Abstract
Cell-cell communication is a crucial component of many biological functions. For example, understanding how immune cells and cancer cells interact, both at the immunological synapse and through cytokine secretion, can help us understand and improve cancer immunotherapy. The study of how cells communicate and form synaptic connections is important in neuroscience, ophthalmology, and cancer. But in order to increase our understanding of these cellular phenomena, better tools need to be developed that allow us to study cell-cell communication in a highly controlled manner. Some technical requirements for better communication studies include manipulating cells spatiotemporally, high resolution imaging, and integrating sensors. Microfluidics is a powerful platform that has the ability to address these requirements and other current limitations. In this review, we describe some new advances in microfluidic technologies that have provided researchers with novel methods to study intercellular communication. The advantages of microfluidics have allowed for new capabilities in both single cell-cell communication and population-based communication. This review highlights microfluidic communication devices categorized as “short distance,” or primarily at the single cell level, and “long distance,” which mostly encompasses population level studies. Future directions and translation/commercialization will also be discussed.
Introduction
The study of cell-cell communication or cell-cell signaling is important in many biological fields, including genetics1, cancer2, immunology3, and more. How two or more cells talk and interact has drastic effects on proliferation, differentiation, migration, and stimulation, while defects in cellular communication can lead to diseases4. The study of cell-cell communication is necessary for both understanding diseases and for creating novel biomedical technologies including immunotherapy5, stem cells6, synthetic biology7, tissue engineering8, neural prosthetics and robotics9, and nanotechnology/nanomedicine10,11. Some examples of cellular communication include immune-tumor cell interactions, both at the immunological synapses and through secretion of cytokines and growth factors, communication within neural networks, mRNA transfer through cellular protrusion, neural and optical synapse formation, and signal propagation. The best method for studying cellular communication is using in vitro tools that allow better isolation and control of the microenvironment. While in vitro studies of cell-cell communication are typically not a good representation of the overall environment, there are many advantages to using in vitro studies that make it worthwhile, such as the opportunity to incorporate gene editing or analyze single cells and subpopulations.
While there is a need to understand cell-cell communication, many challenges exist that prevent scientists’ ability to conduct these studies. These challenges include the ability to manipulate and isolate cells, the ability to track and image cells, and the ability to control and manipulate cells. Integrating all these capabilities into one uniform tool is also very difficult. Another challenge is the different mechanisms of cellular communications and the need to have different techniques to study the multiple types of communication pathways, including gap junction signaling, juxtacrine signaling, paracrine signaling, endocrine signaling, and synaptic/direct signaling12. There exists no singular platform that can lever all these requirements for studying every pathway in cell-cell communication. To better study all of these individual phenomena for a variety of scenarios, specific tools designed for each application need to be available to researchers.
The most common tools and techniques that have been used to study cell-cell communication have been transwell systems and co-culture systems. Transwell inserts are one of the oldest technology for co-culture and are still used today due to the simplicity and robustness of the technology13. Having two separate compartments with multiple surfaces to culture allows for communication studies like secretion14, differentiation15, and migration16. Some of the weaknesses of the transwell system include lack of physiological relevance, flow, difficulty imaging, and limited spatial control, though some of that has been offset by modified transwell systems to incorporate flow17, imaging18, and mechanical forces19. Co-culture systems can include heterogeneous culture on petri dishes13, microcontact printing20, co-culture in gels21, or bioreactors22. However, these methods, while better than traditional petri dishes, lack the ability to be easily customized and versatile for many different scenarios, such as gradient culture, different cell sizes, spatial control, and more. Other tools need to be developed to truly allow controlled studies of cell-cell communication. Interdisciplinary collaborations between biologist and engineers will allow for better tools to be developed.
In the past two decades, microfluidic technology has been used as a tool to enhance biological studies. Microfluidics is the process of precise manipulation of fluids in channels and chambers at micron-level sizes23. Using rapid prototyping techniques that are easily adapted, researchers can design a multitude of microfluidic devices that can be adapted to specific research applications24. The most widely used material for fabrication of microfluidics is polydimethylsiloxane (PDMS) due to its optical properties, permeability, low cost, and straightforward fabrication25. While PDMS is the most commonly used material, other materials, such as paper, hydrogels, thermoplastics, etc, can be utilized for different applications26. Precise manipulation of fluids within microfluidics has allowed for advances in cellular studies27, diagnostics28, chemical synthesis and molecular biology29, and more.
Cell-cell communication studies can be greatly enhanced by microfluidic technology. One of the advantages of microfluidics is the ability to spatially manipulate the cells with precision not found in traditional cell culture, which allows for the ability to spatially control cells individually or collectively30. Another advantage is the ease of introducing flow27 and gradient control31. The uniqueness of microfluidics lies in the ability for both population-based studies and single cell studies32. Because cells are heterogeneous populations, microfluidics has the ability to discover small subpopulations that might be of interest. This could be especially true in studying circulating tumor cells33, immune cells34, and drug development35. Learning how cells can communicate at a single cell level will both help us understand better communication pathways and how special subpopulations of cells might communicate differently, which could have implications in cancer metastasis, immunotherapy, and more.
Microfluidics and other microscale techniques have also been utilized to study many different types of cell-cell communications for almost two decades. One of the earliest studies involved using micropatterning techniques to study co-culture interactions36, which are still being developed today37,38. After micropatterning came culturing cells inside the microfluidic devices39, which soon allowed the field to boom40. More complex microfluidic systems have subsequently been developed over the years for a wide range of applications. Previous reviews of microfluidic cell-cell communication devices have largely focused on either functional aspects of the device41,42 or the signaling aspect during communication12.
In this review, we will mainly highlight more recent microfluidic devices to study cell-cell communication, and focus mostly on several biological applications of interest. We categorize the microfluidic devices as either “short distance” communication or “long distance” communication devices. Following previous definitions of short/long distance cell-cell communications from the cell biology field, in the lab-chip systems, we suggest that’s short distance represents any microfluidic device where the cells are either physically contacting each other or bound within the same microchamber or microwell. These devices would mainly be used for studying gap junction signaling, juxtacrine signaling, or paracrine signaling (<50um), and are typically at the single cell or few cells regime1,43–45. On the other hand, long distance represents a microfluidic device where the cells are physically further apart and communicate via paracrine signaling (>50um), endocrine signaling, or synaptic/direct signaling1,43–45. These devices feature cells that are typically compartmentalized or separated, instead of within the same chamber or channel, and focus more on population-based cell communication. We will highlight devices for both short distance and long distance cell-cell communication and their recent applications in biology. Lastly, we will discuss the future perspective of these devices and their ability to be adopted by researchers in biological sciences.
Short distance Communication
One ability microfluidics has that was never seen before is the ability to spatially pair two cells next to or near each other. Cell pairing in microfluidics can be paired in two orientations, horizontal or vertical. Horizontal cell pairing is the most common type of pairing method. This method is useful for cell tracking and time-lapsed imaging. Because both cells lie in the same plane of view, it is simple to monitor both cells simultaneously and their interactions between each other. Another advantage of this method is the ability to track the migration of cells. In microfluidics, this method will also be easier to fabricate because of the single-layer nature of many of these devices. Most cellular applications will rely on horizontal cell pairing. Vertical cell pairing, however, is specifically useful for imaging the interface between two cells, typically gap junctions46. Imaging the interface of cells in horizontal cell pairing has low resolution because the synapse is oriented in the Z-direction. To image this synapse at high resolution would require a confocal microscope and z-stacking. Vertical stacking of the cells, however, would orient the cellular interface in the horizontal direction. This change in orientation allows imaging of the interface in a single plane. This also allows for a cleaner point-spread function(PSF), which increases the imaging resolution47. Because of the niched nature of these studies, devices for pairing cells vertically have not been developed as widely as horizontal methods have. It also can be more difficult to operate and fabricate in nature. Both pairing of cells horizontally or vertically have different benefits and can be used in a wide range of different studies.
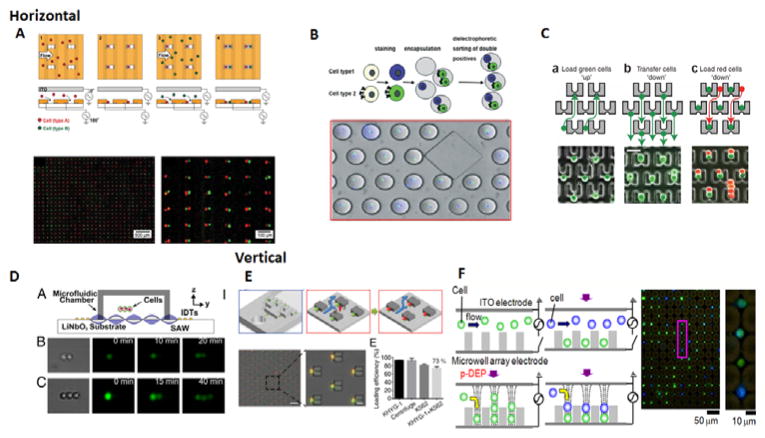
There are a variety of methods and techniques to pair cells in both the horizontal and vertical direction using microfluidics. Many devices rely on using microfluidic structures to trap and capture cells. The simplest method for cell pairing is using microwells. Previous reports have demonstrated that microwell size can control the number of cells per well48. Using the microwell, one can rely on probability to capture the two correct cells together inside the same well49. A Poisson distribution predicts approximately how many wells will have the correct number and type of cells50. It can also be useful when more than two cells need to be studied. A method for vertical cell pairing using microwells was also developed47. The three common microfluidic methods of cell pairing include using electrodes, droplets, or traps/structures. Integrated electrodes use dielectrophoresis to trap the cells at the electrode interface, and various groups have designed devices to pair two different cells together (Fig 1A)51,52. Furthermore, cells can be paired together in separate droplets53–55, which have the added benefit of isolating them from other cells(Fig 1B). Another common method for pairing cells is using a microfluidic cell trap or structure to control the pairing of cells (Fig 1C). These traps commonly utilize either hydrodynamic traps56–58 or some type of structure to hold the cell59,60. Another method of manipulating cells, either vertically or horizontally, is using acoustofluidics61,62. By using surface acoustic waves, these researchers demonstrated the ability to manipulate cells in three dimensions, allowing for horizontal, vertical, and 3-dimensional pairing and subsequent communication studies (Fig 1D). For vertical cell pairing, an electrode-based approach was used to trap cells inside pits(Fig 1E)63, while a microstructure was created on top of a micropit that trapped the cell to drop into the micropit, then trap another cell on top(Fig 1F)46. In essence, there are many microfluidic and microstructure tools that allow for cell pairing for two cells together. We will now discuss some of the applications that have utilized short distance cell communication techniques.
Figure 1.
Horizontal and vertical cell pairing methods. A) Electrode based method of creating horizontal pairs. Positive dielectrophoresis(DEP) is used to trap each cell type(red and green) into the well wall, and negative DEP is used to manipulate the distances between cell pairs52. B) Droplet-based approach for encapsulating two cells. Cell types 1 and 2 are stained and encapsulated, and the correct double positives can then be sorted out through DEP53. C) Trap or structure based approached for horizontal cell pairing. The first cell(green) is captured upward into the gap and then transferred down into the trap. The second cell(red) is then flowed down to occupy the remaining trap space to be paired horizontally59. D) Surface acoustic waves allow for control and manipulation of cell positions61. A microfluidic chamber on top of the acoustic substrate with interdigital transducers allows for surface acoustic waves to travel into the device and connect the two or three cells together. E) Vertical cell pairing using DEP. Cells fall into the well using DEP and excess cells are removed away with a sucrose wash. Then the second cell is introduced and trapped using DEP on top of the first cell63. F) Structure approach for vertical cell pairing. Cells are captured at the trap and fall into the micropit, which clears the trap. This allows for a second cell to be captured at the trap on top of the first cell46. 1A. Reproduced from Ref 52 with permission from the Royal Society of Chemistry. 1B. Reproduced from Ref 53 with permission from the Royal Society of Chemistry. 1C. Reproduced from Ref 59 with permission from Nature Publishing Group. 1D. Reproduced from Ref 61 from the National Academy of Sciences. 1E. Reprinted with permission from Ref 63 Copyright 2014 American Chemical Society 1F. Reproduced from Ref 46 from The American Association of Immunologist.
Application I: Immune cell heterogeneity
Many of the studies using microfluidics to spatially control cells have been aimed at observing immune cells. With microfluidics, one can limit and confine how an immune cell and a target cell interact and process individual action information rather than bulk information64. One interest is immune cell heterogeneity; with single cell-pair studies researchers can analyze the differences within an immune cell population and possibly find rare cell subtypes65. Single cell-pair analysis might lead to a discovery of a specific subset of immune cells that have a higher killing potential. Using microwells, Vanherberghen et al. monitored individual natural killer(NK) cell migration, binding, and killing of target cells.66. They classified NK cells to 5 distinct classes, with a small subpopulation of NK cells they dubbed “serial killers” that were responsible for the majority of cytolytic occurrences. Another group looked at multiple NK cells in a single well and discovered that NK cells did not cooperate when killing target cells49. Their system also allowed for monitoring of secreted proteins, and they demonstrated that NK cell secretion of cytokines was independent of cytolytic ability. Dura et al. used a microfluidic device to pair T-cells to target cells and monitored different scenarios for lymphocyte activation and stimulation67. They were capable of profiling Ca2+ dynamics in response to different stimulation (B cells, antibodies, or chemical agents) over time and demonstrated how T-cell receptors (TCR) affinity affects Ca2+ spikes and the cytokines secreted(Fig 2A). That same group then transited their studies to NK cells68. They compared the calcium flux to cytotoxicity and determined that the strength of Ca2+ activation can correlate with subsequent lysis and interferon-gamma (IFN-γ) production. Other studies have been done to monitor interactions between T-cells and dendritic cells69, analyze activation of lymphocyte killing70,71, and migration72,73. Microfluidics and microscale systems are growing to become a powerful tool to study immunology and researchers are starting to adopt the techniques more frequently, especially to study immune cell heterogeneity.
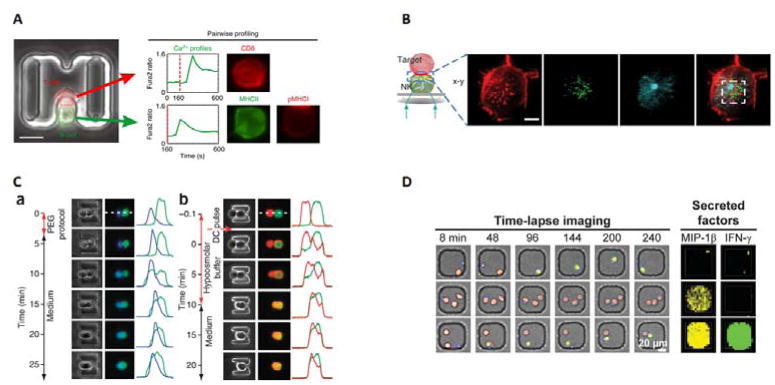
Figure 2.
Applications of cell pairing for communication studies. A) Immune cell heterogeneity studies. Single cell studies conducted on Ca2+ flux and activation between T cells(red) and B cells(green), where 225 individual cell pairs were tracked and analyzed based on CD8 expression(T cells) and MHCII and pMHC1 expression(B cells)67. The Ca2+ expression is measured by fluorescent signal over time. B) Imaging of the immunological synapse between a natural killer cell and a target cell. The authors were able to image the changes in localization between actin(red), perforin(green) and tubulin(blue) during the killing process46. C) Cell fusion of cells using both chemical and electrical techniques59. (a)Chemical fusion of mouse embryonic stem cells(green) to mouse embryonic(blue). (b)Electrical fusion of two 3T3s cells stained with DsRed and eGFP. Fusion is measured by the distance between fluorescence. D) Time lapse imaging and secretion analysis of NK cells interacting with target cells. NK cells(blue) can contact target cells(red) and subsequently kill the target cells(dead cells turn green through Sytox Green)49 Secreted factors are quantified by microengraving, where antibodies for MIP-1β and IFN-γ are bound to the bottom of the microwell. Secreted proteins are captured by the antibodies, and primary and fluorescent secondary antibodies for MIP-1β(yellow) and IFN-γ(green) bind. Concentration can be quantified by fluorescence intensity. 2A. Reproduced from Ref 67 with permission from Nature Publishing Group. 2B. Reproduced from Ref 46 from The American Association of Immunologist. 2C. Reproduced from Ref 59 with permission from Nature Publishing Group. 2D. Reproduced from Ref 49 with permission from the Royal Society of Chemistry.
With microfluidics, researchers are better able to manipulate and control cells for imaging74. It also has allowed for researchers to image biological phenomenon previously difficult to accomplish. One example of this is the immunological synapse, of which understanding has increased as imaging technologies progressed75. As mentioned before, pairing of cells in the vertical direction allows for the synapse plane to be in the horizontal direction and a better PSF. One study demonstrated using micropits to stack T-cells on antigen presenting cells(APCs) to image the immunological synapse47. They were able to image and show how the TCR clustered at the immunological interface to create a central supramolecular activation cluster (cSMAC). Around the cSMAC, they saw the majority of molecules were adhesion molecules and podosomes. Another device developed combined micropits with single cell trap arrays to increase the efficiency and throughput of vertical cell pairing46. The group used this device to look at the inhibitory synapse of NK cells to Programmed cell death protein 1 (PD-1) as well as F-actin and cytolytic granule distribution (Fig 2B). They discovered that PD-1/PD-L1 would first be dispersed, centralized, then dispersed again in the majority of cells, while the minority had different receptor reorganization dynamics. They also demonstrated with their device that they could also look at the heterogeneity of single cell NK killing dynamics. The advantages of these devices are the ability to vertically orient the cells together and prohibit the migration of cells, allowing high-throughput, time-lapsed imaging to be possible.
Application II: Fusion
Cell fusion is a necessary function of many cell types in development and regeneration76. It also has been used as a theory for cancer development and metastasis77,78. To induce cell fusion, cells must be adjacent and communicating with each other for the membranes to start fusion. Previous methods have been shown to have varying efficiency79. Using microfluidic pairing to place the cells together can help increase the efficiency of cell fusion. The Voldman group first used their microfluidic pairing device to increase efficiency of cell fusion using polyethylene glycol(PEG) to 40% and electrofusion up to almost 80%, while also demonstrating the functionality of the fused cells59(Fig 2C). The same group also developed a fusion device based off of deformability60. This device demonstrated electrofusion, while claiming to also be able to use chemical fusion or laser-induced fusion to be a versatile platform for cell fusion. Another group also demonstrated a microstructure device compatible with laser-induced fusion80. The advantages of microfluidic-based cell fusion are the increased efficiency in pairing cells together. Another interesting possibility is fusing more than two cells together, as the Voldman group demonstrated by fusing three cells together60. Cell fusion studies have been limited before by efficiency and throughput, which microfluidics can now solve. With these microfluidic tools, researchers can better study cell fusion, and possibly address questions like the cell fusion metastasis theory81.
Application III: Sensors
With microfluidics, researchers can couple monitoring cell-cell interactions and measure secreted proteins and cytokines at the same time. Many devices to monitor single cell secretions have been previously reported82–85. Coupled with cell pairing, the secretions that cells produce after cell-cell interactions can be monitored. Yamanaka et al. used their microengraving system to monitor the secretions of NK cells while killing target cells49. They demonstrated that when NK cells contact the target cell, they started producing macrophage inflammatory protein 1β (MIP-1β) and interferon gamma (IFN-γ) (Fig 2D). With cell surface sensors attached to a cell, mesenchymal stem cells were capable of detecting platelet derived growth factor (PDGF) produced by MDA-MB-231 breast cancer cells inside microwells86. Another study examined how umbilical cord blood cells survived when co-cultured with MBA2 cells and IL-3 in droplets87. Coupling a sensing technique with single cell analysis allows for quantitative information of secretions due to cellular interactions.
Long Distance Communication
In addition to short distance communication, microfluidic devices have also been developed to study how cells communicate at a longer distance. The most common long distance communication devices look to measure paracrine or endocrine signaling through secretions. Instead of directly contacting cells or cells within the same microwell, single cells or cell populations are compartmentalized and separated by channels88, valves and membranes89,90, permeable gels,91 or gases92. These gels and valves are made permeable so communication proteins like cytokines or growth factors can diffuse through93. Other devices have coupled with sensors when measuring intercellular communication of how cytokines can affect cellular processes in downstream culture94,95(Fig 3A). Another possibility of these devices is coupling them with other external cues simultaneously, including substrate stiffness96 or roughness97, biochemical gradients98,99 or even migration inhibitor factors100. Three-dimensional devices have also been created to study longer distance cell-cell signaling similar to endocrine signaling, where signaling can induce migration, proliferation, epithelial-mesenchymal transition (EMT), or more101–103. Many of these 3D devices utilize hydrogels like traditional 3D culture methods to compartmentalize the cells and allow signaling proteins to pass between104(Fig 3B). Besides paracrine and endocrine, devices can also be created to induce synaptic communication between single cells105. The advantages of these type of devices are the ability to induce synapse formation105 and induce directed migration106. Long distance communication devices were developed earlier than short distance devices and therefore are more established. Here we will highlight some long distance devices and their applications to biology.
Figure 3.
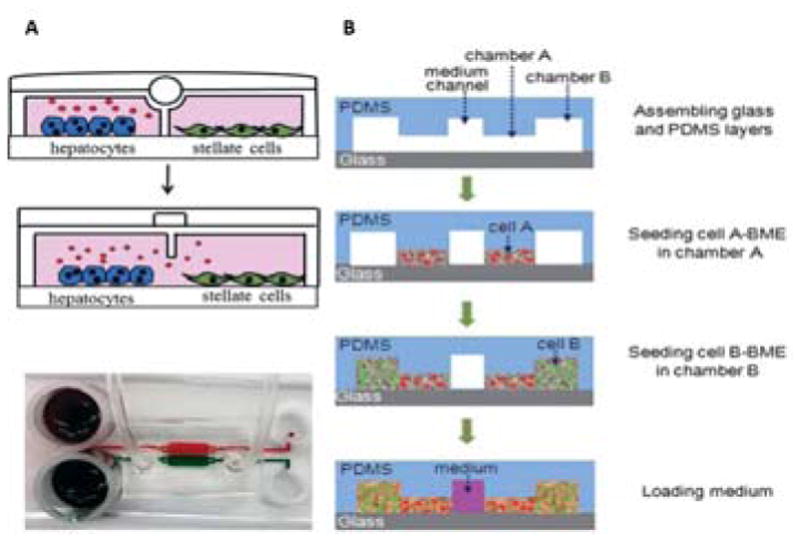
Long distance 2D(A) or 3D(B) co-culture devices for secretion based communication. A) Cells are divided by a valve that can separate the two cells and control the secretion of cytokines(A, top)95. To make the cells communicate, the valve is opened so that secreted proteins and conditioned media can be shared between the populations(A, middle). This device could then be coupled with electrode sensors to monitor cell behavior. A schematic of the chip is shown to demonstrate compartments don’t mix when the valve is turned on(A, bottom) B) 3D cell co-culture using hydrogel culture. Cells are loaded in basement membrane extract(BME) gel to compartmentalize the cell104. First, cell A(red) is introduced, then cell B(green) is introduced adjacent to cell A, but without mixing. The middle channel is filled with media to feed the cells. Cytokines, growth factors, and other secreted proteins can then pass through from one cell population to the next by diffusing through the gel. This also allows for cells to migrate through from one gel to the next. 3A. Reproduced from Ref 95 with permission from the Royal Society of Chemistry. 3B. Reproduced from Ref 104 with permission from the Royal Society of Chemistry.
Application I: Neuron Synapses
One such application for long distance communication is the study of neuron synapses. Neuron cells, unlike other cells, never divide and are in most cases not replaceable after their loss. Neurons use synaptic connections to cover large distances crossing diverse extracellular matrixes and process information transmission. The need to control a multitude of neuronal microenvironments to investigate the cell synapses makes microfluidics a perfect candidate to study the ordered connectivity of neurons. In 2006, Park et al107 described the fabrication of a microfluidic culture chip to compartment hippocampal and other central nervous system neurons. The device used two chambers that could be loaded independently, which were connected with an array of microchannels. By using different hydrostatic pressures, distinct chemical environments could be created in the chambers while allowing for interaction between the neuron populations through the use of their axonal projections. In 2010, Taylor et al108 developed a microfluidic device for the visualization and manipulation of synaptic, presynaptic, and postsynaptic bodies independently. This was accomplished by adapting their previously developed design109,110 and adding a perfusion chamber perpendicular to the microgrooves, thus allowing for the access to the synaptic region with high special and temporal resolution (Fig 4A). The location of the perfusion chamber, located closer to one of the latter compartments, ensured that mostly dendrites from the closer chamber and only axons from the further chamber were perfused. The independent way in which each chamber can be manipulated also allowed for the study of two neuronal populations in an autonomous manner. More recently, Coquinco et al111 also used a more advanced three compartment microfluidic device to create three isolated microfluidic environments using the same principles as Park et al107 to study synaptic competition. Unlike the Park et al device, they used three, instead of two, chemically isolated compartments which allowed them to place the target neurons in the center compartment and the competing neurons on compartments located on either side (Fig 4B). The chemical isolation of the compartments meant that only axons were able to cross the microgrooves, enabling the study of synaptic competition between inhibited and untreated neurons in the lateral compartments and the target neurons located in the center compartment. They discovered that axons from untreated neurons reached the center chamber faster than threated neurons, resulting in increased synaptic events. The study of astrocytes has revealed a parallel development of astrocytes and the formation of synapses.112 Facilitating the co-culture of glia and neurons in the same microfluidic device can provide important information about the development of dendritic spines and synapses. Shi et al89 developed the first microfluidic device for the co-culture of neurons and glia that permitted reversible separation. By using a vertically-layered co-culture scheme, they could culture glia cells on a PLL-coated PDMS roof and neuron cells on a PLL-coated glass surface on the bottom by simply reversing the chip. Using a valve, they could isolate neurons in adjacent chambers by using a pressure chamber. This vertical design provided a working mechanism to study the influence of glia cells in synaptic contacts between adjacent neuronal populations. In this paper, they also presented a four chamber microfluidic that used the same valve isolation principle but with cultured glia cell on the outside chambers with differentiated neuronal populations. Both devices provided new technology for co-culturing differentiated populations of neurons and glia cells. Previously, Majundar et al90 had also reported a valve isolated microfluidic device which they used for the co-culture of one population of hippocampal neurons and a population of glia cells using a microfabricated valve to act as a separating hurdle between the chambers. However, due to having only two chambers, it could not observe the effects of glia on neuron-neuron interactions. One device created to study the interactions between astrocytes and neurons, and their response to glutamate stimulation, was published by Gao et al88. This was the first study combining surface patterning with polyethylene glycol, used as a way to specifically place cells and guide neurite development, microgrooves to separate the two chambers113 and the use of genetically encoded calcium indicators that allowed to monitor neuron-astrocyte interactions. The combination of these technologies in the same device allowed for the first time to study the real time signaling between neurons and astrocytes (Fig 4C). The field of neuroscience has benefitted greatly from microfluidics and will continue to offer unique opportunities for neuron synapses and communication studies.
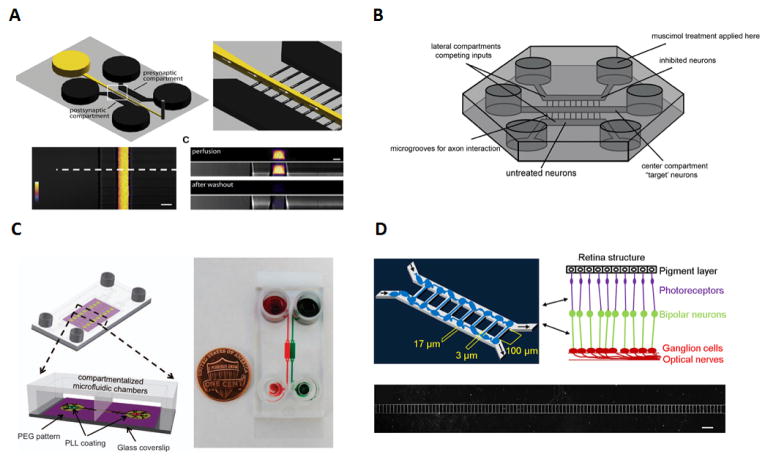
Figure 4.
Applications of neuron and ocular synapses studies A) Schematic of the microfluidic chip used for the visualizations and manipulation of synapses, where flow direction is achieved with the use of negative pressure thus by preventing diffusion into the microgrooves. The use of Alexa Fluor 488 allows for the observation of the difference in profile between perfusion and the washout step108. B) Experimental setup of the 3 compartment microfluidic device used for synaptic competition. The use of microgrooves on the side chambers and ‘target’ neurons in the centre establishes the synaptic competition between axons to reach the central chamber111. C) Schematics and actual image of neuron-astrocytes interaction microfluidic chip using food dyes to show the chambers and channels. This platform used for the first time simultaneously a combination of PEG, (polyethylene glycol), compartmentalized chambers and gen etically encoded calcium indicators for the analyses of neuron-neuron and neuron-astrocytes interaction88. D) Microfluidic device schematics for retinal synapse regeneration above the retinal structure mimicked with the use of two chambers connected by 108 microchannels. Scale bar, 200μm116. 4A. Reproduced from Ref 108 with permission from Elsevier. 4B. Reproduced from Ref 111 with permission from Elsevier. 4C. Reproduced from Ref 88 with permission from Nature Publishing Group. 4D. Reproduced from Ref 116 under a CC-BY license through Nature Publishing Group.
Application II: Ocular Synapses
Ocular synapses activity is another application where the use of microfluidic devices has been recently developed. For example, the study of the cellular communication between donor and host retinal cells is still, for the most part, poorly comprehended, and a better understanding of this integration could allow for an improvement on the transplantation of retinal precursors derived from postnatal retinal cells. These precursors have been able to achieve repair on rod-mediated vision in mice and in clinical trials114,115 To address retinal synaptic regeneration and artificial retina studies, Ping-Jung Su et al116 used a microfluidic device named retinal synaptic regeneration chip (RSR-chip). With the intent to reconstruct retinal neuron synapses, they used retinal precursor cells cultured in a microfluidic chip possessing several arrays of microchannels. By forming a network of aligned synapses and using immunostaining of retinal precursor cells, this microfluidic device allowed for automatic and high throughput quantification and analyses of synaptic regeneration events. To conduct this study, the RSR-Chip was designed with two microchambers connected by a multitude of microchannels arrays. By using 50 microchannels with lengths of 50 or 100um long with a width of 3 or 4um and a height of 17um, this study was able to mimic the long-range interaction of retinal cells by forming a network of oriented synapses in the chip. The design of two independent microchambers connected to each other with a multitude of microchannels allows for the loading of each individual chamber with the desired retina neurons population, and therefore to study the interaction between two or more populations of retina neurons (Fig 4D).The synaptic connections formed in response to secreted cytokine generated a larger number of observable synaptic regeneration events in the shorter channels rather than the longer channels, with the width of the channels producing identical results. Though there are few studies on microfluidic devices for ophthalmology and ocular research117–119, we believe the field could have many advantages in adopting microfluidic studies and that this is an attractive avenue of collaboration for future researchers.
Application III: Cell to cell protrusions
The study of material transfers through gap junction intercellular communication (GJIC) regulates the degree of intercellular coupling, and the use of such channels is critical to several physiological activities ranging from impulse propagation in the heart and neurons to regulation of cellular proliferation. The study of GJIC at the single cell level can be carried out from measuring dye transfer or electrical conductance and metabolic cooperation 120. These measurements are typically not high throughput and controlling the gap junction formation is hard. However, the microfluidic device developed by Zhang et al105, allowed for the formation of cell pairs to occur in a more controllable and high throughput manner. The microfluidic chip named “Block-Cell-Printing” (BloC-Printing) (Fig 5A) consisted of a symmetrical network of microfluidic channels with microarrays of traps present in each channel to capture cells loaded in the device by the use of negative pressure (Fig 5B). The structure of the device allowed for great flexibility in controlling the distance between cell pairs since the cell traps could be designed to be the desired distance from each other. The design of the traps itself was also highly effective in obtaining a single cell trapping efficiency close to 100%. From the moment cells were trapped, it would take over two hours for them to establish protrusions which occur in both directions along the side of the microchannel wall closer to the cells. By aligning traps next to each other with both possible directions of flow, this device was able to study intercellular communication, either body to body (Fig 5C) or protrusion to protrusion (Fig 5D), amongst two different types of cells by observing dye transfer between the forward protrusion of a donor cell and the backward protrusion of a recipient cell or two cell bodies. Cells would develop both a forward and a backward protrusion and were allowed to spread their protrusions typically for periods of time in excess of two hours (Fig 5E). Since the printing of cells, and their transfer to a number of different substrates such as polystyrene or glass, is a controllable characteristic in this device, and because studies of signal transduction between primary neurons require their precise individual positioning, the utilization of this device to print individual cortical neurons was also used after to obtain single and paired neurons with highly branched dendrites. The use of protrusion enhances the specificity for cells to communicate between each other121. Chemical cell-cell communication is not the only way in which cells use protrusions in nature. In fact, cells can use protrusion for several different biological roles that range from expansion of their surface area to cell motility and more122. Therefore, the use of microfluidics devices for these studies provides new and important tools to help better understand the role of cell protrusions in several cellular processes, specifically cellular signaling and communication121.
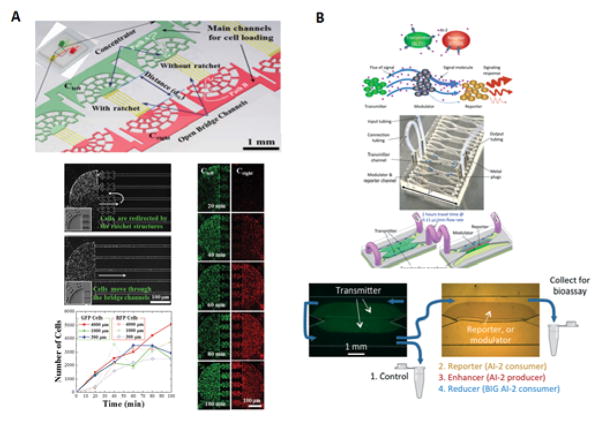
Figure 5.
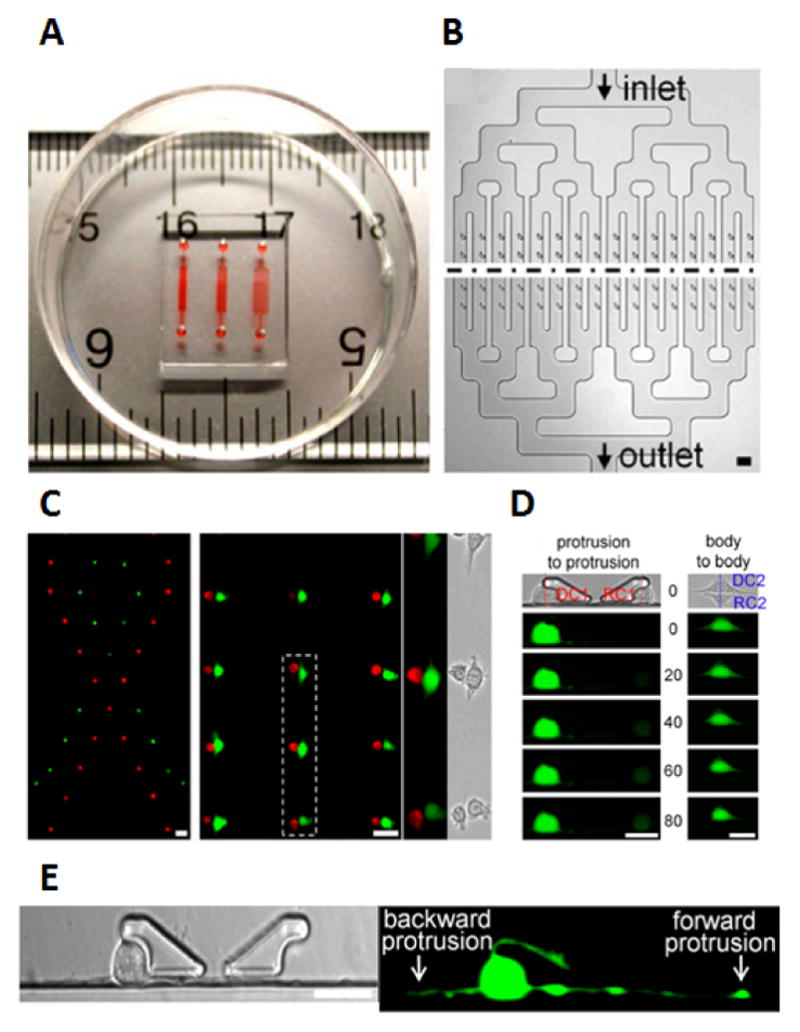
Applications of cell-to-cell protrusions in intercellular communication A) Image of the BloC-printing device displayed with ruler for scale and colored die for the visualization the channels B) Schematics of chip design with the symmetrical channel networks C) Flexibility of cell trapping with ribbon pattern as an example and body to body cell pairing with red and green cells with enlarged micrograph of cell pairs within dotted line D) Calcein transfer via GJIC in cell pairs with protrusion-to-protrusion and body-to-body pairing methods E) Forward and backwards protrusions generated by the fibroblasts after three hours of trapping105. Fig 5. Reproduced from Ref 105 with permission from the National Academy of Sciences.
Application IV: Bacteria
Besides mammalian cells, bacterial cell communication has also been an area where microfluidics has advanced as a tool of research. Although populations of bacteria where initially thought to act independently, this has recently been discovered not to be the case. It has been revealed that certain groups of chemical signal molecules are involved in the regulation of gene expression in a cell density manner. This behavior has been termed quorum sensing or cell-cell communication.123 The phenomenon of quorum sensing in bacteria involves the production release and sensing of small signaling molecules and occurs in both Gram-positive and Gram-negative bacteria124. These signaling molecules known as quorum sensing molecules are responsible for regulating a number of functions including bio-film formation125 and motility126. Redfield127 proposed an alternative to this quorum sensing which he labeled diffusion sensing, where he proposed that these chemical signaling molecules could provide a means to detect the degree of diffusion and mixing in the cell environment127. It has also been proposed that merging both concepts in a term dubbed “efficiency sensing” could provide a better understanding of bacterial cells communication, unifying not only the “what cells sense” theory but also the “why cells sense it” theory with the evolutionary hypotheses of the benefits of cell autoinducer sensing.124 It seems clear that microbial cell-to-cell communication studies can benefit from the use of microfluidic devices with strategies that can provide new tools to observe cell-cell behavior for coordinated bacterial cell communication30. One of those microfluidic device, which was inspired by existing microfluidic devices128,129, had the ability to control the positioning and communication between three different populations of wild-type soil bacteria. The device consisted of a double layered microfluidic structures separated by a nanoporous membrane and reported the necessity and adequacy of the existence of a defined microscale spatial structure between the bacterial communities for a stable coexistence of interacting synthetic bacterial communities.130 Park et al131 developed a microfluidic chip that used a ratchet structure integrated concentrator microfluidic device to develop assays for bacterial cell-to-cell communication. The use of ratchet structures allowed for the controlled concentration of bacterial cells. By using two different channels for cell loading, they could load different pairs of cells on the right and left side of the device while the physical connection between both sides of the device occurred via open microchannels that connected the cells chemically. (Fig 6A) By using receiver cells on the left main channel and sender cells that produced acyl homoserine lactones, which activates receiver cells to activate GFP, on the right, it was discovered that after one hour of concentrating cells, the receiver cells started to show GFP signals while no fluorescence signals were detected on the sender cells. This easy single layer PDMS device facilitated the research on the effect not only of spacing distances but also of populational density in bacterial cell-to-cell communication. More recently, Luo et al132 published a paper in which they demonstrate a device that uses microfluidics to modulate communication between proximal cells and distant populations. The design used multiple microchannels connected in pairs with a flexible tube for the signaling experiments and three distinct cell populations. The transmitter cell community was located upstream and signaled a molecule autoinducer-2 to a reporter population that receive the signal after it was either amplified or attenuated by a “modulator” cell population (Fig 6B). For the assembly of the cell population within the chip, a chitosane membrane was used allowing for the positioning of reporter cells adjacent to the membrane, while the reporter and modulator cells were assembled in calcium alginate hydrogels. This design enabled the modulation of the longitudinal transport of small molecules transmitted by non-pathogenic E. coli mimicking long distal signaling among in the human intestinal track which in live species is hard to access. The development of microfluidic devices to the study of bacteria and other small organisms will continue to increase the possibility for future developments in this field.
Figure 6.
Applications of bacterial cell communications A) Microfluidic device with a ratchet structure used bacterial cell-cell communication between two distinct motile bacterial cell types, the communication between cells was established via bridge-channels that connected both concentrator structures. The use of a ratchet structure prevents cells from moving through the bridge channels and insures only cell signaling molecules are able to cross the bridge. Both GFP and RFP cells were loaded at the same time and saw their concentration increased gradually over time131 B) Schematics of near cell-cell signaling with enhancement or attenuation of the signal molecules from the transmitter to reporter via modulating cells. The use of a 10cm flexible PTFE tube connecting the upstream transmitter cells with the reporter and enhancer/reducer cells downstream was essential for the detection gap time to mimic in vitro human digestion modules. The effluent solutions were collected either immediately after the transmitter cells upstream or allowed to flow downstream were they would encounter the remaining cell populations: test strain, reporter CT104, enhancer LW5, or reducer LW8132. 6A. Reproduced from Ref 131 with permission from the Royal Society of Chemistry. 6B. Reproduced from Ref 132 with permission from the Royal Society of Chemistry.
Application V: Wound Healing
Microfluidic devices have been used as an alternative to the traditional scratch wound healing assay. In the classical scratch assay, cells are physically removed by a razor-like device, and the remaining cells are responsible for closing the gap left by the scratch device. This scratch is the “wound,” and the cells are monitored to close the scratched area. Despite being a simple and fast method to perform, the scratch assay has several disadvantages. For example, this method does not account for the influence that different chemical gradients might pose. The physical characteristics of the wound area are difficult to replicate since the manual method is highly dependent on the user. The use of microfluidic devices, where cells can be patterned with precise and constant distances regardless of the operator’s ability, combined with the smaller structural device size for an easy visualization, has helped overcome some of the disadvantages. One of the earliest applications was to study the influence of a surface for cells to grow free from cell damage caused by the earlier scratching methods. This device used a microstencil technique to culture cells in physically-separated open structures to enable the study of cell migration without inflicting any damage to the cells133. Another application involves the culturing of cells within closed microfluidic channels, where the wound is produced by exposing the cells in one channel to trypsin; this effectively removes cells from the device and creates a gap or wound in between the cells in the other channels and allows for the study of NIH-3T3 fibroblasts migration to close the wound134. This approach has provided the concept for numerous studies to quantify endothelial cell migration135, the effects of ethanol on MCF-7 human breast cancer cells136, the regenerative effect of HGF on wounded alveolar epithelium137 and vascular smooth cell migration138. The major problem with wound healing studies is that despite these advances in devices, the assay itself has not undergone significant changes in the last 20 years. New techniques of inducing wounds, monitoring wound healing, and controlling the wound microenvironment are needed to make wound healing studies more physiologically relevant. More recently, a microfluidic device was developed that used a membrane to induce the wound and allowed repeated injury139. Continual advances need to be made in this field if it will truly have an impact in understanding in vivo wound healing.
Conclusion and Future Perspectives
We briefly detailed various microfluidic devices for cell-cell communication and some applications that researchers have used these devices for. The unique ability to study single cell signaling or small populations in microfluidics with be important in future understanding of cell heterogeneity, and it will have wide implications in many topics including cancer stem cells, metastasis, immunology, neuroscience, and more. Immunology has been one of the biggest directions for cell-cell communication and single cell analysis devices34, while neuroscience has been widely popular for long-distance communication140–142. Despite this, there are still many unexplored biological areas where research could benefit from microfluidic cell-cell signaling devices. Entosis, or when a cell invades another cell’s cytoplasm, could be studied with short distance microfluidic devices143,144. For long distance devices, the role of exosomes in cell-cell communication and subsequent reactions still needs to be investigated145, especially because microfluidics can be used for exosome enrichment and detection146. Exosomes have recently become a topic of interest, and their role in cellular communication has just briefly been touched147,148 We believe that soon researchers will leverage the advantages of microfluidic cell-cell communication devices to exosome communication research. Another area that is in the early stages of research is gene editing and manipulation during cell-cell communication149. The ability to study how cells interact after gene manipulation can lead to better understanding of cell-cell communication at the genetic level, as well as help in fields like synthetic biology7. Studying cell-cell communication through gene editing could also be useful for cell-based screening of subpopulations of interest, similar to work our group has done on cell screening based on deformability150. With novel strategies for gene editing using microfluidics recently being developed151, future studies combining gene editing and cell-cell communication studies are expected to follow. Based on these insights, there are still many avenues for researchers where microfluidics would help increase information and understanding in cell-cell communication.
While many devices have been presented, more devices and designs still need to be developed. Of note is the lack of cell-cell migration devices, which allow for cells to migrate towards signaling of other cells. The challenge in this is having effector cells produce enough signals to form a noticeable gradient over long periods of time that the target cell can sense. Another area of need is the ability to pair more than 2 single cells together with high spatial resolution, high efficiency, and high throughput. Technologies that are competing with that include single cell bioprinters152 and laser writing153,154. The disadvantage of these technologies lies in the low throughput of these technologies, while microfluidics has the potential for high throughput printing while retaining high viability. Single cell long distance devices could also be developed to isolate and examine individual protrustions/synapses88. Another opportunity would be to further develop devices that allow cell-cell communication integrated into 3D tissue or 3D environments, to monitor how single cells communicate in different or more native environments155. As mentioned above, these devices only focus on the in vitro devices and neglected the impact that the 3D microenvironment. To account for and better replicate the microenvironment, organ-on-chip systems have been developed that better mimic in vivo factors156. There are many organ-on-chip technologies and techniques already developed that can be used to study cell-cell communication. Another technique is stacking paper-culture platforms to simplify 3D culture157–159. By being able to stack different layers of culture, the Whitesides group has been able to monitor migration/invasion and develop 3D models and organ-on-chip models160,161. The paper-based culture is a platform that can be used for monitoring cell-cell interactions and may have many advantages in reducing the cost and difficulty associated with materials and 3D culture161. Overall, the needs and opportunities exist for both biologists to utilize microfluidic devices to enhance their research, as well as engineers to develop better cell-cell communication tools to aide biologist.
The true hurdle for advancing these studies is the usability of microfluidics for traditional biology labs. While there are countless opportunities that biologists could use these microfluidic platforms for, the limiting factor is the ease and adoptability of these devices towards non-microfluidic researchers. Lack of standard designs and the difficulty for new users to start using microfluidics means more traditional, but technically lacking systems are still used. Other microfluidic devices are designed with pump systems (syringe, peristaltic, or pressure pumps) that make implementing microfluidic systems costly. In order to push the potentials of microfluidics to advance biological research, simple devices which are easily utilized and require no external equipment are necessary. These devices also need to pursue commercialization. Indeed, some companies have already started commercialization for cell-cell communication devices, with applications in endocrine systems162, neuroscience163, and general co-culture164. Even some larger companies have started distributing microfluidic systems and are helping make microfluidics a more universal research tool165. The goal should be to create a device as simple as the widespread transwell system, with standard protocols and procedures, but with technical features that will allow researchers to expand their research. Engineers need to keep in mind specific biological questions that require advanced technology to solve, as well as creating simple systems that allow for ease of utility for the scientific population as a whole.
Supplementary Material
Table 1.
Types of Microfluidic Devices and their applications
| Type of device | Applications | Advantages | Disadvantages | ||
|---|---|---|---|---|---|
| Short Distance | Horizontal cell pairing | Structure traps and microwells49,56–60,67,69,82,86 Electrode-Based51,52 Droplet53–55 Acoustofluidic61 |
Immunology Fusion Secretions |
|
|
| Vertical cell pairing | Structure traps and microwells46,47 Electrode Based63 Acoustofluidic62 |
Imaging |
|
|
|
| Long Distance | Synapse devices | Single cell block printing105 Neuron and ocular synapse devices88,89,107–109,111,116,142 |
Neurosciences Ocular sciences Synaptic-communication |
|
|
| Migration devices | Channels106,131,137 Gels91,101–104 |
Migration studies Wound Healing |
|
|
|
| Co-culture devices | Channels94,95 Gels91,102 |
Co-culture studies |
|
|
|
Acknowledgments
Our research is funded by NIH-R01 DA035868, R01 CA180083, R56 AG049714, and R21 CA191179.
References
- 1.Mittelbrunn M, Sánchez-Madrid F. Nat Rev Mol Cell Biol. 2012;13:328–335. doi: 10.1038/nrm3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brücher BLDM, Jamall IS. Cell Physiol Biochem. 2014;34:213–243. doi: 10.1159/000362978. [DOI] [PubMed] [Google Scholar]
- 3.Hodgkin PD, Rush J, Gett AV, Bartell G, Hasbold J. Immunol Cell Biol. 1998;76:448–453. doi: 10.1046/j.1440-1711.1998.00776.x. [DOI] [PubMed] [Google Scholar]
- 4.Lai EC. Development. 2004;131:965 LP-973. doi: 10.1242/dev.01074. [DOI] [PubMed] [Google Scholar]
- 5.Malhotra A, Shanker A. Immunotherapy. 2011;3:1143–1166. doi: 10.2217/imt.11.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xin T, Greco V, Myung P. Cell. 2016;164:1212–1225. doi: 10.1016/j.cell.2016.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hennig S, Rödel G, Ostermann K. J Biol Eng. 2015;9:13. doi: 10.1186/s13036-015-0011-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rossello RA, DH Commun Integr Biol. 2010;3:53–56. doi: 10.4161/cib.3.1.9863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taghva A, Song D, Hampson RE, Deadwyler SA, Berger TW. World Neurosurg. 2012;78 doi: 10.1016/j.wneu.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrati S, McConnell KI, Mack AC, Sirisaengtaksin N, Diaz R, Bean AJ, Ferrari M, Serda RE. Nanomedicine (Lond) 2014;9:581–592. doi: 10.2217/nnm.13.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arnoldussen YJ, Anmarkrud KH, Skaug V, Apte RN, Haugen A, Zienolddiny S. J Cell Commun Signal. 2016;10:153–162. doi: 10.1007/s12079-016-0323-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nahavandi S, Tang S-Y, Baratchi S, Soffe R, Nahavandi S, Kalantar-zadeh K, Mitchell A, Khoshmanesh K. Small. 2014;10:4810–4826. doi: 10.1002/smll.201401444. [DOI] [PubMed] [Google Scholar]
- 13.Goers L, Freemont P, Polizzi KM. J R Soc Interface. 2014;11:20140065. doi: 10.1098/rsif.2014.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar R, Harris-Hooker S, Kumar R, Sanford G. Vasc Cell. 2011;3:1–15. doi: 10.1186/2045-824X-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith MP, Young H, Hurlstone A, Wellbrock C. Bio-protocol. 2015;5:e1638. doi: 10.21769/bioprotoc.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mukherjee S, Kim J, Mooren OL, Shahan ST, Cohan M, Cooper JA. PLoS One. 2015;10:1–23. doi: 10.1371/journal.pone.0118153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slattery MJ, Dong C. Int J Cancer. 2003;106:713–722. doi: 10.1002/ijc.11297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watkins S, Sontheimer H. J Neurosci. 2011;31:17250–17259. doi: 10.1523/JNEUROSCI.3938-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lü D, Liu X, Gao Y, Huo B, Kang Y, Chen J, Sun S, Chen L, Luo X, Long M. PLoS One. 2013;8:e74563. doi: 10.1371/journal.pone.0074563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Javaherian S, O’Donnell KA, McGuigan AP. PLoS One. 2011;6:e20909. doi: 10.1371/journal.pone.0020909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albrecht DR, Underhill GH, Wassermann TB, Sah RL, Bhatia SN. Nat Meth. 2006;3:369–375. doi: 10.1038/nmeth873. [DOI] [PubMed] [Google Scholar]
- 22.Williams C, Wick TM. Ann Biomed Eng. 2005;33:920–928. doi: 10.1007/s10439-005-3238-0. [DOI] [PubMed] [Google Scholar]
- 23.Whitesides GM. Nature. 2006;442:368–373. doi: 10.1038/nature05058. [DOI] [PubMed] [Google Scholar]
- 24.Duffy DC, McDonald JC, Schueller OJA, Whitesides GM. Anal Chem. 1998;70:4974–4984. doi: 10.1021/ac980656z. [DOI] [PubMed] [Google Scholar]
- 25.McDonald JC, Whitesides GM. Acc Chem Res. 2002;35:491–499. doi: 10.1021/ar010110q. [DOI] [PubMed] [Google Scholar]
- 26.Ren K, Zhou J, Wu H. Acc Chem Res. 2013;46:2396–2406. doi: 10.1021/ar300314s. [DOI] [PubMed] [Google Scholar]
- 27.Halldorsson S, Lucumi E, Gómez-Sjöberg R, Fleming RMT. Biosens Bioelectron. 2015;63:218–231. doi: 10.1016/j.bios.2014.07.029. [DOI] [PubMed] [Google Scholar]
- 28.Chin CD, Laksanasopin T, Cheung YK, Steinmiller D, Linder V, Parsa H, Wang J, Moore H, Rouse R, Umviligihozo G, Karita E, Mwambarangwe L, Braunstein SL, van de Wijgert J, Sahabo R, Justman JE, El-Sadr W, Sia SK. Nat Med. 2011;17:1015–1019. doi: 10.1038/nm.2408. [DOI] [PubMed] [Google Scholar]
- 29.Theberge AB, Courtois F, Schaerli Y, Fischlechner M, Abell C, Hollfelder F, Huck WTS. Angew Chemie Int Ed. 2010;49:5846–5868. doi: 10.1002/anie.200906653. [DOI] [PubMed] [Google Scholar]
- 30.Velve-Casquillas G, Le Berre M, Piel M, Tran PT. Nano Today. 2010;5:28–47. doi: 10.1016/j.nantod.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chung BG, Choo J. Electrophoresis. 2010;31:3014–3027. doi: 10.1002/elps.201000137. [DOI] [PubMed] [Google Scholar]
- 32.Reece A, Xia B, Jiang Z, Noren B, McBride R, Oakey J. Curr Opin Biotechnol. 2016;40:90–96. doi: 10.1016/j.copbio.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen X-X, Bai F. Cancer Biol Med. 2015;12:184–192. doi: 10.7497/j.issn.2095-3941.2015.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chattopadhyay PK, Gierahn TM, Roederer M, Love JC. Nat Immunol. 2014;15:128–135. doi: 10.1038/ni.2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heath JR, Ribas A, Mischel PS. Nat Rev Drug Discov. 2016;15:204–216. doi: 10.1038/nrd.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhatia SN, Yarmush ML, Toner M. J Biomed Mater Res. 1997;34:189–199. doi: 10.1002/(sici)1097-4636(199702)34:2<189::aid-jbm8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 37.Lunova M, Zablotskii V, Dempsey NM, Devillers T, Jirsa M, Sykova E, Kubinova S, Lunov O, Dejneka A. Integr Biol. 2016:8. doi: 10.1039/c6ib00125d. [DOI] [PubMed] [Google Scholar]
- 38.Li CY, Stevens KR, Schwartz RE, Alejandro BS, Huang JH, Bhatia SN. Tissue Eng Part A. 2014;20:2200–12. doi: 10.1089/ten.tea.2013.0667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takayama S, McDonald JC, Ostuni E, Liang MN, Kenis PJA, Ismagilov RF, Whitesides GM. Proc Natl Acad Sci. 1999;96:5545–5548. doi: 10.1073/pnas.96.10.5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park TH, Shuler ML. Biotechnol Prog. 2003;19:243–253. doi: 10.1021/bp020143k. [DOI] [PubMed] [Google Scholar]
- 41.Guo F, French JB, Li P, Zhao H, Chan CY, Fick JR, Benkovic SJ, Huang TJ. Lab Chip. 2013;13:3152–62. doi: 10.1039/c3lc90067c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Konry T, Sarkar S, Sabhachandani P, Cohen N. Annu Rev Biomed Eng. 2016;18:259–84. doi: 10.1146/annurev-bioeng-090215-112735. [DOI] [PubMed] [Google Scholar]
- 43.Kumar NM, Gilula NB. Cell. 1996;84:381–388. doi: 10.1016/s0092-8674(00)81282-9. [DOI] [PubMed] [Google Scholar]
- 44.Walker GM, Zeringue HC, Beebe DJ. Lab Chip. 2004;4:91–97. doi: 10.1039/b311214d. [DOI] [PubMed] [Google Scholar]
- 45.Brownlee C. Curr Opin Plant Biol. 2002;5:396–401. doi: 10.1016/s1369-5266(02)00286-8. [DOI] [PubMed] [Google Scholar]
- 46.Jang JH, Huang Y, Zheng P, Jo MC, Bertolet G, Zhu MX, Qin L, Liu D. J Immunol. 2015;195:1320–1330. doi: 10.4049/jimmunol.1403143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Biggs MJP, Milone MC, Santos LC, Gondarenko a, Wind SJ. J R Soc Interface. 2011;8:1462–1471. doi: 10.1098/rsif.2011.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rettig JR, Folch A. Anal Chem. 2005;77:5628–5634. doi: 10.1021/ac0505977. [DOI] [PubMed] [Google Scholar]
- 49.Yamanaka YJ, Berger CT, Sips M, Cheney PC, Alter G, Love JC. Integr Biol. 2012;4:1175. doi: 10.1039/c2ib20167d. [DOI] [PubMed] [Google Scholar]
- 50.Zaretsky I, Polonsky M, Shifrut E, Reich-Zeliger S, Antebi Y, Aidelberg G, Waysbort N, Friedman N. Lab Chip. 2012;12:5007. doi: 10.1039/c2lc40808b. [DOI] [PubMed] [Google Scholar]
- 51.Yin Z, Noren D, Wang CJ, Hang R, Levchenko A. Mol Syst Biol. 2008;4:232. doi: 10.1038/msb.2008.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Şen M, Ino K, Ramón-Azcón J, Shiku H, Matsue T. Lab Chip. 2013;13:3650–2. doi: 10.1039/c3lc50561h. [DOI] [PubMed] [Google Scholar]
- 53.Hu H, Eustace D, Merten CA. Lab Chip. 2015;15:3989–3993. doi: 10.1039/c5lc00686d. [DOI] [PubMed] [Google Scholar]
- 54.Lagus TP, Edd JF. RSC Adv. 2013;3:20512. [Google Scholar]
- 55.Schoeman RM, Kemna EWM, Wolbers F, van den Berg A. Electrophoresis. 2014;35:385–392. doi: 10.1002/elps.201300179. [DOI] [PubMed] [Google Scholar]
- 56.Lee PJ, Hung PJ, Shaw R, Jan L, Lee LP. Appl Phys Lett. 2005;86:1–3. [Google Scholar]
- 57.Chen Y-C, Cheng Y-H, Kim HS, Ingram PN, Nor JE, Yoon E. Lab Chip. 2014;14:2941–7. doi: 10.1039/c4lc00391h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Frimat J-P, Becker M, Chiang Y-Y, Marggraf U, Janasek D, Hengstler JG, Franzke J, West J. Lab Chip. 2011;11:231–237. doi: 10.1039/c0lc00172d. [DOI] [PubMed] [Google Scholar]
- 59.Skelley AM, Kirak O, Suh H, Jaenisch R, Voldman J. Nat Methods. 2009;6:147–152. doi: 10.1038/nmeth.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dura B, Liu Y, Voldman J. Lab Chip. 2014;14:2783. doi: 10.1039/c4lc00303a. [DOI] [PubMed] [Google Scholar]
- 61.Guo F, Li P, French JB, Mao Z, Zhao H, Li S, Nama N, Fick JR, Benkovic SJ, Huang TJ. Proc Natl Acad Sci U S A. 2015;112:43–8. doi: 10.1073/pnas.1422068112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guo F, Mao Z, Chen Y, Xie Z, Lata JP, Li P, Ren L, Liu J, Yang J, Dao M, Suresh S, Huang TJ. Proc Natl Acad Sci. 2016;113:1522–1527. doi: 10.1073/pnas.1524813113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yoshimura Y, Tomita M, Mizutani F, Yasukawa T. Anal Chem. 2014;86:6818–6822. doi: 10.1021/ac5015996. [DOI] [PubMed] [Google Scholar]
- 64.Junkin M, Tay S. Lab Chip. 2014;14:1246–60. doi: 10.1039/c3lc51182k. [DOI] [PubMed] [Google Scholar]
- 65.Satija R, Shalek AK. Trends Immunol. 2014;35:219–229. doi: 10.1016/j.it.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vanherberghen B, Olofsson PE, Forslund E, Sternberg-simon M, Khorshidi MA. Blood. 2016;121:1326–1335. doi: 10.1182/blood-2012-06-439851. [DOI] [PubMed] [Google Scholar]
- 67.Dura B, Dougan SK, Barisa M, Hoehl MM, Lo CT, Ploegh HL, Voldman J. Nat Commun. 2015;6:5940. doi: 10.1038/ncomms6940. [DOI] [PubMed] [Google Scholar]
- 68.Dura B, Servos MM, Barry RM, Ploegh HL, Dougan SK. Proc Natl Acad Sci. 2016 doi: 10.1073/pnas.1515364113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Faley S, Seale K, Hughey J, Schaffer DK, VanCompernolle S, McKinney B, Baudenbacher F, Unutmaz D, Wikswo JP. Lab Chip. 2008;8:1700–1712. doi: 10.1039/b719799c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Romain G, Senyukov V, Rey-Villamizar N, Merouane A, Kelton W, Liadi I, Mahendra A, Charab W, Georgiou G, Roysam B, Lee DA, Varadarajan N. Blood. 2014;124:3241–3249. doi: 10.1182/blood-2014-04-569061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liadi I, Singh H, Romain G, Rey-Villamizar N, Merouane A, Adolacion JRT, Kebriaei P, Huls H, Qiu P, Roysam B, Cooper LJN, Varadarajan N. Cancer Immunol Res. 2015;3:473–482. doi: 10.1158/2326-6066.CIR-14-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tauriainen JM, Gustafsson K, Göthlin M, Gertow J, Buggert M, Frisk TW, Karlsson AC, Uhlin M, Önfelt B. Front Immunol. 2015:6. doi: 10.3389/fimmu.2015.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Forslund E, Sohlberg E, Enqvist M, Olofsson PE, Malmberg K-J, Önfelt B. J Immunol. 2015;195:3374–3381. doi: 10.4049/jimmunol.1500171. [DOI] [PubMed] [Google Scholar]
- 74.Halldorsson S, Lucumi E, Gómez-Sjöberg R, Fleming RMT. Biosens Bioelectron. 2015;63:218–231. doi: 10.1016/j.bios.2014.07.029. [DOI] [PubMed] [Google Scholar]
- 75.Huppa JB, Davis MM. Nat Rev Immunol. 2003;3:973–983. doi: 10.1038/nri1245. [DOI] [PubMed] [Google Scholar]
- 76.Ogle BM, Cascalho M, Platt JL. Nat Rev Mol Cell Biol. 2005;6:567–575. doi: 10.1038/nrm1678. [DOI] [PubMed] [Google Scholar]
- 77.Lu X, Kang Y. Cancer Res. 2009;69:8536–8539. doi: 10.1158/0008-5472.CAN-09-2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Noubissi FK, Harkness T, Alexander CM, Ogle BM. FASEB J. 2015;29:4036–4045. doi: 10.1096/fj.15-271098. [DOI] [PubMed] [Google Scholar]
- 79.Rems L, Ušaj M, Kandušer M, Reberšek M, Miklavčič D, Pucihar G. Sci Rep. 2013;3:3382. doi: 10.1038/srep03382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang P-F, Wang C-H, Lee G-B. Sci Rep. 2016;6:22036. doi: 10.1038/srep22036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pawelek JM, Chakraborty AK. In: Advances in Cancer Research. BT-A, editor. Vol. 101. C. Research, Academic Press; 2008. pp. 397–444. [DOI] [PubMed] [Google Scholar]
- 82.Ogunniyi AO, Story CM, Papa E, Guillen E, Love JC. Nat Protoc. 2009;4:767–782. doi: 10.1038/nprot.2009.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lu Y, Xue Q, Eisele MR, Sulistijo ES, Brower K, Han L, Amir ED, Pe’er D, Miller-Jensen K, Fan R. Proc Natl Acad Sci. 2015;112:E607–E615. doi: 10.1073/pnas.1416756112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yamanaka YJ, Szeto GL, Gierahn TM, Forcier TL, Benedict KF, Brefo MSN, Lauffenburger DA, Irvine DJ, Love JC. Anal Chem. 2012;84:10531–10536. doi: 10.1021/ac302264q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Deng Y, Zhang Y, Sun S, Wang Z, Wang M, Yu B, Czajkowsky DM, Liu B, Li Y, Wei W, Shi Q. Sci Rep. 2014;4:7499. doi: 10.1038/srep07499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhao W, Schafer S, Choi J, Yamanaka YJ, Lombardi ML, Bose S, Carlson AL, Phillips JA, Teo W, Droujinine IA, Cui CH, Jain RK, Lammerding J, Love JC, Lin CP, Sarkar D, Karnik R, Karp JM. Nat Nanotechnol. 2011;6:524–531. doi: 10.1038/nnano.2011.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tumarkin E, Tzadu L, Csaszar E, Seo M, Zhang H, Lee A, Peerani R, Purpura K, Zandstra PW, Kumacheva E. Integr Biol. 2011;3:653–662. doi: 10.1039/c1ib00002k. [DOI] [PubMed] [Google Scholar]
- 88.Gao Y, Broussard J, Haque A, Revzin A, Lin T. Microsystems Nanoeng. 2016;2:15045. doi: 10.1038/micronano.2015.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shi M, Majumdar D, Gao Y, Brewer BM, Goodwin CR, McLean Ja, Li D, Webb DJ. Lab Chip. 2013;13:3008–21. doi: 10.1039/c3lc50249j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Majumdar D, Gao Y, Li D, Webb DJ. J Neurosci Methods. 2011;196:38–44. doi: 10.1016/j.jneumeth.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shin Y, Han S, Jeon JS, Yamamoto K, Zervantonakis IK, Sudo R, Kamm RD, Chung S. Nat Protoc. 2012;7:1247–1259. doi: 10.1038/nprot.2012.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen Y-C, Ingram P, Yoon E. Analyst. 2014;139:6371–6378. doi: 10.1039/c4an01282h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wong AP, Perez-Castillejos R, Christopher Love J, Whitesides GM. Biomaterials. 2008;29:1853–1861. doi: 10.1016/j.biomaterials.2007.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kwa T, Zhou Q, Gao Y, Rahimian A, Kwon L, Liu Y, Revzin A. Lab Chip. 2014:1695–1704. doi: 10.1039/c4lc00037d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhou Q, Patel D, Kwa T, Haque A, Matharu Z, Stybayeva G, Gao Y, Diehl AM, Revzin A. Lab Chip. 2015;15:4467–4478. doi: 10.1039/c5lc00874c. [DOI] [PubMed] [Google Scholar]
- 96.Menon NV, Chuah YJ, Phey S, Zhang Y, Wu Y, Chan V, Kang Y. ACS Appl Mater Interfaces. 2015;7:17095–17103. doi: 10.1021/acsami.5b03753. [DOI] [PubMed] [Google Scholar]
- 97.Han J, Menon NV, Kang Y, Tee S-Y. J Mater Chem B. 2015;3:1565–1572. doi: 10.1039/c4tb01783h. [DOI] [PubMed] [Google Scholar]
- 98.Chen Y-C, Allen SG, Ingram PN, Buckanovich R, Merajver SD, Yoon E. Sci Rep. 2015;5:9980. doi: 10.1038/srep09980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Berthier E, Beebe DJ. Lab Chip. 2014:3241–3247. doi: 10.1039/c4lc00448e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang Y, Zhang W, Qin L. Angew Chemie - Int Ed. 2014;53:2344–2348. doi: 10.1002/anie.201309885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Byrne MB, Trump L, Desai AV, Schook LB, Gaskins HR, Kenis PJA. Biomicrofluidics. 2014;8:1–9. doi: 10.1063/1.4887098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Aref AR, Huang RY-J, Yu W, Chua K-N, Sun W, Tu T-Y, Bai J, Sim W-J, Zervantonakis IK, Thiery JP, Kamm RD. Integr Biol (Camb) 2013;5:381–9. doi: 10.1039/c2ib20209c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bai J, Tu T, Kim C, Thiery JP, Kamm RD. Oncotarget. 2015:6. doi: 10.18632/oncotarget.5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu T, Lin B, Qin J, Toh Y-C, Zhang C, Zhang J, Khong YM, Chang S, Samper VD, van Noort D, Hutmacher DW, Yu H. Lab Chip. 2007;7:302–309. doi: 10.1039/b614872g. [DOI] [PubMed] [Google Scholar]
- 105.Zhang K, Chou C-K, Xia X, Hung M-C, Qin L. Proc Natl Acad Sci. 2014;111:2948–2953. doi: 10.1073/pnas.1313661111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Businaro L, De Ninno A, Schiavoni G, Lucarini V, Ciasca G, Gerardino A, Belardelli F, Gabriele L, Mattei F. Lab Chip. 2013;13:229–239. doi: 10.1039/c2lc40887b. [DOI] [PubMed] [Google Scholar]
- 107.Park JW, Vahidi B, Taylor AM, Rhee SW, Jeon NL. Nat Protoc. 2006;1:2128–36. doi: 10.1038/nprot.2006.316. [DOI] [PubMed] [Google Scholar]
- 108.Taylor AM, Dieterich DC, Ito HT, Kim SA, Schuman EM. Neuron. 2010;66:57–68. doi: 10.1016/j.neuron.2010.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Taylor AM, Rhee SW, Tu CH, Cribbs DH, Cotman CW. Langmuir. 2010;19:1551–1556. doi: 10.1021/la026417v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Taylor AM, Blurton-Jones M, Rhee SW, Cribbs DH, Cotman CW, Jeon NL. Nat Methods. 2005;2:599–605. doi: 10.1038/nmeth777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Coquinco A, Kojic L, Wen W, Wang YT, Jeon NL, Milnerwood AJ, Cynader M. Mol Cell Neurosci. 2014;60:43–52. doi: 10.1016/j.mcn.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 112.Ullian EM, Sapperstein SK, Christopherson KS, Barres Ba. Science. 2001;291:657–661. doi: 10.1126/science.291.5504.657. [DOI] [PubMed] [Google Scholar]
- 113.Cohen MS, Orth CB, Kim HJ, Jeon NL, Jaffrey SR. Proc Natl Acad Sci U S A. 2011;108:11246–11251. doi: 10.1073/pnas.1012401108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Uchholz DAEB, Ennington BROP, Roze ROHC. Stem Cells Transl Med. 2013:384–393. doi: 10.5966/sctm.2012-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gonzalez-Cordero A, West EL, Pearson RA, Duran Y, Carvalho LS, Chu CJ, Naeem A, Blackford SJ, Georgiadis A, Lakowski J, Hubank M, Smith AJ, Bainbridge JW, Sowden JC, Ali RR. Nat Biotechnol. 2013;31:741–747. doi: 10.1038/nbt.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Su P-J, Liu Z, Zhang K, Han X, Saito Y, Xia X, Yokoi K, Shen H, Qin L. Sci Rep. 2015;5:13591. doi: 10.1038/srep13591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Peterman MC, Mehenti NZ, Bilbao KV, Lee CJ, Leng T, Noolandi J, Bent SF, Blumenkranz MS, Fishman HA. Artif Organs. 2003;27:975–985. doi: 10.1046/j.1525-1594.2003.07307.x. [DOI] [PubMed] [Google Scholar]
- 118.Dodson KH, Echevarria FD, Li D, Sappington RM, Edd JF. Biomed Microdevices. 2015;17:114. doi: 10.1007/s10544-015-0019-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Winkler M, Simon MG, Vu T, Gartner TL, Jester JV, Lee AP, Brown DJ. Biomed Microdevices. 2014;16:255–267. doi: 10.1007/s10544-013-9829-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Abbaci M, Barberi-Heyob M, Blondel W, Guillemin F, Didelon J. Biotechniques. 2008;45:33–62. doi: 10.2144/000112810. [DOI] [PubMed] [Google Scholar]
- 121.Buszczak M, Inaba M, Yamashita YM. Trends Cell Biol. 2016;xx:1–9. doi: 10.1016/j.tcb.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mattila PK, Lappalainen P. Nat Rev Mol Cell Biol. 2008;9:446–454. doi: 10.1038/nrm2406. [DOI] [PubMed] [Google Scholar]
- 123.Keller L, Surette MG. Nat Rev Microbiol. 2006;4:249–58. doi: 10.1038/nrmicro1383. [DOI] [PubMed] [Google Scholar]
- 124.Hense Ba, Kuttler C, Müller J, Rothballer M, Hartmann A, Kreft J-U. Nat Rev Microbiol. 2007;5:230–239. doi: 10.1038/nrmicro1600. [DOI] [PubMed] [Google Scholar]
- 125.Hammer BK, Bassler BL. Mol Microbiol. 2003;50:101–114. doi: 10.1046/j.1365-2958.2003.03688.x. [DOI] [PubMed] [Google Scholar]
- 126.Hoang HH, Gurich N, Gonz??lez JE. J Bacteriol. 2008;190:861–871. doi: 10.1128/JB.01310-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Redfield RJ. Trends Microbiol. 2002;10:365–370. doi: 10.1016/s0966-842x(02)02400-9. [DOI] [PubMed] [Google Scholar]
- 128.Abhyankar VV, Beebe DJ. Anal Chem. 2007;79:4066–4073. doi: 10.1021/ac062371p. [DOI] [PubMed] [Google Scholar]
- 129.Chueh B-H, Huh D, Kyrtsos CR, Houssin TE, Futai N, Takayama S. Anal Chem. 2007;79:3504–3508. doi: 10.1021/ac062118p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kim HJ, Boedicker JQ, Choi JW, Ismagilov RF. Proc Natl Acad Sci U S A. 2008;105:18188–18193. doi: 10.1073/pnas.0807935105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Park S, Hong X, Choi WS, Kim T. Lab Chip. 2012;12:3914. doi: 10.1039/c2lc40294g. [DOI] [PubMed] [Google Scholar]
- 132.Luo X, Tsao C-Y, Wu H-C, Quan DN, Payne GF, Rubloff GW, Bentley WE. Lab Chip. 2015;15:1842–1851. doi: 10.1039/c5lc00107b. [DOI] [PubMed] [Google Scholar]
- 133.Poujade M, Grasland-Mongrain E, Hertzog a, Jouanneau J, Chavrier P, Ladoux B, Buguin a, Silberzan P. Proc Natl Acad Sci U S A. 2007;104:15988–15993. doi: 10.1073/pnas.0705062104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nie F-Q, Yamada M, Kobayashi J, Yamato M, Kikuchi A, Okano T. Biomaterials. 2007;28:4017–4022. doi: 10.1016/j.biomaterials.2007.05.037. [DOI] [PubMed] [Google Scholar]
- 135.van der Meer AD, Vermeul K, Poot Aa, Feijen J, Vermes I. Am J Physiol Heart Circ Physiol. 2010;298:H719–H725. doi: 10.1152/ajpheart.00933.2009. [DOI] [PubMed] [Google Scholar]
- 136.Huang X, Li L, Tu Q, Wang J, Liu W, Wang X, Ren L, Wang J. Microfluid Nanofluidics. 2011;10:1333–1341. [Google Scholar]
- 137.Felder M, Sallin P, Barbe L, Haenni B, Gazdhar A, Geiser T, Guenat O. Lab Chip. 2012;12:640–646. doi: 10.1039/c1lc20879a. [DOI] [PubMed] [Google Scholar]
- 138.Wei Y, Chen F, Zhang T, Chen D, Jia X, Wang J, Guo W, Chen J. Sci Rep. 2015;5:14049. doi: 10.1038/srep14049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sticker D, Lechner S, Jungreuthmayer C, Zanghellini J, Ertl P. Anal Chem. 2017 doi: 10.1021/acs.analchem.6b03886. [DOI] [PubMed] [Google Scholar]
- 140.Iourov IY, Vorsanova SG, Yurov YB. Curr Genomics. 2012;13:477–488. doi: 10.2174/138920212802510439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Park JW, Kim HJ, Kang MW, Jeon NL. Lab Chip. 2013;13:509–521. doi: 10.1039/c2lc41081h. [DOI] [PubMed] [Google Scholar]
- 142.Renault R, Sukenik N, Descroix S, Malaquin L, Viovy J-L, Peyrin J-M, Bottani S, Monceau P, Moses E, Vignes M. PLoS One. 2015;10:e0120680. doi: 10.1371/journal.pone.0120680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lugini L, Matarrese P, Tinari A, Lozupone F, Federici C, Iessi E, Gentile M, Luciani F, Parmiani G, Rivoltini L, Malorni W, Fais S. Cancer Res. 2006;66:3629 LP-3638. doi: 10.1158/0008-5472.CAN-05-3204. [DOI] [PubMed] [Google Scholar]
- 144.Overholtzer M, Mailleux AA, Mouneimne G, Normand G, Schnitt SJ, King RW, Cibas ES, Brugge JS. Cell. 2016;131:966–979. doi: 10.1016/j.cell.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 145.Bang C, Thum T. Int J Biochem Cell Biol. 2012;44:2060–2064. doi: 10.1016/j.biocel.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 146.Zhao Z, Yang Y, Zeng Y, He M. Lab Chip. 2016;16:489–496. doi: 10.1039/c5lc01117e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Record M, Carayon K, Poirot M, Silvente-Poirot S. Biochim Biophys Acta - Mol Cell Biol Lipids. 2014;1841:108–120. doi: 10.1016/j.bbalip.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 148.Sharghi-Namini S, Tan E, Ong L-LS, Ge R, Asada HH. Sci Rep. 2014;4:4031. doi: 10.1038/srep04031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kuhn E, Naschberger E, Konrad A, Croner RS, Britzen-Laurent N, Jochmann R, Munstedt H, Sturzl M. Lab Chip. 2012;12:1363–1372. doi: 10.1039/c2lc20724a. [DOI] [PubMed] [Google Scholar]
- 150.Han X, Liu Z, Zhao L, Wang F, Yu Y, Yang J, Chen R, Qin L. Angew Chem Int Ed Engl. 2016;55:8561–8565. doi: 10.1002/anie.201601984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Stewart MP, Sharei A, Ding X, Sahay G, Langer R, Jensen KF. Nature. 2016;538:183–192. doi: 10.1038/nature19764. [DOI] [PubMed] [Google Scholar]
- 152.Yusof A, Keegan H, Spillane CD, Sheils OM, Martin CM, O’Leary JJ, Zengerle R, Koltay P. Lab Chip. 2011;11:2447–2454. doi: 10.1039/c1lc20176j. [DOI] [PubMed] [Google Scholar]
- 153.Schiele NR, Corr DT, Huang Y, Raof NA, Xie Y, Chrisey DB. Biofabrication. 2010;2:32001. doi: 10.1088/1758-5082/2/3/032001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gruene M, Pflaum M, Hess C, Diamantouros S, Schlie S, Deiwick A, Koch L, Wilhelmi M, Jockenhoevel S, Haverich A, Chichkov B. Tissue Eng Part C Methods. 2011;17:973–982. doi: 10.1089/ten.tec.2011.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pampaloni F, Reynaud EG, Stelzer EHK. Nat Rev Mol Cell Biol. 2007;8:839–845. doi: 10.1038/nrm2236. [DOI] [PubMed] [Google Scholar]
- 156.Huh D, Hamilton GA, Ingber DE. Trends Cell Biol. 2011;21:745–754. doi: 10.1016/j.tcb.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mosadegh B, Lockett MR, Minn KT, Simon KA, Gilbert K, Hillier S, Newsome D, Li H, Hall AB, Boucher DM, Eustace BK, Whitesides GM. Biomaterials. 2015;52:262–271. doi: 10.1016/j.biomaterials.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 158.Simon KA, Park KM, Mosadegh B, Subramaniam AB, Mazzeo AD, Ngo PM, Whitesides GM. Biomaterials. 2014;35:259–268. doi: 10.1016/j.biomaterials.2013.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Derda R, Laromaine A, Mammoto A, Tang SKY, Mammoto T, Ingber DE, Whitesides GM. Proc Natl Acad Sci U S A. 2009;106:18457–18462. doi: 10.1073/pnas.0910666106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Camci-Unal G, Newsome D, Eustace BK, Whitesides GM. Adv Healthc Mater. 2016;5:641–647. doi: 10.1002/adhm.201500709. [DOI] [PubMed] [Google Scholar]
- 161.Mosadegh B, Dabiri BE, Lockett MR, Derda R, Campbell P, Parker KK, Whitesides GM. Adv Healthc Mater. 2014;3:1036–1043. doi: 10.1002/adhm.201300575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Aim Biotech. [Accessed Nov 2016]; http://www.aimbiotech.com/
- 163.Xona Microfluid. [Accessed Nov 2016]; http://xonamicrofluidics.com/
- 164.Ananda Devices. [Accessed Nov 2016]; http://www.anandadevices.com/
- 165.Ibidi. [Accessed Nov 2016]; http://ibidi.com/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.