Abstract
OBJECTIVES
Limited data support how to safely and effectively treat refractory pain and agitation in neonates and infants. Gabapentin has been used in this patient population and has shown promising results, yet there is still a paucity of data about its clinical efficacy. There is a need for a larger evaluation to determine its effectiveness. This study describes one institution's experience with gabapentin for the treatment of refractory pain and agitation in the neonatal intensive care unit (NICU).
METHODS
This was a retrospective, observational evaluation of patients who received gabapentin in the level IV NICU at the Cleveland Clinic Children's Hospital. Changes in neonatal pain, agitation, and sedation scale (N-PASS) scores and analgesic and sedative medication requirements were analyzed, as were gabapentin dose requirements and adverse reactions.
RESULTS
Between January 2012 and November 2015, 22 patients received gabapentin and were included in this study. The average gabapentin starting dose was 10.2 mg/kg/day, with maximum doses up to 25.5 mg/kg/day. The median N-PASS score at gabapentin therapy initiation was 3.1 and after gabapentin initiation the last N-PASS score documented was 0 in all but 5 patients. Gabapentin use reduced the need for analgesic or sedative medications. The drug was well tolerated, and only 1 patient experienced an adverse reaction to gabapentin (i.e., nystagmus).
CONCLUSIONS
Gabapentin was well tolerated and associated with decreases in pain scores. It's use resulted in decreased requirements for analgesic and sedative medications. Gabapentin therapy appears to be an effective option for neonates and infants with refractory pain and agitation.
Keywords: agitation, gabapentin, infant, neonate, pain
Introduction
Neonates and infants are exposed to many painful events during hospital admissions to the neonatal intensive care unit (NICU). Standard therapy for pain and agitation in this population includes opiates and benzodiazepines. However, pain and agitation that does not respond to first-line efforts can be diffcult to manage. Unfortunately, evidence-based guidelines and recommendations are not currently available, and prior reports and studies are hampered by small numbers.1 Furthermore, there are few alternative therapeutic options currently available to the practitioner.
Gabapentin is a gamma-aminobutyric acid analog that binds the α2-δ subunit on voltage-gated calcium channels of the dorsal root ganglion in the central nervous system.2 In patients experiencing chronic painful stimuli, there is a proposed upregulation of the α2-δ subunit receptors associated with tactile allodynia.3 These channels may activate the release of excitatory neurotransmitters, leading to nociception or the processing of potential painful stimuli. Gabapentin binds and inhibits these currents, preventing the transmission of painful stimuli.3 It appears to be an attractive option for the management of refractory pain and agitation in pediatrics as it is highly lipophilic, penetrates well through the blood-brain barrier, and has a relatively tame adverse effect profile compared to that of the sedative and addictive properties of opiates and benzodiazepines. Despite this, the clinical experience regarding dosage and efficacy for the treatment of neonatal and infant pain and agitation with gabapentin is relatively limited.4–7 This study sought to describe one institution's experience with the use of gabapentin for the treatment of refractory pain and agitation in 22 patients in the NICU.
Materials and Methods
This was a retrospective review of medical records of neonates and infants admitted to the 17- bed, level IV NICU at Cleveland Clinic Children's Hospital in Cleveland, Ohio, between January 2012 and November 2015. Appropriate approval from the Cleveland Clinic's institutional review board was obtained, and patient consent was not required due to the minimal risk nature of the evaluation. Records of all patients who received gabapentin for the treatment of refractory pain and agitation not relieved by other agents in the NICU were collected from a pharmacy department report and were included in this evaluation; no patients were excluded.
Baseline characteristics collected on the day of gabapentin initiation included gestational age (GA) at birth, day of life, gender, weight, height, renal function, neonatal pain, agitation and sedation scale (N-PASS) scores,8 and indications for NICU admittance. All gabapentin dose changes were noted, in addition to N-PASS scores at every dose change. Gabapentin doses are recorded as mg/kg/day. The use of dexmedetomidine, morphine, midazolam, and lorazepam was also collected, as these are the agents most often used for neonatal pain and agitation clinically at our institution.
Changes in N-PASS scores and analgesic and sedative medication use and dosages as well as adverse reactions due to gabapentin therapy were evaluated. Descriptive statistics were used to describe all results.
Results
During the study period, 22 patients received gabapentin in the NICU for refractory pain and agitation and were included in this evaluation. The median age of patients at baseline (gabapentin initiation) was 87 days old, and their median weight was 3.5 kg. Two patients had been started on gabapentin therapy at an outside hospital prior to transfer. The remaining 20 patients were initiated on gabapentin during the evaluated hospital admission. Complete baseline characteristics are detailed in Table 1.
Table 1.
Summary of Patient Characteristics on Day 1 of Gabapentin Therapy *
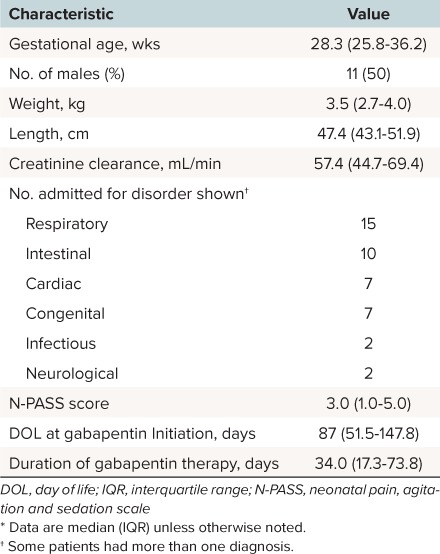
The average gabapentin starting dosage was 10.2 mg/kg/day (range, 4.6 to 16.3 mg/kg/day), and most regimens were divided 3 times daily. The average maximum gabapentin dose after dose titration was 16.4 mg/kg/day (range, 9 to 25.5 mg/kg/day). The average dosage in patients discharged from the NICU receiving gabapentin was 15.9 mg/kg/day. Twenty patients had a median N-PASS score of 3 charted at baseline. After gabapentin therapy, the median last evaluable N-PASS score was 0 (interquartile range [IQR], 0 to 0.25). N-PASS scores were 0 in all but 5 patients (3 patients had N-PASS scores of 1; 1 patient had an N-PASS score of 5; and one had a score of 3). Selected individual patient characteristics including analgesic or sedative agent use and N-PASS score changes are detailed further in Table 2.
Table 2.
Individual Patient Characteristics and Results
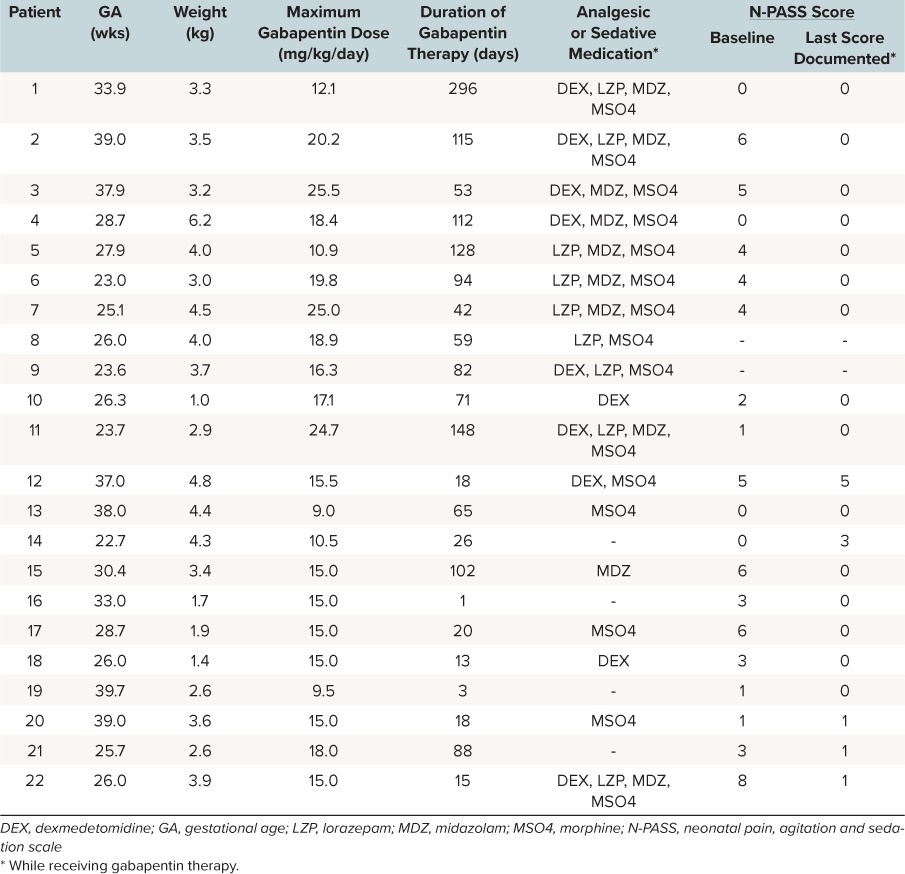
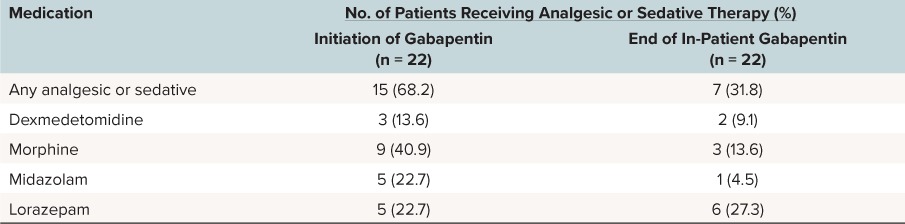
Fifteen patients were receiving additional sedative and analgesic medications at the time of gabapentin initiation (Table 3). Six of these patients were receiving more than one agent. The most common medication given at the time of gabapentin initiation was morphine. At the end of inpatient gabapentin therapy, 7 patients were receiving analgesic or sedative medications (2 patients were receiving more than 1 agent); the most common agent was lorazepam. All patients who were receiving lorazepam at the end of gabapentin therapy were receiving it on an as-needed basis or as a tapering dose to discontinuation regimen.
Table 3.
Analgesic and Sedative Medications Prescribed During Gabapentin Therapy
Of the 22 patients, 11 patients were discharged from the NICU on gabapentin therapy, 5 had gabapentin therapy discontinued while in the NICU, 4 remained in the hospital receiving gabapentin therapy at the time of study completion, and 2 expired in the NICU while receiving gabapentin. One patient experienced nystagmus, a non–sedation-related adverse reaction, likely due to gabapentin therapy.
Discussion
This is the largest report to date of the use of gabapentin in NICU patients for the treatment of refractory pain and agitation. Dosages were initiated at an average of 10.2 mg/kg/day, higher than that reported in previously published reports. Currently, there is no dosage protocol at our institution for gabapentin in the NICU, and doses are prescribed according to physician preference. However, in our series, dosages as high as 25.5 mg/kg/day were well tolerated, with only 1 episode of nystagmus reported. In regard to efficacy, this study showed improvements in N-PASS scores as well as a decrease in the number of patients who required analgesic and sedative medications after the initiation of gabapentin. Interestingly, we did not observe a decrease in the use of lorazepam in our series. This is likely due to the practice at our institution of weaning patients from sedatives and analgesic agents, which is to first wean patients from intravenous analgesics and then from continuous intravenous sedation medications. Patients who require ongoing sedation then typically receive lorazepam, from which they are slowly tapered or weaned as tolerated, resulting in the high rates seen in our study. Overall, however, there was a reduction in the use of dexmedetomidine and midazolam after gabapentin initiation.
Previous data regarding gabapentin use in this population are limited to 2 case series and 2 case studies.4–7 Behm et al2 reported the use of gabapentin for the treatment of pain and agitation in a 3-week-old male born at 36.7 weeks GA with amyoplasia congenital, which resulted in severe contractures and multiple dislocated joints and agitation. The neonatal toxicology screen was positive for marijuana (Δ9-THC) and opiates, and the infant received routine resuscitation at birth. Pain had been managed with ibuprofen and acetaminophen. Initiation of extemporaneous oral liquid formulation of gabapentin at a dosage of 7.0 mg/kg given once daily. Gabapentin was associated with improvements in pain scores over the first 72 hours. After 5 days of therapy, the dosage was increased to 10 mg/kg once daily, and the patient was discharged home.
Haney et al4 described 1 neurologically impaired male infant born at 39 weeks GA with a rare chromosomal microduplication resulting in neurological impairments in the fourth month of life. Multiple interventions including morphine, lorazepam and phenobarbital for the treatment of irritability failed for the infant. On day 98 of life, the infant was started on 5 mg/kg of gabapentin at bedtime. The infant's tone and pain scale showed significant improvement. After 6 days, the gabapentin dosage was increased to 10 mg/kg at bedtime. Ten days later, it was increased to 5 mg/kg in the morning and 10 mg/kg at bedtime. The patient was slowly weaned from gabapentin therapy, and it was ultimately discontinued at 11 months of age. The infant tolerated gabapentin well but did develop nystagmus 31 days after starting the drug. Nystagmus resolved following discontinuation of gabapentin.
Hauer et al5 retrospectively evaluated the management of 9 non-verbal children with severe neurologic impairments, ranging from 9 months to 22 years of age. All patients experienced recurrent crying episodes that lasted at least 1 hour every day with no identifiable cause. Gabapentin was prescribed when agitation could no longer be controlled by other therapeutic strategies. The regimen was started at 5 mg/kg at bedtime and increased every 3 to 7 days, with the final dosage ranging from 15 to 35 mg/kg/day divided 3 or 4 times a day. After titration of gabapentin up to 35 mg/kg/day, patients and caregivers reported significant improvements, such as decreased irritability, decreased crying; and improved comfort, feeding tolerance, and better sleeping patterns. A single case of nystagmus was reported.
Edwards et al3 recently reported a study of 11 infants (8 preterm) with visceral hyperalgesia treated with gabapentin. Initial dosages were 5 mg/kg once daily (n = 1), 5 mg/kg every 8 hours (n = 3), 5 mg/kg every 12 hours (n = 6), and 10 mg/kg every 12 hours (n = 1). Gabapentin therapy was associated with improved feeding tolerance, decreased irritability, and decreased requirements for opioids and/or benzodiazepines while patients received gabapentin. Overall, 5 patients experienced an adverse event related to gabapentin therapy, with the most common adverse event being either bradycardia or tachycardia. Tachycardia was likely related to abrupt withdrawal of gabapentin, but the event resolved when gabapentin was resumed.
Our study adds to the growing body of data for the effectiveness of gabapentin for the treatment of refractory pain and irritability in a diverse patient population of newborns and infants admitted to a NICU. This series is the largest cohort of NICU patients reported to date and is similar to previous reports. We observed an improvement in the patient's symptoms with a low incidence of adverse reactions.
The current study has limitations that should be noted. This was a single-center, retrospective, observational study without a control group and was limited by a small sample size, which preclude us from performing meaningful inferential statistics. As in other studies, the definition of the indication for initiation of gabapentin is difficult to define objectively, and we were limited to the attending physician's definition of refractory pain and irritability. However, our study used well-validated scales for the evaluation of pain and sedation, and we documented a clinically significant improvement in all patients in whom the scores were completed. Finally, due to retrospective methodology, side effects were determined based on physician-documented notes, and the occurrence of sedation was unable to be obtained.
Overall, our experience indicates that gabapentin appears to be an effective treatment option for neonates and infants with refractory pain and agitation, and its use was associated with decreasing N-PASS scores and less need for analgesic and sedative medications. There is a need for larger, prospective, controlled studies to further evaluate the efficacy and safety of gabapentin and to determine the most appropriate dose and duration for therapy in this patient population.
Abbreviations
- GA
gestational age
- NICU
neonatal intensive care unit
- N-PASS
neonatal pain, agitation, and sedation scale
Footnotes
Disclosure The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria. Dr. Sacha had full access to all data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
Copyright Published by the Pediatric Pharmacy Advocacy Group. All rights reserved. For permissions, email: matthew.helms@ppag.org.
REFERENCES
- 1. Hauer J. . Identifying and managing sources of pain and distress in children with neurological impairment. Pediatric Annals. 2010; 39( 4): 198– 205. [DOI] [PubMed] [Google Scholar]
- 2. Behm MO, Kearns GL. . Treatment of pain with gabapentin in a neonate. Pediatrics. 2001; 108( 2): 482– 484. [DOI] [PubMed] [Google Scholar]
- 3. Edwards L, DeMeo S, Hornik CD, . et al. Gabapentin use in the neonatal intensive care unit. J Pediatr. 2016; 169: 310– 312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Haney AL, Garner SS, Cox TH. . Gabapentin therapy for pain and irritability in a neurologically impaired infant. Pharmacotherapy. 2009; 29( 8): 997– 1001. [DOI] [PubMed] [Google Scholar]
- 5. Hauer JM, Wical BS, Charnas L. . Gabapentin successfully manages chronic unexplained irritability in children with severe neurologic impairment. Pediatrics. 2007; 119( 2): e519– 522. [DOI] [PubMed] [Google Scholar]
- 6. Hummel P, Puchalski M, Creech SD, Weiss MG. . Clinical reliability and validity of the N-PASS: neonatal pain, agitation and sedation scale with prolonged pain. J Perinatol. 2008; 28( 1): 55– 60. [DOI] [PubMed] [Google Scholar]


