Abstract
Purpose
The purpose of this review is to identify emerging developmental toxicants that are understudied in children’s health. Exposures may arise from new products designed to improve utility, to reduce toxicity, or to replace undesirable chemicals. Exposures to less toxic chemicals may also be significant if they are very commonly used, thereby generating widespread exposure. Sources of exposure include the workplace, personal, home and office products; food, water, and air.
Recent Findings
We describe eight exposure categories that contain numerous potential developmental toxicants. References are discussed if reported in PubMed during the past decade at least ten-times more frequently than in 1990–2000. Examples included phthalates, phenols, sunscreens, pesticides, halogenated flame retardants, perfluoroalkyl coatings, nanoparticles, e-cigarettes, and dietary polyphenols. Replacements are often close structural homologs of their precursors. We suggest biomonitoring as preferred means of exposure assessment to emerging chemicals. Some existing analytic methods would require minimal modification to measure these exposures, but others require toxicokinetic and analytic investigation.
Summary
A deliberate strategy for biomonitoring of emerging replacement chemicals is warranted, especially in view of concerns regarding developmental toxicity. To prevent adverse health effects, it is important to characterize such exposures before they become widely disseminated.
Keywords: Child health, environmental exposures, exposure biomarkers, orphan exposures
Introduction
Emerging exposures are defined as environmental agents with potential for human exposure, where there is reason for concern about health effects. There may be limited toxicological and biomonitoring data but ample information on possible sources. Developmental toxicants may derive from parental occupational exposures, from use of personal or consumer products that contain toxic chemicals, or from contaminated food, water, or air. Estimating personal exposures to toxicants can sometimes be challenging in the absence of validated biomarkers. However, in some circumstances they may be reliably estimated by compiling a personal record of products utilized, foods consumed, or aspects of the home environment. Environmental exposure biomarkers are often desirable to quantify individual level exposure and monitor changes in exposure over time. As toxic, and, in particular, persistent, chemicals have been discovered in the environment, replacement materials have been developed by industry in response to consumer concerns or regulatory changes. Other new chemicals have arisen from modern technology. Some of these agents are suspected to be toxic, but are overlooked in research because there are no readily available exposure assessment and biomonitoring methods. This paper aims to identify emerging exposures and to suggest means of measuring them in people with a focus on organic chemicals.
Methods
We assembled in Table 1 a selected list of chemicals and products that are emerging toxicants, based on several kinds of use, including compounds that are being phased-out or replaced. We selected representative chemicals that are of increasing interest based on having been cited in the past ten years. We looked for existing analytic methods that could be used to detect these chemicals in the body with minimal modifications. We focus on this means of exposure assessment, as there are no comprehensive means of using retrospective recall of product use, diet, home environment, etc. that can capture sufficient information.
Table 1.
EMERGING EXPOSURES–Chemicals of concern with little human data, reported from 1/1/1990 – 12/31/1999 and in the past 10 years.
| Exposure Class | Search terms | # Citations, 1990–2000 | # Citations, last 10 years |
|---|---|---|---|
| PHTHALATES AND PHENOLS | |||
| Phthalate | urine | 14 | 449 |
| Phthalate replacements (long chain: decyl, nonyl, isononyl) | urine | 0 | 28 |
| DINCH (1,2-cyclohexane dicarboxylic acid diisononyl ester) | exposure | 1 | 16 |
| DEHT (bis-(2-ethylhexyl)-terephthalate) | exposure | 0 | 5 |
| DEHA (bis-(2-ethylhexyl)-adipate) | exposure | 0 | 10 |
| Bis Phenol A | urine | 9 | 399 |
| Bisphenol F, Bisphenol S | urine | 0 | 16 |
| Nonyl phenol | exposure | 1 | 6 |
| Parabens | urine | 1 | 84 |
| Triclosan, trichlocarban | urine, exposure | 9 | 143 |
| 2,5-Dichlorophenol (2,4-dichlorobenzene metabolite) | exposure | 3 | 36 |
| SUNSCREENS | |||
| Benzophenone-3 or oxybenzone | exposure | 5 | 59 |
| Other terpenes (Limonene, citronella, linalool, mexoryl) | exposure | 22 | 68 |
| Avobenzene (Butyl methoxydibenzoylmethane, methoxydibenzoylmethane) b | exposure | 2 | 13 |
| POLYPHENOLS, including phytoestrogens | urine | 84 | 386 |
| PESTICIDES | |||
| Neonicotinoids | urine, exposure | 0 | 10 |
| HALOGENATED FLAME RETARDANTS | |||
| PBDE | Exposure, plasma or serum | 4 1 |
445 179 |
| TBBP-A | exposure | 0 | 12 |
| Hexabromocyclododecane | exposure | 0 | 96 |
| Brominated organophosphates | exposure | 1 | 13 |
| Brominated phthalates clusters (2-Ethylhexyl tetrabromobenzoate, bis(2-ethylhexyl) tetrabromophthalate) | exposure | 0 | 13 |
| Chlorinated flame retardants | exposure | 3 | 59 |
| COATINGS, PFOA REPLACEMENTS | |||
| Long chain perfluoroalkyl acids | exposure | 1 | 14 |
| PFOA | exposure | 4 | 428 |
| NANO PARTICLES OR NANOPARTICLES OR NANOPARTICLE | exposure | 0 | 2220 |
| Nano titanium, Zn, Ag, Au | exposure | 0 | 523 |
| Nano organics | 0 | 60 | |
| e-CIGARETTES | exposure | 1 | 208 |
| e-cigarettes, Flavorings | Exposure | 0 | 11 |
| Nicotine, cotinine, e-cigarettes | Urine | 0 | 17 |
| Volatiles (acetoin/3-hydroxybutanone, diacetyl, pentanedione/acetyl propionyl) | e-cigarettes | 3 | 70 |
Search was done in October 2016, limited to Humans; items for these 2 intervals are listed if citations in “past 10 years” (a PubMed option) were ten times more frequent than in 1990–2000. Search parameters were as shown, e.g. “PFOA exposure”, limited to Humans, Limited to [date].
Avobenzene included because of its potential wide exposure to humans, although the number of citations is slightly different than ten-times in past 10 years.
Searched and not listed because recent references were too few (past ten or past two years): PAH, fuel additives, cotinine, Pb, Mn, Ti, PBB, PCB, DDT, DEET, nitrophenol, chlorpyrifos, pentachlorophenol, 2,4,5-TCP, 2,4-D, atrazine, DAPs, 4-t-octyl- and ortho-phenyl- phenols, benzophenone-2 or -4, octyl methoxycinnamate (octinoxate), homosalates (trimethylcyclohexenyl salicylate), octisalate (octyl salicylate), hydrofluoropolyether, perfluoroalkyl acids, perfluoroether carboxylic acids (PFECAs) and perfluoroether sulfonic acids (PFESAs including GenX — CF3CF2CF2OCF(CF3)COOH.NH3, Adona 3H-perfluoro-3-[(3-methoxy-propoxy)propanoic acid, ADONA, 3(4-methylbenzylidene)camphor (4-MBC), glyphosate
We searched for known, replacement, and other chemicals in PubMed during three timeframes: 1990–2000, the “past 10 years” (a PubMed option), using the terms including “chemical name”, “urine” and/or “exposure”, limited to humans. Items were included in Table 1 if citations in “past 10 years” were at least ten times more frequent than in 1990–2000 and if they were also cited in the past two years. To compare with the overall rise in publications, a search for “exposure” alone found that the number of references doubled in the earlier vs later 10-yr periods (90,312 vs 185,167), and 35,385 references appeared for the past 2 years. Publications on “exposure and urine” increased about 1.5 times for the 10-yr intervals (4018 vs 6366), with 1340 in the past 2 years. This suggests that higher numbers for reported agents in Table 1 are not publication bias. Reference count changed over the months of assembling the information, and the Table enumeration is that retrieved on October 1, 2016. Not all references were reviewed individually, so some that do not directly address human exposure may be included in the count. We recognize that PubMed citations are lagged relative to the identification, measurement, and research on contaminants of concern; therefore, there is a need to identify additional exposures using knowledge of prevalent chemicals such as those in commonly used products [1].
Results and Discussion
Table 1 lists selected potential emerging exposures in eight categories. Many of these chemicals occur together in products thus presenting exposure to a mixture of chemicals from their use [1]. Moreover, multiple chemicals also exist in many exposure sources, such as swimming pools (>40 chemicals including 14 UV filters) [2]. Many emerging exposures are close structural homologs of the replaced substance that were developed to reduce persistence and absorption in the body. However, replacement chemicals often do not represent an improvement in terms of health effects and may have substantial data gaps from which to estimate toxicity or population prevalence [3]. Almost all of these exposures are nonpersistent organic chemicals. It is remarkable that these exposures with citations before 2000 are also those with reported health effects research.
Phthalates and phenols
Both phthalates and phenols have been associated with health outcomes in children, including neurodevelopment, allergy, and obesity [4,5,6]. For phthalates, health effects have been observed for exposures prenatally through childhood. Phthalates are used in a wide variety of consumer items including personal care products, food packaging, and building materials, resulting in widespread population exposure. More than 20 phthalate replacements have been reported [3]. Three of the newer ones have been found in human biomonitoring (DINCH, DEHT, DEHA; figure 1) [3,7]. It is unclear whether the replacements are less toxic or less persistent. Of some concern, brominated phthalates are being used as flame retardants [8].
Figure 1.
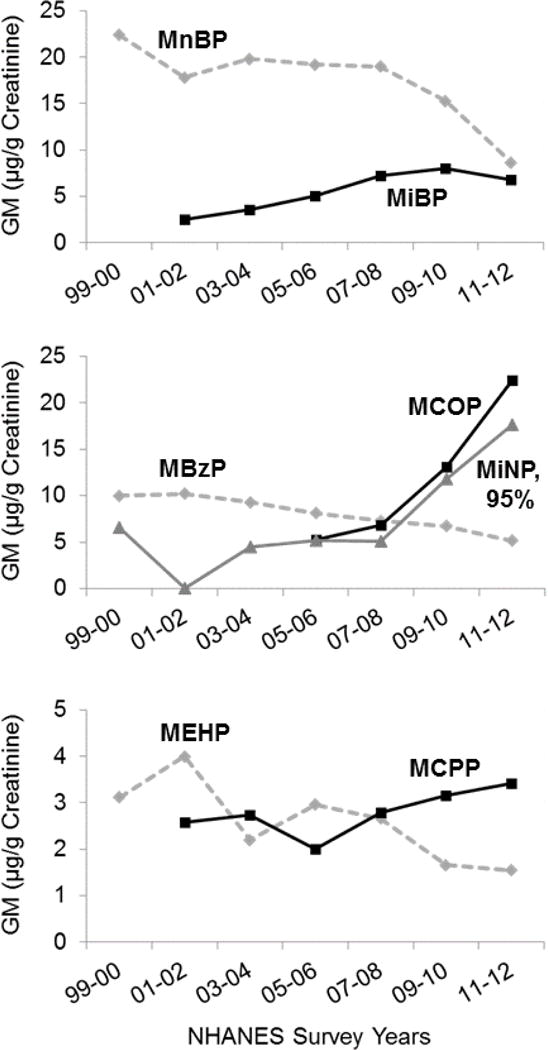
Phthalate urinary metabolites and long-chain replacements measured in the US (geometric means, all NHANES 1999–2012 [11]).
Parabens are phenol preservatives that are extensively used in personal care products, food, and medications. Parabens are commonly detected in urine as the parent compound (largely conjugated). Newly identified specific oxidative metabolites may be more specific indicators of exposure than the parent compounds [9]. Triclosan and triclocarban have been banned recently for soaps, but still exist as biocides in many products [10]. 2,5-Dichlorophenol is the metabolite of 1,4-dichlorobenzene, a putative carcinogen used in mothballs and air and toilet fresheners; levels in the U.S. population are still considerable 10 years after the ban for use in schools [11]. bis-Phenol A (BPA) is a monomer found in dental sealants and food containers. It and several homologs have been measured in indoor environments and urine [12,13]. The BPA family of chemicals possesses strong estrogenic properties [14,15].
Existing analytic methods can be used to measure the phthalate monoester metabolites and many phenols in urine [16]. Urine is the preferred matrix, as phthalate and phenol metabolites are most highly concentrated there and there is less possibility of specimen contamination by extraneous sources. For nonyl phenol, there is not yet a valid method, as it is metabolized to multiple oxidative phenols [17].
Sunscreens (UV filters)
Sunscreens typically contain a mixture of 2–8 UV filtering agents, including multiple phenols (e.g. benzophenone-3 [BP3], avobenzene and paraben) as well as zinc oxide and titanium dioxide, the nanoparticle UV filters. While BP3, a phenol UV filter, has been measured in many epidemiology studies, the others have not. They do not appear in Pub Med searches. Limited findings on development and somatic growth in children have been reported [18]. BP3, its analogs, and other chemicals from personal products are found in swimming pools and in the Pacific Ocean, where Hawaii has asked bathers not to use them in order to protect coral [19]. For many of the sunscreen ingredients, pharmacokinetic and metabolism information is needed to guide design of biomonitoring methods.
Other polyphenols including phytoestrogens
Phytoestrogens are natural polyphenols homologous molecularly to hormones and environmental phenols. They share biological activity with synthetic phenols, such as hormone antagonism and obesogenicity. They include isoflavones (soy), quercetin (fruits), and lignan metabolites (flax), and have long been considered as healthy micronutrients. Recently more than 80 phytoestrogens in urine were measured in a large European study, and in one report 4/37 urinary biomarkers had concentrations exceeding 10 um in urine [20]. Their dietary sources were mainly six foods [21].
Pesticides and herbicides
Pesticide residues such as DDE and the herbicide contaminant tetrachlorodibenzodioxin have declined steeply in recent decades in developed countries. For example, breast-milk DDE and PCBs in Sweden declined 10–20-fold over the past 30 years (Figure 2; [22]). Pesticides that replaced the persistent halogenated compounds, such as organophosphates, have also declined [11], but newer, less persistent pesticides are in use, with limited information on human exposure. Neonicotinoids have been measured in urine in a few human studies [23,24]. 2,4-Dichlorophenoxyacetic acid and glyphosate have also long been in continuous use as herbicides, with recent concerns about toxicity and carcinogenesis [25]. Urinary 2,4-dichlorophenoxyacetic acid levels increased from 1999–2010 in NHANES [16]. Glyphosate is the most commonly used pesticide in the United States, however, few methods exist to measure it in urine and those that do exist are extremely cumbersome. Newer methods should be developed to make the measurement of glyphosate more accessible and conducive to adding in other analytes for measurement. Another area of interest is the burgeoning use of thiazole fungicides where almost no studies have been done to understand toxicokinetics or exposure.
Figure 2.
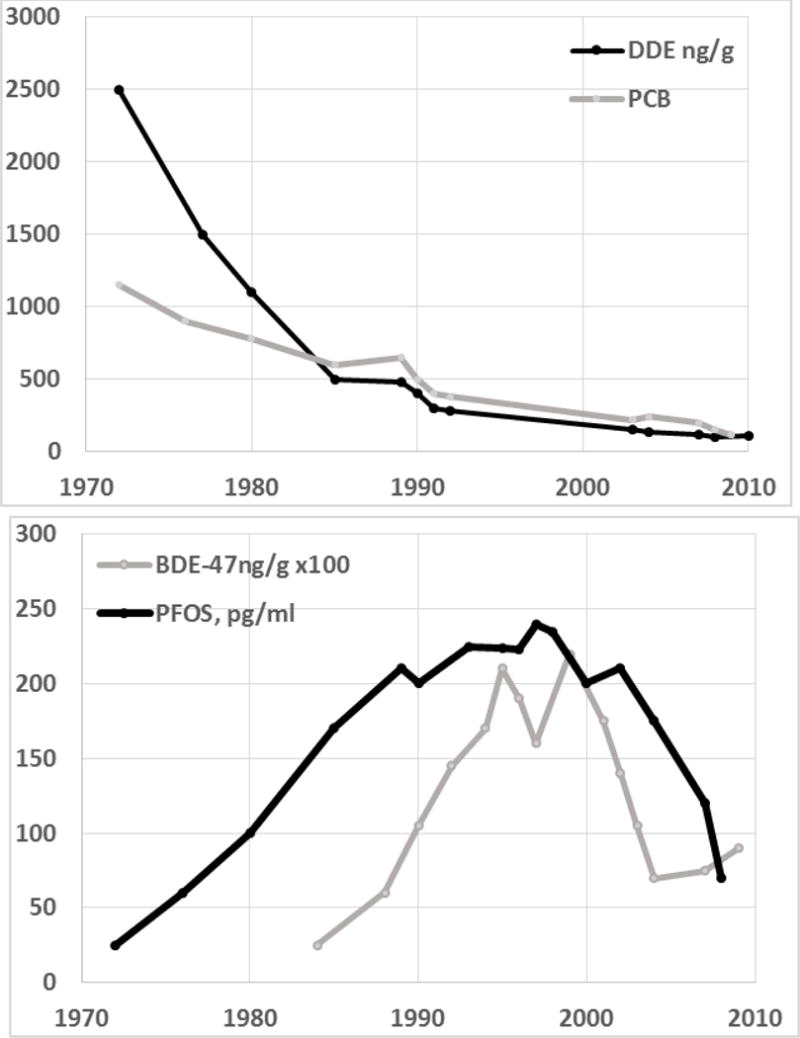
Levels of POPs and PFOS in breastmilk in Sweden, data extracted from Fang et al., [22].
Halogenated Flame Retardants
PBDE flame retardants replaced molecularly homologous polybrominated biphenyls after a tragic accident contaminated cattle and the entire state of Michigan during 1974–76 [26]. PBDE levels began to increase world-wide (Figure 2), and newer replacements have come into being. While PBB and PBDE are persistent with half-lives in humans of 4–40 years, newer fire retardants are less persistent, and they have polar metabolites that can be detected in urine. These chemicals are also being controlled in the United States particularly in products used by children, such as sleeping mats [27]. Multiple halogenated organophosphates have been found in human biomonitoring studies, suggesting that they co-occur in home items or are often used in items in the home [28].
Perfluorinated Coatings
Perfluorinated alkyl carboxylic acids (PFCs) are used in oil and water resistant coatings for fabric and cookware, fire-retardant foam, and floor polish. Following the typical manufacturing practice, PFCs including PFOA and PFOS are being replaced with homologous longer chain chemicals. In Sweden and the US biomonitoring levels of PFOS have declined since 2000 after increasing in the 1990s (Figure 2). More than 20 replacement compounds have been reported, many with structures similar to PFCs [29]. It is not clear that these are less toxic or less persistent [30].
Nanoparticles
Nanomaterials are sub-micron sized fibers, tubes and large molecules made of metal, polymer, or carbon materials. They are controversial in terms of human exposure and toxicity, as it is contended that they are poorly absorbed from sunscreens and clothing [31]. However, they are also being applied as drug delivery systems including to the brain, suggesting that absorption or penetration is possible. Nanoparticles may be inhaled. Immune effects, inflammation, and possible developmental toxicity have been reported [31,32,33]. Validated methods for measuring nanoparticles in humans are limited to respiratory intake. However, limited literature suggests that they are absorbed, as they have been measured in body fluids, including Au-U [34]. Their disparate use makes use of recall methods almost impossible.
e-Cigarettes
Electronic- or e-cigarettes were introduced into the United States tobacco market in 2007; ever-use rapidly increased after 2010, especially among youth, to include 16% of high school students by 2015 [35]. The active ingredient in e-cigarette liquid is nicotine. E-cigarette use results in nicotine urinary metabolites similar in level and pattern to those from users of tobacco and smokeless tobacco products. However, the oxidative nicotine metabolites are lower in users of e-cigarettes [36]. Diacetyl, 2,3-pentadione, and acetoin are structurally similar compounds that have been shown to cause bronchiolitis obliterans in exposed workers or laboratory animals [37]. They are also ubiquitous in fruity and sweet e-liquid flavorings used in marketing to children, as well as in traditional tobacco flavorings such as menthol [38,39,40]. While these compounds are safe for ingestion, they cause lung toxicity when inhaled at concentrations likely to be generated by commercially available e-cigarette liquids. Other flavor ingredients, that are “generally recognized as safe” (GRAS) to ingest by the Flavor and Extract Manufacturers Association (FEMA), and that are commonly added to food, have been identified by FEMA as potentially toxic to the lung, if inhaled [41,42]. There has been limited research to determine whether these aldehydes and other reactive flavorings are present in e-cigarette liquids. However, the implication by manufacturers that flavor ingredients used in e-cigarettes and related devices (e.g. hookahs) are safe for inhalation because they have FEMA GRAS™ status for use in food has been stated to be “false and misleading” by FEMA [43]. Unintentional nicotine poisoning of children as a result of e-cigarette liquid exposure has emerged recently as a public health problem [44, 45].
Multiple and mixed exposures
Various chemicals we have described can occur in many different products, so that multiple exposures exist with everyday use. In NHANES exposure biomarker surveys, a large proportion of >250 measured chemicals are >50% detectable, meaning that many co-exist in the bodies of most people. Multiple sources of exposure exist; e.g., parabens might be absorbed from food, sunscreen, lotion, and lipstick. Plus, as noted above, many products contain more than one ingredient, which constitutes a mixture, so that users would be exposed to several chemicals at once (e.g. organic and nanoparticle UV filters with paraben in sunscreen). When 38,975 products were surveyed for developmental toxicant contents, 30% had one or more chemical of interest and 1,059 contained three phenols [1].
Fundamental/Overarching needs
Before adequate biomonitoring methods can be developed for emerging chemicals, we need to conduct toxicological studies in animals or in silico to understand the toxicokinetics of these chemicals. It is imperative to know what metabolites are formed from non-persistent chemicals before we begin trying to monitor them in biological matrices. Experience, for example with high molecular weight phthalates, taught us that even similar chemicals in the same class may not metabolize similarly resulting in measurement of inappropriate or less useful biomarkers. In addition, one of the biggest challenges in methods’ development for emerging chemicals is the lack of authentic standards for measurement. It would be quite useful if industries introducing new chemicals into manufacture could conduct toxicokinetic studies to inform exposure scientists about potential biomarkers and to synthesize or isolate standards for the measurement of these biomarkers. Without metabolic information and standards, valid biomarker methods cannot be developed.
Conclusion
Many developmental toxicants can be determined using biomarkers. A number of these have been widely detected in the United States with levels rising in recent years [11]. Overall, however, attention has focused on a limited number of chemicals that have validated biomarkers or that have been most convenient to analyze. Historically, comprehensive health information has followed the discovery of widespread exposure, for example to lead, PCBs, and PFOA. We cited a number of exposures that have been introduced to replace or to improve other agents. Many products have more than one additive, and little is known about how mixtures or multiple chemicals interact. Biomonitoring or personal exposure assessment can characterize individual body burden more efficiently than information using recalled exposure sources.
Key points.
Biomonitoring of emerging exposures has often lagged peak population exposures, resulting in lapses of health risk assessment.
Agents without exposure monitoring also lack sufficient information on health effects.
Valid exposure biomarkers are not available for many well-known exposures of interest for child development.
Acknowledgments
Sources of funding: P30ES023515, U2ES026555, U2ES026561, U01ES019454, P30ES019776, U2CES026560, R21HD084812, P01ES02284, EPA RD-83544101, R21ES024707, R21HD084812, R01ES021777, P30ES010126
Abbreviations
- UV
ultraviolet
- DDE
1,1′-dichloro-2,2′-bis(4-chlorophenyl)ethylene
- PCBs
polychlorinated biphenyls
- PFOA/S
perfluorooctanoic acid
- PFOS
perfluorooctanesulfonic acid
- DINCH
1,2-cyclohexane dicarboxylic acid diisononyl ester
- DEHT
bis-(2-ethylhexyl)-terephthalate
- DEHA
bis-(2-ethylhexyl)-adipate
- BPA
bis-phenol A
- NHANES
National Health and Nutrition Examination Survey
- PBDE
polybrominated diphenyl ethers
Footnotes
COI: None declared.
Contributor Information
Mary S. Wolff, Icahn School of Medicine at Mount Sinai, New York, NY, USA
Jessie Buckley, University of North Carolina, Chapel Hill, NC, USA.
Stephanie M. Engel, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD, USA
Rob Scot McConnell, Keck School of Medicine, University of Southern California, Los Angeles, CA, USA.
Dana B. Barr, Rollins School of Public Health, Emory University, Atlanta, GA, USA
Reference List
Papers of particular interest have been highlighted as:
*of special interest
- 1*.Gabb HA, Blake C. An Informatics Approach to Evaluating Combined Chemical Exposures from Consumer Products: A Case Study of Asthma-Associated Chemicals and Potential Endocrine Disruptors. Environ Health Perspect. 2016;124:1155–1165. doi: 10.1289/ehp.1510529. In the moment when epidemiology is realizing that more than asingle chemical exists in the body, this paper codifies the presence of multiple chemicals in mnay products – true mixed exposures. Thhsu while exposure biomarkers mesure one at a time, their sources and their biological activity might be better characterized if synchronous exposures. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ekowati Y, Buttiglieri G, Ferrero G, Valle-Sistac J, Diaz-Cruz MS, Barcelo D, Petrovic M, Villagrasa M, Kennedy MD, Rodriguez-Roda I. Occurrence of pharmaceuticals and UV filters in swimming pools and spas. Environ Sci Pollut Res Int. 2016;23:14431–14441. doi: 10.1007/s11356-016-6560-1. [DOI] [PubMed] [Google Scholar]
- 3.Bui TT, Giovanoulis G, Cousins AP, Magner J, Cousins IT, de Wit CA. Human exposure, hazard and risk of alternative plasticizers to phthalate esters. Sci Total Environ. 2016;541:451–67. doi: 10.1016/j.scitotenv.2015.09.036. Epub;%2015 Sep 26.; 451–467. [DOI] [PubMed] [Google Scholar]
- 4.Ejaredar M, Nyanza EC, Ten EK, Dewey D. Phthalate exposure and childrens neurodevelopment: A systematic review. Environ Res. 2015;142:51–60. doi: 10.1016/j.envres.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 5.Goodman M, Lakind JS, Mattison DR. Do phthalates act as obesogens in humans? A systematic review of the epidemiological literature. Crit Rev Toxicol. 2014;44:151–175. doi: 10.3109/10408444.2013.860076. [DOI] [PubMed] [Google Scholar]
- 6.North ML, Takaro TK, Diamond ML, Ellis AK. Effects of phthalates on the development and expression of allergic disease and asthma. Ann Allergy Asthma Immunol. 2014;112:496–502. doi: 10.1016/j.anai.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 7.Fromme H, Schutze A, Lahrz T, Kraft M, Fembacher L, Siewering S, Burkardt R, Dietrich S, Koch HM, Volkel W. Non-phthalate plasticizers in German daycare centers and human biomonitoring of DINCH metabolites in children attending the centers (LUPE 3) Int J Hyg Environ Health. 2016;219:33–39. doi: 10.1016/j.ijheh.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Ma Y, Venier M, Hites RA. 2-Ethylhexyl tetrabromobenzoate and bis(2-ethylhexyl) tetrabromophthalate flame retardants in the Great Lakes atmosphere. Environ Sci Technol. 2012 Jan 3;46:204–208. doi: 10.1021/es203251f. [DOI] [PubMed] [Google Scholar]
- 9.Moos RK, Angerer J, Dierkes G, Bruning T, Koch HM. Metabolism and elimination of methyl, iso- and n-butyl paraben in human urine after single oral dosage. Arch Toxicol. 2015;90:2699–2709. doi: 10.1007/s00204-015-1636-0. [DOI] [PubMed] [Google Scholar]
- 10.Erickson B. FDA bans triclosan and triclocarban in consumer soaps. C&E News. 2016;94:16. [Google Scholar]
- 11.CDC. Fourth National Report on Human Exposure to Environmental Chemicals. CDC; 2015. Updated Tables, February 2015. http://www.cdc.gov/exposurereport. [PubMed] [Google Scholar]
- 12.Liao C, Liu F, Guo Y, Moon HB, Nakata H, Wu Q, Kannan K. Occurrence of eight bisphenol analogues in indoor dust from the United States and several Asian countries: implications for human exposure. Environ Sci Technol. 2012;46:9138–9145. doi: 10.1021/es302004w. [DOI] [PubMed] [Google Scholar]
- 13.Ye X, Wong LY, Kramer J, Zhou X, Jia T, Calafat AM. Urinary Concentrations of Bisphenol A and Three Other Bisphenols in Convenience Samples of U.S. Adults during 2000–2014. Environ Sci Technol. 2015;49:11834–11839. doi: 10.1021/acs.est.5b02135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rochester JR, Bolden AL. Bisphenol S and F: A Systematic Review and Comparison of the Hormonal Activity of Bisphenol A Substitutes. Environ Health Perspect. 2015;123:643–650. doi: 10.1289/ehp.1408989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenmai AK, Dybdahl M, Pedersen M, Alice van Vugt-Lussenburg BM, Wedebye EB, Taxvig C, Vinggaard AM. Are structural analogues to bisphenol a safe alternatives? Toxicol Sci. 2014;139:35–47. doi: 10.1093/toxsci/kfu030. [DOI] [PubMed] [Google Scholar]
- 16.CDC. Fourth National Report on Human Exposure to Environmental Chemicals. 2015 Updated Tables, February 2015. http://www.cdcgov/exposurereport. [PubMed]
- 17.Zalko D, Costagliola R, Dorio C, Rathahao E, Cravedi JP. In vivo metabolic fate of the xeno-estrogen 4-n-nonylphenol in Wistar rats. Drug Metab Dispos. 2003;31:168–178. doi: 10.1124/dmd.31.2.168. [DOI] [PubMed] [Google Scholar]
- 18.Krause M, Klit A, Blomberg JM, Soeborg T, Frederiksen H, Schlumpf M, Lichtensteiger W, Skakkebaek NE, Drzewiecki KT. Sunscreens: are they beneficial for health? An overview of endocrine disrupting properties of UV-filters. Int J Androl. 2012;35:424–436. doi: 10.1111/j.1365-2605.2012.01280.x. [DOI] [PubMed] [Google Scholar]
- 19.Hogue C. Hawaii targets sunscreens with oxybenzone. C&E News. 2016;94:16. [Google Scholar]
- 20.Achaintre D, Bulete A, Cren-Olive C, Li L, Rinaldi S, Scalbert A. Differential Isotope Labeling of 38 Dietary Polyphenols and Their Quantification in Urine by Liquid Chromatography Electrospray Ionization Tandem Mass Spectrometry. Anal Chem. 2016;88:2637–2644. doi: 10.1021/acs.analchem.5b03609. [DOI] [PubMed] [Google Scholar]
- 21*.Edmands WM, Ferrari P, Rothwell JA, Rinaldi S, Slimani N, Barupal DK, Biessy C, Jenab M, Clavel-Chapelon F, Fagherazzi G, Boutron-Ruault MC, Katzke VA, Kuhn T, Boeing H, Trichopoulou A, Lagiou P, Trichopoulos D, Palli D, Grioni S, Tumino R, Vineis P, Mattiello A, Romieu I, Scalbert A. Polyphenol metabolome in human urine and its association with intake of polyphenol-rich foods across European countries. Am J Clin Nutr. 2015;102:905–913. doi: 10.3945/ajcn.114.101881. This research including referenced papers by this group on a number of methods is remarkable because it shows a wide range of polyphenols, including some with urinary concentrations above 1 micormular. Thus, focusing only on subgroups such as isoflavones may miss biologically effective exposures. [DOI] [PubMed] [Google Scholar]
- 22*.Fang J, Nyberg E, Winnberg U, Bignert A, Bergman A. Spatial and temporal trends of the Stockholm Convention POPs in mothers’ milk – a global review. Environ Sci Pollut Res Int. 2015;22:8989–9041. doi: 10.1007/s11356-015-4080-z. This comprehensive review of 50 years of POPs exposure shows patterns of rise and fall in humans, and thus the response of population health to regulatory action. IT is an invaluable reference. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morgan MK. Predictors of urinary levels of 2,4-dichlorophenoxyacetic acid, 3,5,6-trichloro-2-pyridinol, 3-phenoxybenzoic acid, and pentachlorophenol in 121 adults in Ohio. Int J Hyg Environ Health. 2015;218:479–488. doi: 10.1016/j.ijheh.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 24.Osaka A, Ueyama J, Kondo T, Nomura H, Sugiura Y, Saito I, Nakane K, Takaishi A, Ogi H, Wakusawa S, Ito Y, Kamijima M. Exposure characterization of three major insecticide lines in urine of young children in Japan-neonicotinoids, organophosphates, and pyrethroids. Environ Res. 2016;147:89–96. doi: 10.1016/j.envres.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 25.Loomis D, Guyton K, Grosse Y, El GF, Bouvard V, Benbrahim-Tallaa L, Guha N, Mattock H, Straif K. Carcinogenicity of lindane, DDT, and 2,4-dichlorophenoxyacetic acid. Lancet Oncol. 2015;16:891–892. doi: 10.1016/S1470-2045(15)00081-9. [DOI] [PubMed] [Google Scholar]
- 26.Alaee M, Arias P, Sjodin A, Bergman A. An overview of commercially used brominated flame retardants, their applications, their use patterns in different countries/regions and possible modes of release. Environ Int. 2003;29:683–689. doi: 10.1016/S0160-4120(03)00121-1. [DOI] [PubMed] [Google Scholar]
- 27.Erickson B. California targets flame retardants in kids’ sleeping mats. C&E News. 2016;94:19. [Google Scholar]
- 28.Dodson RE, Van den Eede N, Covaci A, Perovich LJ, Brody JG, Rudel RA. Urinary biomonitoring of phosphate flame retardants: levels in California adults and recommendations for future studies. Environ Sci Technol. 2014;48:13625–13633. doi: 10.1021/es503445c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Z, Cousins IT, Scheringer M, Hungerbuhler K. Fluorinated alternatives to long-chain perfluoroalkyl carboxylic acids (PFCAs), perfluoroalkane sulfonic acids (PFSAs) and their potential precursors. Environ Int. 2013;60:242–248. doi: 10.1016/j.envint.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 30.Wang Z, Cousins IT, Scheringer M, Hungerbuehler K. Hazard assessment of fluorinated alternatives to long-chain perfluoroalkyl acids (PFAAs) and their precursors: status quo, ongoing challenges and possible solutions. Environ Int. 2015;75:172–9. doi: 10.1016/j.envint.2014.11.013. Epub;%2014 Nov 27.; 172–179. [DOI] [PubMed] [Google Scholar]
- 31*.WHO. Nanotechnology human health: Scientific evidence and risk governance. 2016 http://www.euro.who.int/en/health-topics/environment-and-health/health-impact-assessment/publications/2013/nanotechnology-and-human-health-scientific-evidence-and-risk-governance WHO compiled many reports on nanotechnology in this monograph. Of particular interest to child health are potential immunotoxic and inflammatory potential of various materials.
- 32.Ali A, Suhail M, Mathew S, Shah MA, Harakeh SM, Ahmad S, Kazmi Z, Alhamdan MA, Chaudhary A, Damanhouri GA, Qadri I. Nanomaterial Induced Immune Responses and Cytotoxicity. J Nanosci Nanotechnol. 2016;16:40–57. doi: 10.1166/jnn.2016.10885. [DOI] [PubMed] [Google Scholar]
- 33.Ema M, Gamo M, Honda K. Developmental toxicity of engineered nanomaterials in rodents. Toxicol Appl Pharmacol. 2016;299:47–52. doi: 10.1016/j.taap.2015.12.015. Epub;%2015 Dec 22.; 47–52. [DOI] [PubMed] [Google Scholar]
- 34.Hadrup N, Sharma AK, Poulsen M, Nielsen E. Toxicological risk assessment of elemental gold following oral exposure to sheets and nanoparticles - A review. Regul Toxicol Pharmacol. 2015;72:216–221. doi: 10.1016/j.yrtph.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 35.Singh T, Marynak K, Arrazola RA, Cox S, Rolle IV, King BA. Vital Signs: Exposure to Electronic Cigarette Advertising Among Middle School and High School Students - United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;64:1403–1408. doi: 10.15585/mmwr.mm6452a3. [DOI] [PubMed] [Google Scholar]
- 36.Hecht SS, Carmella SG, Kotandeniya D, Pillsbury ME, Chen M, Ransom BW, Vogel RI, Thompson E, Murphy SE, Hatsukami DK. Evaluation of toxicant and carcinogen metabolites in the urine of e-cigarette users versus cigarette smokers. Nicotine Tob Res. 2015;17:704–709. doi: 10.1093/ntr/ntu218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.NIOSH. Preventing Lung Disease in Workers Who Use or Make Flavorings. 2004 https://www.cdc.gov/niosh/docs/2004-110/pdfs/2004-110.pdf.
- 38*.Allen JG, Flanigan SS, LeBlanc M, Vallarino J, MacNaughton P, Stewart JH, Christiani DC. Flavoring Chemicals in E-Cigarettes: Diacetyl, 2,3-Pentanedione, and Acetoin in a Sample of 51 Products, Including Fruit-, Candy-, and Cocktail-Flavored E-Cigarettes. Environ Health Perspect. 2016;124:733–739. doi: 10.1289/ehp.1510185. While it is known that e-cigs may contain hundreds of flavoring an sweeterners, this paper carefully quanitifies a cnumber of quite toxic additrives as well as the fact that several are present at once in the same product, a mixture. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farsalinos KE, Kistler KA, Gillman G, Voudris V. Evaluation of electronic cigarette liquids and aerosol for the presence of selected inhalation toxins. Nicotine Tob Res. 2015;17:168–174. doi: 10.1093/ntr/ntu176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miao S, Beach ES, Sommer TJ, Zimmerman JB, Jordt SE. High-Intensity Sweeteners in Alternative Tobacco Products. Nicotine Tob Res. 2016 doi: 10.1093/ntr/ntw141. pii: ntw141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barrington-Trimis JL, Samet JM, McConnell R. Flavorings in electronic cigarettes: an unrecognized respiratory health hazard? JAMA. 2014;312:2493–2494. doi: 10.1001/jama.2014.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.FEMA. Respiratory Health and Safety in the Flavor Manufacturing Workplace: 2012 Update. Washington DC: The Flavor and Extract Manufacturers Association of the United States; 2012. http://www.femaflavororg/respiratory-health-and-safety-flavor-manufacturing-workplace-2012-update. [Google Scholar]
- 43.Hallagan J. Safety Assessment and Regulatory Authority to Use Flavors - Focus on Electronic Nicotine Delivery Systems and Flavored Tobacco Products. Flavor Extract and Manufacturers Association of the United States; 2016. https://www.femaflavor.org/sites/default/files/FEMAGRAS%20Ecig%20092616.pdf. [Google Scholar]
- 44.Kamboj A, Spiller HA, Casavant MJ, Chounthirath T, Smith GA. Pediatric Exposure to E-Cigarettes, Nicotine, and Tobacco Products in the United States. Pediatrics. 2016;137(6) doi: 10.1542/peds.2016-0041. [DOI] [PubMed] [Google Scholar]
- 45*.McConnell R, Barrington-Trimis JL, Wang KMPH, Urman R, Hong H, Unger J, Samet J, Leventhal A, Berhane K. Electronic-cigarette Use and Respiratory Symptoms in Adolescents. Am J Respir Crit Care Med. 2016 Nov 2; doi: 10.1164/rccm.201604-0804OC. [Epub ahead of print] One of the first papers to show chronic health effects of children using e-cigarettes. [DOI] [PMC free article] [PubMed] [Google Scholar]


