Abstract
Despite its controversial nature, the use of medical marijuana and cannabis-derived medicinal products grows more popular with each passing year. As of November 2016, over 40 states have passed legislation regarding the use of either medical marijuana or cannabidiol products. Many providers have started encountering patients experimenting with cannabis products for a wide range of conditions. While the debate continues regarding these agents for both medicinal and recreational use in the general population, special consideration needs to be made for pediatric use. This review will deliver the history of marijuana use and legislation in the United States in addition to the currently available medical literature to equip pediatric health care providers with resources to provide patients and their parents the best recommendation for safe and appropriate use of cannabis-containing compounds.
Keywords: CBD, cannabidiol; cannabis; epilepsy; pediatrics; pharmacy
Introduction
Over the past several years, medical marijuana use has become a controversial topic not only within the medical community but also at state and national legislative levels. Although marijuana and its derivatives are currently Schedule 1 substances per the federal Controlled Substances Act (CSA), many states have relaxed their legislation to allow use. More recently, the use of cannabidiol (CBD) products in pediatrics has sparked additional debate, and pediatric providers have started encountering patients experimenting with these products in their daily practice, necessitating an understanding of the history and available medical literature on this topic.
Many of the misconceptions regarding medical marijuana in the pediatric population stem from negative connotations associated with the term marijuana owing to its psychoactive effects. Therefore, it is important to define the various terms associated with products that are currently being used by the public as well as by pediatric researchers. Cannabis is a general term that refers to the 3 species of hemp plants (Cannabis sativa, Cannabis indica, Cannabis ruderalis).1 Marijuana is a term that describes the dried leaves, flowers, stems, and seeds from the hemp plant that are often smoked for recreational and medicinal use. Marijuana contains various different chemicals called cannabinoids. Cannabinoids are the chemicals found within cannabis that interact with specific receptors, namely, cannabinoid (CB) receptors, within the body. The over 60 types of cannabinoids currently identified differ by the degree to which they are psychoactive.2 While delta-9-tetrahydrocannabinol (THC), the cannabinoid most commonly associated with marijuana as a drug of abuse, is psychoactive, other cannabinoids including CBD are not. THC has been linked to the development of schizophrenia, and a contributor to neurodevelopment deficits in adolescents.3,4 Different marijuana strains will have varying amounts of both THC and CBD, and thus the concentrations and ratios of these different cannabinoids within a product, especially for pediatric use, has been a subject of interest not only for medical professionals but also for state legislators as well.
History and Regulation
Dating back as far as 2000 BC, hemp plants had been used for various medicinal and industrial purposes. In 1851, the United States Pharmacopeia (USP) classified marijuana as a legitimate medical compound and many physicians supported its use for conditions such as epilepsy, chronic migraines, and pain.5 Reports of Victorian-era neurologists using Indian hemp to treat epilepsy were also promising.6 However, when phenobarbital and phenytoin came to the market in the early 1900s, the use of marijuana-based products declined.
In the 1930s, political propaganda sought to associate marijuana use, specifically by minority and low-income populations, with psychosis, addiction, and violent crime. Many believe this was influenced by several prominent businessmen in competing synthetic fiber industries in attempts to reduce the size of the growing hemp industry.5 Marijuana soon became labeled as a drug of abuse and to discourage its use, Congress passed the Marijuana Tax Act of 1937 placing a heavy tax on cannabis and hemp use for both medicinal and industrial purposes. Despite opposition from the American Medical Association (AMA) and physicians who believed in the medical efficacy of marijuana, by 1941, all cannabis preparations were removed from the USP and National Formulary.
In the 1960s and early 1970s, marijuana soon became associated with recreational use by anti-establishment groups further adding to the stigma associated with its usage. By 1970, the CSA labeled cannabis as a Schedule 1 substance. This relatively short era of recreational marijuana use has influenced how the public perceives the drug. Since that time, there have been repeated unsuccessful attempts to reconsider its Schedule 1 status to allow for easier investigation.5 The AMA and the American Academy of Pediatrics (AAP) have reaffirmed their opposition to the legalization of medical and recreational marijuana use outside of any US Food and Drug Administration (FDA) regulatory process.7
The AAP also supports further research into the indications and correct dosage for cannabinoids in addition to developing policy around how to verify purity and formulations.8 In the meantime, the AAP has suggested good practices to follow when considering the use of marijuana, recreationally or medically (Table 1).
Table 1.
Recommendations from the American Academy of Pediatrics8

To date, however, 8 states and the District of Columbia have passed legislation to legalize recreational marijuana use, with an additional 20 states allowing for some form of medical cannabis. Fourteen nonmedical marijuana states have specific legislation regarding CBD (Figure).9–11 The changing legislative and regulatory landscape has significantly impacted the use of cannabinoid products in this country. Discussion about the safe and efficacious use of these products in a responsible way that protects vulnerable populations, including pediatrics, is necessary.
Figure.
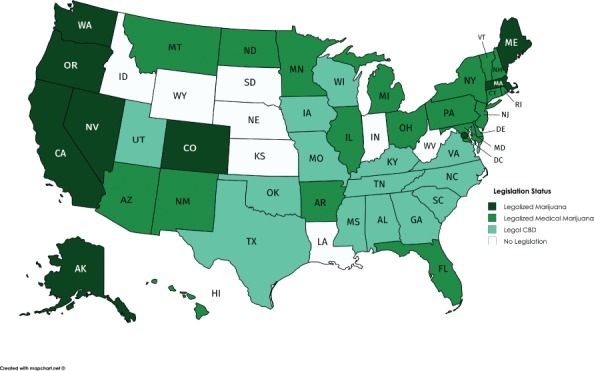
2017 Legislative status of marijuana in the United States
Pharmacology
Similar to endogenous opioids, a human's central nervous system is impregnated with cannabinoid receptors and endocannabinoids. In the early 1990s, 2 receptors were discovered, cannabinoid type 1 (CB1) and cannabinoid type 2 (CB2). Both CB1 and CB2 are G-coupled protein receptors located presynaptically and control the release of neurotransmitters at both inhibitory and excitatory synapses. CB1 is mostly expressed on presynaptic peripheral and central nerve terminals and is believed to be responsible for psychologic effects on pleasure, memory, thought, concentration, sensory and time perceptions, and coordinated movement. CB2 receptors, concentrated in peripheral tissues and immune cells, may play an anti-inflammatory and immunosuppressive role. In addition to directing the release of various neurotransmitters, this receptor regulates the release of certain cytokines. Innervation of both these receptors results in both physiological (tachycardia, hypertension, dry mouth and throat) as well as psychological (elation, euphoria, heightened perception, irritability, poor coordination and balance) effects.3,5
Additionally, endocannabinoids N-arachidonoylethanolamine (anandamide) and 2-arachidonoylglycerol, both arachidonic acid derivatives, bind with CB1 and CB2. While the function of these endogenous ligands is not fully understood, their action may be attributed as antiemetic, antianalgesic, and anti-inflammatory. Endocannabinoids can also play a role in excitation of the neuronal networks, thus having effect on the quality of a seizure. Previous studies have documented deficiencies in endocannabinoids in temporal lobe epilepsy patients as well as a rise in anandamide concentrations post seizures in mice, suggesting an antiseizure activity profile.6
The 2 most studied exogenous cannabinoids include THC and CBD. THC is a partial agonist at both CB1 and CB2 receptors and achieves its psychoactive properties likely through modulation of gamma-aminobutyric acid (GABA) and glutamine. THC seems to possess antiseizure activity but may be a proconvulsant in certain species.12 CBD, however, does not appear to bind to either CB1 or CB2 but does possess neuroprotective and anti-inflammatory effects.5 Several possible mechanisms of CBD have been proposed: inhibition of cyclooxygenase and lipoxygenase, inverse agonism at CB1/CB2 receptors, and enhancement of anandamide.3 It is proposed that CBD may be effective in epilepsy through modulation of the endocannabinoid system. CBD halts the degradation of the endocannabinoid anandamide, which may have a role in inhibiting seizures. Additionally, research demonstrates that CBD may play a role with the regulation of T-type calcium channels and nuclear peroxisome proliferator-activated receptor-γ, both of which have been implicated in seizure activity.12 Because CBD is one of the most abundant cannabinoids within cannabis resin and its mechanism is still unclear, there is peaked interest in the possible clinical indications that it could treat including epilepsy, pain, and inflammatory disorders.
Several other synthetic forms of cannabinoids have been available for use in some countries, including dronabinol, nabilone, and nabiximols (Table 2). These products are being used to treat nausea and vomiting associated with chemotherapy, anorexia and weight loss in patients with acquired immune deficiency syndrome (AIDS), and relief of spasticity and neuropathic pain associated with multiple sclerosis (MS).13–16 Epidiolex (GW Pharmaceuticals, Cambridge, United Kingdom) is a CBD product currently in clinical trials.17
Table 2.
Synthetic Cannabinoid Products

Pharmacokinetics
Historically, patients and recreational users have inhaled or vaporized marijuana, resulting in a quick onset and higher peak concentrations. Owing to first-pass metabolism, the enteral route decreases the bioavailability of THC to from 5% to 20% and CBD to from 6% to 19% and increases the time to onset.3,18,19 Differences in absorption between various age groups, populations, and individual people make it difficult to recommend a one-size-fits-all dosage strategy. Interpatient variability may affect which blood concentrations will be effective, and tolerance is known to occur owing to downregulation of CB1 receptors.3,18
Both THC and CBD are highly lipophilic with long half-lives, 30 hours versus 9 to 32 hours, respectively.3,18,20 CBD is also highly protein bound and is both metabolized by and a potent inhibitor of the CYP450 enzymes (2C19, 3A4), potentially causing significant medication interactions.3,18,20,21 While CYP inducers such as phenytoin and carbamezapine may decrease CBD concentrations, CBD is known to increase concentrations of clobazam, an antiepileptic drug approved by the FDA in 2011 for the treatment of Lennox-Gastaut syndrome (LGS). CBD inhibits CYP3A4 and CYP2C19, preventing the degradation of clobazam and its active metabolite, N-desmethylclobazam. In an expanded access trial, patients with concomitant clobazam and CBD use had increases in clobazam concentrations of > 60% and N-desmethylclobazam, of 500%.22 At this time it is not clear what other drug interactions may exist and what dosage manipulations may be necessary.
Clinical Data
The debate about the use of cannabinoid products in pediatric patients has persisted owing to the lack of well-developed and published randomized controlled trials. There has been a wide variety of mostly case series and international studies for adult indications, such as chronic pain, MS, headache, and various neuropsychiatric disorders, which are beyond the scope of this review but have been reviewed elsewhere.20 The pediatric literature lacks the same breadth owing to public stigma and restrictions on investigational use. This has resulted in retrospective and parentally reported data in epilepsy and behavioral conditions. Despite the overall lack of published data on CBD in pediatric patients, most of the literature is devoted to its use in epilepsy. Current large prospective trials are underway for different epilepsy indications, and recent animal studies researching use in perinatal brain injury and neuroblastoma may open new avenues to consider cannabinoids for pediatrics.
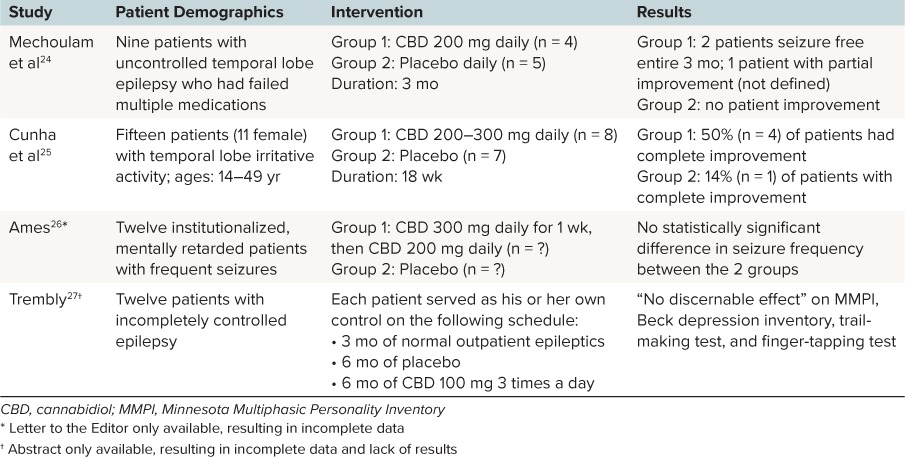
Epilepsy. The data in pediatric epilepsy have been surrounding the use of CBD products as well as unregulated THC/CBD products from private dispensaries. A Cochrane review23 was conducted in 2012 to assess the safety and efficacy of cannabinoid use in patients with epilepsy. The authors included blinded and unblinded randomized controlled trials. Only 4 studies met their criteria, including 1 abstract and 1 letter to the editor (Table 3). All 4 trials were of low quality with small sample sizes and variations in product, dose, frequency, and duration.24–27 The authors summarized the finding that a CBD dose of 200 mg to 300 mg daily was safely administered over a short period. The only reasonable conclusion made was that the efficacy of CBD use could not be confirmed, but the rate of adverse reactions in each of the studies was low over a short period.
Table 3.
Included Studies in Cochrane Review23
The American Academy of Neurology conducted a systematic review in 2014 which included 34 studies that used medical marijuana to treat MS, epilepsy, and movement disorders.28 The authors included 2 studies to assess the role of cannabinoids in decreasing seizure frequency.25,26 Of note, these studies were also evaluated in the Cochrane review. The authors28 concluded that “data are insufficient to support or refute the efficacy of cannabinoids for reducing seizure frequency” and thus, there is not sufficient evidence to advise patients to use cannabinoid products in epilepsy.
Despite this, parents and patients are making the decision to use these products for 3 reasons according to Cilio et al:12 1) prominent Internet and nation media attention; 2) reports of cases of children successfully treated with CBD products; and 3) the belief that treatments derived from natural products are safer or more effective.12 National attention has been on those patients who have moved to states where CBD use is legal and researchers have sought to gather data from parental observations. It is important to note that the following studies are based on parental perceptions and thus we cannot draw definitive conclusions.
The most famous case was presented on a CNN special, “Weed.”29 Charlotte is a little girl from Colorado who was diagnosed with Dravet syndrome at the age of 3 months. She suffered from frequent status epilepticus. Charlotte failed multiple medications, and at 5 years of age, she had significant cognitive delay and required help with all of her activities of daily living.30 Her parents sought out a group in Colorado that created an oral, liquid, high-concentration CBD-to-THC strain of cannabis. Once her parents started giving her this strain, dubbed “Charlotte's Web”, within 3 months Charlotte had a > 90% reduction in her seizure frequency and by month 20, Charlotte was able to perform most of her daily activities independently with only 2 to 3 nocturnal tonic-clonic seizures per month. Stories like Charlotte's have prompted parents across the country in similar situations to move their families across the country to gain access to these products.
In a retrospective chart review of 75 children and adolescents younger than 18 years who were given oral cannabis extracts for treatment of their epilepsy, 57% of parents reported some improvement in seizure frequency with 33% reporting a >50% reduction in seizures.31 Dosage information was not reported and parents used various formulations and concentrations of CBD and THC. Parents also described improvements in behavior and alertness (33%), language (11%), and motor skills (11%). Major adverse effects noted were somnolence (12%) and gastrointestinal symptoms (11%).
Investigators at Stanford University administered a survey to 150 parents on Facebook to identify parentally reported effects of CBD on their child's seizures.32 Of 19 respondents aged 2 to 16 years, 18 had treatment-resistant epilepsy for more than 3 years before CBD use. Based on parental response, 84% reported a reduction in child's seizure frequency with 50% having a greater than 80% reduction in seizure frequency. Twelve of these 19 patients were also able to be weaned from another antiepileptic drug. In addition, parents reported overall better mood, increased alertness, and better sleep. Parents reported oral CBD dosages of 0.5 mg/kg/day to 28.6 mg/kg/day and THC of 0 to 0.8 mg/kg/day.
In a similar Facebook survey administered by researchers at the University of California, Los Angeles, the authors33 similarly reported an 85% reduction in seizure frequency among 117 respondents, with an average age of 6 years. Most patients (86%) conveyed that changes in frequency occurred within 14 days. As with previous surveys, dosage and formulations were varied but based on parental report of formulation used. Overall, most parents (83.5%) reported using an oral CBD product with at least a 15:1 ratio of CBD to THC. Of the 40% of respondents who provided dosages, the median weight-based dose of CBD was 4.3 mg/kg/day given in 2 to 3 oral doses. As mentioned above, these surveys should be evaluated carefully given the inability to verify dose, formulation, and response. The conclusion that can be made is that there is a rather strong positive parental perception regarding the efficacy of cannabinoids, specifically CBD.
Most orphan drug designations for CBD are for pediatric seizure disorders (Table 4).34 A search of ClinicalTrials.gov in November 2016 identified 4 completed Phase 2 and Phase 3 protocols for pediatric seizure disorders, as well as 14 ongoing treatment trials, including intermediate-size expanded access protocols (up to 50 patients each). Published findings from open-label use of CBD for treatment-resistant epilepsy under an expanded-access program at 11 epilepsy centers in the United States suggest that CBD might reduce seizure frequency and might have an adequate safety profile in children and young adults with this condition.35 Congressional testimony in June 2015 indicated that 20 intermediate-size expanded access Investigational New Drug Applications had been authorized to treat approximately 420 children with 1 CBD product; most of these are not listed on ClinicalTrials.gov.36
Table 4.
Orphan Drug Designations for Cannabidiol in the Treatment of Pediatric Conditions34 *

After announcing positive results from 2 pivotal randomized, double-blind, Phase 3 trials for the treatment of seizures related to LGS, and a third for seizures associated with Dravet syndrome in 2016, GW Pharmaceuticals expects to submit a single New Drug Application for both indications to the FDA in the first half of 2017 for its proprietary pharmaceutical-grade CBD product Epidiolex.37 In the second LGS study, patients randomized to the investigational product 20 mg/kg/day (n = 76) or 10 mg/kg/day (n = 73) added to their current antiepileptic treatment, experienced a median reduction in monthly drop seizures of 42% and 37%, compared to 17% in the placebo group (n = 73); a difference that was statistically and clinically significant (p = 0.0047 and p = 0.0016, respectively).37 These data confirmed the results of the first LGS trial in which 86 patients receiving Epidiolex 20 mg/kg/day achieved a 44% mean reduction in monthly drop seizures as compared to 22% for 85 patients in the placebo group (p = 0.0135).38 Patients with Dravet syndrome receiving the GW Pharmaceuticals' CBD in addition to their baseline antiepileptic regimen (n = 61) achieved the primary endpoint of a significant reduction in convulsive seizures (p = 0.01, median reduction of 39%) assessed over the 14-week treatment period as compared with the addition of placebo (n = 59).39 Insys Therapeutics (Phoenix, AZ) has reported that their synthetic pharmaceutical CBD in a nonalcoholic, medium-chain triglyceride-based formulation was generally well tolerated in a completed Phase 1/Phase 2 safety and pharmacokinetic study in 61 pediatric patients with treatment-resistant epilepsy at total daily doses up to 40 mg/kg.40
Behavioral Conditions. Cannabinoids and CBD use in this patient population is a growing interest on social media sites. While the data for these indications are limited to case reports using dronabinol, some of the benefits of CBD on behavior and motor skills reported in the aforementioned retrospective studies in epilepsy may be transferable to this population as well. A 6-year-old patient with early infant autism received enteral dronabinol drops titrated up to 3.62 mg/day. He had improvements in hyperactivity, irritability, lethargy, stereotype, and speech.41 In a published abstract, Kruger et al42 report on the effect of dronabinol use in treating self-injurious behavior in 10 mentally retarded adolescents. The dronabinol dose ranged from 2.5 mg twice daily to 5 mg 4 times a day. Seven of the 10 patients had significant improvement in their self-injurious behavior that lasted through the follow-up at 6 months. Two of the 10 patients experienced agitation and the drug was discontinued. An Israeli single-center, double-blind, placebo-controlled cross-over trial of CBD and THC in a 20:1 mixture for behavioral problems in children with autistic spectrum disorder is scheduled to start in January 2017.43
Perinatal Brain Injury. Perinatal brain injury can be induced by neonatal asphyxia, stroke-induced focal ischemia, and neonatal hypoxia-ischemic encephalopathy, among other things. These conditions lead to long-lasting functional impairment due to neuroinflammation, apoptotic-necrotic cell death, and brain lesions.44 Several adjunctive medication therapies in addition to hypothermia, include magnesium sulfate and minocycline which may play a role in modulating neuroinflammation and apoptosis. The endocannabinoid system responds early to neuronal damage, working to prevent glutamate excitotoxicity and regulate the inflammatory response. While there are no current human studies, results from mice and pig models demonstrate that CBD can reduce the density of necrotic neurons and modulate cytokine release.45,46
Neuroblastoma. Most recently, researchers have reported on the use of CBD in both in vitro and in vivo animal studies of neuroblastoma (NBL), a common childhood cancer.47 Investigators are proposing that antitumor activity is achieved by action at vanilloid and peroxisome proliferator-activated receptors. In vitro, they found that both CBD and THC reduced the viability of NBL cells in a dose- and time-dependent manner. When comparing the two, CBD had a significantly better response in reducing viability of NBL cells than THC. They next treated mice with daily intraperitoneal injections of THC, CBD, or ethanol, or gave no treatment. Tumor growth in both the THC and CBD groups was significantly reduced.
What's The Harm?
Worldwide, marijuana is the most commonly abused illegal substance and adolescent daily use is on the rise.18 Adolescents perceive that marijuana use is not as much of a risk owing to legalization and decriminalization, leading to its use both recreationally and to self-treat anxiety and other psychiatric conditions. Unfortunately, the neurocognitive and behavioral effects of marijuana use in pediatric patients, including its effects on psychological dysfunction, amotivation syndrome, and carcinogenic risk, have been widely reported.4,21
Evolving legislation and the increased use of cannabinoid products outside of investigational studies have also impacted our health care delivery and emergency resources. The state of Colorado has been on the forefront of the medicinal and recreational use of cannabis debate. Wang et al48 reported the occurrences of pediatric emergency department visits associated with marijuana exposure before and after changes in drug enforcement in 2009. A total of 1378 patients younger than 12 years were evaluated for unintentional ingestions from January 1, 2005, to December 31, 2011. Before 2009, no patients (0/790; 0%) sought care at this emergency department for accidental marijuana ingestions as compared with 14 patients (14/588; 2.4%) after 2009 (p < 0.001). Patients ranged in age from 8 months to 12 years and presented with symptoms of lethargy, ataxia, and respiratory insufficiency. While the dosages were not reported, 7 patients ingested a marijuana edible. Eight of the 14 patients were admitted to the hospital with 2 admissions to the pediatric intensive care unit. Prior to diagnosis, these 14 patients received routine testing such as urinalyses, complete blood counts, and complete metabolic panels. Some of these patients also received more invasive testing including computed tomography, activated charcoal, lumbar punctures, and intravenous antibiotics. All of these contribute to higher hospital and emergency room costs, increased lengths of stay, and potential harm to the patients.
In addition to increased emergency room visits, from 2005 to 2011, the call volume at Poison Control Centers for pediatric marijuana exposures had increased by 30.3% in states where marijuana has been decriminalized as compared to a steady rate in states that have not adopted marijuana decriminalization legislation.49 While marijuana and CBD products are becoming more available, these products remain in DEA (Drug Enforcement Administration) Schedule 1 status and are therefore not regulated in manufacturing, packaging, and labeling outside of clinical trials. As seen in the Colorado case study, 50% of the unintentional ingestions were secondary to an edible, which children can easily mistake for food if not supervised by parents. None of these products are required to have safety packaging to prevent accidental ingestion by children. In addition, no warning labels or verification of product ingredients is required, leaving the medical community caught between providing safe medical care and allowing patient autonomy. As mentioned previously, the AAP has published recommendations to limit the access of marijuana to children.
Pharmacist's Role
In 2007, amidst medical marijuana legalization in several states, Seamon et al21 identified that pharmacists needed to be attentive to the legislative changes going on at the state and federal levels. Pharmacists are uniquely poised to understand the medicinal chemistry as well as the practical implications associated with decriminalization and legalization. Pharmacists can continue to educate both medical professionals and lay people about the differences among cannabinoids, and help to remove the stigma around appropriate and legal use of CBD products. At the same time, medical professionals need to remember the documented deleterious effects of acute marijuana intoxication on neurocognitive development and psychiatric issues.
Many health care facilities are working through processes that address patient use of these medications. Because use of cannabis products outside of approved clinical trials is not legal under federal law, thus not permitted under Centers for Medicare & Medicaid Services (CMS) Conditions of Participation, there are significant challenges in managing hospitalized patients. Whatever the state and situation, pharmacists need to be aware of the external factors associated with allowing a patient to use CBD in an inpatient setting.
Pharmacists are also poised to participate in the design and evaluation of current and future research in this area. The importance of drug interactions between CBD and other antiepileptics remains uncertain both for the efficacy and safety of CBD products. The difference in concentrations, dosages, and formulations of various products sold at private dispensaries is not standardized or regulated. Differences in state legislation on allowable concentrations and amounts can be confusing for patients and their families, and pharmacists can help to provide that information. Various organizations have been helpful in updating and summarizing this information.9
Conclusions
Cannabis and its ingredients have had a fascinating history over the past 4000 years, but lack of published data precludes fully recommending its use for medicinal purposes in pediatrics. Further study is underway and will add to our knowledge of the efficacy and safety of CBD in pediatrics. Long-term studies to assess neurocognitive development with CBD will need to be assessed as well. As pharmacists, it is our duty to provide our patients and their parents with the most accurate, safe, and legally appropriate advice.
Abbreviations
- AAP
American Academy of Pediatrics
- AIDS
acquired immune deficiency syndrome
- AMA
American Medical Association
- CB
cannabinoid
- CB1
cannabinoid type 1 receptor
- CB2
cannabinoid type 2 receptor
- CBD
cannabidiol
- CMS
Centers for Medicare & Medicaid Services
- CNN
Cable News Network
- CSA
Controlled Substances Act
- DEA
Drug Enforcement Administration
- FDA
US Food and Drug Administration
- GABA
gamma-aminobutyric acid
- LGS
Lennox-Gastaut syndrome
- MS
multiple sclerosis
- NBL
neuroblastoma
- THC
delta-9-tetrahydrocannabinol
- USP
United States Pharmacopeia
Footnotes
Disclosures The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria. Of note, both Augusta University (ClinicalTrials.gov Identifier: NCT02397863) and the University of Florida (ClinicalTrials.gov Identifier: NCT02461706) are sponsors of expanded access clinical trials of cannabidiol and drug-resistant epilepsy in children.
Copyright Published by the Pediatric Pharmacy Advocacy Group. All rights reserved. For permissions, email: matthew.helms@ppag.org.
REFERENCES
- 1. Drugabuse.gov [Internet]. Washington, DC: National Institute on Drug Abuse; National Institutes of Health; US Department of Health and Human Services; Updated March 2016. Cited June 8, 2016. https://www.drugabuse.gov/publications/drugfacts/marijuana. Accessed March 19, 2017. [Google Scholar]
- 2. University of Washington Alcohol and Drug Abuse Institute [Internet]. Seattle: University of Washington; Updated June 2013. Cited June 8, 2016. http://learnaboutmarijuanawa.org/factsheets/cannabinoids.htm. Accessed March 19, 2017. [Google Scholar]
- 3. Borgelt LM, Franson KL, Nussbaum AM, Wang GS. . The pharmacologic and clinical effects of medical cannabis. Pharmacotherapy. 2013; 33( 2): 195– 209. [DOI] [PubMed] [Google Scholar]
- 4. Meier MH, Caspi A, Ambler A, . et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci U S A. 2012; 109( 40): E2657– E2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baron EP. . Comprehensive review of medicinal marijuana, cannabinoids, and therapeutic implications in medicine and headache: what a long strange trip it's been… Headache. 2015; 55( 6): 885– 916. [DOI] [PubMed] [Google Scholar]
- 6. Friedman D, Devinsky O. . Cannabinoids in the treatment of epilepsy. N Engl J Med. 2015; 373( 11): 1048– 1058. [DOI] [PubMed] [Google Scholar]
- 7. ProCon.Org [Internet]. Santa Monica: history of the American Medical Association (AMA) and marijuana. Updated October 24, 2016. Cited January 27, 2017. http://medicalmarijuana.procon.org/view.resource.php?resourceID=006641. Accessed March 19, 2017. [Google Scholar]
- 8. AAP.org [Internet]. American Academy of Pediatrics; American Academy of Pediatrics reaffirms opposition to legalizing marijuana for recreational or medical use. Updated January 26, 2015. Cited November 18, 2016. https://www.aap.org/en-us/about-the-aap/aap-press-room/pages/American-Academy-of-Pediatrics-Reaffirms-Opposition-to-Legalizing-Marijuana-for-Recreational-or-Medical-Use.aspx. Accessed March 19, 2017. [Google Scholar]
- 9. ProCon.Org [Internet]. Santa Monica: 28 legal medical marijuana states and DC. Updated November 9, 2016. Cited November 18, 2016. http://medicalmarijuana.procon.org/view.resource.php?resourceID=000881. Accessed March 19, 2017. [Google Scholar]
- 10. ProCon.Org [Internet]. Santa Monica: 16 states with laws specifically about legal cannabidiol (CBD). Updated March 17, 2016. Cited November 18, 2016. http://medicalmarijuana.procon.org/view.resource.php?resourceID=006473. Accessed March 19, 2017. [Google Scholar]
- 11. BusinessInsider.com [Internet]. New York City: this map shows every state that legalized marijuana on Election Day. Updated November 9, 2016. Cited January 28, 2017. http://www.businessinsider.com/where-is-marijuana-legal-2016-11. Accessed March 19, 2017. [Google Scholar]
- 12. Cilio MR, Thiele EA, Devinsky O. . The case for assessing cannabidiol in epilepsy. Epilepsia. 2014; 55( 6): 787– 790. [DOI] [PubMed] [Google Scholar]
- 13. Marinol (dronabinol) [package insert]. Unimed Pharmaceuticals Inc; September 2004. Cited January 27, 2017. http://www.fda.gov/ohrms/dockets/dockets/05n0479/05N-0479-emc0004-04.pdf. Accessed March 19, 2017. [Google Scholar]
- 14. Cesamet (nabilone) [package insert]. Valeant Pharmaceuticals International; May 2006. Cited January 27, 2017. http://www.accessdata.fda.gov/drugsatfda_docs/label/2006/018677s011lbl.pdf. Accessed March 19, 2017. [Google Scholar]
- 15. Sativex (nabiximols) [package insert]. Canada: Bayer Pharmaceuticals Inc; March 2015. Cited January 27, 2017. http://omr.bayer.ca/omr/online/sativex-pm-en.pdf. Accessed March 19, 2017. [Google Scholar]
- 16. Russo EB. . Cannabinoids in the management of difficult to treat pain. Ther Clin Risk Manag. 2008; 4( 1): 245– 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. GWPharm.com [Internet]. Cambridge: FW's Epidiolex Clinical Program; 2016. Cited January 27, 2017. https://www.gwpharm.com/patients-caregivers/patients. Accessed March 19, 2017. [Google Scholar]
- 18. Hadland SE, Knight JR, Harris SK. . Medical marijuana: review of the science and implications for developmental-behavioral pediatric practice. J Dev Behav Pediatr. 2015: 36( 2): 115– 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fasinu PS, Phillips S, ElSohly MA, . et al. Current status and prospects for cannabidiol preparations as new therapeutic agents. Pharmacotherapy. 2016; 36( 7): 781– 796. [DOI] [PubMed] [Google Scholar]
- 20. Whiting PF, Wolff RF, Deshpande S, . et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015; 313( 24): 2456– 2473. [DOI] [PubMed] [Google Scholar]
- 21. Seamon MJ, Fass JA, Maniscaolco-Feichtl M, Abu-Shraie NA. . Medical marijuana and the developing role of the pharmacist. Am J Health Syst Pharm. 2007; 64( 10): 1037– 1044. [DOI] [PubMed] [Google Scholar]
- 22. Geffrey AL, Pollack SF, Bruno PL, Thiele EA. . Drug-drug interaction between clobazam and cannabidiol in children with refractory epilepsy. Epilepsia. 2015; 56( 8): 1246– 1251. [DOI] [PubMed] [Google Scholar]
- 23. Gloss D, Vickrey B. . Cannabinoids for epilepsy. Cochrane Database Syst Rev. 2014;( 3): CD009270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mechoulam R, Carlini EA. . Toward drugs derived from cannabis. Naturwissenschaften. 1978; 65( 4): 174– 179. [DOI] [PubMed] [Google Scholar]
- 25. Cunha JM, Carlini EA, Pereira AE, . et al. Chronic administration of cannabidiol to healthy volunteers and epileptic patients. Pharmacology. 1980; 21( 3): 175– 185. [DOI] [PubMed] [Google Scholar]
- 26. Ames FR, Cridland S. . Anticonvulsant effect of cannabidiol. S Afr Med J. 1985; 69( 1): 14. [PubMed] [Google Scholar]
- 27. Trembly B, Sherman M. . Double-blind clinical study of cannabidiol as a secondary anticonvulsant. Marijuana '90 International Conference on Cannabis and Cannabinoids; July 8–11, 1990; Kolympari, Crete International Association for Cannabinoid Medicines; 1990: section 2, p 5. [Google Scholar]
- 28. Koppel BS, Brust JC, Fife T, . et al. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2014; 82( 17): 1556– 1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. WEED: a Dr. Sanjay Gupta investigation [transcript]. Sanjay Gupta MD. CNN television. August 11, 2013. [Google Scholar]
- 30. Maa E, Figi P. . The case for medical marijuana in epilepsy. Epilepsia. 2014; 55( 6): 783– 786. [DOI] [PubMed] [Google Scholar]
- 31. Press CA, Knupp KG, Chapman KE. . Parental reporting of response to oral cannabis for treatment of refractory epilepsy. Epilepsy Behav. 2015; 45: 49– 52. [DOI] [PubMed] [Google Scholar]
- 32. Porter BE, Jacobson C. . Report of a parent survey of cannabidiol-enriched cannabis use in pediatric treatment-resistant epilepsy. Epilepsy Behav. 2013; 29: 574– 577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hussain SA, Zhou R, Jacobson C, . et al. Perceived efficacy of cannabidiol—enriched cannabis extracts for treatment of pediatric epilepsy: a potential role for infantile spasms and Lennox-Gastaut syndrome. Epilsepsy Behav. 2015; 47: 138– 141. [DOI] [PubMed] [Google Scholar]
- 34. US Food and Drug Administration [Internet]. Washington, DC: Search Orphan Drug designations and approvals. Cited November 8, 2016. http://www.accessdata.fda.gov/scripts/opdlisting/oopd/. Accessed March 19, 2017. [Google Scholar]
- 35. Devinsky O, Marsh E, Friedman D, Thiele E, . et al. Cannabidiol in patients with treatment-resistant epilepsy: an open-label intervention trial. Lancet Neurol. 2016; 15( 3): 270– 278. [DOI] [PubMed] [Google Scholar]
- 36. Throckmorton DC. . Cannabidiol: barriers to research and potential medical benefits. June 24, 2015 testimony before the Caucus on International Narcotics Control, United States Senate. FDA.gov [Internet]. June 24, 2015. Cited November 20, 2016. http://www.fda.gov/NewsEvents/Testimony/ucm453989.htm. Accessed March 19, 2017. [Google Scholar]
- 37. GW Pharmaceuticals [Internet]. GW Pharmaceuticals announces second positive phase 3 pivotal trial for Epidiolex (cannabidiol) in the treatment of Lennox-Gastaut syndrome (press release, September 26, 2016). Cited November 8, 2016. https://www.gwpharm.com/about-us/news/gw-pharmaceuticals-announces-second-positive-phase-3-pivotal-trial-epidiolex. Accessed March 19, 2017
- 38. GW Pharmaceuticals [Internet]. GW Pharmaceuticals announces positive phase 3 pivotal trial results for Epidiolex (cannabidiol) in the treatment of Lennox-Gastaut syndrome (press release June 27, 2016). Cited November 8, 2016. https://www.gwpharm.com/about-us/news/gw-pharmaceuticals-announces-positive-phase-3-pivotal-trial-results-epidiolex. Accessed March 19, 2017.
- 39. GW Pharmaceuticals [Internet]. GW Pharmaceuticals announces positive phase 3 pivotal study results for Epidiolex (cannabidiol) (press release March 14, 2016). Cited November 8, 2016. https://www.gwpharm.com/about-us/news/gw-pharmaceuticals-announces-positive-phase-3-pivotal-study-results-epidiolex. Accessed March 19, 2017.
- 40. Insys Therapeutics [Internet]. Insys Therapeutics successfully completes safety and pharmacokinetic (PK) study of cannabidiol oral solution in pediatric epilepsy patients (press release, May 24, 2016). Cited November 20, 2016. http://investors.insysrx.com/phoenix.zhtml?c=115949&p=irol-newsArticle&ID=2171675. Accessed March 19, 2017. [Google Scholar]
- 41. Kurz R, Blass K. . Use of dronabinol (delta-9-THC) in autism: a prospective single-case-study with an early infantile autistic child. Cannabinoids. 2010; 5( 4): 4– 6 [Google Scholar]
- 42. Kruger T, Christophersen E. . An open label study of the use of dronabinol (marinol) in the management of treatment-resistant self-injurious behavior in 10 retarded adolescent patients. J Dev Behav Pediatr. 2006; 27( 5): 433. [Google Scholar]
- 43. ClinicalTrials.gov [Internet]. Cannabinoids for behavioral problems in children with ASD (CBA). Updated November 2016. Cited November 6, 2016. https://www.clinicaltrials.gov/ct2/show/NCT02956226. Accessed March 19, 2017.
- 44. Fernandez-Lopez D, Lizasoain I, Moro MA, . et al. Cannabinoids: well-suited candidates for the treatment of perinatal brain injury. Brain Sci. 2013; 3( 3): 1043– 1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Castillo A, Tolon MR, Fernandez-Ruiz J, . et al. The neuroprotective effect of cannabidiol in an in vitro model of newborn hypoxic-ischemic brain damage in mice is mediated by CB2 and adenosine receptors. Neurobiol Dis. 2010; 37( 2): 434– 440. [DOI] [PubMed] [Google Scholar]
- 46. Pazos MR, Mohammed N, Lafuente H, . et al. Mechanisms of cannabidiol neuroprotection in hypoxic-ischemic newborn pigs: role of 5HT(1A) and CB2 receptors. Neuropharmacology. 2013; 71: 282– 291. [DOI] [PubMed] [Google Scholar]
- 47. Fisher T, Golan H, Schiby G. . In vitro and in vivo efficacy of non-psychoactive cannabidiol in neuroblastoma. Curr Oncol. 2016; 23( 2): S15– S22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang GS, Roosevelt G, Heard K. . Pediatric marijuana exposures in a medical marijuana state. JAMA Pediatr. 2013; 167( 7): 630– 633. [DOI] [PubMed] [Google Scholar]
- 49. Berger E. . Legal marijuana and pediatric exposure. Ann Emerg Med. 2014; 64( 4): 19A– 21A. [DOI] [PubMed] [Google Scholar]


