Abstract
The susceptibilities of nonduplicate isolates to six antifungal agents were determined for 391 blood isolates of seven Candida species, 70 clinical isolates (from blood or cerebrospinal fluid) of Cryptococcus neoformans, and 96 clinical isolates of four Aspergillus species, which were collected in seven different hospitals in Taiwan (as part of the 2003 program of the study group Surveillance of Multicenter Antimicrobial Resistance in Taiwan). All isolates of Candida species other than C. glabrata and C. krusei were susceptible to fluconazole. Among the 59 C. glabrata isolates, 16 (27%) were not susceptible to fluconazole, and all were dose-dependently susceptible or resistant to itraconazole. For three (5.1%) C. glabrata isolates, voriconazole MICs were 2 to 4 μg/ml, and for all other Candida species isolates, voriconazole MICs were ≤0.5 μg/ml. The proportions of isolates for which amphotericin B MICs were ≥2 μg/ml were 100% (3 isolates) for C. krusei, 11% (23 of 207 isolates) for Candida albicans, 3.0% (2 of 67 isolates) for Candida tropicalis, 20% (12 of 59 isolates) for C. glabrata, and 0% for both Candida parapsilosis and Candida lusitaniae. For three (4%) Cryptococcus neoformans isolates, fluconazole MICs were ≥16 μg/ml, and two (3%) isolates were not inhibited by 1 μg of amphotericin B/ml. For four (4.2%) of the Aspergillus isolates, itraconazole MICs were 8 μg/ml. Aspergillus flavus was less susceptible to amphotericin B, with the MICs at which 50% (1 μg/ml) and 90% (2 μg/ml) nsrsid417869\delrsid7301351 of isolates were inhibited being twofold greater than those for Aspergillus fumigatus and Aspergillus niger. All Aspergillus isolates were inhibited by ≤1 μg of voriconazole/ml, including isolates with increased resistance to amphotericin B and itraconazole. This study revealed the emergence in Taiwan of decreased susceptibilities of Candida species to amphotericin B and of C. neoformans to fluconazole and amphotericin B. Voriconazole was the most potent agent against the fungal isolates tested, including fluconazole- and amphotericin B-nonsusceptible strains.
The incidence of invasive fungal infections, particularly those caused by Candida species, Cryptococcus neoformans, and Aspergillus species, has increased over the past few decades (5-7, 14, 17, 18, 28, 32, 35). These infections are major complications in immunocompromised patients, such as bone marrow or solid-organ transplant recipients, and in patients with profound neutropenia due to hematological malignancies or chemotherapy, and these infections are usually associated with a high attributable mortality (7, 18, 19, 28, 32, 35). The treatment of choice for infected patients includes amphotericin B, the antifungal azoles fluconazole, itraconazole, and voriconazole, and alternative agents with activities against these pathogens which have recently become available (16, 28, 32, 33, 35).
Although several reports revealed the maintenance of good in vitro activities of fluconazole against Candida species despite the dramatic rise in fluconazole use in the past decade, decreased susceptibilities of several Aspergillus and Candida species and C. neoformans to currently available antifungal agents, such as fluconazole, itraconazole, voriconazole, and amphotericin B, have also been detected (2, 11, 17, 21, 23, 25, 32, 33). Moreover, there have been increases in the numbers of reported cases of both primary and secondary resistance among strains causing human mycoses (11).
Although several reports from Taiwan have described the resistance of Candida and Cryptococcus species to azoles (8, 9, 17, 37, 38), there have been no nationwide surveillance studies of Aspergillus species (7, 17, 19). The aim of this study was to determine the in vitro activities of four to six antifungal agents against clinically important fungi, i.e., Candida species, C. neoformans, and Aspergillus species.
This study was one of the programs of the study group Surveillance of Multicenter Antimicrobial Resistance in Taiwan (SMART) in 2003.
MATERIALS AND METHODS
Organisms.
A total of 391 nonduplicate isolates of Candida species were obtained between March 2003 and August 2003 from patients treated at seven teaching hospitals located in different parts of Taiwan (Table 1). The seven teaching hospitals included the following hospitals from different regions of Taiwan: National Taiwan University Hospital and Taipei Veterans General Hospital, Taipei (northern Taiwan); Taichung Veterans General Hospital and China Medical College Hospital, Taichung (central Taiwan); Kaohsiung Veterans General Hospital, Kaohsiung, and Chi-Mei Medical Center, and National Cheng-Kung University Hospital, Tainan (southern Taiwan). The following numbers of Candida isolates were recovered from blood samples: 207 isolates of C. albicans, 67 isolates of C. tropicalis, 59 of C. glabrata, 41 of C. parapsilosis, 11 of C. lusitaniae, and 3 each of C. krusei and C. guilliermondii.
TABLE 1.
In vitro susceptibilities of 385 isolates of Candida species and 70 isolates of C. neoformans to six antifungal agentsa
| Organism (no. of isolates) and agent | MIC (μg/ml)b
|
% of isolates in each susceptibility categoryc
|
||||
|---|---|---|---|---|---|---|
| Range | 50% | 90% | S | S-DD | R | |
| C. albicans (207) | ||||||
| Fluconazole | 0.12-4 | 0.25 | 0.5 | 100 | 0 | 0 |
| Itraconazole | 0.03-0.5 | 0.03 | 0.12 | 97 | 3 | 0 |
| Ketoconazole | 0.03-0.25 | 0.03 | 0.06 | |||
| 5-Flucytosine | 0.12->64 | 0.12 | 0.5 | 98 | 2 | |
| Voriconazole | 0.06-0.25 | 0.12 | 0.12 | |||
| Amphotericin B | 0.25-4 | 1 | 2 | |||
| C. tropicalis (67) | ||||||
| Fluconazole | 0.12-4 | 1 | 2 | 100 | 0 | 0 |
| Itraconazole | 0.03-0.5 | 0.12 | 0.25 | 72 | 28 | 0 |
| Ketoconazole | 0.03-0.5 | 0.06 | 0.25 | |||
| 5-Flucytosine | 0.12-4 | 0.25 | 0.5 | 100 | 0 | |
| Voriconazole | 0.12-0.25 | 0.12 | 0.12 | |||
| Amphotericin B | 0.25-2 | 1 | 1 | |||
| C. glabrata (59) | ||||||
| Fluconazole | 4->64 | 8 | 16 | 72 | 14 | 14 |
| Itraconazole | 0.5->64 | 1 | 4 | 0 | 15 | 85 |
| Ketoconazole | 0.25-4 | 1 | 2 | |||
| 5-Flucytosine | 0.12-8 | 0.12 | 0.12 | 98 | 2d | 0 |
| Voriconazole | 0.12-4 | 0.25 | 0.5 | |||
| Amphotericin B | 0.25-2 | 1 | 2 | |||
| C. parapsilosis (41) | ||||||
| Fluconazole | 0.25-4 | 0.5 | 1 | 100 | 0 | 0 |
| Itraconazole | 0.06-0.5 | 0.12 | 0.25 | 63 | 37 | 0 |
| Ketoconazole | 0.03-0.25 | 0.12 | 0.25 | |||
| 5-Flucytosine | 0.12-0.25 | 0.12 | 8 | 100 | 0 | |
| Voriconazole | 0.12-0.5 | 0.12 | 0.12 | |||
| Amphotericin B | 0.5-1 | 1 | 1 | |||
| C. lusitaniae (11) | ||||||
| Fluconazole | 0.12-0.5 | 0.25 | 0.5 | 100 | 0 | 0 |
| Itraconazole | 0.03-0.12 | 0.06 | 0.06 | 100 | 0 | 0 |
| Ketoconazole | 0.03-0.06 | 0.03 | 0.03 | |||
| 5-Flucytosine | 0.12 | 0.12 | 0.12 | 100 | 0 | |
| Voriconazole | 0.12-0.5 | 0.12 | 0.12 | |||
| Amphotericin B | 0.25-1 | 0.5 | 1 | |||
| C. neoformans (70) | ||||||
| Fluconazole | 0.12-16 | 2 | 8 | |||
| Itraconazole | 0.03-0.25 | 0.12 | 0.25 | |||
| Ketoconazole | 0.03-0.5 | 0.12 | 0.25 | |||
| 5-Flucytosine | 0.12-8 | 1 | 8 | |||
| Voriconazole | 0.12-0.5 | 0.12 | 0.12 | |||
| Amphotericin B | 0.12-2 | 0.5 | 1 | |||
Data are from a program carried out in 2003 by the SMART study group. They were obtained by the broth microdilution method.
50% and 90%, MICs at which 50 and 90% of isolates are inhibited, respectively.
S, susceptible; S-DD, susceptible-dose dependent; R, resistant.
These isolates were intermediately susceptible.
Seventy clinical isolates of C. neoformans and 96 isolates of Aspergillus species were also collected for analysis in this study. These nonduplicate isolates were recovered during 2003 from patients treated at the National Taiwan University Hospital, a 2,000-bed teaching hospital in northern Taiwan. Among the 70 isolates of C. neoformans, 58 were recovered from cerebrospinal fluid and 12 were recovered from blood samples. Among the 96 Aspergillus isolates, 60 were recovered from respiratory secretions (sputum, bronchial-washing samples, pleural effusions, and lung biopsy specimens), 20 were recovered from external ear discharges, 12 were recovered from wound pus, and 4 were recovered from other sources (2 from continuous ambulatory peritoneal dialysis drainage specimens and 1 each from liver abscess and epidural drainage specimens) (Table 2).
TABLE 2.
In vitro susceptibility of 96 isolates of Aspergillus species to three antifungal agentsa
| Organism (no. of isolates) and agent | MIC (μg/ml)b
|
||
|---|---|---|---|
| Range | 50% | 90% | |
| A. fumigatus (40) | |||
| Ketoconazole | 0.06-8 | 2 | 4 |
| Itraconazole | 0.06-8 | 0.25 | 1 |
| Voriconazole | 0.06-0.5 | 0.25 | 0.5 |
| Amphotericin B | 0.25-2 | 0.5 | 1 |
| A. flavus (24) | |||
| Ketoconazole | 0.25-4 | 1 | 2 |
| Itraconazole | 0.06-8 | 0.5 | 1 |
| Voriconazole | 0.25-1 | 0.5 | 1 |
| Amphotericin B | 0.25-2 | 1 | 2 |
| A. niger (20) | |||
| Ketoconazole | 0.5-8 | 2 | 4 |
| Itraconazole | 0.5-2 | 0.5 | 2 |
| Voriconazole | 0.12-0.5 | 0.25 | 0.5 |
| Amphotericin B | 0.25-1 | 0.25 | 1 |
| A. terreus (12) | |||
| Ketoconazole | 0.5-2 | 1 | 1 |
| Itraconazole | 0.06-2 | 0.5 | 1 |
| Voriconazole | 0.25-1 | 0.5 | 1 |
| Amphotericin B | 0.06-1 | 1 | 1 |
| Aspergillus species (96) | |||
| Ketoconazole | 0.5-2 | 1 | 1 |
| Itraconazole | 0.06-8 | 0.5 | 1 |
| Voriconazole | 0.25-1 | 0.5 | 1 |
| Amphotericin B | 0.06-2 | 0.5 | 1 |
Data are from a program carried out in 2003 by the SMART study group. They were obtained by the broth microdilution method.
50% and 90%, MICs at which 50 and 90% of isolates are inhibited, respectively.
These isolates were identified to the species level by conventional methods (15, 34). The identifications of C. neoformans and Candida species were confirmed by using the API 20C and Vitek YBC systems (bioMerieux Vitek, St. Louis, Mo.). Isolates were stored at −70°C in Trypticase soy broth supplemented with 15% glycerol until they were tested. These isolates were passaged twice on potato dextrose agar (Difco Laboratories, Detroit, Mich.) at 35°C prior to testing.
Antifungal agents.
Standard powders of the following antifungal agents were obtained from their respective manufacturers: fluconazole and voriconazole (Pfizer, Inc., New York, N.Y.), itraconazole (Janssen), flucytosine and ketoconazole (Sigma Chemical Co., St. Louis, Mo.), and amphotericin B (Bristol-Myers Squibb, Princeton, N.J.).
Antifungal susceptibility testing.
Antifungal susceptibility testing of the Candida species and C. neoformans isolates was performed by the reference broth microdilution method as outlined in the National Committee for Clinical Laboratory Standards (NCCLS) document M27-A2 (26). The tested concentrations of these agents ranged from 0.03 to 64 μg/ml. Final dilutions were made in RPMI 1640 medium (Sigma) buffered to pH 7.0 with 0.165 M 3-(N-morpholino)propanesulfonic acid (MOPS) buffer (Sigma).
For susceptibility testing of Aspergillus species isolates, a broth microdilution method was used by following the NCCLS reference method (27). RPMI 1640 medium (with l-glutamine and without bicarbonate) with 0.165 M MOPS and 10 M NaOH was used, and the pH of the medium was 7.0. The final inoculum was 0.4 × 104 to 5 × 104 CFU/ml. C. parapsilosis ATCC 22019 and C. krusei ATCC 6258 were used as the control strains for susceptibility testing of Candida species isolates, and C. neoformans ATCC 90112 was used as the control for the C. neoformans isolates (26). For susceptibility testing of Aspergillus species isolates, A. fumigatus ATCC 204305 and A. flavus ATCC 204304 were used as reference strains (27).
Following incubation at 35°C for 48 h for Candida and Aspergillus species and for 72 h for C. neoformans, the MICs for these organisms were determined according to the criteria provided by the NCCLS (26, 27).
For amphotericin B, itraconazole, and voriconazole, the MIC was defined as the lowest drug concentration that prevented any discernible growth (numerical score of 0). For ketoconazole, fluconazole, and flucytosine, the MIC was defined as the lowest concentration in which a prominent reduction in growth, or an approximately 50% reduction compared to the growth of the control (numerical score of 2), was observed (26, 27). The MIC interpretive criteria for the susceptibilities of Candida species isolates to fluconazole, flucytosine, and itraconazole were those published by the NCCLS (26). Voriconazole, ketoconazole, and amphotericin B have not been assigned interpretive breakpoints. For purposes of comparison, a susceptibility breakpoint of ≤1 μg/ml was employed for both voriconazole and amphotericin B (13, 26). Interpretive MIC breakpoints for C. neoformans and Aspergillus isolates for six of the agents tested have not been defined by the NCCLS (26, 27).
Statistical analysis.
Data were analyzed by the chi-square test. A P value of ≤0.05 was considered statistically significant.
RESULTS
The MICs of the antifungal agents tested for the control strains were consistent within twofold dilutions. The ranges of the MICs for the control strains obtained in this study for the agents tested were within the ranges provided by the NCCLS.
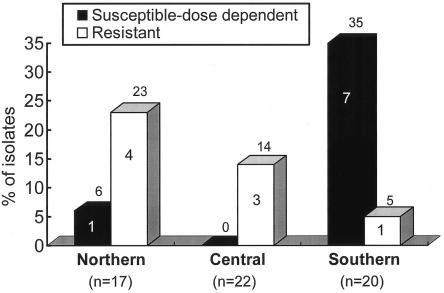
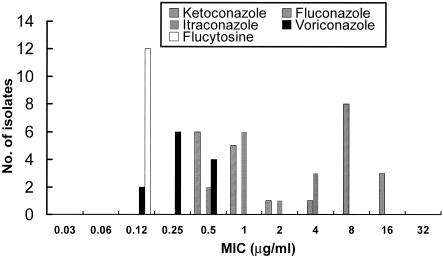
The MICs of six antifungal agents for the isolates of Candida species and C. neoformans are shown in Table 1. All isolates of Candida species other than C. glabrata were susceptible to fluconazole. All 59 C. glabrata isolates were dose-dependently susceptible (9 isolates) or resistant (50 isolates) to itraconazole, and 16 isolates (27%) were dose-dependently susceptible (8 isolates) or resistant (8 isolates) to fluconazole. Forty percent of C. glabrata isolates from the southern region of Taiwan were not susceptible to fluconazole, compared with 14% from the central region (P = 0.22). There were no statistically significant differences among the rates of dose-dependently susceptible or resistant C. glabrata isolates in the three regions (Fig. 1). The proportions of isolates for which amphotericin B MICs were ≥2 μg/ml were 11% (23 isolates) for C. albicans, 3.0% (2 isolates) for C. tropicalis, 20% (12 isolates) for C. glabrata, and 0% for both C. parapsilosis and C. lusitaniae. Among the 12 C. glabrata isolates for which amphotericin B MICs were 2 μg/ml, 4 (33%) were dose-dependently susceptible to fluconazole (MICs, 16 μg/ml), 10 (83%) were resistant to itraconazole, 11 (92%) were inhibited by ketoconazole at 1 μg/ml, and all were susceptible to 5-flucytosine and voriconazole (Fig. 2).
FIG. 1.
Geographical distribution in Taiwan of C. glabrata isolates according to their susceptibilities to fluconazole. The numbers on the bars represent the numbers of specified isolates; the numbers above the bars represent the percentages of specified isolates relative to the numbers tested.
FIG. 2.
MIC distribution of five antifungal agents against 12 C. glabrata isolates for which amphotericin B MICs were 2 μg/ml.
For all Candida species isolates, voriconazole MICs were ≤0.5 μg/ml, except one (1.7%) C. glabrata isolate for which the MIC was 2 μg/ml and four (6.8%) C. glabrata isolates for which the MIC was 4 μg/ml. All isolates of Candida species were susceptible to 5-flucytosine except for one isolate of C. glabrata. Ketoconazole exhibited in vitro activities against C. glabrata isolates four- to eightfold lower than those against other Candida species.
MICs of fluconazole, itraconazole, ketoconazole, 5-flucytosine, voriconazole, and amphotericin B for the three C. guilliermondii isolates were 4, 0.12 to 0.5, 0.06 to 0.5, 0.12 to 0.25, 0.12 to 0.25, and 0.5 μg/ml, respectively. MICs of ketoconazole, itraconazole, 5-flucytosine, amphotericin B, and voriconazole for the three C. krusei isolates were 0.5 to 1, 0.5, 16 to 64, 2, and 0.25 to 0.5 μg/ml, respectively.
For three (4%) of the C. neoformans isolates, the fluconazole MICs were ≥16 μg/ml, and all but two isolates were inhibited by 1 μg of amphotericin B/ml. Voriconazole, ketoconazole, and 5-flucytosine were considerably more active than fluconazole against C. neoformans isolates.
The in vitro activities of ketoconazole, itraconazole, voriconazole, and amphotericin B against Aspergillus species isolates are shown in Table 2. All of the Aspergillus isolates tested were inhibited by ≤8 μg of ketoconazole/ml, ≤8 μg of itraconazole/ml, and ≤1 μg of voriconazole/ml. For two isolates of A. fumigatus (5%) and two isolates of A. flavus (8%), itraconazole MICs were 8 μg/ml, and these were all inhibited by ≤1 μg of voriconazole/ml. All of the 96 isolates tested were inhibited by ≤2 μg of amphotericin B/ml. Among the four species tested, A. flavus was the least susceptible to amphotericin B; the MICs at which 50 and 90% of A. flavus isolates were inhibited were twofold greater than those for A. fumigatus and Aspergillus niger.
DISCUSSION
This is a nationwide survey of the in vitro activities of agents against clinical isolates of seven Candida species, C. neoformans, and four Aspergillus species collected recently (2003) in Taiwan. C. albicans was the most common species causing candidemia, followed by C. tropicalis, C. glabrata, and C. parapsilosis. This finding was in agreement with several previous reports in Taiwan (8, 14, 17, 37, 38). Fluconazole retained potent in vitro activities against recent isolates of Candida species other than C. glabrata and C. krusei despite a dramatic increase in fluconazole use in the hospitals where these isolates were collected (data not shown). This observation supports the results of several previous studies, in Taiwan and in other countries, indicating the existence of stable or increasing susceptibilities of Candida blood isolates to fluconazole despite the increasing use of fluconazole (8, 9, 15, 17, 22, 30, 31), although two nationwide surveillance reports in Taiwan in 1999 showed that about 12% of C. parapsilosis and 15% of C. tropicalis isolates were resistant to fluconazole (37, 38). The reason for the difference in the fluconazole susceptibilities of C. glabrata isolates in different geographic regions of Taiwan is not clear. The small numbers of C. glabrata isolates collected in the three regions might partly contribute to these discrepancies.
The emergence of decreased susceptibility to amphotericin B in some Candida species is important, particularly among several C. glabrata isolates which were also not susceptible to fluconazole. Previous studies demonstrated amphotericin B MICs ranging from 0.016 to 12 μg/ml, with 0.4 to 7% of Candida isolates causing bloodstream infections exhibiting potential resistance at concentrations of ≥2 μg/ml (14, 18). These isolates included C. glabrata, C. krusei, Candida famata, C. tropicalis, and C. lusitaniae isolates, with amphotericin B MICs of up to 12 μg/ml reported for C. glabrata isolates (14, 18, 38). In the present study from Taiwan, the good in vitro activities of amphotericin B against C. lusitaniae isolates and the decreased susceptibilities of C. glabrata and C. krusei isolates to amphotericin B were consistent with results from previous studies of these organisms from France and the United States, respectively (12, 14, 18). Furthermore, our results suggested a trend toward a decreased potency of amphotericin B, which was found in 11% of C. albicans isolates. This finding was rarely described in previous reports (14, 17, 18, 29-31, 38).
The recent emergence of C. neoformans isolates with decreased susceptibilities to fluconazole and amphotericin B is of great concern because these agents are recommended as the initial drugs of choice for the treatment of cryptococcosis (2, 32). Previous studies suggest that a more favorable clinical outcome after fluconazole maintenance therapy for cryptococcosis can be predicted when the fluconazole MIC is <16 μg/ml (2, 32). All but 4% of our C. neoformans isolates were inhibited by fluconazole at concentrations of <16 μg/ml, indicating that fluconazole remains the drug of choice for empirical therapy of cryptococcosis. However, the emergence of C. neoformans isolates with reduced susceptibility to fluconazole is alarming. Routine susceptibility testing of C. neoformans isolates for guidance during initial antifungal therapy is warranted.
Susceptibilities of Aspergillus species to antifungal agents were not previously reported in other national surveys in Taiwan. In the present survey, isolates of A. flavus and A. fumigatus (MICs, 2 μg/ml) with reduced susceptibilities to amphotericin B were found. Amphotericin B MICs of up to 4 μg/ml for A. flavus and 16 μg/ml for Aspergillus terreus were found in a previous study from Spain (13). In vivo resistance to amphotericin B has been reported for A. fumigatus, A. flavus, and A. terreus (1, 3, 20, 24, 36). There are very few data available regarding correlations between MIC and outcome of treatment with amphotericin B for infections caused by Aspergillus species. A study by Lass-Florl et al. demonstrated that amphotericin B MICs of ≥2 μg/ml were associated with treatment failure among patients with invasive aspergillosis (20). Mosquera et al. demonstrated a lack of correlation between susceptibility to amphotericin B in vitro and clinical outcome for A. flavus and A. fumigatus infections in vivo by using different susceptibility testing methods, including the NCCLS M-38A methods (24). Data on the clinical evaluation of infections caused by Aspergillus isolates in Taiwanese patients who were treated with amphotericin B are lacking, particularly for Aspergillus isolates for which the MICs were 2 μg/ml.
Another important issue is the azole resistance of Aspergillus species (1, 10, 13, 23). Aspergillus species with itraconazole resistance have presumptively been defined as isolates for which the MIC is ≥8 μg/ml (13, 23). According to these criteria, resistance to itraconazole was found in 4.2% (4 of 96) of our isolates of Aspergillus species, including two A. fumigatus isolates and two A. flavus isolates. Similar rates of resistance were found among isolates included in previous studies (13, 23, 25). Balajee et al. further demonstrated the presence of a genetically unique and poorly sporulating variant of A. fumigatus isolates which were associated with decreased susceptibilities to several antifungals, including itraconazole (4). Voriconazole had potent in vitro activity against these isolates with reduced susceptibilities to amphotericin B and itraconazole, indicating its suitability as an option for the initial treatment of aspergillosis.
In conclusion, this study identified several types of emerging antifungal resistance among clinical isolates of Candida species, C. neoformans, and Aspergillus species in Taiwan. Further studies are needed to investigate better the correlation of these types of resistance with the patterns of usage of antifungal agents, as well as with clinical outcome, in Taiwan and in other countries. Periodic surveillance is needed to monitor trends of resistance to azole and amphotericin B among these commonly encountered fungi in hospitals. The potent in vitro activity of voriconazole in this study indicates that this agent now has a clinically important role in the treatment of mycosis in settings with increasing incidences of antifungal resistance.
Acknowledgments
All authors of this study are members of SMART.
REFERENCES
- 1.Abraham, O. C., E. K. Manavathu, J. L. Cutright, and P. H. Chandrasekar. 1999. In vitro susceptibilities of Aspergillus species to voriconazole, itraconazole, and amphotericin B. Diagn. Microbiol. Infect. Dis. 33:7-11. [DOI] [PubMed] [Google Scholar]
- 2.Aller, A. I., E. Martin-Mazuelos, F. Lozano, J. Gomez-Mateos, L. Steele-Moore, W. J. Holloway, M. J. Gutiérrez, F. J. Recio, and A. Espinel-Ingroff. 2000. Correlation of fluconazole MICs with clinical outcome in cryptococcal infection. Antimicrob. Agents Chemother. 44:1544-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arikan, S., M. Lozano-Chiu, V. Paetznick, S. Nangia, and J. H. Rex. 1999. Microdilution susceptibility testing of amphotericin B, itraconazole, and voriconazole against clinical isolates of Aspergillus and Fusarium species. J. Clin. Microbiol. 37:3946-3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balajee, S. A., M. Weaver, A. Imhof, J. Gribskov, and K. A. Marr. 2004. Aspergillus fumigatus variant with decreased susceptibility to multiple antifungals. Antimicrob. Agents Chemother. 48:1197-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berrouane, Y. F., L. A. Herwaldt, and M. A. Pfaller. 1999. Trends in antifungal use and epidemiology of nosocomial yeast infections in a university hospital. J. Clin. Microbiol. 37:531-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chandrasekar, P. H., J. L. Cutright, and E. K. Manavathu. 2001. Aspergillus: rising frequency of clinical isolation and continued susceptibility to antifungal agents, 1994-1999. Diagn. Microbiol. Infect. Dis. 41:211-214. [DOI] [PubMed] [Google Scholar]
- 7.Chen, K. Y., S. C. Ko, P. R. Hsueh, K. T. Luh, and P. C. Yang. 2001. Pulmonary fungal infection: emphasis on microbiological spectra, patient outcome, and prognostic factors. Chest 120:177-184. [DOI] [PubMed] [Google Scholar]
- 8.Chen, Y. C., S. C. Chang, K. T. Luh, and W. C. Hsieh. 2003. Stable susceptibility of Candida blood isolates to fluconazole despite increasing use during the past 10 years. J. Antimicrob. Chemother. 52:71-77. [DOI] [PubMed] [Google Scholar]
- 9.Cheng, M. F., K. W. Yu, R. B. Tang, Y. H. Fan, Y. L. Yang, K. S. Hsieh, M. Ho, and H. J. Lo. 2004. Distribution and antifungal susceptibility of Candida species causing candidemia from 1996 to 1999. Diagn. Microbiol. Infect. Dis. 48:33-37. [DOI] [PubMed] [Google Scholar]
- 10.Dannaoui, E., J. Meletiadis, A. M. Tortorano, F. Symoens, N. Nolard, M. A. Viviani, M. A. Piens, B. Lebeau, P. E. Verweij, R. Grillot, and EBGA Network. 2004. Susceptibility testing of sequential isolates of Aspergillus fumigatus recovered from treated patients. J. Med. Microbiol. 53:129-134. [DOI] [PubMed] [Google Scholar]
- 11.Denning, D. W., K. Venkateswarlu, K. L. Oakley, M. J. Anderson, N. J. Manning, D. A. Stevens, D. W. Warnock, and S. L. Kelly. 1997. Itraconazole resistance in Aspergillus fumigatus. Antimicrob. Agents Chemother. 41:1364-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Favel, A., A. Michl-Nguyen, A. Datry, S. Challier, F. Leclerc, C. Chastin, K. Fallague, and P. Regli. 2004. Susceptibility of clinical isolates of Candida lusitaniae to five systemic antifungal agents. J. Antimicrob. Chemother. 53:526-529. [DOI] [PubMed] [Google Scholar]
- 13.Gomez-Lopez, A., G. Garcia-Effron, E. Mellado, A. Monzon, J. L. Rodriguez-Tudela, and M. Cuenca-Estrella. 2003. In vitro activities of three licensed antifungal agents against Spanish clinical isolates of Aspergillus spp. Antimicrob. Agents Chemother. 47:3085-3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hajjeh, R. A., A. N. Sofair, L. H. Harrison, G. M. Lyon, B. A. Arthington-Skaggs, S. A. Mirza, M. Phelan, J. Morgan, W. Lee-Yang, M. A. Ciblak, L. E. Benjamin, L. T. Sanza, S. Huie, S. F. Yeo, M. E. Brandt, and D. W. Warnock. 2004. Incidence of bloodstream infections due to Candida species and in vitro susceptibilities of isolates collected from 1998 to 2000 in a population-based active surveillance program. J. Clin. Microbiol. 42:1519-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hazen, K. C., and S. A. Howell. 2003. Candida, Cryptococcus, and other yeasts of medical importance, p. 1693-1711. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. A. Pfaller, and R. H. Yolken (ed.), Manual of clinical microbiology, 8th ed., vol. 2. American Society for Microbiology, Washington, D.C.
- 16.Hospenthal, D. R., C. K. Murray, and M. G. Rinaldi. 2004. The role of antifungal susceptibility testing in the therapy of candidiasis. Diagn. Microbiol. Infect. Dis. 48:153-160. [DOI] [PubMed] [Google Scholar]
- 17.Hsueh, P. R., L. J. Teng, P. C. Yang, S. W. Ho, and K. T. Luh. 2002. Emergence of nosocomial candidemia at a teaching hospital in Taiwan from 1981 to 2000: increased susceptibility of Candida species to fluconazole. Microb. Drug Resist. 8:311-319. [DOI] [PubMed] [Google Scholar]
- 18.Kao, A. S., M. E. Brandt, W. R. Pruitt, L. A. Conn, B. A. Perkins, D. S. Stephens, W. S. Baughman, A. L. Reingold, G. A. Rothrock, M. A. Pfaller, R. W. Pinner, and R. A. Hajjeh. 1999. The epidemiology of candidemia in two U.S. cities: results of a population-based active surveillance. Clin. Infect. Dis. 29:1164-1170. [DOI] [PubMed] [Google Scholar]
- 19.Ko, S. C., K. Y. Chen, P. R. Hsueh, K. T. Luh, and P. C. Yang. 2000. Fungal empyema thoracis: an emerging clinical entity. Chest 117:1672-1678. [DOI] [PubMed] [Google Scholar]
- 20.Lass-Florl, C., G. Kofler, G. Kropshofer, J. Hermans, A. Kreczy, M. P. Dierich, and D. Niederwieser. 1998. In-vitro testing of susceptibility to amphotericin B is a reliable predictor of clinical outcome in invasive aspergillosis. J. Antimicrob. Chemother. 42:497-502. [DOI] [PubMed] [Google Scholar]
- 21.Marco, F., M. A. Pfaller, S. Messer, and R. N. Jones. 1998. In vitro activities of voriconazole (UK-109,496) and four other antifungal agents against 394 clinical isolates of Candida spp. Antimicrob. Agents Chemother. 42:161-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meis, J., M. Petrou, J. Bille, D. Ellis, D. Gibbs, et al. 2000. A global evaluation of the susceptibility of Candida species to fluconazole by disk diffusion. Diagn. Microbiol. Infect. Dis. 36:215-223. [DOI] [PubMed] [Google Scholar]
- 23.Moore, C. B., N. Sayers, J. Mosquera, J. Slaven, and D. W. Denning. 2000. Antifungal drug resistance in Aspergillus. J. Infect. 41:203-220. [DOI] [PubMed] [Google Scholar]
- 24.Mosquera, J., P. A. Warn, J. Morrissey, C. B. Moore, C. Gil-Lamaignere, and D. W. Denning. 2001. Susceptibility testing of Aspergillus flavus: inoculum dependence with itraconazole and lack of correlation between susceptibility to amphotericin B in vitro and outcome in vivo. Antimicrob. Agents Chemother. 45:1456-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mosquera, J., and D. W. Denning. 2002. Azole cross-resistance in Aspergillus fumigatus. Antimicrob. Agents Chemother. 46:556-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth microdilution antifungal susceptibility testing of yeasts. Approved standard M27-A2. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 27.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard M38-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 28.Pappas, P. G., J. H. Rex, J. D. Sobel, S. G. Filler, W. E. Dismukes, T. J. Walsh, and J. E. Edwards. 2004. Guidelines for treatment of candidiasis. Clin. Infect. Dis. 38:161-189. [DOI] [PubMed] [Google Scholar]
- 29.Pfaller, M. A., D. J. Diekema, S. A. Messer, L. Boyken, R. J. Hollis, R. N. Jones, and the International Fungal Surveillance Participant Group. 2003. In vitro activities of voriconazole, posaconazole, and four licensed systemic antifungal agents against Candida species infrequently isolated from blood. J. Clin. Microbiol. 41:78-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfaller, M. A., D. J. Diekema, S. A. Messer, L. Boyken, R. J. Hollis, and R. N. Jones. 2004. In vitro susceptibilities of rare Candida bloodstream isolates to ravuconazole and three comparative antifungal agents. Diagn. Microbiol. Infect. Dis. 48:101-105. [DOI] [PubMed] [Google Scholar]
- 31.Pfaller, M. A., S. A. Messer, R. J. Hollis, R. N. Jones, and D. J. Diekema. 2002. In vitro activities of ravuconazole and voriconazole compared with those of four approved systemic antifungal agents against 6,970 clinical isolates of Candida spp. Antimicrob. Agents Chemother. 46:1723-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saag, M. S., R. J. Graybill, R. A. Larsen, P. G. Pappas, J. R. Perfect, W. G. Powderly, J. D. Sobel, and W. E. Dismukes. 2000. Practice guidelines for the management of cryptococcal disease. Clin. Infect. Dis. 30:710-718. [DOI] [PubMed] [Google Scholar]
- 33.Sheehan, D. J., C. A. Hitchcock, and C. M. Sibley. 1999. Current and emerging azole antifungal agents. Clin. Microbiol. Rev. 12:40-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sigler, L., and P. E. Verweij. 2003. Aspergillus, Fusarium, and other opportunistic moniliaceous fungi, p. 1726-1760. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. A. Pfaller, and R. H. Yolken (ed.), Manual of clinical microbiology, 8th ed., vol. 2. American Society for Microbiology, Washington, D.C.
- 35.Stevens, D. A., V. L. Kan, M. A. Judson, V. A. Morrison, S. Dummer, D. W. Denning, J. E. Bennett, T. J. Walsh, T. F. Patterson, and G. A. Pankey. 2000. Practice guidelines for diseases caused by Aspergillus. Clin. Infect. Dis. 30:696-709. [DOI] [PubMed] [Google Scholar]
- 36.Verweij, P. E., D. T. A. Te Dorsthorst, A. J. M. M. Rijs, H. G. De Vries-Hospers, and J. F. G. M. Meis. 2002. Nationwide survey of in vitro activities of itraconazole and voriconazole against clinical Aspergillus fumigatus isolates cultured between 1945 and 1998. J. Clin. Microbiol. 40:2648-2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang, Y. L., H. H. Cheng, Y. A. Ho, C. F. Hsiao, and H. J. Lo. 2003. Fluconazole resistance rate of Candida species from different regions and hospital types in Taiwan. J. Microbiol. Immunol. Infect. 36:187-191. [PubMed] [Google Scholar]
- 38.Yang, Y. L., Y. A. Ho, H. H. Cheng, M. Ho, and H. J. Lo. 2004. Susceptibilities of Candida species to amphotericin B and fluconazole: the emergence of fluconazole resistance in Candida tropicalis. Infect. Control Hosp. Epidemiol. 25:60-64. [DOI] [PubMed] [Google Scholar]