 33:1173. [Google Scholar]
- 15.Hirai, K., S. Suzue, T. Irikura, S. Iyobe, and S. Mitsuhashi. 1987. Mutations producing resistance to norfloxacin in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 31:582-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kohler, T., M. Michea-Hamzehpour, P. Plesiat, A. L. Kahr, and J.-C. Pechere. 1997. Differential selection of multidrug efflux systems by quinolones in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 41:2540-2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kohler, T., C. vanDelden, L. K. Curty, M. Michea-Hamzehpour, and J. C. Pechere. 2001. Overexpression of the MexEF-OprN multidrug efflux system affects cell-to-cell signaling in Pseudomonas aeruginosa. J. Bacteriol. 183:5212-5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Legakis, N. J., L. S. Tzouvelekis, A. Makris, and H. Kotsifaki. 1989. Outer membrane alterations in multiresistant mutants of Pseudomonas aeruginosa selected by ciprofloxacin. Antimicrob. Agents Chemother. 33:124-127.2496655 [Google Scholar]
- 19.Leger, R., T. E. Renau, J. Zhang, P. Belej, E. Flamme, W. McMillan, J. Sangalang, M. W. She, R. Yen, S. Chamberland, C. L. Gannon, O. Lomovskaya, and V. J. Lee. 2000. Peptidomimetics of dipeptide amide efflux pump inhibitors potentiate the activity of levofloxacin in Pseudomonas aeruginosa, abstr. 1494. Program Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., Toronto, Canada.
- 20.Li, Y., T. Mima, Y. Komori, Y. Morita, T. Kuroda, T. Mizushima, and T. Tsuchiya. 2003. A new member of the tripartite multidrug efflux pumps, MexVW-OprM, in Pseudomonas aeruginosa. J. Antimicrob. Chemother. 52:572-575. [DOI] [PubMed] [Google Scholar]
- 21.Lomovskaya, O., A. Lee, K. Hoshino, H. Ishida, A. Mistry, M. S. Warren, E. Boyer, S. Chamberland, and V. J. Lee. 1999. Use of a genetic approach to evaluate the consequences of inhibition of efflux pumps in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 43:1340-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lomovskaya, O., M. S. Warren, A. Lee, J. Galazzo, R. Fronko, M. Lee, J. Blais, D. Cho, S. Chamberland, T. E. Renau, R. Leger, S. J. Hecker, W. Watkins, K. Hoshino, H. Ishida, and V. J. Lee. 2001. Identification and characterization of inhibitors of multidrug resistance efflux pumps in Pseudomonas aeruginosa: novel agents for combination therapy. Antimicrob. Agents Chemother. 45:105-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masuda, N., and S. Ohya. 1992. Cross-resistance to meropenem, cephems, and quinolones in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 36:1847-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masuda, N., E. Sakagawa, S. Ohya, N. Gotoh, H. Tsujimoto, and T. Nishino. 2000. Substrate specificities of MexAB-OprM, MexCD-OprJ, and MexXY-OprM efflux pumps in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 44:3322-3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCaig, L. F., R. E. Besser, and J. M. Hughes. 2003. Antimicrobial drug prescriptions in ambulatory care settings. United States, 1992-2000. Emerg. Infect. Dis. 9:432-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.NCCLS. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed. Approved standard M7-A5. NCCLS, Wayne, Pa.
- 27.Neuhauser, M. M., R. A. Weinstein, R. Rydman, L. H. Danziger, K. George, and J. P. Quinn. 2003. Antibiotic resistance among gram-negative bacilli in US intensive care units: implications for fluoroquinolone use. JAMA 289:885-888. [DOI] [PubMed] [Google Scholar]
- 28.Neyfakh, A. A. 1997. Natural functions of multidrug transporters. Trends Microbiol. 5:309-313. [DOI] [PubMed] [Google Scholar]
- 29.Okamoto, K., N. Gotoh, and T. Nishino. 2002. Alterations of susceptibility of Pseudomonas aeruginosa by overproduction of multidrug efflux systems, MexAB-OprM, MexCD-OprJ, and MexXY-OprM to carbapenems: substrate specificities of the efflux systems. J. Infect. Chemother. 8:371-373. [DOI] [PubMed] [Google Scholar]
- 30.Pearson, J. P., C. vanDelden, and B. H. Iglewski. 1999. Active efflux and diffusion are involved in transport of Pseudomonas aeruginosa cell-to-cell signals. J. Bacteriol. 181:1203-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poole, K. 2000. Efflux-mediated resistance to fluoroquinolones in gram-negative bacteria. Antimicrob. Agents Chemother. 44:2233-2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poole, K., K. Krebes, C. McNally, and S. Neshat. 1993. Multiple antibiotic resistance in Pseudomonas aeruginosa: evidence for involvement of an efflux operon. J. Bacteriol. 175:7363-7372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Radberg, G., L. E. Nilsson, and S. Svensson. 1990. Development of quinolone-imipenem cross resistance in Pseudomonas aeruginosa during exposure to ciprofloxacin. Antimicrob. Agents Chemother. 34:2142-2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Renau, T. E., R. Leger, L. Filonova, E. Flamme, M. Wang, R. Yen, D. Madsen, D. Griffith, S. Chamberland, M. N. Dudley, V. J. Lee, O. Lomovskaya, W. Watkins, T. Ohta, K. Nakayama, and Y. Ishida. 2003. Conformationally-restricted analogues of efflux pump inhibitors that potentiate the activity of levofloxacin in Pseudomonas aeruginosa. Bioorg. Med. Chem. Lett. 13:2755-2758. [DOI] [PubMed] [Google Scholar]
- 35.Renau, T. E., R. Leger, E. M. Flamme, J. Sangalang, M. W. She, R. Yen, C. L. Gannon, D. C. Griffith, S. Chamberland, O. Lomovskaya, S. J. Hecker, V. J. Lee, T. Ohta, and K. Nakayama. 1999. Inhibitors of efflux pumps in Pseudomonas aeruginosa potentiate the activity of fluoroquinolone antibacterial levofloxacin. J. Med. Chem. 42:4928-4931. [DOI] [PubMed] [Google Scholar]
- 36.Renau, T. E., R. Leger, E. M. Flamme, M. W. She, C. L. Gannon, K. M. Mathias, O. Lomovskaya, S. Chamberland, V. J. Lee, T. Ohta, K. Nakayama, and Y. Ishida. 2001. Addressing the stability of C-capped dipeptide efflux pump inhibitors that potentiate the activity of levofloxacin in Pseudomonas aeruginosa. Bioorg. Med. Chem. Lett. 11:663-667. [DOI] [PubMed] [Google Scholar]
- 37.Richards, M. J., J. R. Edwards, D. H. Culver, and R. P. Gaynes. 1999. Nosocomial infections in medical intensive care units in the United States. Crit. Care Med. 27:887-892. [DOI] [PubMed] [Google Scholar]
- 38.Sefton, A. M. 2002. Mechanisms of antimicrobial resistance: their clinical relevance in the new millennium. Drugs 62:557-566. [DOI] [PubMed] [Google Scholar]
- 39.Van Bambeke, F., Y. Glupczynski, P. Plesiat, J. C. Pechere, and P. Tulkens. 2003. Antibiotic efflux pumps in prokaryotic cells: occurrence, impact on resistance and strategies for the future of antimicrobial therapy. J. Antimicrob. Chemother. 51:1055-1065. [DOI] [PubMed] [Google Scholar]
- 40.Watkins, W. J., Y. Landaverry, R. Leger, R. Litman, T. E. Renau, N. Williams, R. Yen, J. Z. Zhang, S. Chamberland, D. Madsen, D. Griffith, V. Tembe, K. Huie, and M. N. Dudley. 2003. The relationship between physicochemical properties, in vitro activity and pharmacokinetic profiles of analogues of diamine-containing efflux pump inhibitors. Bioorg. Med. Chem. Lett. 13:4241-4244. [DOI] [PubMed] [Google Scholar]