Abstract
Aim:
To investigate the correlation between the status of interstitial cells of Cajal (ICC) in ureteropelvic junction (UPJ) and the resected ureteric margin and the postoperative outcome of Anderson-Hynes pyeloplasty in UPJ obstruction (UPJO) and to compare the ICC in the UPJ and the resected margin of the normal ureter.
Materials and Methods:
An observational study was conducted over a period of 2 years at the Department of Pediatric Surgery at Niloufer Institute of Women and Child Health. Children with intrinsic UPJO who underwent Anderson-Hynes dismembered pyeloplasty were included in the study. Six months postoperatively, the patients were divided into two groups based on diuretic isotopic renogram using technetium-99m-labeled diethylene triaminepentaacetic acid. Group 1 comprised patients with good surgical outcome. Group 2 comprised patients with a poor outcome. The histologic specimens were evaluated for ICC, and the immunohistochemical findings were correlated with the outcome.
Results:
Twenty-five patients were included in this study (19 male and 6 female). Seventy-six percent of patients were under the age of 1 year. Group 1 had 23 cases and Group 2 had 2 cases. Out of the two patients with a poor outcome, one had negative grading at the UPJ and one had positive grading. Both these patients had a negative grading at the lower resected margins. More number of patients (24%) had +++ grading at the lower resected margin when compared to the UPJ (8%).
Conclusion:
This is the first study which correlates the status of ICC in UPJ with the outcome of pyeloplasty in pediatric patients. Both the cases with bad outcome had no ICC at the lower margin of the resected specimen and one case had no ICC at the UPJ. There is a statistically significant difference (P = 0.001) in the number of ICC at the UPJ and the resected margin.
KEYWORDS: Anderson-Hynes pyeloplasty, immunohistochemistry, interstitial cells of Cajal, ureteropelvic junction, ureteropelvic junction obstruction
INTRODUCTION
Ureteropelvic junction obstruction (UPJO) is the most common congenital urinary tract obstruction with an incidence of 1 in 1000 newborns.[1] The underlying mechanism of UPJO remains unclear.
The various theories proposed as the cause of UPJO include an increase in the collagen between the muscle bundles and elastin in the adventitia,[2] infolding of ureteral mucosa and crossing vessels. Murakumo et al. postulated a combined neurogenic and myogenic theory for UPJO.[3] A decrease in smooth muscle cells at the UPJ, abnormal muscle orientation, and collagen deposition, as well as a reduction in Cajal cells,[4] have been suggested to play a role in the pathogenesis of congenital UPJO. Newer molecular techniques and immunohistochemical analysis have helped to identify abnormalities of ureteral innervation and abnormalities of microstructure.
Interstitial cells of Cajal (ICC) were first described as “neuron-like cells” at the motor neuron endings in the gastrointestinal system. Thuneberg suggested that these cells had pacemaker activity in the intestine. These cells have gap junctions with smooth muscle cells which give way to nerve terminals; these cells are called pacemaker cells.[5] Although the embryologic origin is unclear, it has been shown that interstitial cells originate from the mesenchymal cells.[6] ICC express c-kit (CD117), a tyrosine kinase. The c-kit is a membrane receptor protein, a growth factor, and a proto-oncogene. It consists of an external ligand-binding component and a cytoplasmic tyrosine kinase component.[7]
The renal calyces, renal pelvis, UPJ, ureters (proximal to distal), ureteral orifices, fundus, and corpus of the bladder in porcines were evaluated by Metzger et al.[8] C-kit expression was examined at the level of mRNA in ureteral tissue and the highest expression of c-kit mRNA was determined to be in the UPJ.[9] Solari et al.[4] were the first to observe C-kit-positive ICC in the normal human UPJ. ICC in the urinary tract are involved in conducting electrical impulses from atypical smooth muscle cells to typical smooth muscle cells (t-SMCs). The result of this is a synchronous contraction of t-SMCs propagated distally to the bladder.
Pyeloplasty is the standard treatment for UPJO, with a high success rate. However, some patients show a postoperative deterioration in the renal function and drainage of the renal pelvis. The deterioration may be due to a technical element or an intrinsic element. In the present study, we aim to find a correlation between the expression of ICC in the obstructed pelviureteric segment and the lower resected margin of the ureter and the outcome of pyeloplasty in cases of UPJO.
Materials and Methods
An observational study was conducted over a period of 2 years. Children who presented with intrinsic UPJO to the Department of Pediatric Surgery, Niloufer Institute of Women and Child Health, were included in this study. Patients with extrinsic or secondary UPJO were excluded from this study. All the included patients underwent Anderson-Hynes dismembered pyeloplasty.
Histopathological specimens obtained from the Anderson-Hynes dismembered pyeloplasty were evaluated with immunohistochemical staining with CD117 to look for the expression of c-kit by the ICC. The DAKO protocol was used for the immunohistochemistry. The samples were incubated at 56°C and then dewaxed and rehydrated. Antigen unmasking was done with ethylenediaminetetraacetic acid buffer in 1:10 concentration, moist heat (98°C–99°C), and cooling to room temperature. To block endogenous peroxidase, Large Volume Hydrogen Peroxide Block was used. Blocking nonspecific reactions was made with Large Volume Ultra V Block. CD117 primary antibody was used at a concentration of 1:600. After visualizing by 3,3-diaminobenzidine, a hematoxylin counterstain was performed.
Ten consecutive high power fields (HPFs) were examined. They were graded according to the number of c-kit positive cells seen in ten consecutive HPFs. The mast cells were used as internal controls [Figure 1]. 0–1 cells were graded as negative, 2–5 cells as +, 6–10 cells as ++, and more than 11 cells as +++[10] [Figure 2 and Table 1]. Location of cells in subepithelial, smooth muscle, and adventitial sites was selected.
Figure 1.
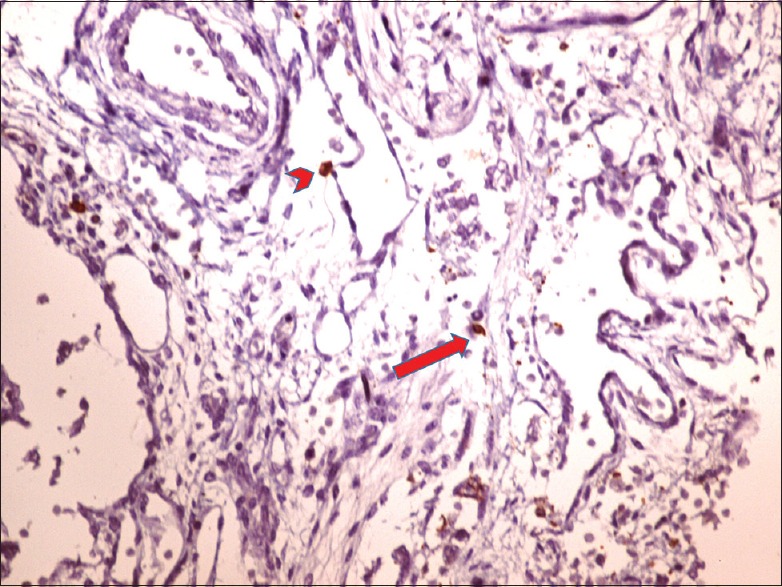
Normal ureteropelvic junction obstruction Arrowhead: mast cell, Arrow: spindle-shaped interstitial cell of Cajal
Figure 2.
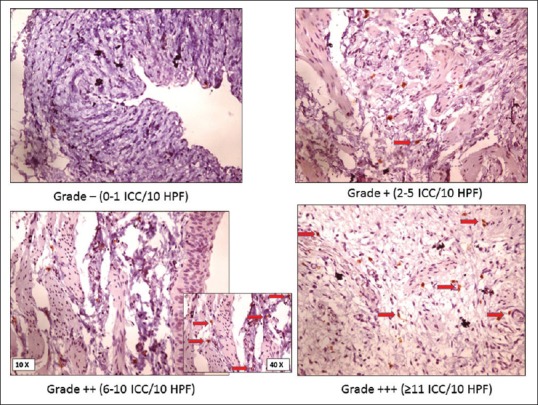
Abnormal ureteropelvic junction
Table 1.
Grading of Interstitial cells of Cajal

Twenty-five patients with intrinsic UPJO were included in this study. There were 19 male and 6 female patients. The age of the patients ranged from 15 days to 4 years [Table 2]. The mean age was 12.2 months. Thirteen patients had right-sided and 12 had left-sided UPJO. All patients underwent Anderson-Hynes dismembered pyeloplasty, and the excised UPJ was sent for histopathologic examination.
Table 2.
Age distribution (n=25)

Patients were followed up for a period ranging from 6 months to 2 years after surgery. Diuretic isotopic renogram using technetium–99m-labeled diethylenetriaminepentaacetic acid (DTPA) was done 6 months after pyeloplasty. Patients were divided into two groups. Group 1 comprised patients with good surgical outcome. This included patients who had an improvement of drainage and renal function. Group 2 comprised patients with a poor outcome, that is, cases which had no improvement or worsening drainage and renal function. Immunohistochemical findings in each group were correlated with the outcome.
RESULTS
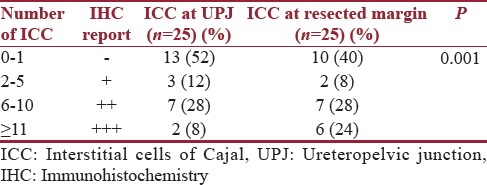
Immunohistochemical evaluation for the presence of ICC was done on all 25 specimens. Nearly 13 specimens had no ICC in the UPJ specimens examined and were reported as negative. Three specimens had 2–5 ICC in ten HPFs and were reported as +. Seven specimens had 6–10 ICC in ten HPFs and were reported as ++. Two specimens had more than 10 ICC in ten HPFs and were reported as +++ [Table 3].
Table 3.
Grading of Interstitial cells of Cajal at the ureteropelvic junction (n=25)

Two specimens had no ICC in the sections from the lower resected margin of the specimen and were reported as negative. Two specimens had 2–5 ICC in ten HPFs and were reported as +. Seven specimens had 6–10 ICC in ten HPFs and were reported as ++. Six specimens had more than 10 ICC in ten HPFs and were reported as +++ [Table 4].
Table 4.
Grading of Interstitial cells of Cajal at the lower resected margin of specimen (n=25)

Patients underwent DTPA scan in the postoperative period and were divided into two groups. Twenty-three patients were free of obstruction and had improved renal function and were classified as Group 1. Two patients did not improve and they were placed in Group 2. The mean age of the patients in Group 1 was 12.7 months and the mean age of patients in Group 2 was 7 months. Both the patients in Group 2 were male. One patient had right-sided and one patient had left-sided UPJO.
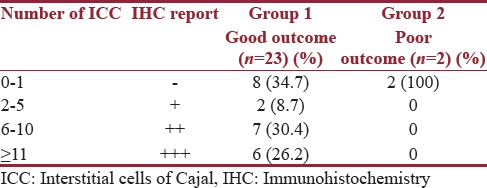
The status of the ICC at the UPJ and the lower resected margin of the specimen were correlated with the outcome. Out of the two patients with a poor outcome, one had negative grading at the UPJ and one had + grading [Table 5]. Both these patients had a negative grading at the lower resected margins [Table 6].
Table 5.
Correlation of Interstitial cells of Cajal at ureteropelvic junction with outcome
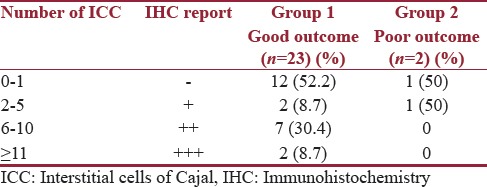
Table 6.
Correlation of Interstitial cells of Cajal at lower resected margin with outcome
Only 8% of specimens had +++ grading at the UPJ, whereas 24% of the specimens had +++ grading at the ureteric margin of the resected specimen. This difference was statistically significant (P =0.001) [Table 7].
Table 7.
Comparison of Interstitial cells of Cajal at ureteropelvic junction and the resected margin of the ureter
DISCUSSION
Pyeloplasty is a successful surgery for the treatment of UPJO. Occasionally, some patients have a poor postoperative outcome with poor drainage or deteriorating renal function necessitating redo surgery. One of the postulates of this study is that adequate ICC are required for a functioning UPJ.
It is well known that ICC are required to generate smooth muscle electrical slow waves.[11,12] As in gastrointestinal motility, ICC may play an important role in the propagation, coordination, and modulation of ureteropelvic peristalsis. Studies have examined ICC in the histopathology examination of UPJ segments. ICC were determined through CD117 tyrosine kinase receptor.
Many studies have compared the histopathological pattern and ICC of normal and obstructed UPJ segments. Yang et al. compared the expression of ICC in histological specimens from UPJ segments between patients with UPJO and controls with Wilms’ tumor. They reported that a reduction of the number of ICC may play an important role in the etiology and pathogenesis of UPJO.[13]
In the study done by Senol et al., ICC in the UPJ were graded as rare (0–3 cells/10 HPF), few (4–6 cells/10 HPF), and many (≥7 cells/HPFs). Nearly 68.4% of cases in their study were graded as rare which was similar to this study where 52% of cases were graded as negative.[14]
The study by Alper Eken used a similar grading system as Senol et al.[14] They reported 74.3% patients with few cells in ten HPFs. They concluded that in UPJO, a decrease in the number of ICC, as well as the changes in the morphologic structure of the ICC, indicates that these cells have a role in the pacemaker system and are associated with ureteral peristalsis.[15] Similar results were reported by Balikci et al.[16]
However, Apoznanski et al. reported no distributional difference in obstructed and unobstructed UPJ and that UPJO is not associated with anomalous distribution of c-kit-positive ICC.[17] Koleda et al.[18] reported that the number of ICC in obstructed UPJ segments was higher than in normal UPJ segments, concluding that the observed increase in ICC compensates for the effects of obstruction.
A study by Issi et al.[10] which has examined the role of the histopathological pattern of UPJO segments in surgical success in 52 adults. They found no differences in the quantity of collagen type 3, elastin, fibrosis, or ICC between the two groups (P > 0.05).
The present study is the first study which was done to find a correlation between ICC in the UPJ and surgical outcome in pediatric age group. Two patients had a poor outcome after pyeloplasty, they had no ICC at the ureteric margin of the resected specimen, and one patient had no ICC at the UPJ. There is a statistically significant difference (P = 0.001) in the number of ICC found at the UPJ and at the lower resected margin.
CONCLUSION
This is the first study which correlates the status of ICC in UPJ with the outcome of pyeloplasty in pediatric patients. At present, the extent of resection is decided depending on the visual impression of the narrow segment and internal mucosal folds by the surgeon. In the future, ICC may provide an objective method to review the adequacy of the extent of resection. It may be useful to review the specimens of the first surgery in cases undergoing redo pyeloplasty. Further larger studies are required to confirm the role of ICC in the pathogenesis of UPJO and prognosis after pyeloplasty.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Wang Y, Puri P, Hassan J, Miyakita H, Reen DJ. Abnormal innervation and altered nerve growth factor messenger ribonucleic acid expression in ureteropelvic junction obstruction. J Urol. 1995;154(2 Pt 2):679–83. doi: 10.1097/00005392-199508000-00091. [DOI] [PubMed] [Google Scholar]
- 2.Notley RG. Electron microscopy of the upper ureter and the pelvi-ureteric junction. Br J Urol. 1968;40:37–52. doi: 10.1111/j.1464-410x.1968.tb11811.x. [DOI] [PubMed] [Google Scholar]
- 3.Murakumo M, Nonomura K, Yamashita T, Ushiki T, Abe K, Koyanagi T. Structural changes of collagen components and diminution of nerves in congenital ureteropelvic junction obstruction. J Urol. 1997;157:1963–8. [PubMed] [Google Scholar]
- 4.Solari V, Piotrowska AP, Puri P. Altered expression of interstitial cells of Cajal in congenital ureteropelvic junction obstruction. J Urol. 2003;170(6 Pt 1):2420–2. doi: 10.1097/01.ju.0000097401.03293.f0. [DOI] [PubMed] [Google Scholar]
- 5.Thuneberg L. Interstitial cells of Cajal: Intestinal pacemaker cells? Adv Anat Embryol Cell Biol. 1982;71:1–130. [PubMed] [Google Scholar]
- 6.Young HM. Embryological origin of interstitial cells of Cajal. Microsc Res Tech. 1999;47:303–8. doi: 10.1002/(SICI)1097-0029(19991201)47:5<303::AID-JEMT1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 7.Maeda H, Yamagata A, Nishikawa S, Yoshinaga K, Kobayashi S, Nishi K, et al. Requirement of c-kit for development of intestinal pacemaker system. Development. 1992;116:369–75. doi: 10.1242/dev.116.2.369. [DOI] [PubMed] [Google Scholar]
- 8.Metzger R, Schuster T, Till H, Stehr M, Franke FE, Dietz HG. Cajal-like cells in the human upper urinary tract. J Urol. 2004;172:769–72. doi: 10.1097/01.ju.0000130571.15243.59. [DOI] [PubMed] [Google Scholar]
- 9.Metzger R, Neugebauer A, Rolle U, Böhlig L, Till H. C-Kit receptor (CD117) in the porcine urinary tract. Pediatr Surg Int. 2008;24:67–76. doi: 10.1007/s00383-007-2043-2. [DOI] [PubMed] [Google Scholar]
- 10.Issi O, Deliktas H, Gedik A, Ozekinci S, Bircan MK, Sahin H. Does the histopathologic pattern of the ureteropelvic junction affect the outcome of pyeloplasty. Urol J. 2015;12:2028–31. [PubMed] [Google Scholar]
- 11.Huizinga JD, Thuneberg L, Klüppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347–9. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- 12.Thomsen L, Robinson TL, Lee JC, Farraway LA, Hughes MJ, Andrews DW, et al. Interstitial cells of Cajal generate a rhythmic pacemaker current. Nat Med. 1998;4:848–51. doi: 10.1038/nm0798-848. [DOI] [PubMed] [Google Scholar]
- 13.Yang X, Zhang Y, Hu J. The expression of Cajal cells at the obstruction site of congenital pelviureteric junction obstruction and quantitative image analysis. J Pediatr Surg. 2009;44:2339–42. doi: 10.1016/j.jpedsurg.2009.07.061. [DOI] [PubMed] [Google Scholar]
- 14.Senol C, Onaran M, Gurocak S, Gonul II, Tan MO. Changes in Cajal cell density in ureteropelvic junction obstruction in children. J Pediatr Urol. 2016;12:89.e1–5. doi: 10.1016/j.jpurol.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 15.Eken A, Erdogan S, Kuyucu Y, Seydaoglu G, Polat S, Satar N. Immunohistochemical and electron microscopic examination of Cajal cells in ureteropelvic junction obstruction. Can Urol Assoc J. 2013;7:E311–6. doi: 10.5489/cuaj.11293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balikci O, Turunç T, Bal N, Çelik H, Özkardes H. Comparison of Cajal-like cells in pelvis and proximal ureter of kidney with and without hydronephrosis. Int Braz J Urol. 2015;41:1178–84. doi: 10.1590/S1677-5538.IBJU.2014.0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Apoznanski W, Koleda P, Wozniak Z, Rusiecki L, Szydelko T, Kalka D, et al. The distribution of interstitial cells of Cajal in congenital ureteropelvic junction obstruction. Int Urol Nephrol. 2013;45:607–12. doi: 10.1007/s11255-013-0454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koleda P, Apoznanski W, Wozniak Z, Rusiecki L, Szydelko T, Pilecki W, et al. Changes in interstitial cell of Cajal-like cells density in congenital ureteropelvic junction obstruction. Int Urol Nephrol. 2012;44:7–12. doi: 10.1007/s11255-011-9970-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


