Abstract
G protein-coupled receptor 30 (GPR30), also called G protein-coupled estrogen receptor 1 (GPER1), is thought to play important roles in breast cancer and cardiometabolic regulation, but many questions remain about ligand activation, effector coupling, and subcellular localization. We showed recently that GPR30 interacts through the C-terminal type I PDZ motif with SAP97 and protein kinase A (PKA)-anchoring protein (AKAP) 5, which anchor the receptor in the plasma membrane and mediate an apparently constitutive decrease in cAMP production independently of Gi/o. Here, we show that GPR30 also constitutively increases ERK1/2 activity. Removing the receptor PDZ motif or knocking down specifically AKAP5 inhibited the increase, showing that this increase also requires the PDZ interaction. However, the increase was inhibited by pertussis toxin as well as by wortmannin but not by AG1478, indicating that Gi/o and phosphoinositide 3-kinase (PI3K) mediate the increase independently of epidermal growth factor receptor transactivation. FK506 and okadaic acid also inhibited the increase, implying that a protein phosphatase is involved. The proposed GPR30 agonist G-1 also increased ERK1/2 activity, but this increase was only observed at a level of receptor expression below that required for the constitutive increase. Furthermore, deleting the PDZ motif did not inhibit the G-1-stimulated increase. Based on these results, we propose that GPR30 increases ERK1/2 activity via two Gi/o-mediated mechanisms, a PDZ-dependent, apparently constitutive mechanism and a PDZ-independent G-1-stimulated mechanism.
Keywords: A-kinase-anchoring protein (AKAP), calcineurin, extracellular signal-regulated kinase (ERK), G protein, G protein-coupled receptor (GPCR), PDZ domain, GPER1, GPR30
Introduction
G protein-coupled receptors (GPCRs)2 classically elicit intracellular signals by interacting with G proteins to stimulate or inhibit various effector pathways (1). Recently, it has become clear that these receptors also interact with numerous additional proteins that either regulate G protein-dependent signals or mediate G protein-independent signals (2). This is particularly obvious in ERK1/2 signaling (3), a pathway involved in a number of cellular events including survival, proliferation, and differentiation. G protein-dependent ERK1/2 signals occur by Gi/o-mediated stimulation of PI3K, phospholipase C (PLC) β, or EGFR transactivation or by Gq-mediated elevation of intracellular Ca2+, leading to Pyk2 activation and Ras recruitment to the focal adhesion complex (3, 4) or to calcineurin activation of kinase suppressor of Ras (KSR) 2, which scaffolds and activates mitogen-activated protein kinase (MAPK) enzymes (5). Conversely, G protein-independent signals occur by receptor recruitment of β-arrestin, which also scaffolds and activates MAPK enzymes (6).
Protein scaffolds such as KSR2 and β-arrestin localize MAPK enzymes in specific cellular microdomains, causing both spatial and temporal differences in receptor-mediated ERK1/2 signaling, yielding unique cellular responses (7). Compartmentalizing receptors through interactions between their C-terminal PDZ motifs and PDZ domain scaffold proteins such as membrane-associated guanylate kinases (MAGUKs) also localize signaling (8). Some MAGUKs in turn interact with other scaffold proteins such as AKAP79/150 or AKAP5, which anchors additional effectors including PKA, protein kinase C, calcineurin, and protein phosphatase 1 (PP1) (9), themselves capable of regulating both receptor localization and ERK1/2 activation.
GPR30 is a GPCR that is currently attracting considerable attention for its potential roles in breast cancer and cardiometabolic regulation. Belonging to the chemokine receptor family based on structural homology, this receptor was reported to mediate rapid non-genomic estrogenic responses (10, 11) and thus named G protein-coupled estrogen receptor 1 (GPER1). Using 17β-estradiol or G-1, a proposed GPR30 agonist (12), as stimuli, the receptor was described as increasing cAMP production via Gαs (10), intracellular Ca2+ via an unknown mechanism (11), and ERK1/2 activity via PTX-sensitive transactivation of EGFR (13, 14). However, considerable controversies exist about receptor activation, effector coupling, and subcellular localization (15–18), suggesting a more complex mechanism of receptor function than originally thought.
A picture is emerging that GPR30 exists in a complex through its C-terminal PDZ motif (-SSAV) with the MAGUK proteins PSD-95 (19–21) and SAP97 (19, 22) and that this complex is important for receptor plasma membrane localization (19, 20) and signaling (19, 21). SAP97-anchored AKAP5 and PKA RII regulatory subunit were reported to favor receptor membrane retention and mediate constitutive inhibition of cAMP production independently of Gi/o (19), and PSD-95 was reported to form an inhibitory complex between the receptor and plasma membrane Ca2+-ATPase 4b (21).
To further clarify the mechanism of GPR30 function, we investigated the activity of the receptor both in the absence of any added stimulus and in the presence of G-1 in HEK293 cells, a well described GPCR model system. Specifically, we studied the effect of the receptor on ERK1/2 activity and the role of the receptor PDZ interaction in this response. Our results show that GPR30 increases ERK1/2 activity through both a PDZ-dependent, apparently constitutive mechanism and a PDZ-independent G-1-stimulated mechanism, and these mechanisms may provide the receptor with a cell context-dependent profile of both agonist responsiveness and signaling.
Results
GPR30 increases ERK1/2 activity both constitutively and in response to G-1
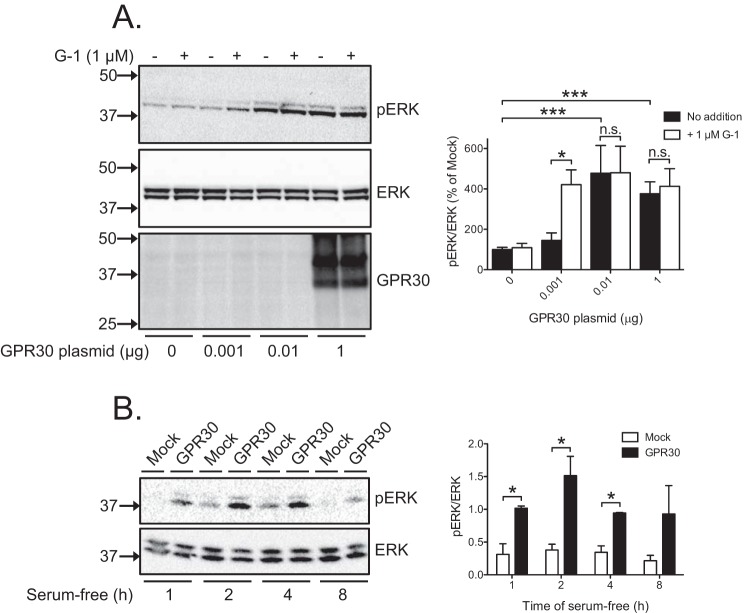
To measure GPR30-dependent ERK1/2 activity, we compared HEK293 cells transfected with different amounts (0.001, 0.01, and 1 μg) of a plasmid expressing N-terminally FLAG-tagged human wild-type (WT) GPR30 (GPR30) with cells transfected with empty plasmid (0 μg) (Fig. 1A). We then assayed receptor expression with a GPR30 antibody previously validated by us for receptor specificity (23). Receptor expression was clearly detected at 1 μg of plasmid, whereas expression at the lower plasmid levels (0.001 and 0.01 μg) was below the limit of antibody detection (Fig. 1A). Basal ERK1/2 activity, assayed as phosphorylated ERK1/2 (pERK), increased with increasing amount of GPR30 plasmid, reaching a maximal level at 0.01 μg of plasmid (Fig. 1A). The increase at 1 μg of plasmid was sustained, being elevated above that in mock-transfected cells for at least 4 h following removal of serum (Fig. 1B). Treatment with the proposed GPR30 agonist G-1 at 1 μm for 10 min also increased ERK1/2 activity. However, the G-1-stimulated increase was only observed at the lowest receptor plasmid level (0.001 μg) below that required for the constitutive increase (Fig. 1A). Thus, GPR30 increases ERK1/2 activity both constitutively and in response to G-1 in HEK293 cells.
Figure 1.
GPR30 stimulates ERK1/2 activity both constitutively and in response to G-1. A, HEK cells were transfected with 1 μg of empty plasmid (0 μg) and with increasing amounts of GPR30 plasmid (0.001, 0.01, and 1 μg), treated without or with 1 μm G-1 for 10 min, and immunoblotted with pERK, ERK, and GPR30 antibodies. B, HEK cells were transfected with 1 μg of empty plasmid (Mock) or GPR30 plasmid, incubated without serum for various times as indicated, and immunoblotted using pERK and ERK antibodies. In all panels, the combined pERK band intensities were normalized to the combined ERK band intensities for each condition, and the data are graphed as either percentage of mock without any additions (A) or the normalized value (B). The results are means of at least three independent experiments with error bars representing S.E. Molecular mass standards are indicated (left side). *, p < 0.05; ***, p < 0.001; n.s., not significant.
Constitutive GPR30-stimulated ERK1/2 activity is mediated by Gi/o and downstream PI3K activity
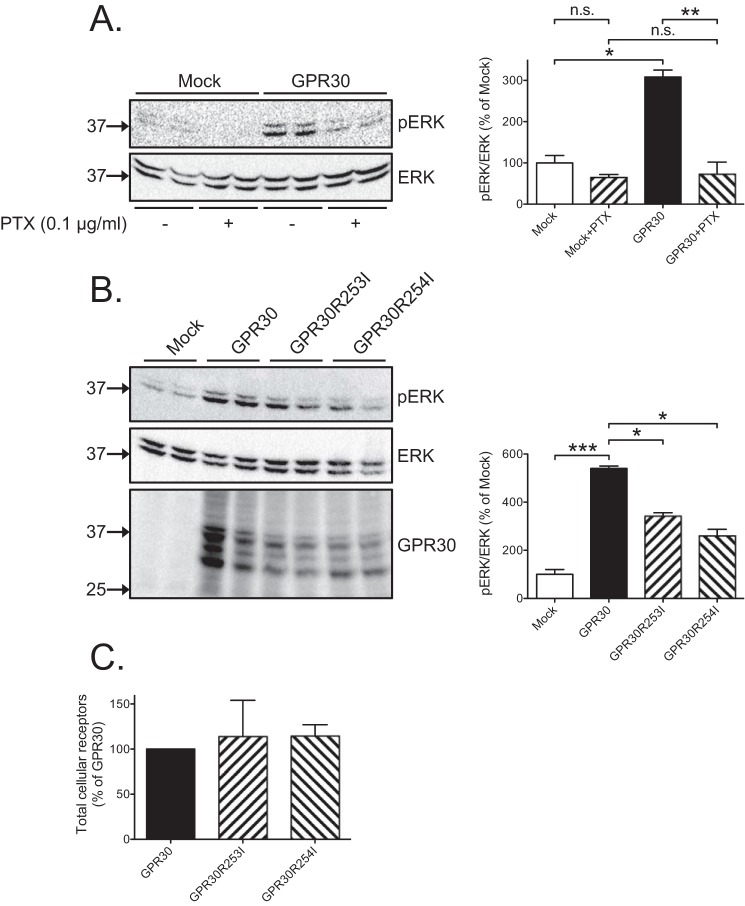
The GPR30-dependent constitutive increase in ERK1/2 activity was then further explored. Treatment with PTX (0.1 μg/ml) completely inhibited the constitutive increase, indicating that Gi/o mediates the increase (Fig. 2A). To further address constitutive GPR30-G protein coupling, we mutated arginine 254 to isoleucine at position 6.30 (Ballesteros-Weinstein numbering (24)) at the bottom of transmembrane domain 6 in the receptor, a position known to participate in an “ionic lock” in GPCR important for receptor-triggered G protein activation (25). This mutation had no effect on the total level of receptor expression as determined both by immunoblotting with GPR30 antibody (Fig. 2B) and flow cytometry with FLAG antibody (Fig. 2C). In contrast, the mutation significantly decreased the constitutive increase in ERK1/2 activity (Fig. 2B), consistent with the effect of mutating this arginine in CCR5, another member of the chemokine receptor family (26). Mutating arginine 253 to isoleucine at position 6.31 in the receptor had a similar effect (Fig. 2, B and C). These results provide further evidence that Gi/o coupling is the mechanism by which GPR30 constitutively increases ERK1/2 activity in HEK293 cells.
Figure 2.
Constitutive GPR30-stimulated ERK1/2 activity is mediated by Gi/o. A, HEK cells were transfected with 1 μg of empty plasmid (Mock) or GPR30 plasmid, treated without or with 0.1 μg/ml PTX for 18 h, and immunoblotted with pERK and ERK antibodies. B, HEK cells were transfected with 1 μg of empty plasmid (Mock) or GPR30, GPR30(R253I), or GPR30(R254I) plasmid and immunoblotted with pERK, ERK, and GPR30 antibodies. C, HEK cells were transfected as in B, stained following permeabilization (Total cellular receptors) with M1 FLAG antibodies, and subjected to flow cytometry. In A and B, the combined pERK band intensities were normalized to the combined ERK band intensities for each condition, and the data are graphed as percentage of mock without any additions. In C, the data are graphed as percentage of GPR30. The results are means of at least three independent experiments with error bars representing S.E. Molecular mass standards are indicated (left side). *, p < 0.05; **, p < 0.01; ***, p < 0.001; n.s., not significant.
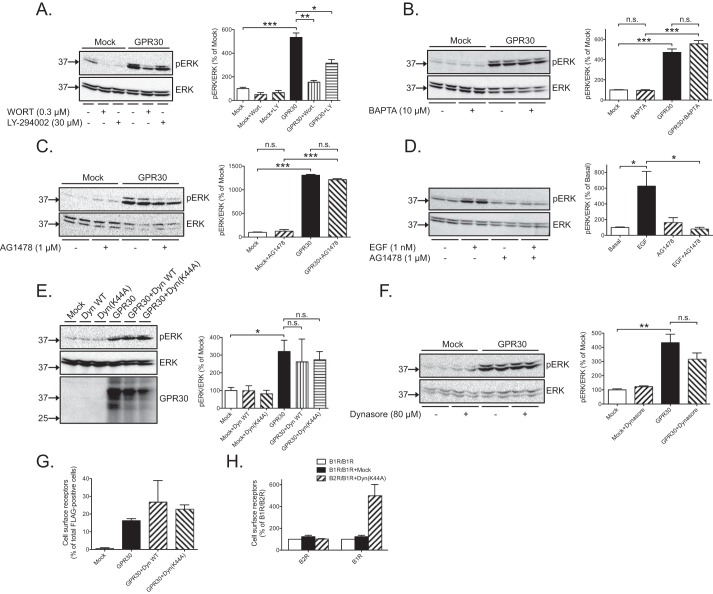
Three effectors have been reported to mediate Gi/o-dependent stimulation of ERK1/2 activity including PI3K, PLCβ, and EGFR transactivation (4, 27). Treatment of cells for 30 min with 0.3 μm wortmannin or 30 μm LY-290002, two general PI3K inhibitors, decreased the GPR30-dependent constitutive increase in ERK1/2 activity (Fig. 3A). Conversely, BAPTA-AM (10 μm), an intracellular Ca2+ chelator, which we previously showed decreases the intracellular Ca2+ level (28) and which would block PLCβ-mediated ERK1/2 activation, had no effect (Fig. 3B). AG1478 (1 μm), a specific EGFR tyrosine kinase inhibitor, also had no effect (Fig. 3C), whereas this inhibitor completely inhibited the increase in ERK1/2 activity in response to 1 nm EGF (Fig. 3D). Thus, PI3K is the effector by which GPR30 constitutively increases ERK1/2 activity downstream of Gi/o in HEK293 cells.
Figure 3.
Constitutive GPR30-stimulated ERK1/2 activity is mediated by PI3K activity. A, HEK cells were transfected with 1 μg of empty plasmid (Mock) or GPR30 plasmid, treated without or with 0.3 μm wortmannin (WORT) or 30 μm LY-294002 for 30 min, and immunoblotted with pERK and ERK antibodies. B, HEK cells were transfected with 1 μg of empty plasmid (Mock) or GPR30 plasmid, treated without or with 10 μm BAPTA-AM (BAPTA) for 30 min, and immunoblotted with pERK and ERK antibodies. C, HEK cells were transfected with 1 μg of empty plasmid (Mock) or GPR30 plasmid, treated without or with 1 μm AG1478 for 30 min, and immunoblotted with pERK and ERK antibodies. D, HEK cells were treated without or with 1 nm EGF and/or 1 μm AG1478 for 30 min and immunoblotted with pERK and ERK antibodies. E, HEK cells were transfected with 1 μg of empty plasmid (Mock) or GPR30 plasmid in the absence or presence of WT dynamin (Dyn WT) or dynamin(K44A) (Dyn(K44A)) plasmid and immunoblotted with pERK, ERK, and GPR30 antibodies. F, HEK cells were transfected with 1 μg of empty plasmid (Mock) or GPR30 plasmid, treated without or with 80 μm Dynasore for 2 h, and immunoblotted with pERK and ERK antibodies. G, HEK cells were transfected with 1 μg of empty plasmid (Mock) or GPR30 plasmid in the absence or presence of WT dynamin (Dyn WT) or dynamin(K44A) (Dyn(K44A)) plasmid, stained live (Cell surface receptors) or following permeabilization (total cellular receptors) with M1 FLAG antibodies, and subjected to flow cytometry. H, HEK cells were transfected with 1 μg of BK B1R or BK B2R plasmid in the absence (Mock) or presence of dynamin(K44A) (Dyn(K44A)) plasmid, and specific radioligand binding was performed using [3H]BK (B2R) and [3H]Lys-des-Arg9-BK (B1R). In A–F, the combined pERK band intensities were normalized to the combined ERK band intensities for each condition, and the data are graphed as either percentage of mock without any additions (A–C, E, and F) or percentage of basal without any additions (D). In G, the data are graphed as percentage of total FLAG-positive cells. In H, the data are graphed as percentage of B1R/B2R. The results are means of at least three independent experiments with error bars representing S.E. Molecular mass standards are indicated (left side). *, p < 0.05; **, p < 0.01; ***, p < 0.001; n.s., not significant.
GPR30 is subject to limited constitutive endocytosis (23, 29), and blocking dynamin-dependent endocytosis of some Gi/o-coupled GPCRs interferes with their ability to increase ERK1/2 activity (30, 31). Although the mechanism of GPR30 endocytosis has not yet been defined, we addressed the role of endocytosis in constitutive GPR30-stimulated ERK1/2 activity by co-expressing receptors with the dominant-negative dynamin mutant dynamin(K44A) or treating cells with the dynamin GTPase inhibitor Dynasore at 80 μm for 2 h. Neither dynamin(K44A) (Fig. 3E) nor Dynasore had any effect on the GPR30-dependent increase in ERK1/2 activity (Fig. 3F). Similarly, dynamin(K44A) had no apparent effect on the cell surface expression of GPR30 as determined with flow cytometry using M1 FLAG antibody (Fig. 3G). In contrast, this mutant dramatically increased the cell surface expression of bradykinin (BK) B1 receptors, a GPCR subject to significant constitutive endocytosis, but had no effect on BK B2 receptors, a GPCR subject to little if any constitutive endocytosis (Fig. 3H) (32). Thus, GPR30 does not seem to depend on any dynamin-dependent event to constitutively increase ERK1/2 activity in HEK293 cells.
Constitutive GPR30-stimulated ERK1/2 activity depends on PDZ-dependent receptor coupling to AKAP5
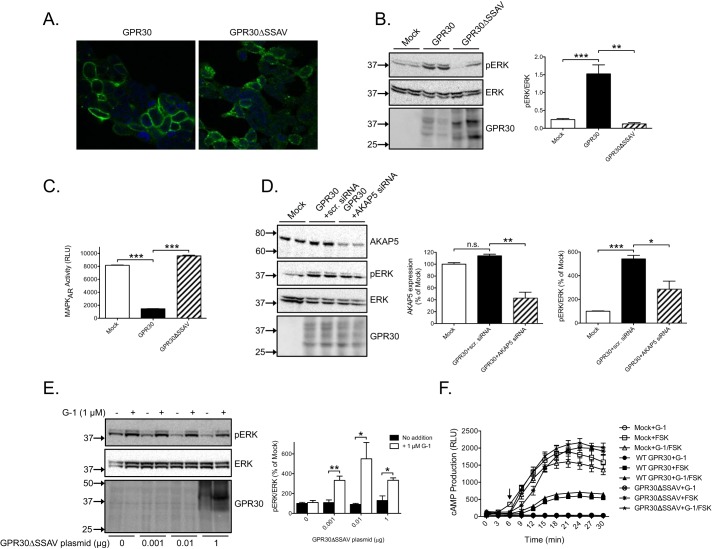
We showed previously that GPR30 interacts through the C-terminal PDZ motif (-SSAV) with SAP97 and AKAP5 and that this interaction is necessary to effectively anchor the receptor in the plasma membrane in HEK293 cells (19). Indeed, deleting the PDZ motif to make GPR30ΔSSAV dramatically increased constitutive receptor endocytosis as determined by confocal immunofluorescence microscopy using M1 FLAG antibody (Fig. 4A). To address whether the PDZ motif is also necessary for the constitutive increase in ERK1/2 activity, the effect of GPR30ΔSSAV on ERK1/2 activity was compared with that of WT GPR30. Deleting the PDZ motif completely prevented the constitutive increase as measured by both pERK immunoblotting (Fig. 4B) and the biosensor MAPK activity reporter (MAPKAR) (Fig. 4C). To determine whether the constitutive increase also involves AKAP5, cells treated with AKAP5-specific siRNA to knock down AKAP5 were compared with cells treated with scrambled siRNA (Fig. 4D). AKAP5 knockdown significantly inhibited the receptor-promoted increase in ERK1/2 activity (Fig. 4D). Thus, GPR30 requires the PDZ-dependent interaction with SAP97 and AKAP5 to constitutively increase ERK1/2 activity in these cells. We then determined the effect of deleting the PDZ motif on G-1-stimulated ERK1/2 activity. Interestingly, G-1 increased ERK1/2 activity at both low and high levels of GPR30ΔSSAV expression (Fig. 4E). We conclude from these results that the G-1-stimulated increase in ERK1/2 activity does not require the PDZ motif, whereas the constitutive increase in ERK1/2 activity is completely dependent on this motif. Fig. 4F shows that GPR30 also constitutively decreased forskolin-stimulated cAMP production in a PDZ-dependent manner, and we reported previously that this is a Gi/o-independent response (19). In contrast to the ERK1/2 response, G-1 did not increase or decrease cAMP production through GPR30 either in the absence or presence of the PDZ motif (Fig. 4F). Therefore, decreased cAMP production appears to be primarily a PDZ-dependent receptor response in these cells.
Figure 4.
Constitutive GPR30-stimulated ERK1/2 activity depends on PDZ-dependent receptor coupling to AKAP5, whereas G-1-stimulated activity does not. A, HEK cells were transfected with 1 μg of GPR30 or GPR30ΔSSAV plasmid and stained live with M1 FLAG antibodies, and fluorescence images were collected using a Nikon Eclipse confocal microscope with 60× objective and 50-μm zoom. B, HEK cells were transfected with 1 μg of empty plasmid (Mock) or GPR30 or GPR30ΔSSAV plasmid and immunoblotted with pERK, ERK, and GPR30 antibodies. C, HEK cells were transfected with MAPKAR with 1 μg of empty plasmid (Mock) or GPR30 or GPR30ΔSSAV plasmid, and MAPKAR activity was assayed by luminescence. D, HEK cells were transfected with 1 μg of empty plasmid (Mock) or GPR30 plasmid, scrambled (scr.) siRNA, and/or AKAP5 siRNA and immunoblotted using pERK, ERK, and GPR30 antibodies. E, HEK cells were transfected with 1 μg of empty plasmid (0 μg) and increasing amounts of GPR30ΔSSAV plasmid (0.001, 0.01, and 1 μg), treated without or with 1 μm G-1 for 10 min, and immunoblotted with pERK, ERK, and GPR30 antibodies. F, HEK cells were transfected with 1 μg of empty plasmid (Mock) or GPR30 or GPR30ΔSSAV plasmid and treated without or with 1 μm G-1 and/or 1 μm forskolin (FSK), and cAMP was measured by luminescence using the GloSensor assay. In B, D, and E, the combined pERK band intensities were normalized to the combined ERK band intensities for each condition, and the data are graphed either as the normalized value (B) or as percentage of mock without any additions (D and E). In D, the AKAP5 band intensity is graphed as percentage of mock. In C and F, the data are graphed as relative light units (RLU). The results are representative of experiments performed at least three times (A), the means of at least three independent experiments with error bars representing S.E. (B–E), or representative of at least six experiments with each data point being the mean of 8–16 measurements and error bars representing S.E. (F). Molecular mass standards are indicated (left side). *, p < 0.05; **, p < 0.01; ***, p < 0.001; n.s., not significant.
A protein phosphatase participates in constitutive GPR30-promoted increase in ERK1/2 activity
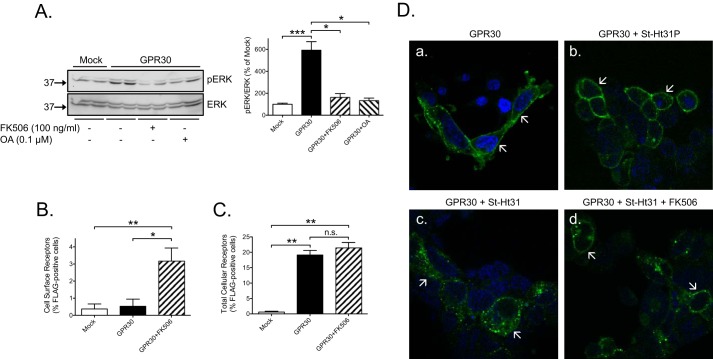
AKAP5 anchors the two protein phosphatases calcineurin and PP1 (9). To address whether either of these enzymes participates in constitutive GPR30-stimulated ERK1/2 activity, cells were treated for 30 min with 0.1 μg/ml FK506, a calcineurin inhibitor, or 0.1 μm okadaic acid (OA), a PP1 inhibitor. Both inhibitors inhibited the constitutive increase in ERK1/2 activity (Fig. 5A). Cells were also assayed by flow cytometry to determine whether FK506 acts by influencing GPR30 membrane trafficking. Treatment of cells with 0.1 μg/ml FK506 for 30 min caused a small but significant increase in the amount of cell surface receptors as determined in non-permeabilized cells (Fig. 5B), whereas no change was observed in the total amount of cellular GPR30 as determined in permeabilized cells (Fig. 5C). We also used confocal immunofluorescence microscopy to determine whether the increase in cell surface receptors may be caused by decreased receptor endocytosis. To more readily view this, cells were first treated with St-Ht31 to increase constitutive GPR30 endocytosis. As shown previously (19), disruption of AKAP-PKA RII interaction with 50 μm St-Ht31 for 15 min increases GPR30 endocytosis (Fig. 5D, panels a and c), whereas the negative control substance, St-Ht31P (50 μm), which does not disrupt this interaction, has no effect (Fig. 5D, panels a and b). Treatment with FK506 for 15 min prior to addition of St-Ht31 for 15 min appeared to decrease receptor endocytosis (Fig. 5D, panels c and d). Although these results suggest that calcineurin favors GPR30 endocytosis, calcineurin probably does not use this mechanism to favor the GPR30-stimulated increase in ERK1/2 activity as this increase was completely insensitive to dynamin inhibition (Fig. 3, E and F).
Figure 5.
A protein phosphatase participates in constitutive GPR30-stimulated ERK1/2 activity. A, HEK cells were transfected with 1 μg of empty plasmid (Mock) or GPR30 plasmid, treated without or with 100 ng/ml FK506 or 0.1 μm OA for 30 min, and immunoblotted with pERK and ERK antibodies. B and C, HEK cells were transfected with 1 μg of empty plasmid (Mock) or GPR30 plasmid, treated without or with 100 ng/ml FK506, stained live (Cell surface receptors) (B) or following permeabilization (Total Cellular Receptors) (C) with M1 FLAG antibodies, and subjected flow cytometry. D, HEK cells were transfected with 1 μg of GPR30 plasmid and stained live with M1 FLAG antibodies in the absence (panel a) or presence of 50 μm St-Ht31P (panel b) or 50 μm St-Ht31 (panel c) for 30 min or 50 μm St-HT31 for 30 min with 100 ng/ml FK506 included during the last 15 min (panel d), and fluorescence images were collected using a Nikon Eclipse confocal microscope with 60× objective and 50-μm zoom. In A, the combined pERK band intensities were normalized to the combined ERK band intensities for each condition, and the data are graphed as percentage of mock without any additions. In B and C, the data are graphed as percentage FLAG-positive cells. In D, areas of interest as discussed in the text are indicated (arrows). The results are the means of at least three independent experiments with error bars representing S.E. (A–C) or representative of experiments performed at least three times (D). Molecular mass standards are indicated (left side). *, p < 0.05; **, p < 0.01; ***, p < 0.001; n.s., not significant.
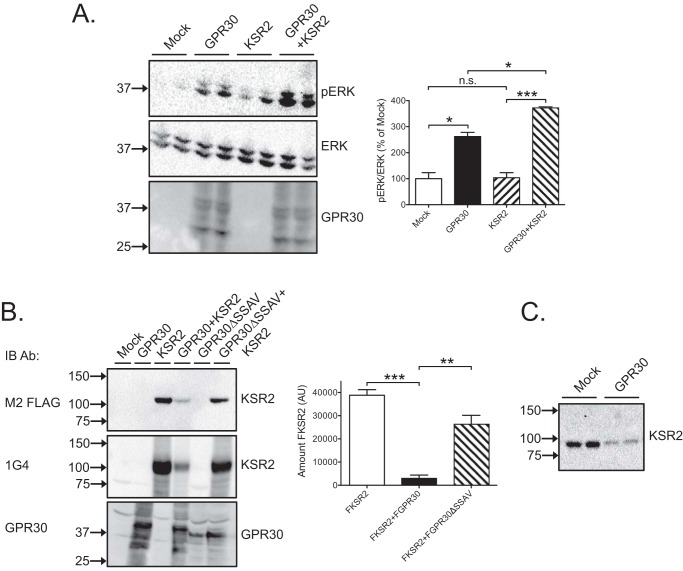
An alternative mechanism by which calcineurin could favor constitutive GPR30-stimulated ERK1/2 activity is through KSR2, a MAPK scaffold that activates ERK1/2 upon calcineurin dephosphorylation (5). Interestingly, overexpression of FLAG-tagged KSR2 caused a small but significant increase in basal ERK1/2 activity in the presence of GPR30, whereas no effect was observed in the absence of GPR30 (Fig. 6A). However, GPR30 PDZ-dependently decreased the amount of cellular KSR2 including both ectopically expressed FLAG-tagged mouse KSR2 (Fig. 6B) and endogenously expressed human KSR2 (Fig. 6C), implying that GPR30 promotes KSR2 degradation. Therefore, this MAPK scaffold is most likely not directly involved in constitutive GPR30-stimulated ERK1/2 activity.
Figure 6.
KSR2 is not involved in constitutive GPR30-stimulated ERK1/2 activity. A, HEK cells were transfected with 1 μg of empty plasmid (Mock) or GPR30 plasmid and/or KSR2 plasmid and immunoblotted with pERK, ERK, and GPR30 antibodies. B, HEK cells were transfected with 1 μg of empty plasmid (Mock) or GPR30, GPR30ΔSSAV, and/or KSR2 plasmid and immunoblotted (IB) with M2 FLAG, 1G4, and GPR30 antibodies (Ab). C, HEK cells were transfected with 1 μg of empty plasmid (Mock) or GPR30 plasmid and immunoblotted with 1G4 antibodies. In A, the combined pERK band intensities were normalized to the combined ERK band intensities for each condition, and the data are graphed as percentage of mock. In B, the FLAG-KSR2 band intensity was graphed as arbitrary units (AU). The results are means of at least three independent experiments with error bars representing S.E. (A and B) or representative of at least three independent experiments (C). Molecular mass standards are indicated (left side). *, p < 0.05; **, p < 0.01; ***, p < 0.001; n.s., not significant.
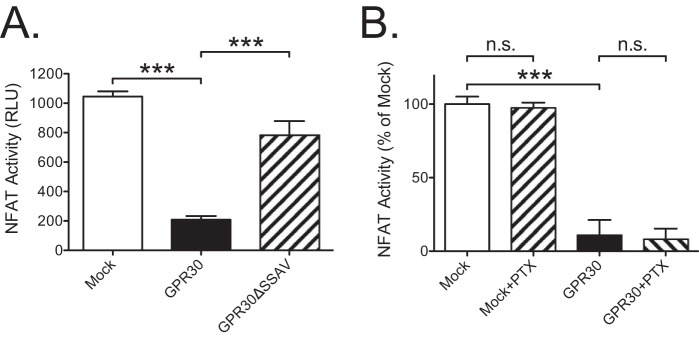
To more directly address the effect GPR30 on calcineurin activity, we measured the activity of NFAT, a specific transcription factor target for calcineurin that depends on calcineurin dephosphorylation to translocate to the nucleus (33). Expression of GPR30 completely inhibited basal NFAT activity as measured with the luciferase promoter-reporter plasmid pGL3-NFAT (Fig. 7A), and the response was sensitive to PDZ motif deletion (Fig. 7A) but insensitive to PTX treatment (Fig. 7B). Although these results suggest that GPR30 inhibits calcineurin activity through the PDZ interaction, the mechanism by which calcineurin participates in constitutive receptor-stimulated ERK1/2 activity remains to be fully clarified.
Figure 7.
GPR30 PDZ-dependently inhibits basal NFAT activity. A, HEK cells were transfected with pGL3-NFAT with 1 μg of empty plasmid (Mock) or GPR30 or GPR30ΔSSAV plasmid, and NFAT activity was assayed as luminescence. B, HEK cells were transfected with pGL3-NFAT with 1 μg of empty plasmid (Mock) or GPR30 plasmid and treated without or with 0.1 μg/ml PTX for 18 h, and NFAT activity was assayed as luminescence. The data are graphed as either relative light units (RLU) (A) or percentage of mock without any addition (B). The results are means of at least three independent experiments with error bars representing S.E. ***, p < 0.001; n.s., not significant.
Discussion
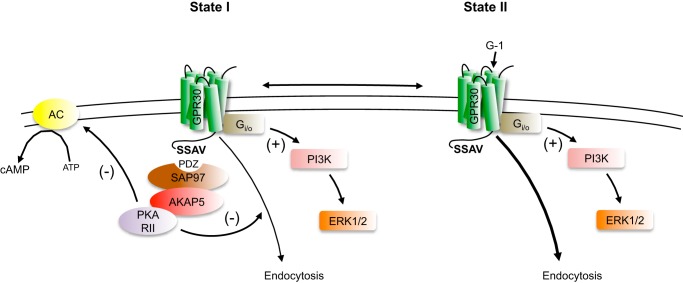
Much confusion has surrounded GPR30 for a considerable length of time regarding the mechanisms of receptor activation, effector coupling, and subcellular distribution. To address this, we recently took an unbiased approach by studying receptor activity in the absence of any added stimulus in HEK293 cells, a well defined GPCR model system. Doing so, we found that GPR30 interacts through the receptor C-terminal PDZ motif (-SSAV) with SAP97 and the SAP97-anchored protein AKAP5. In turn, AKAP-anchored PKA RII acts to retain the receptor in the plasma membrane and mediate apparently constitutive receptor inhibition of cAMP production independently of Gi/o (19). Several groups have now shown that GPR30 interacts PDZ-dependently with both SAP97 and PSD-95 in both recombinant (19–21) and native systems (22), indicating the general importance of this interaction. In this study, we show that GPR30 also apparently constitutively increases ERK1/2 activity and that this increase also depends on the PDZ motif and AKAP5 expression. However, unlike the receptor-promoted decrease in cAMP production, the increase in ERK1/2 activity depends on Gi/o. The increase was also sensitive to mutation of position 6.30 at the bottom of transmembrane domain 6 in the receptor, a position important for GPCR-promoted G protein activation (25), providing additional evidence that GPR30-Gi/o coupling is necessary for the increase. Therefore, we propose that the PDZ-dependent interaction with the SAP97-AKAP5 complex positions GPR30 in a plasma membrane-associated functional state (state I; Fig. 8) through which the complex constitutively decreases cAMP production and at the same time favors Gi/o coupling to constitutively increase ERK1/2 activity.
Figure 8.
Model of PDZ-dependent and -independent GPR30 coupling to ERK1/2 and cAMP signaling. In functional state I, GPR30 interacts via its C-terminal PDZ motif (-SSAV) with a MAGUK and AKAP5, and via AKAP-PKA RII this receptor complex inhibits receptor endocytosis and constitutively inhibits cAMP production. The complex also favors receptor coupling to Gi/o through which the receptor constitutively stimulates ERK1/2 activity. Calcineurin also participates in this functional state, but the mechanism of this participation remains to be determined. In functional state II, GPR30 does not interact with MAGUK/AKAP5, which favors GPR30 endocytosis and prevents constitutive inhibition of cAMP production and stimulation of ERK1/2 activity. However, in this state, the agonist G-1 can stimulate receptor coupling to Gi/o to specifically favor increased ERK1/2 activity. States I and II are thought to exist in equilibrium with their relative amounts depending on the amount and type of MAGUK expressed in the cell. AC, adenylate cyclase.
Constitutive GPCR activity is normally explained as an increased amount of inherently generated activated receptor state (1). However, considering that the GPR30-dependent increase in ERK1/2 activity saturated at higher levels of receptor expression and that deleting the PDZ motif prevented the increase, a more likely explanation is that the increase is a consequence of a PDZ-dependent cell-derived stimulus. Although the identity of such a stimulus is currently unknown, one possibility is that it is the SAP97-AKAP5 complex itself that acts as an intracellular stimulus to favor receptor-Gi/o coupling, which is in line with the theory that GPCRs are highly allosteric proteins through which both extracellular and intracellular ligands can act to stabilize effector coupling (34). Another possibility relates to the ability of SAP97-AKAP5 to retain the receptor in the plasma membrane, which could direct the receptor to a membrane-bound stimulus that favors Gi/o coupling.
The G-1-stimulated increase in ERK1/2 activity was apparent only at a receptor level below that required for the constitutive increase, and the two responses were not additive, suggesting that a common receptor species is involved in both cases. However, although the constitutive increase in ERK1/2 activity was completely dependent on the PDZ motif, the G-1-stimulated increase was not. Considering that G-1-stimulated ERK1/2 activity is also Gi/o-dependent (14), we propose that the absence of the PDZ-dependent interaction with SAP97-AKAP5 positions GPR30 in a unique functional state (state II; Fig. 8) that is less anchored in the membrane but through which G-1 allows the receptor to increase ERK1/2. We also propose that the PDZ-coupled state I and the PDZ-uncoupled state II exist in equilibrium with their relative amounts determined by the amounts of MAGUKs and their interacting proteins expressed in the cell. Considering that the expression of these proteins varies between different cell types (8), this mechanism would therefore provide the receptor with a cell context-dependent profile of both signaling and G-1 responsiveness. As GPR30 interacts with both SAP97 and PSD-95, the type of MAGUK expressed may further refine this profile.
About 70% of all GPCRs contain a negatively charged residue at position 6.30 that participates in an ionic lock with a positively charged residue at position 3.60 that is important for G protein activation (25). The remaining 30% contain a positively charged residue at position 6.30, suggesting a different mechanism of G protein activation. GPR30 harbors an arginine at position 6.30. Interestingly, this is also the case for all chemokine receptors, which are also Gi/o-coupled and with which GPR30 also shows the highest structural homology. Furthermore, mutation of this residue decreased G protein-dependent signaling by both GPR30 and the typical chemokine receptor CCR5 (26). Although the molecular basis for this decrease remains unclear, this result is interesting as it further argues that GPR30 and chemokine receptors are related.
AKAP5 anchors the protein phosphatases calcineurin and PP1 (9), which prompted us to investigate the effect of these phosphatases on the GPR30-dependent constitutive increase in ERK1/2 activity. Several observations suggested that one or both of these phosphatases participates in this increase. First, both the calcineurin inhibitor FK506 and the PP1 inhibitor OA inhibited the increase. Second, FK506 increased the amount of receptors at the cell surface apparently by decreasing receptor endocytosis. Therefore, calcineurin may favor GPR30-stimulated ERK1/2 activity by favoring receptor endocytosis, a mechanism previously proposed for some other Gi/o-coupled GPCRs (30, 31). However, this mechanism is unlikely as 1) it contradicts the effect of deleting the receptor PDZ motif, which increases receptor endocytosis and decreases ERK1/2 activity, and 2) neither dynamin(K44A) nor Dynasore perturbed the receptor response. Another way that calcineurin could favor receptor-stimulated ERK1/2 activity is by dephosphorylating KSR2, a MAPK scaffold that activates ERK1/2 upon calcineurin dephosphorylation (5). Interestingly, KSR2 overexpression increased basal ERK1/2 activity only in the presence of GPR30. However, GPR30 also increased KSR2 degradation, arguing against this scaffold as a direct contributor to the constitutive receptor response.
We also investigated the direct effect of GPR30 on calcineurin by measuring the basal activity of NFAT, an established target for this enzyme (33). GPR30 decreased NFAT activity, arguing that the receptor inhibits calcineurin. The decrease was sensitive to PDZ motif deletion but not PTX treatment, implying that the PDZ interaction is specifically involved. Ectopic expression of AKAP5 inhibits NFAT activity (35, 36), which originally led investigators to conclude that AKAP5-anchored calcineurin is enzymatically inactive. However, it is now known that this interaction is dynamic in native systems, allowing AKAP5-anchored calcineurin to dephosphorylate and direct NFAT to the nucleus in a compartmentalized manner (37, 38). Nevertheless, to propose that GPR30 uses AKAP5-anchored calcineurin in a similar manner would require evidence of a direct physical interaction between these proteins in a native system. Thus, although several observations argue that calcineurin participates in constitutive GPR30-stimulated ERK1/2 activity, the mechanism by which this occurs is still unclear.
In summary, we propose that GPR30 exists in an equilibrium between two Gi/o-coupled receptor states. One state is anchored in the plasma membrane by SAP97 and AKAP5, through which the receptor elicits an apparently constitutive AKAP-PKA RII-mediated decrease in cAMP production and a Gi/o-mediated increase in ERK1/2. The other state of GPR30 does not interact with SAP97 and AKAP5 and is therefore less anchored in the membrane but allows G-1 to stimulate Gi/o coupling to increase ERK1/2 activity. As the amounts of MAGUKs and their interacting proteins vary between different cell systems, we propose that this mechanism provides the receptor with a cell context-dependent profile of both signaling and G-1 responsiveness. These results may explain some of the controversies regarding GPR30 effector coupling and ligand activation and will be important when exploiting this receptor for therapeutic benefit.
Experimental procedures
Cell culture and DNA constructs
HEK293 cells (American Type Culture Collection, Manassas, VA) were grown in phenol red-free Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) in 10% CO2 at 37 °C. N-terminally FLAG-tagged human GPR30, B2 BK receptor, and B1 BK receptor cDNAs in pcDNA3.1 were made as described previously (23, 32). The GPR30ΔSSAV deletion mutant in which the four C-terminal residues in GPR30 (-SSAV) were deleted was produced by PCR (18). The R254I and R253I mutations in GPR30 were introduced using Phusion polymerase-based site-directed mutagenesis essentially as described previously (39). Human AKAP5-specific and scrambled siRNAs were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The ERK1 biosensor plasmid MAPKAR was a gift from Dr. K. Swann (Cardiff University, UK). pGL3-NFAT luciferase plasmid was a gift from Jerry Crabtree (Addgene plasmid number 17870). pCDNA3.1 KSR2, tagged with the FLAG epitope at the C terminus, was a gift from Rob Lewis (Addgene plasmid number 25968). WT HA-dynamin 1 pcDNA3.1 was a gift from Sandra Schmid (Addgene plasmid number 34682). K44A HA-dynamin 1 pcDNA3.1 was a gift from Sandra Schmid (Addgene plasmid number 34683). TransIT-LT1 and TransIT-X2 (Mirus Bio LLC, Madison, WI) were used to transfect plasmid DNA and siRNA. Cells transfected with plasmid containing receptor constructs were always compared with cells transfected with empty plasmid alone (mock).
Immunoblotting
Immunoblotting was done as described previously (19), and blots were stained with goat GPR30 antibody (R&D Systems, Minneapolis, MN; 1:200), mouse FLAG M2 antibody (Sigma-Aldrich; 1:1,000), mouse AKAP5 antibody (BD Bioscience; 1:1,000), and KSR2 antibody 1G4 (Abnova, Taipei City, Taiwan; 1:500). Immunoreactive bands were visualized with a chemiluminescence immunodetection kit using peroxidase-labeled secondary antibody (Invitrogen) according to the procedure described by the supplier (PerkinElmer Life Sciences).
ERK activity
ERK1/2 activity was assayed by immunoblotting as described above using a pERK antibody (Santa Cruz Biotechnology; 1:200) for ERK1/2 phosphorylation and an ERK1/2 (ERK) antibody (Santa Cruz Biotechnology; 1:1,000) for total ERK1/2. Briefly, transfected cells were grown in 60-mm dishes in DMEM with 10% FBS, washed, incubated without serum for 1 h, and then treated in the absence or presence of various substances as indicated in figure legends. The cells were then washed, lysed, and subjected to immunoblotting, and immunoreactive bands were visualized as described above. Films were scanned using a Chemidoc XRS+ imager (Bio-Rad), and the combined band density of ERK1 and ERK2 was quantified using ImageJ software. ERK1/2 activity was expressed as the ratio between the combined pERK band densities and the combined ERK band densities for each condition.
ERK1 activity was also assayed in cells transfected with the ERK1 biosensor MAPKAR plasmid. As described previously (40), MAPKAR is composed of two tandem ERK1 monomers placed between the N- and C-terminal halves of click beetle luciferase and separated by a short linker. Upon activation, a conformational change occurs in the ERK1 dimer that separates the luciferase halves, yielding no bioluminescence emission, whereas ERK1 inactivation combines the two halves, yielding bioluminescence emission. Transfected cells were seeded in a white-bottom 96-well dish at a density of ∼25,000 cells/well. At 48 h post-transfection, the medium was replaced with 100 μl/well DMEM, and at 72 h post-transfection, the medium was replaced with 90 μl/well HEPES-buffered DMEM containing 3% GloSensor luciferin (Promega, Madison, WI). The cells were then incubated for 2–3 h at room temperature and subsequently analyzed in a Victor2 luminometer (PerkinElmer Life Sciences).
NFAT promoter activity
NFAT promoter activity was measured in cells transfected with pGL3-NFAT luciferase plasmid. Transfected cells in 96-well plates (20,000 cells/well) were grown in medium overnight and then in serum-free medium for ∼20 h. Cells were then lysed with 10 μl/well reporter lysis buffer (Promega). Following addition of 35 μl/well luciferin reagent (Biothema, Handen, Sweden) and ATP, NFAT promoter activity was measured as luminescence in a Victor2 luminometer.
Immunofluorescence microscopy
Immunofluorescence microscopy was done as described previously (19, 23, 32). For live staining of GPR30, we took advantage of the fact that mouse M1 FLAG antibody (Sigma-Aldrich; 1:500) labels specifically the receptor extracellular N-terminal FLAG epitope. Therefore, “feeding” live transfected cells with this antibody for 30 min at 37 °C monitored exclusively cell surface receptor-antibody complexes and complexes that had subsequently undergone endocytosis. Cells were then fixed, permeabilized, and incubated with secondary Alexa Fluor 488-labeled mouse IgG2b antibodies (Life Technologies). 4′,6-Diamidino-2-phenylindole (DAPI) was used for nuclear staining. Images were collected using a Nikon Eclipse confocal fluorescence microscope.
Analysis of cell surface receptors
Cell surface expression of GPR30 was analyzed in transfected cells by flow cytometry as reported previously (19) using mouse M1 FLAG antibody (1:200) or mouse IgG (Dako, Glostrup, Denmark) as primary antibody and phycoerythrin-labeled goat anti-mouse antibody (Dako; 1:2,000) as secondary antibody. The cells were analyzed using an Accuri C6 or BD FACSCanto cytometer and FACSDiva software (BD Immunocytometry Systems). Forward and side scatter measurements were attained with gain settings in linear mode. Cell surface expression of BK B1 receptor (B1R) and B2 receptor (B2R) was assayed by equilibrium radioligand binding on intact transfected cells at 4 °C using [3H]BK (B2R) and [3H]Lys-des-Arg9-BK (B1R) as described previously (41).
Statistical analysis
Data are presented as means ± S.E. and were statistically analyzed by the Student's t test. p values less than 0.05 were regarded as statistically significant. Data analysis was performed using the Prism program (GraphPad, La Jolla, CA).
Author contributions
E. G. d. V. and S. B. conducted most of the experiments including cell culture, mutagenesis, immunoblotting, and confocal immunofluorescence microscopy and analyzed results. R. K. conducted experiments and analyzed flow cytometry results. B. O. and L. M. F. L.-L. conceived the idea of the project, and L. M. F. L.-L. wrote the article. All authors reviewed the results and approved the final version of the manuscript.
Acknowledgments
We thank Joanna Daszkiewicz-Nilsson and Birgitta Gullstrand for expert technical assistance.
This work was supported by Swedish Cancer Foundation (Grant CAN 2016/423), Swedish Research Council (Grant 2016-02427), the NovoNordisk Foundation, the Alfred Österlund Foundation, and the Gunnar Nilsson Cancer Foundation (to L. M. F. L.-L.). The authors declare that they have no conflicts of interest with the contents of this article.
- GPCR
- G protein-coupled receptor
- GPR30
- G protein-coupled receptor 30
- GPER1
- G protein-coupled estrogen receptor 1
- PDZ
- PSD-95/Discs-large/ZO-1 homology
- MAGUK
- membrane-associated guanylate kinase
- SAP97
- synapse-associated protein 97
- PSD-95
- postsynaptic density-95
- AKAP5
- protein kinase A-anchoring protein 5
- PP1
- protein phosphatase 1
- EGFR
- epidermal growth factor receptor
- KSR
- kinase suppressor of Ras
- NFAT
- nuclear factor of activated T cells
- OA
- okadaic acid
- PTX
- pertussis toxin
- BAPTA-AM
- 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl ester)
- PLC
- phospholipase C
- PKA RII
- PKA regulatory subunit II
- pERK
- phosphorylated ERK1/2
- BK
- bradykinin
- B1R
- B1 receptor
- B2R
- B2 receptor
- MAPKAR
- MAPK activity reporter
- ERK
- ERK1/2.
References
- 1. Pierce K. L., Premont R. T., and Lefkowitz R. J. (2002) Seven-transmembrane receptors. Nat. Rev. Mol. Cell Biol. 3, 639–650 [DOI] [PubMed] [Google Scholar]
- 2. Ritter S. L., and Hall R. A. (2009) Fine-tuning of GPCR activity by receptor-interacting proteins. Nat. Rev. Mol. Cell Biol. 10, 819–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Luttrell L. M. (2005) Composition and function of G protein-coupled receptor signalsomes controlling mitogen-activated protein kinase activity. J. Mol. Neurosci. 26, 253–264 [DOI] [PubMed] [Google Scholar]
- 4. Goldsmith Z. G., and Dhanasekaran D. N. (2007) G protein regulation of MAPK networks. Oncogene 26, 3122–3142 [DOI] [PubMed] [Google Scholar]
- 5. Dougherty M. K., Ritt D. A., Zhou M., Specht S. I., Monson D. M., Veenstra T. D., and Morrison D. K. (2009) KSR2 is a calcineurin substrate that promotes ERK cascade activation in response to calcium signals. Mol. Cell 34, 652–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Luttrell L. M., Roudabush F. L., Choy E. W., Miller W. E., Field M. E., Pierce K. L., and Lefkowitz R. J. (2001) Activation and targeting of extracellular signal-regulated kinases by β-arrestin scaffolds. Proc. Natl. Acad. Sci. U.S.A. 98, 2449–2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kolch W. (2005) Coordinating ERK/MAPK signalling through scaffolds and inhibitors. Nat. Rev. Mol. Cell Biol. 6, 827–837 [DOI] [PubMed] [Google Scholar]
- 8. Dunn H. A., and Ferguson S. S. (2015) PDZ protein regulation of G protein-coupled receptor trafficking and signaling pathways. Mol. Pharmacol. 88, 624–639 [DOI] [PubMed] [Google Scholar]
- 9. Gold M. G., Stengel F., Nygren P. J., Weisbrod C. R., Bruce J. E., Robinson C. V., Barford D., and Scott J. D. (2011) Architecture and dynamics of an A-kinase anchoring protein 79 (AKAP79) signaling complex. Proc. Natl. Acad. Sci. U.S.A. 108, 6426–6431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thomas P., Pang Y., Filardo E. J., and Dong J. (2005) Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology 146, 624–632 [DOI] [PubMed] [Google Scholar]
- 11. Revankar C. M., Cimino D. F., Sklar L. A., Arterburn J. B., and Prossnitz E. R. (2005) A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 307, 1625–1630 [DOI] [PubMed] [Google Scholar]
- 12. Bologa C. G., Revankar C. M., Young S. M., Edwards B. S., Arterburn J. B., Kiselyov A. S., Parker M. A., Tkachenko S. E., Savchuck N. P., Sklar L. A., Oprea T. I., and Prossnitz E. R. (2006) Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat. Chem. Biol. 2, 207–212 [DOI] [PubMed] [Google Scholar]
- 13. Filardo E. J., Quinn J. A., Bland K. I., and Frackelton A. R. Jr. (2000) Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol. Endocrinol. 14, 1649–1660 [DOI] [PubMed] [Google Scholar]
- 14. Albanito L., Madeo A., Lappano R., Vivacqua A., Rago V., Carpino A., Oprea T. I., Prossnitz E. R., Musti A. M., Andò S., and Maggiolini M. (2007) G protein-coupled receptor 30 (GPR30) mediates gene expression changes and growth response to 17β-estradiol and selective GPR30 ligand G-1 in ovarian cancer cells. Cancer Res. 67, 1859–1866 [DOI] [PubMed] [Google Scholar]
- 15. Pedram A., Razandi M., and Levin E. R. (2006) Nature of functional estrogen receptors at the plasma membrane. Mol. Endocrinol. 20, 1996–2009 [DOI] [PubMed] [Google Scholar]
- 16. Otto C., Rohde-Schulz B., Schwarz G., Fuchs I., Klewer M., Brittain D., Langer G., Bader B., Prelle K., Nubbemeyer R., and Fritzemeier K. H. (2008) G protein-coupled receptor 30 localizes to the endoplasmic reticulum and is not activated by estradiol. Endocrinology 149, 4846–4856 [DOI] [PubMed] [Google Scholar]
- 17. Langer G., Bader B., Meoli L., Isensee J., Delbeck M., Noppinger P. R., and Otto C. (2010) A critical review of fundamental controversies in the field of GPR30 research. Steroids 75, 603–610 [DOI] [PubMed] [Google Scholar]
- 18. Kang L., Zhang X., Xie Y., Tu Y., Wang D., Liu Z., and Wang Z. Y. (2010) Involvement of estrogen receptor variant ER-alpha36, not GPR30, in nongenomic estrogen signaling. Mol. Endocrinol. 24, 709–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Broselid S., Berg K. A., Chavera T. A., Kahn R., Clarke W. P., Olde B., and Leeb-Lundberg L. M. (2014) G protein-coupled receptor 30 (GPR30) forms a plasma membrane complex with membrane-associated guanylate kinases (MAGUKs) and protein kinase A-anchoring protein 5 (AKAP5) that constitutively inhibits cAMP production. J. Biol. Chem. 289, 22117–22127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Akama K. T., Thompson L. I., Milner T. A., and McEwen B. S. (2013) Post-synaptic density-95 (PSD-95) binding capacity of G-protein-coupled receptor 30 (GPR30), an estrogen receptor that can be identified in hippocampal dendritic spines. J. Biol. Chem. 288, 6438–6450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tran Q. K., VerMeer M., Burgard M. A., Hassan A. B., and Giles J. (2015) Hetero-oligomeric complex between the G protein-coupled estrogen receptor 1 and the plasma membrane Ca2+-ATPase 4b. J. Biol. Chem. 290, 13293–13307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Waters E. M., Thompson L. I., Patel P., Gonzales A. D., Ye H. Z., Filardo E. J., Clegg D. J., Gorecka J., Akama K. T., McEwen B. S., and Milner T. A. (2015) G-protein-coupled estrogen receptor 1 is anatomically positioned to modulate synaptic plasticity in the mouse hippocampus. J. Neurosci. 35, 2384–2397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sandén C., Broselid S., Cornmark L., Andersson K., Daszkiewicz-Nilsson J., Mårtensson U. E., Olde B., and Leeb-Lundberg L. M. (2011) G protein-coupled estrogen receptor 1/G protein-coupled receptor 30 localizes in the plasma membrane and traffics intracellularly on cytokeratin intermediate filaments. Mol. Pharmacol. 79, 400–410 [DOI] [PubMed] [Google Scholar]
- 24. Ballesteros J. A., and Weinstein H. (1995) Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G protein-coupled receptors. Methods Neurosci. 25, 366–428 [Google Scholar]
- 25. Ballesteros J. A., Jensen A. D., Liapakis G., Rasmussen S. G., Shi L., Gether U., and Javitch J. A. (2001) Activation of the β2-adrenergic receptor involves disruption of an ionic lock between the cytoplasmic ends of transmembrane segments 3 and 6. J. Biol. Chem. 276, 29171–29177 [DOI] [PubMed] [Google Scholar]
- 26. Springael J. Y., de Poorter C., Deupi X., Van Durme J., Pardo L., and Parmentier M. (2007) The activation mechanism of chemokine receptor CCR5 involves common structural changes but a different network of interhelical interactions relative to rhodopsin. Cell. Signal. 19, 1446–1456 [DOI] [PubMed] [Google Scholar]
- 27. Smrcka A. V. (2008) G protein βγ subunits: central mediators of G protein-coupled receptor signaling. Cell. Mol. Life Sci. 65, 2191–2214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Leeb-Lundberg L. M., Song X. H., and Mathis S. A. (1994) Focal adhesion-associated proteins p125FAK and paxillin are substrates for bradykinin-stimulated tyrosine phosphorylation in Swiss 3T3 cells. J. Biol. Chem. 269, 24328–24334 [PubMed] [Google Scholar]
- 29. Cheng S. B., Quinn J. A., Graeber C. T., and Filardo E. J. (2011) Down-modulation of the G-protein-coupled estrogen receptor, GPER, from the cell surface occurs via a trans-Golgi-proteasome pathway. J. Biol. Chem. 286, 22441–22455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Holstein D. M., Berg K. A., Leeb-Lundberg L. M., Olson M. S., and Saunders C. (2004) Calcium-sensing receptor-mediated ERK1/2 activation requires Gαi2 coupling and dynamin-independent receptor internalization. J. Biol. Chem. 279, 10060–10069 [DOI] [PubMed] [Google Scholar]
- 31. García Lopez M. A., Aguado Martínez A., Lamaze C., Martínez-A C., Fischer T. (2009) Inhibition of dynamin prevents CCL2-mediated endocytosis of CCR2 and activation of ERK1/2. Cell. Signal. 21, 1748–1757 [DOI] [PubMed] [Google Scholar]
- 32. Enquist J., Skröder C., Whistler J. L., and Leeb-Lundberg L. M. (2007) Kinins promote B2 receptor endocytosis and delay constitutive B1 receptor endocytosis. Mol. Pharmacol. 71, 494–507 [DOI] [PubMed] [Google Scholar]
- 33. Clipstone N. A., and Crabtree G. R. (1992) Identification of calcineurin as a key signaling enzyme in T-lymphocyte activation. Nature 357, 695–697 [DOI] [PubMed] [Google Scholar]
- 34. Kenakin T. P. (2009) 7TM receptor allostery: putting numbers to shapeshifting proteins. Trends Pharmacol. Sci. 30, 460–469 [DOI] [PubMed] [Google Scholar]
- 35. Coghlan V. M., Perrino B. A., Howard M., Langeberg L. K., Hicks J. B., Gallatin W. M., and Scott J. D. (1995) Association of protein kinase A and protein phosphatase 2B with a common anchoring protein. Science 267, 108–111 [DOI] [PubMed] [Google Scholar]
- 36. Kashishian A., Howard M., Loh C., Gallatin W. M., Hoekstra M. F., and Lai Y. (1998) AKAP79 inhibits calcineurin through a site distinct from the immunophilin-binding region. J. Biol. Chem. 273, 27412–27419 [DOI] [PubMed] [Google Scholar]
- 37. Li H., Pink M. D., Murphy J. G., Stein A., Dell'Acqua M. L., and Hogan P. G. (2012) Balanced interactions of calcineurin with AKAP79 regulate Ca2+-calcineurin-NFAT signaling. Nat. Struct. Mol. Biol. 19, 337–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Murphy J. G., Sanderson J. L., Gorski J. A., Scott J. D., Catterall W. A., Sather W. A., and Dell'Acqua M. L. (2014) AKAP-anchored PKA maintains neuronal L-type calcium channel activity and NFAT transcriptional signaling. Cell Rep. 7, 1577–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rabhi I., Guedel N., Chouk I., Zerria K., Barbouche M. R., Dellagi K., and Fathallah D. M. (2004) A novel simple and rapid PCR-based site-directed mutagenesis method. Mol. Biotechnol. 26, 27–34 [DOI] [PubMed] [Google Scholar]
- 40. Gonzalez-Garcia J. R., Bradley J., Nomikos M., Paul L., Machaty Z., Lai F. A., and Swann K. (2014) The dynamics of MAPK inactivation at fertilization in mouse eggs. J. Cell Sci. 127, 2749–2760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Phagoo S. B., Poole S., and Leeb-Lundberg L. M. (1999) Autoregulation of bradykinin receptors: agonists in the presence of interleukin-1β shift the repertoire of receptor subtypes from B2 to B1 in human lung fibroblasts. Mol. Pharmacol. 56, 325–333 [DOI] [PubMed] [Google Scholar]