Abstract
Background and Purpose:
The mobility of individuals who are obese can be limited compared with their healthy weight counterparts. Lower limb strength has been associated with mobility, and reduced strength may contribute to mobility limitation among individuals who are obese. However, our understanding of the effects of obesity on lower limb strength is limited. The purpose of this study was to investigate the effects of obesity and age on extension and flexion strength at the hip, knee, and ankle.
Methods:
Using a cross-sectional design, 10 younger (18-30 years) healthy weight (body mass index = 18-24.9 kg/m2), 10 younger obese (body mass index >30 kg/m2), 10 older (65-80 years) healthy weight, and 10 older obese female participants performed isokinetic maximum voluntary contractions in ankle plantar flexion (PF), ankle dorsiflexion (DF), knee extension (KE), knee flexion (KF), hip extension (HE), and hip flexion (HF).
Results and Discussion:
Absolute strength among obese participants was 29% higher in DF (P = .002), 27% higher in KE (P = .004), and 23% higher in HF (P = .001), compared with healthy weight participants. Strength relative to body mass among obese participants was 31% lower in PF (P < .001), 14% lower in DF (P = .042), 16% lower in KE (P = .015), 27% lower in KF (P < .001), 29% lower in HE (P < .001), and 19% lower in HF (P = .001).
Conclusions:
Obese females exhibited lower relative strength at the ankle and hip, similar to the lower relative strength exhibited at the knee. Obese females also exhibited higher absolute strength, but only for 3 of 6 lower limb exertions investigated. This lack of uniformity across the 6 exertions is likely due to the still unclear underlying biomechanical mechanism responsible for these strength differences, which may also be influenced by aging. The effects of obesity on lower limb strength were also generally consistent between the 2 age groups investigated.
Keywords: aging, lower extremity strength, obesity
INTRODUCTION
More than one-third of adults in the United States are obese.1 One of the many problems associated with obesity is limited mobility,2–4 which can include difficulty rising from a chair,5,6 difficulty ascending or descending from stairs,7 lower gait speed,8,9 and poorer balance.10–12 This limited mobility has been related to having less lower limb strength than body mass and higher lower limb strength demands due to having additional body mass.13
Individuals who are obese exhibit altered lower limb strength compared with healthy weight individuals.14–18 Lower limb strength can be characterized in terms of absolute strength and relative strength. Absolute strength can be defined as the maximum force or net muscle moment that can be generated at a joint and is expressed in units of newtons or Newton·meters (N·m). Relative strength is typically determined as absolute strength divided by body mass14,18 or fat-free mass.15,17 Knee extensor strength among individuals who are obese is higher when expressed as absolute strength14–17 but lower when expressed as relative strength.14,17 The higher absolute strength is thought to be a neuromuscular adaptation to the long-term exposure to increased body weight14,15 since the knee extensors play a major role in supporting the body against gravity while standing and walking. Consistent with this hypothesis, absolute strength of the knee flexors, which do not play a major role in supporting the body against gravity while standing or walking, is not higher among individuals who are obese.14,15 Despite the importance of hip and ankle strength on mobility,19–21 no studies to our knowledge have investigated obesity-related differences in strength at these joints.
The prevalence of obesity among adults older than 60 years has increased from 31% in 2003-2004 to 35% in 2011-2012.1 This growth in the older obese population provides motivation for understanding how obesity affects strength among older adults, particularly because of the association between lower limb strength and mobility.22 Although absolute knee extensor strength is higher among individuals who are obese, the typical loss of strength associated with aging23,24 may result in obesity-related differences in absolute strength, being smaller among older adults than among young adults. Although relative knee extensor strength is lower among individuals who are obese, the typical loss of strength associated with aging may result in obesity-related differences in relative strength, being larger among older adults than among young adults. Obesity is associated with higher absolute knee extensor strength25 and lower relative knee extensor strength among older adults,18 but no comparison of these effects was performed between young and older adults. Additional studies are needed to more fully understand the interaction of obesity and age on lower limb strength.
The purpose of this study was to investigate the effects of obesity and age on extension and flexion strength at the hip, knee, and ankle. These data will help elucidate whether the effects of obesity on strength differ between lower limb joints, between extension and flexion directions, and between younger and older adults. Such information may be useful in developing strength training interventions among obese/older adults for maintaining mobility. We focused on females because of their higher prevalence of obesity,1 higher prevalence of obesity-related mobility limitation,4 and higher rate of falls and fall-related injuries.26 The specific hypotheses investigated were that (1) absolute strength would be higher among obese females than among the healthy weight females, (2) relative strength would be lower among obese females than among the healthy weight females, (3) the effects of obesity on absolute strength would be smaller among older females than among younger females, and (4) the effects of obesity on relative strength would be larger among older females than among younger females.
METHODS
Participants
This cross-sectional study was conducted in a university biomechanics research laboratory over a period of 11 months. Forty adult women completed the study, including 10 younger (18-30 years) and healthy weight (body mass index [BMI] = 18-24.9 kg/m2), 10 younger and obese (BMI >30 kg/m2), 10 older (65-80 years) and healthy weight, and 10 older and obese (Table 1). The number of participants in each group was based upon a sample size analysis using data from 2 studies investigating obesity-related differences in isokinetic knee extension (KE) strength among young adults.14,17 These analyses indicated 10 healthy weight and 10 obese participants were needed to detect a main effect of obesity with 0.70 power. To be conservative, we recruited 20 healthy weight and 20 obese participants (equally split between younger and older age groups). Participants were recruited from the university and local community using Web and e-mail announcements, flyers, and newspaper advertisements. Participants were required to pass a screening to exclude individuals with self-reported neurologic, cardiac, or musculoskeletal conditions that might jeopardize their safety during testing. In addition, all participants were body mass stable (<2.3 kg) for the prior 6 months and had no obvious balance problems. Participants completed the Godin Leisure-Time Exercise Questionnaire27 to quantify their habitual physical activity level, given that higher physical activity levels may influence lower limb strength. Participants who performed 1 hour or more of moderate exercise more than 3 to 4 times a week were excluded from the study. Body fat percentage was measured using a Lange skinfold caliper (Cambridge Scientific Industries, Cambridge, Massachusetts) at 4 locations including superficial to the triceps and biceps, inferior to the scapula, and superior to the lateral iliac crest. These measurements were summed, and body fat percentage was predicted from this sum according to the caliper manufacturer's specifications. The study was approved by the university institutional review board, and written informed consent was obtained from all participants prior to participation.
Table 1. Participant Characteristics (Median [IQR]).
| Group | Younger Healthy Weight | Younger Obese | Older Healthy Weight | Older Obese |
|---|---|---|---|---|
| Age,a y | 20.5 (4.5) | 22.0 (5.8) | 69.0 (8.8) | 68.5 (8.5) |
| Height,a cm | 165.0 (5.7) | 168.5 (7.3) | 161.0 (10.4) | 161.8 (9.0) |
| Mass,a,b kg | 62.1 (10.3) | 94.1 (9.5) | 59.0 (8.8) | 87.8 (8.5) |
| BMI,b kg/m2 | 22.4 (3.2) | 33.1 (4.3) | 22.2 (5.8) | 32.5 (4.2) |
| Body fat,a,b,c % | 23.4 (1.9) | 35.3 (4.3) | 33.8 (7.7) | 42.6 (4.2) |
| Godin score | 32.0 (19.8) | 24.0 (30.0) | 23.5 (20.0) | 25.5 (20.0) |
Abbreviations: BMI, body mass index; IQR, interquartile range.
aA main effect of age group (P < .05).
bA main effect of obesity group (P < .05).
cBody fat was determined with a Lange skinfold caliper (Cambridge Scientific Industries, Cambridge, Massachusetts).
Instrumentation and Procedure
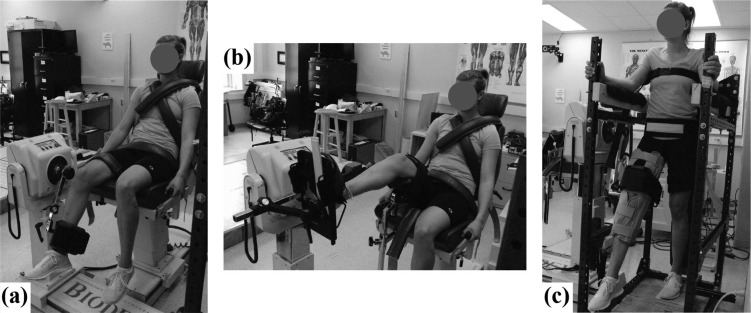
Participants completed 2 sessions. Knee strength was measured during the first session, and ankle and hip strength was measured during the second session. All strength measurements were performed on the right lower limb, which was the preferred limb to kick a ball. The strength testing protocol was adapted from prior work.28 Strength was measured as the maximum net muscle moment during concentric isokinetic maximum voluntary contractions (MVCs). These MVCs were performed using a Biodex System 3 dynamometer (Biodex Medical Systems, Inc, Shirley, New York). The setup can be seen in Figure 1.
Figure 1.
Setup for strength measurements at the (A) knee, (B) ankle, and (C) hip.
In the first session, knee strength was measured while participants were secured, using straps, in a seated posture with their hip flexed approximately 70°.28 Relaxed trials were performed first to measure the passive elastic/gravitational moment over the entire joint range of motion. Participants were instructed to remain relaxed while the Biodex attachment moved at 5°/s through the range of motion at least 3 times. Participants then performed concentric isokinetic MVCs in KE and knee flexion (KF) at 75°/s. This velocity was chosen on the basis of prior studies.15,17,28 A total of 4 MVCs were completed for each exertion direction. Prior to data collection, participants performed 1 practice trial for each exertion.
In the second session, ankle and hip strength was measured in this order. The general testing protocol was similar to that used for the knee but with different body positions and isokinetic velocities. Concentric isokinetic MVCs in ankle plantar flexion (PF) and dorsiflexion (DF) were performed at 60°/s in a seated position while the knees and hip were flexed 50° and 80°, respectively. Concentric isokinetic MVCs in hip extension (HE) and hip flexion (HF) were performed at 60°/s in a standing position while the knees were held in a near fully extended position. These joint angles and angular velocities were chosen on the basis of prior studies.28,29
Joint angle, angular velocity, and moment were sampled from the dynamometer at 200 Hz and low-pass filtered at 5 Hz (fourth-order Butterworth filter). The passive elastic/gravitational moment was estimated by fitting a curvilinear line (least square) to moment data from relaxed trials throughout the range of motion and was subtracted from each MVC trial.28 The isokinetic region was identified for each trial, defined by where the acceleration was negligible, and the peak moment in that region was determined. The maximum moment across the 4 trials for each joint/extension-flexion combination was used as the absolute strength in subsequent analyses. Relative strength was determined by normalizing this strength measurement to body mass. All postprocessing was performed in Matlab (MathWorks Inc, Natick, Massachusetts).
Statistical Analysis
Separate 2-way analyses of covariance were used to determine the effects of obesity (healthy weight or obese), age (younger or older), and their interaction on each strength measurement. Strength measurements included absolute and relative strength in PF, DF, KE, KF, HE, and HF. Godin score was used as a covariate. The first and second hypotheses were tested using the main effects of obesity. The main effects of age are also reported, but these were not a major focus since they have been reported in previous studies. The third and fourth hypotheses were tested using the age × obesity interaction. In the event of a significant obesity × age interaction, simple effects testing was used to assess the effects of obesity within each age group and the effects of age within each obesity group. No data were missing during the analyses. Effect sizes were reported using the partial eta squared (ηp2). Percent differences and absolute differences reported in the “Results” section were least-squares means to account for the influence of the Godin score on strength measurements. Statistical analyses were performed using JMP Pro 10 (SAS Institute, Inc, Cary, North Carolina), with a significance level of P < .05.
RESULTS
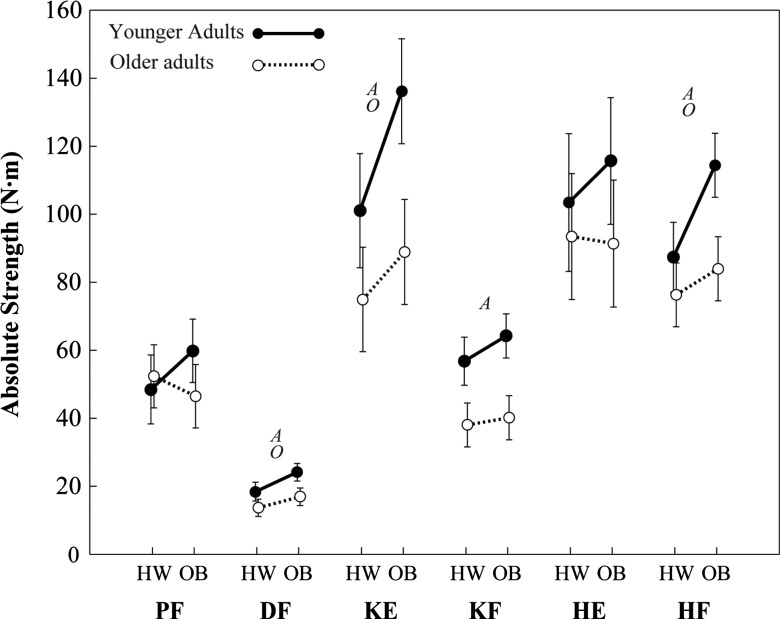
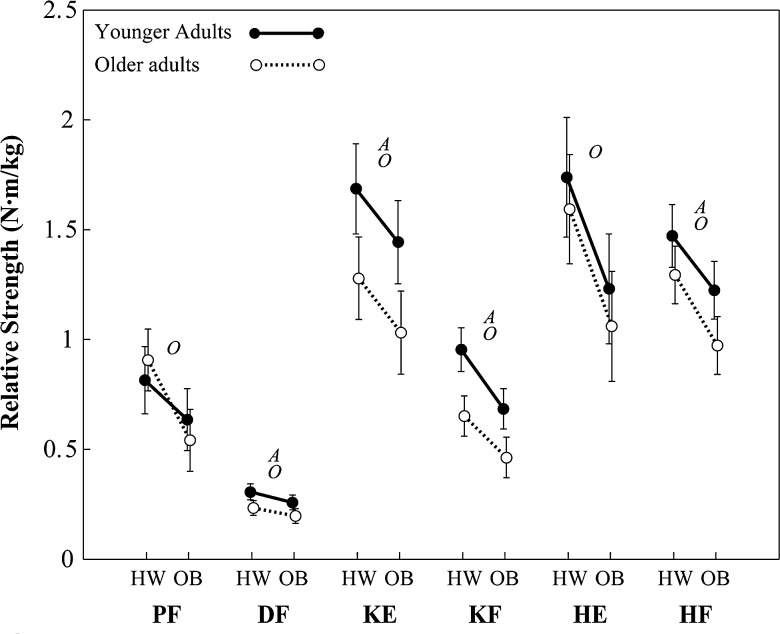
Absolute and relative strength among younger and older groups is shown in Figures 2 and 3. Several main effects of obesity were observed on both absolute strength and relative strength (see Table 2 for P values and effect sizes). No strength variables exhibited a significant obesity × age interaction. Absolute strength among obese participants was 29% (4.7 N·m) higher in DF (P = .002), 27% (24.1 N·m) higher in KE (P = .004), and 23% (18.5 N·m) higher in HF (P < .001), compared with healthy weight participants. Relative strength among obese participants was 31% (0.26 N·m/kg) lower in PF (P < .001), 14% (0.04 N·m/kg) lower in DF (P = .042), 16% (0.24 N·m/kg) lower in KE (P = .015), 27% (0.21 N·m/kg) lower in KF (P < .001), 29% (0.48 N·m/kg) lower in HE (P < .001), and 19% (0.25 N·m/kg) lower in HF (P = .001), compared with healthy weight participants.
Figure 2.
Least-squares means of absolute strength (error bars indicate upper and lower 95th confidence intervals). HW indicates healthy weight; OB, obese; A, main effect of age; O, main effect of obesity; PF, plantar flexors; DF, dorsiflexors; KE, knee extensors; KF, knee flexors; HE, hip extensors; and HF, hip flexors. The actual values plotted here are available from the corresponding author upon request.
Figure 3.
Least-squares means of relative strength (error bars indicate upper and lower 95th confidence intervals). HW indicates healthy weight; OB, obese; A, main effect of age; O, main effect of obesity; PF, plantar flexors; DF, dorsiflexors; KE, knee extensors; KF, knee flexors; HE, hip extensors; and HF, hip flexors. The actual values plotted here are available from the corresponding author upon request.
Table 2. Effects of Obesity, Age, and Obesity × Age on Exertions (P value [Effect Size]).
| Muscle Group | Absolute Strength, N·m | Relative Strength, N·m/kg | ||||
|---|---|---|---|---|---|---|
| Obese vs Healthy Weight | Older vs Younger Adults | Obesity × Age | Obese vs Healthy Weight | Older vs Younger Adults | Obesity × Age | |
| Plantar flexor | .54 (0.011) | .19 (0.048) | .13 (0.065) | <.01 (0.292) | .83 (0.001) | .26 (0.036) |
| Dorsiflexor | <.01 (0.252) | <.01 (0.401) | .35 (0.025) | .04 (0.113) | <.01 (0.324) | .82 (0.001) |
| Knee extensor | <.01 (0.214) | <.01 (0.429) | .32 (0.029) | .01 (0.158) | <.01 (0.383) | .80 (0.002) |
| Knee flexor | .087 (0.082) | <.01 (0.570) | .36 (0.025) | <.01 (0.390) | <.01 (0.467) | .47 (0.015) |
| Hip extensor | .45 (0.016) | .05 (0.103) | .44 (0.017) | <.01 (0.299) | .21 (0.045) | .88 (0.001) |
| Hip flexor | <.01 (0.275) | <.01 (0.367) | .07 (0.089) | <.01 (0.265) | <.01 (0.228) | .6 (0.008) |
Several main effects of age were also observed on both absolute strength and relative strength (Table 2). Absolute strength among older participants was 30% (6.6 N·m) lower in DF (P < .001), 33% (39.7 N·m) lower in KE (P < .001), 35% (20.9 N·m) lower in KF (P < .001), and 22% (22.8 N·m) lower in HF (P < .001), compared with younger participants. Relative strength among older participants was 25% (0.07 N·m/kg) lower in DF (P < .001), 27% (0.44 N·m/kg) lower in KE (P < .001), 31% (0.25 N·m/kg) lower in KF (P < .001), and 17% (0.23 N·m/kg) lower in HF (P = .003), compared with younger participants.
DISCUSSION
The purpose of this study was to investigate the effects of obesity and age on extension and flexion strength at the hip, knee, and ankle. Our first hypothesis was that absolute strength would be higher among obese females than among healthy weight females. This hypothesis was supported for DF, KE, and HF since all 3 were higher among obese participants. Our second hypothesis was that relative strength would be lower among obese females than among healthy weight females. This hypothesis was supported for all 6 joint/extension-flexion combinations since relative strength was lower among obese participants. Our third hypothesis was that the effects of obesity on absolute strength would be smaller among older females than among younger females. This hypothesis was not supported for any joint/extension-flexion combination since no obesity × age interaction effect was observed. Our fourth hypothesis was that the effects of obesity on relative strength would be larger among older females than among younger females. This hypothesis was also not supported. These results provide 3 general findings. First, obese females exhibited lower relative strength at the ankle (PF and DF) and hip (HE and HF) similar to the lower relative strength they exhibited at the knee (KE and KF) found here and reported elsewhere. Second, the magnitude of these differences in relative strength, and the existence of differences in absolute strength, differed between the 6 joints/extension-flexion combinations investigated. Third, the effects of obesity on lower limb strength were generally consistent between the 2 age groups investigated.
Strength differences at the knee found here were consistent with those of previous reports but differed in magnitude. Absolute KE strength was 27% higher among obese females than among Healthy weight females, which was slightly larger than 12% to 20% differences reported elsewhere.14,15,17,18 Relative KE strength was 16% lower among obese females than among Healthy weight females, which is moderate compared with 32% and 7% differences reported elsewhere.14,17,18 Absolute KF strength did not differ between obese and healthy weight females or between obese and healthy weight participants reported elsewhere.14,15 Relative KF strength was 27% lower among obese females than among Healthy weight females, which was somewhat smaller than the 41% difference reported elsewhere.14 At least 3 factors are present that may contribute to the differences in magnitude between the current and prior studies. The first factor is a difference in age of participants between these studies. General age ranges in the noted prior studies included exclusively younger adults (eg, 20-40 years),14,17 or essentially the entire adult age range (eg, 20-79 years).15,18 The second factor is a difference in BMI of participants between these studies. Prior studies have included obese participants with BMIs of 31 to 43 kg/m2,14 over 35 kg/m2,17 and over 26.4 kg/m2,18 whereas the present study included obese participants with a range in BMI of 30 to 37 kg/m2. The third factor is a difference in gender of participants between these studies. Prior studies have included only female participants,14,15 only male participants,17 and both genders,18 and the effects of obesity on lower limb strength may be influenced by gender. Despite these discrepancies in participant characteristics and the magnitude of strength differences between studies, the differences in absolute and relative KE and KF between obese and healthy weight participants appear relatively consistent across studies.
The underlying cause for the higher absolute strength among obese participants remains unclear. The higher KE absolute strength among obese participants has been attributed to a neuromuscular training effect from long-term exposure to higher body weight.14,15 This seems reasonable, given that the KE muscle group plays a major role in supporting the body against gravity, for example, while standing and walking. However, DF and HF also exhibited higher absolute strength among obese participants, but these exertions are not typically associated with body support during standing or gait.30 Instead, PF and HE have been associated with body support during gait30,31 but did not exhibit higher absolute strength among obese participants. Peak net muscle moments in HE and HF are fairly similar during gait,32 suggesting that any increase in strength, if mediated by net muscle moments during gait, would not seem to differ between these 2 exertions. This seems inconsistent with our results that absolute strength increased for HF but not for HE. One possible explanation for these findings at the hip is that the higher HF absolute strength among obese participants could be a secondary effect of the higher KE absolute strength since the rectus femoris muscle contributes to both KE and HF. However, no such biarticular muscle can help explain the unexpected increase in DF but not PF. Peak net muscle moments in DF during standing and gait are typically much smaller than in PF and not traditionally thought to play a major role in supporting the body against gravity. It is also interesting to note that the 3 exertions that exhibited higher absolute strength among obese participants (HF, KE, and DF) exhibited smaller effect sizes for relative strength than their respective antagonist muscle groups at the same joint (HE, KF, and PF). Although no obesity × age interaction was found to be statistically significant, our mean results (Figure 2) suggest a greater trend toward an obesity × age interaction for PF and HE, as indicated by lower mean absolute strength among older obese participants than among older healthy weight participants. This was the opposite of the trend seen among young participants and may have moderated the overall main effects of obesity for these 2 exertions. Indeed, prior work has shown older adults to exhibit a neuromuscular adaptions during gait that deemphasize the use of PF net muscle moments and emphasize the use of HE net muscle moments.33 In light of these results, long-term exposure to higher body weight may still play a major role in explaining the higher absolute strength among individuals who are obese, but that neuromuscular changes associated with aging may modify these effects among older adults. Additional research is needed to better understand the underlying biomechanical mechanisms responsible for the higher absolute strength associated with obesity.
A novel contribution of this study is the finding that lower limb relative strength at the hip (HE and HF) and ankle (PF and DF) was lower among obese participants than among healthy weight participants. These findings are important because both ankle strength and hip strength, in addition to knee strength, are important for mobility19–21 and suggest that strength training interventions aimed at improving or maintaining mobility with obesity should include not only the knee, based upon prior reports, but also the hip and ankle. These data also provide informative strength values for young and older obese and healthy weight adults that could be used to identify individuals with potentially more drastic strength differences. However, the relative importance of the joint/extension-flexion combinations for improving or maintaining mobility among individuals who are obese remains unclear and would be useful to know in the event that it is deemed more advantageous to focus strength training interventions on the joint/extension-flexion combinations that are most critical for mobility.
Age-related differences in strength were in general agreement with prior work. Harbo et al34 reported isokinetic strength at the hip, knee, and ankle for different age and gender groups. Absolute strength of healthy weight females of age (mean (standard deviation)) 63 (3) years was, compared with healthy weight females of age 25 (4) years, was 24% lower in PF, 18% lower in DF, 26% lower in KE, 35% lower in KF, 16% lower in HE, and 14% lower in HF. These differences in absolute strength compare favorably with the knee and hip data reported here when comparing older healthy weight participants with younger healthy weight participants. For example, differences in KE (30%), KF (32%), HE (11%), and HF (15%) exhibited a similar magnitude to those reported by Harbo et al.34 Differences in PF (2%) and DF (28%) between older and younger participants did not agree as well between studies and may be due to differences in participant characteristics and strength protocols.
This study had a few limitations that should be acknowledged. First, and as with all cross-sectional experimental designs, the effects of obesity that we report may not be solely attributed to obesity. Other factors, for example, may include differences in motivation level to generate maximum contractions, or lower limb muscle mass secondary to differences in body shape/mass distribution. Second, the number of subjects in each group was based upon a sample size analysis using obesity-related differences in knee extensor strength data among young adults. Because the obesity-related differences at some joint/extension-flexion combinations may have been smaller, we may not have had sufficient statistical power to detect all effects that we investigated. Third, the PF strength measurement setup, the standard setup recommended by the dynamometer manufacturer, was susceptible to influence by KE effort, although participants were instructed to only use their ankle muscles. Fourth, the results presented here do not necessarily generalize to other populations, or other types of muscle contractions, besides those tested. Fifth, only sagittal plane strength was measured and the findings may differ for other movements.
CONCLUSIONS
In conclusion, obese females exhibited lower relative strength at the ankle and hip, similar to the lower relative strength exhibited at the knee. Obese females also exhibited higher absolute strength, but only for 3 of 6 lower limb exertions investigated. This lack of uniformity across the 6 exertions is likely due to the still unclear underlying biomechanical mechanism responsible for these strength differences, which may also be influenced by aging. The effects of obesity on lower limb strength were also generally consistent between the 2 age groups investigated. These findings provide a better understanding of how obesity influences lower limb strength and lends insight into the causes of limited mobility among individuals who are obese.
Footnotes
This work was supported by award R01OH009880 from the Centers for Disease Control and Prevention (CDC). The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of CDC.
Portions of this work have been presented in abstract form at 2 scientific meetings:
Madigan ML, Koushyar H, Nussbaum MA, Davy KP. Effects of obesity on lower extremity strength are joint specific. Paper presented at: Annual Meeting of the American College of Sports Medicine; May 26-30, 2015; San Diego, CA.
Koushyar H, Matrangola SL, Nussbaum MA, Madigan ML. Effects of obesity on lower extremity strength in young females: preliminary findings. Paper presented at: Annual Meeting of the American Society of Biomechanics; September 4-7, 2013; Omaha, NE.
The authors declare no conflicts of interest.
Richard W Bohannon was the Decision Editor.
REFERENCES
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311(8):806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hue O, Simoneau M, Marcotte J, et al. Body weight is a strong predictor of postural stability. Gait Posture. 2007;26(1):32–38. [DOI] [PubMed] [Google Scholar]
- 3.Koster A, Penninx BWJH, Newman AB, et al. Lifestyle factors and incident mobility limitation in obese and non-obese older adults. Obesity. 2007;15(12):3122–3132. [DOI] [PubMed] [Google Scholar]
- 4.Vincent HK, Vincent KR, Lamb KM. Obesity and mobility disability in the older adult. Obes Rev. 2010;11(8):568–579. [DOI] [PubMed] [Google Scholar]
- 5.Galli M, Crivellini M, Sibella F, Montesano A, Bertocco P, Parisio C. Sit-to-stand movement analysis in obese subjects. Int J Obes Relat Metab Disord. 2000;24(11):1488–1492. [DOI] [PubMed] [Google Scholar]
- 6.Pataky Z, Armand S, Müller-Pinget S, Golay A, Allet L. Effects of obesity on functional capacity. Obesity. 2014;22(1):56–62. [DOI] [PubMed] [Google Scholar]
- 7.Stickles B, Phillips L, Brox WT, Owens B, Lanzer WL. Defining the relationship between obesity and total joint arthroplasty. Obes Res. 2001;9(3):219–223. [DOI] [PubMed] [Google Scholar]
- 8.Lai PPK, Leung AKL, Li ANM, Zhang M. Three-dimensional gait analysis of obese adults. Clin Biomech. 2008;23(suppl 1):S2–S6. [DOI] [PubMed] [Google Scholar]
- 9.Spyropoulos P, Pisciotta JC, Pavlou KN, Cairns M, Simon SR. Biomechanical gait analysis in obese men. Arch Phys Med Rehabil. 1991;72(13):1065–1070. [PubMed] [Google Scholar]
- 10.Dutil M, Handrigan G, Corbeil P, et al. The impact of obesity on balance control in community-dwelling older women. Age. 2013;35(3):883–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jadelis K, Miller ME, Ettinger WH, Messier SP. Strength, balance, and the modifying effects of obesity and knee pain: results from the observational arthritis study in seniors (oasis). J Am Geriatr Soc. 2001;49(7):884–891. [DOI] [PubMed] [Google Scholar]
- 12.Menegoni F, Galli M, Tacchini E, Vismara L, Cavigioli M, Capodaglio P. Gender-specific effect of obesity on balance. Obesity. 2009;17(10):1951–1956. [DOI] [PubMed] [Google Scholar]
- 13.Capodaglio P, Castelnuovo G, Brunani A, Vismara L, Villa V, Capodaglio EM. Functional limitations and occupational issues in obesity: a review. Int J Occup Saf Ergon. 2010;16(4):507–523. [DOI] [PubMed] [Google Scholar]
- 14.Capodaglio P, Vismara L, Menegoni F, Baccalaro G, Galli M, Grugni G. Strength characterization of knee flexor and extensor muscles in Prader-Willi and obese patients. BMC Musculoskelet Disord. 2009;10(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hulens M, Vansant G, Lysens R, Claessens A, Muls E, Brumagne S. Study of differences in peripheral muscle strength of lean versus obese women: an allometric approach. Int J Obes Relat Metab Disord. 2001;25(5):676–681. [DOI] [PubMed] [Google Scholar]
- 16.Lafortuna CL, Maffiuletti NA, Agosti F, Sartorio A. Gender variations of body composition, muscle strength and power output in morbid obesity. Int J Obes Relat Metab Disord. 2005;29(7):833–841. [DOI] [PubMed] [Google Scholar]
- 17.Maffiuletti NA, Jubeau M, Munzinger U, et al. Differences in quadriceps muscle strength and fatigue between lean and obese subjects. Eur J Appl Physiol. 2007;101(1):51–59. [DOI] [PubMed] [Google Scholar]
- 18.Miyatake N, Fujii M, Nishikawa H, et al. Clinical evaluation of muscle strength in 20-79-years-old obese Japanese. Diabetes Res Clin Pract. 2000;48(1):15–21. [DOI] [PubMed] [Google Scholar]
- 19.Portney LG, Watkins MP. Foundations of Clinical Research: Applications to Practice. Vol 2 Upper Saddle River, NJ: Prentice Hall; 2000. [Google Scholar]
- 20.Rantanen T, Guralnik JM, Izmirlian G, et al. Association of muscle strength with maximum walking speed in disabled older women. Am J Phys Med Rehabil. 1998;77(4):299–305. [DOI] [PubMed] [Google Scholar]
- 21.Barrett RS, Lichtwark GA. Effect of altering neural, muscular and tendinous factors associated with aging on balance recovery using the ankle strategy: a simulation study. J Theor Biol. 2008;254(3):546–554. [DOI] [PubMed] [Google Scholar]
- 22.Payette H, Hanusaik N, Boutier V, Morais J, Gray-Donald K. Muscle strength and functional mobility in relation to lean body mass in free-living frail elderly women. Eur J Clin Nutr. 1998;52(1):45–53. [DOI] [PubMed] [Google Scholar]
- 23.Dean JC, Kuo AD, Alexander NB. Age-related changes in maximal hip strength and movement speed. J Gerontol A Biol Sci Med Sci. 2004;59(3):M286–M292. [DOI] [PubMed] [Google Scholar]
- 24.Hunter SK, Thompson MW, Adams RD. Relationships among age-associated strength changes and physical activity level, limb dominance, and muscle group in women. J Gerontol A Biol Sci Med Sci. 2000;55(6):B264–B273. [DOI] [PubMed] [Google Scholar]
- 25.Rolland Y, Lauwers-Cances V, Pahor M, Fillaux J, Grandjean H, Vellas B. Muscle strength in obese elderly women: effect of recreational physical activity in a cross-sectional study. Am J Clin Nutr. 2004;79(4):552–557. [DOI] [PubMed] [Google Scholar]
- 26.Stevens JA, Sogolow ED. Gender differences for non-fatal unintentional fall related injuries among older adults. Inj Prev. 2005;11(2):115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Godin G, Shephard R. Godin Leisure-Time Exercise Questionnaire. Med Sci Sports Exerc. 1997;29(6s):S36. [Google Scholar]
- 28.Anderson DE, Madigan ML, Nussbaum MA. Maximum voluntary joint torque as a function of joint angle and angular velocity: model development and application to the lower limb. J Biomech. 2007;40(14):3105–3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perry MC, Carville SF, Smith ICH, Rutherford OM, Newham DJ. Strength, power output and symmetry of leg muscles: effect of age and history of falling. Eur J Appl Physiol. 2007;100(5):553–561. [DOI] [PubMed] [Google Scholar]
- 30.Sadeghi H, Sadeghi S, Prince F, Allard P, Labelle H, Vaughan CL. Functional roles of ankle and hip sagittal muscle moments in able-bodied gait. Clin Biomech. 2001;16(8):688–695. [DOI] [PubMed] [Google Scholar]
- 31.Honeine JL, Schieppati M, Gagey O, Do MC. The functional role of the triceps surae muscle during human locomotion. PLoS One. 2013;8(1):e52943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eng JJ, Winter DA. Kinetic analysis of the lower limbs during walking: what information can be gained from a three-dimensional model? J Biomech. 1995;28(6):753–758. [DOI] [PubMed] [Google Scholar]
- 33.DeVita P, Hortobagyi T. Age causes a redistribution of joint torques and powers during gait. J Appl Physiol (1985). 2000;88(5):1804–1811. [DOI] [PubMed] [Google Scholar]
- 34.Harbo T, Brincks J, Andersen H. Maximal isokinetic and isometric muscle strength of major muscle groups related to age, body mass, height, and sex in 178 healthy subjects. Eur J Appl Physiol. 2012;112(1):267–275. [DOI] [PubMed] [Google Scholar]