Abstract
Leydig cell tumors (LCTs) are rare testicular tumors. Incidence is 1%–3% of all testicular neoplasms, bilateral in 10%. They are frequently hormonally active, leading to feminizing or virilizing syndromes. LCTs can be either pure or mixed with germ cell tumors or other sex cord-stromal tumors. Here, we are reporting a benign pure LCT in a 6-year-old boy presented with pseudopuberty.
KEYWORDS: Calretinin, pseudopuberty, testicular tumor
INTRODUCTION
Leydig cell tumors (LCTs) are rare testicular tumors arising from male gonadal interstitium[1] and most common type of testicular sex cord-stromal tumor.[2,3] Adult male between 20- and 60-year age group usually affected[4], prepubertal (most often between 5 and 10 years of age)[5] children are also affected in 20% of cases.[6] Testicular swelling, decreased libido (20%), and gynecomastia (15%) are common symptoms in adults, but in children only a few cases had been reported and that are associated with pseudopuberty.[2]
CASE REPORT
A 6-year-old boy presented with precocious puberty [Figure 1]. On isotope scan, bone age was >12 and <14 years. Ultrasound reveals a heterogeneous echogenic space-occupying lesions involving whole left testis with many micro- and macrocalcification and increased vascularity. The volume of left testis was 12 cc. Hormonal assay showed luteinizing hormone (LH) levels was <0.07 U; normal human chorionic gonadotropin level <1 mIU/ml; alpha-fetoprotein (AFP) level 0.97 IU/ml; serum testosterone 17.9 nmol/L; and serum cortisol, adrenocorticotropic hormone, and 17 OH progesterone level were 8.86 μ/dl, 37.9 pg/ml, and 31.56 ng/ml, respectively. All levels are within normal limits except testosterone levels are raised. Computed tomography abdomen was normal. The patient underwent orchiectomy. Grossly, testis with scrotum was 5.5 cm × 3.5 cm × 2.5 cm, testis was 4 cm × 3 cm × 2 cm, and attached spermatic cord was 5.5 cm in length. Cut section shows lobulated, yellow well-circumscribed mass [Figure 2]. On microscopy, sections show polygonal cells with abundant eosinophilic cytoplasm and prominent nucleoli arranged in sheets and nodular pattern [Figure 3]. Pleomorphism present at places. Immunohistochemical staining with calretinin done, showed positive staining [Figure 4]. Diagnosis of benign LCT was made from clinical, hormonal, pathological, and immunohistochemical study.
Figure 1.
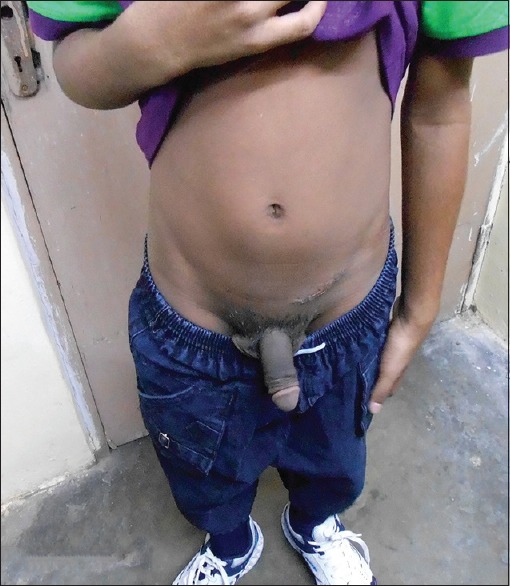
Boy with precocious puberty
Figure 2.
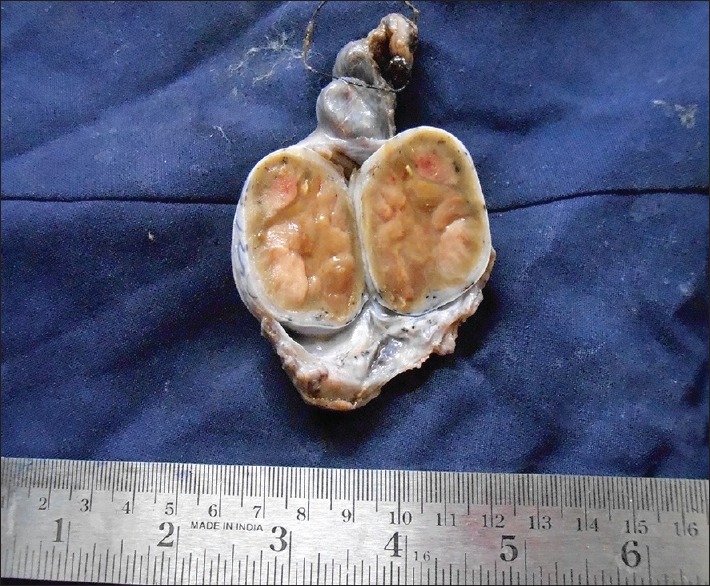
Gross picture of lobulated yellow well-circumscribed mass
Figure 3.
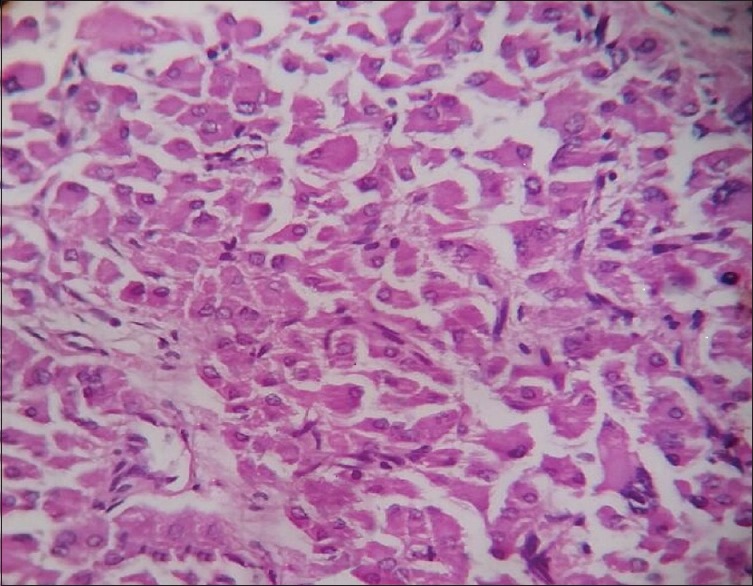
Polygonal cells with abundant eosinophilic cytoplasm, prominent nucleoli (×400)
Figure 4.
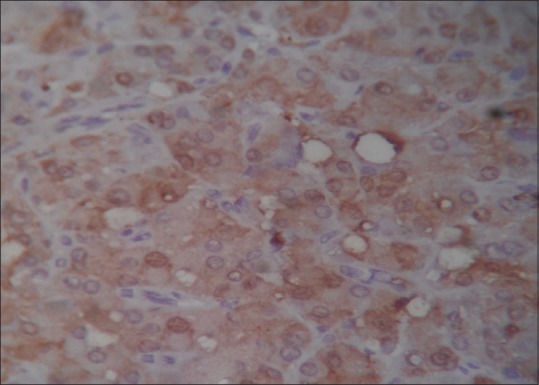
Immunohistochemistry positivity for calretinin (×400)
DISCUSSION
Leydig cells are named after Franz Leydig. They are interstitial cells located between the seminiferous tubules. They are involved in the development of secondary male characteristics and maintenance of spermatogenesis as they produce testosterone when stimulated by LH.[5] The origin of tumor is still unknown. The excess LH may lead to disruption of the hypothalamic-pituitary testicular axis leading to excessive stimulation of Leydig cells.[7] Rare cases are reported associated with cryptorchidism and Klinefelter Syndrome[5] although cryptorchidism is not considered as a risk factor.[5] Grossly, the tumor is small (3–5 cm), sharply circumscribed, solid mass within the testicle. The color of the tumor is characteristic ranging from brown to golden brown to gray-white (depending on the lipid or lipofuscin content of the tumor). Large size (>5 cm), infiltrative margins, areas of hemorrhage, and necrosis extending beyond testicular parenchyma suggest malignancy.[5] Microscopically, large polygonal tumor Cells, abundant eosinophilic granular cytoplasm with indistinct border is most commonly arranged in nests or sheets, separated by delicate fibrovascular septa. Nucleus is round to oval sometimes with a prominent nucleolus. Other cell types can be found small cells with scant, densely eosinophilic cytoplasm, and grooved nuclei and spindle cells. Some cells may have pale cytoplasm due to the presence of glycogen. Abundant lipofuscin (also called lipochrome) pigments which appear golden yellow to brown cytoplasmic (mainly perinuclear) granules on hematoxylin and eosin stain. It also can be demonstrated by periodic acid–Schiff stain with or without diastase. Reinke crystals are pale-staining, refractile, cylindrical, rectangular, or rhomboid intracytoplasmic and intranuclear structures (or inclusions) arranged in a linear fashion.[5] Presence of reinke crystals has no significance. Rare cases of LCT with adipocyte differentiation, ossification, calcification,[8] fibrous or myxoid stroma,[9] or microcystic growth pattern have also been reported.[5] LCT should be differentiated from Leydig cell hyperplasia, granular cell tumor, malakoplakia, the testicular nodules of adrenogenital syndrome,[5] and large cell calcifying Sertoli cell tumor which commonly has a retiform pattern.[5] Malignancy is suspected in the presence of a large tumor size (>5.7 cm), nuclear atypia, a mitotic count of >3/10 HPF, foci of necrosis, infiltrative margins, angiolymphatic invasion, an increased expression of Ki67/MIB-1 and p53, and DNA aneuploidy.[5] LCTs show diffuse cytoplasmic positivity for calretinin, inhibin, vimentin, Melan-A, and negative immunostaining with cytokeratin AFP.[5] In case of malignant and borderline cases (positive) of LCT, bcl-2 could be helpful.[8] Radical inguinal orchidectomy is the classical treatment, but now testis-sparing surgery by tumor enucleation with clear margins is considered treatment of choice, especially in young patients, to maintain their fertility.[9] For malignant LCTs, orchiectomy with retroperitoneal lymphadenectomy is required as metastasis most commonly involves retroperitoneal nodes other than liver (45%), lungs (40%), and bone (25%).[10] Survival time is reduced to approximately 3 years after surgery. That is in contrast for benign LCTs. Prognosis is good with subsidence of clinical and hormonal manifestations 90% of patients following orchidectomy.[11] Sönmez et al.[3] and Ilkhanizadeh et al.[6] reported LCT in adult male, whereas Singh and Chauhan et al.[1] reported a case in a 13-year-old boy, but none found this entity like us in a 6-year-boy.
CONCLUSION
LCTs are uncommon neoplasm arising from gonadal stroma. It can be early detected by self-examination of testicles. Early detection and management of these tumors is necessary to preserve the reproductive capacity with long-term follow-up for recurrence or metastasis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Singh N, Chauhan JS. Leydig cell tumors of the testis: A case report. Int J Enhanced Res Med Dent Care. 2015;2:12–4. [Google Scholar]
- 2.Rosai J. Ackerman's Surgical Pathology. Edinburgh: Mosby; 2004. pp. 1436–7. [Google Scholar]
- 3.Sternberg SS, Antonioli DA, Carter D. Diagnostic Surgical Pathology. Philadelphia: Lippincott Williams & Wilkins; 1999. pp. 2004–6. [Google Scholar]
- 4.Sönmez N, Ton O, Arisan S, Kilinç F, Eken K, Güney S. Bilateral Leydig cell tumor of the testis: A case report. Contemp Oncol (Pozn) 2012;16:356–9. doi: 10.5114/wo.2012.30069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Agha OM, Axiotis CA. An in-depth look at Leydig cell tumor of the testis. Arch Pathol Lab Med. 2007;131:311–7. doi: 10.5858/2007-131-311-AILALC. [DOI] [PubMed] [Google Scholar]
- 6.Ilkhanizadeh B, Taghizadieh M, Mahzad-Sadaghiani M, Noroozinia F, Jahandideh B. Bilateral Leydig cell tumor and male infertility: A case report. Iran J Reprod Med. 2005;3:47–9. [Google Scholar]
- 7.Holm M, Rajpert-De Meyts E, Andersson AM, Skakkebaek NE. Leydig cell micronodules are a common finding in testicular biopsies from men with impaired spermatogenesis and are associated with decreased testosterone/LH ratio. J Pathol. 2003;199:378–86. doi: 10.1002/path.1309. [DOI] [PubMed] [Google Scholar]
- 8.Hekimgil M, Altay B, Yakut BD, Soydan S, Ozyurt C, Killi R. Leydig cell tumor of the testis: Comparison of histopathological and immunohistochemical features of three azoospermic cases and one malignant case. Pathol Int. 2001;51:792–6. doi: 10.1046/j.1440-1827.2001.01278.x. [DOI] [PubMed] [Google Scholar]
- 9.Chandak P, Shah A, Taghizadeh A, Tiptaft R, Dasgupta P. Testis-sparing surgery for benign and malignant testicular tumours. Int J Clin Pract. 2003;57:912–3. [PubMed] [Google Scholar]
- 10.Kim I, Young RH, Scully RE. Leydig cell tumors of the testis. A clinicopathological analysis of 40 cases and review of the literature. Am J Surg Pathol. 1985;9:177–92. doi: 10.1097/00000478-198503000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Walsh PC. Campbell's Urology. Elsevier, Philadelphia: Saunders; 2007. pp. 925–7. [Google Scholar]


