Abstract
OBJECTIVES
Extended-infusion piperacillin/tazobactam (TZP) has been associated with positive clinical outcomes in adults, but similar data in children are lacking. The objective of this study was to describe efficacy outcomes with pediatric patients receiving extended-infusion TZP.
METHODS
This was a retrospective case series of children aged 1 month to 17 years who had documented Gram-negative infection and received extended-infusion TZP between April 2011 and March 2012. The primary outcome was 21-day clinical cure defined as negative follow-up cultures, where available, and infection resolution.
RESULTS
Fifty children with a median (interquartile range [IQR]) age of 5 (2–9) years were included in the study. Patients received a median (IQR) TZP dose of 111.4 (100–112.5) mg/kg administered every 8 hours over 4 hours. Clinical and microbiologic cure were observed in 74% and 100% of patients, respectively. Patients not meeting criterial for 21-day clinical cure were younger (1 vs 7 years, p = 0.087) and had a longer length of hospital stay (23 vs 11 days, p = 0.037).
CONCLUSIONS
The majority of children in this cohort achieved 21-day clinical cure with extended-interval TZP. Those without clinical cure tended to be younger and critically ill. Additional comparative studies evaluating traditional and extended-infusion TZP in children are needed.
Keywords: beta-lactam, extended infusion, outcomes, pediatrics, piperacillin/tazobactam
Introduction
Piperacillin/tazobactam (TZP) is a beta-lactam/beta-lactamase inhibitor combination frequently used to treat suspected and documented Gram-negative infections in pediatric patients. Increased prevalence of bacterial resistance is being reported in adults and children, and resistant infections have been associated with negative outcomes.1–4 Suboptimal antimicrobial dosing is also associated with poorer clinical outcomes, and antimicrobial dose optimization using pharmacokinetic and pharmacodynamic (PK/PD) principles is one potential way to improve clinical outcomes.5–7 For beta-lactam antimicrobials such as TZP it has been demonstrated that the time during which the non–protein bound concentration exceeds the minimum inhibitory concentration (fT>MIC) of the organism at the site of the infections is the best predictor of bactericidal activity.6,8 For penicillin antibiotics, including TZP, the desired fT>MIC is ≥50% of the dosing interval to ensure maximal bactericidal effect.5,6 Extending the duration of infusion is one way to increase the fT>MIC without using more drug per day or tying up intravenous access continuously. Extended-infusion TZP dosing involves infusion of a standard dose over 3 to 4 hours, which may result in fewer daily doses (every 8 hours vs every 6 hours).
Numerous descriptions of extended-infusion TZP dosing strategies are available in adult populations.7–14 In adults, extended-infusion TZP results in the achievement of pharmacodynamic targets more reliably than does traditional dosing, and it has been associated with better clinical outcomes when compared to traditional infusion TZP.7,9 There are fewer reports of this strategy being applied to the pediatric population, with many describing Monte Carlo simulations using PK data derived from traditional TZP dosing administered over 30 minutes.15–19 Nichols and colleagues18 obtained serial blood samples from 12 critically ill children receiving extended-infusion TZP to determine the population PK of this regimen. Monte Carlo simulations built using the extended-infusion TZP population PK data demonstrated that TZP doses of ≥80 mg/kg/dose (piperacillin component) administered every 8 hours over 4 hours achieved a probability of target attainment (PTA) of greater than 90% for bacteria with MIC values of ≤16 mg/L.18 Implementing extended-infusion TZP as standard of care in pediatric patients is suggested to be feasible, but there is an absence of clinical outcomes data associated with the dosing strategy.19 The objective of this study was to describe efficacy outcomes in children who were treated with an extended-infusion TZP dosing regimen. Children at our institution received 112.5 mg/kg intravenously (IV) every 8 hours infused over 4 hours as part of routine clinical practice based on published Monte Carlo simulations by Courter et al15 and to keep total daily dose consistent with previous practice.
Materials and Methods
This was a retrospective case series of patients who received TZP 112.5 mg/kg IV every 8 hours infused over 4 hours between April 1, 2011, and March 31, 2012. Patients aged 1 month through 17 years with a documented Gram-negative infection who received extended-infusion TZP for at least 48 hours were identified through a pharmacy computer system report. Patients were excluded if they received more than 1 dose of an additional antimicrobial with Gram-negative activity (with the exception of double coverage with an aminoglycoside or a fluoroquinolone), received multiple TZP dosage regimens, were inadequately treated for Gram-positive or fungal infection, were cared for in the neonatal intensive care unit, or received any type of renal replacement therapy. Patients with cystic fibrosis were also excluded.
The primary outcome was clinical cure. Patients were categorized as achieving clinical cure if at 21 days following TZP initiation they were afebrile, had experienced complete symptomatic resolution, if their white blood cell count had normalized (if initially abnormal), and if they had negative follow-up cultures, when available. Secondary outcomes included length of stay and duration of TZP treatment, 30-day readmission, and 30-day mortality. Descriptive statistics were used for baseline characteristics and for primary and secondary outcomes. Groupwise comparisons were made using Chi-square analyses and Mann-Whitney tests for non-parametric data. Statistical analyses were conducted using Statistical Package for Social Sciences, version 23.0 (SPSS Inc, Chicago, IL). The study was approved by the Indiana University and Butler University institutional review boards.
Results
Fifty of 1004 screened patient encounters were eligible for inclusion. The most common reasons that screened patients were not included were lack of documented Gram-negative infection and receipt of an extended-infusion TZP regimen for less than 48 hours. Eleven patients were excluded as the result of a diagnosis of cystic fibrosis, leaving 39 patients in the final analysis. Patients receiving extended-infusion TZP in this cohort had a median (interquartile range [IQR]) age of 5 (2–9) years and a weight of 19.5 (11.5–36) kg. Forty-nine percent (n = 19) of patients were male. Patients were most commonly cared for by the general surgery (30.8%) and oncology (25.6%) services. The median (IQR) dose of extended-infusion TZP was 111.4 (100–112.5) mg/kg administered every 8 hours, and the median (IQR) of 4 (3–6) days, with minimum and maximum durations of therapy, was 2 and 16 days, respectively. Concomitant aminoglycoside therapy was used in 28.2% (n = 11) of patients. Median (IQR) length of stay was 12 (5–28) days.
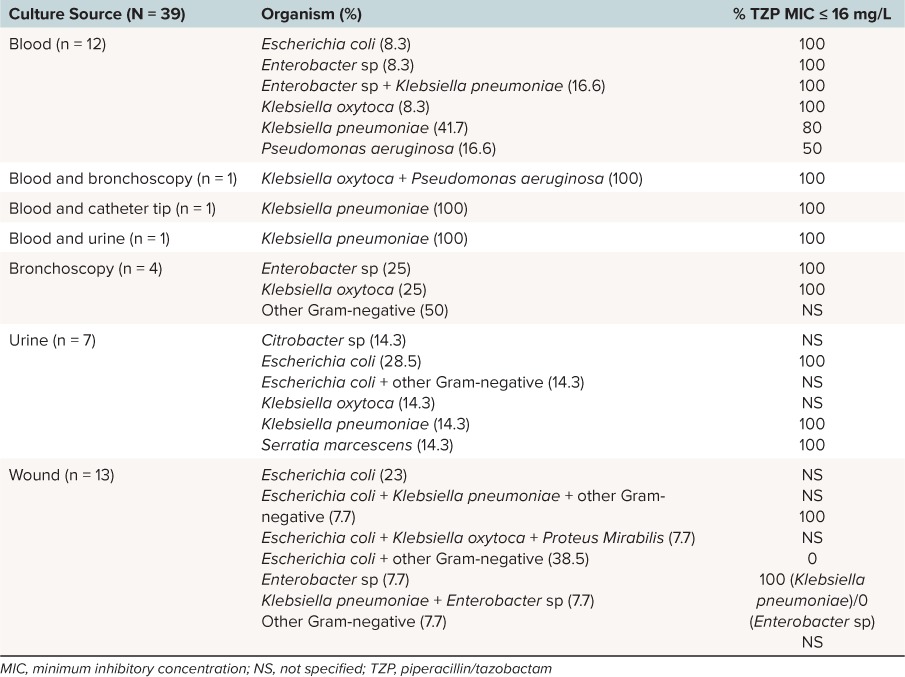
Blood (38.5%) and wound (33.3%) were the most common culture sites, with Escherichia coli and Klebsiella pneumoniae the most commonly isolated organisms. Infection was polymicrobial in 35.9% (n = 14) of patients, with 2 Gram-negative organisms in 30.8% of all patients and 3 Gram-negative organisms in 5.1% (n = 2). Culture site and organism distribution are summarized in Table 1. Follow-up cultures were obtained in 35.9% (n = 14) of patients, and these were follow-up of blood (n = 13) and wound culture (n = 1). All follow-up cultures resulted in no growth. Follow-up cultures were not obtained in patients with bacterial growth isolated from bronchoscopy or urine. Gram-positive and fungal organisms in addition to Gram-negative organisms were isolated in 35.9% and 7.7% of patients, respectively, and patients with concurrent infections received adequate therapy.
Table 1.
Culture and Susceptibility Data
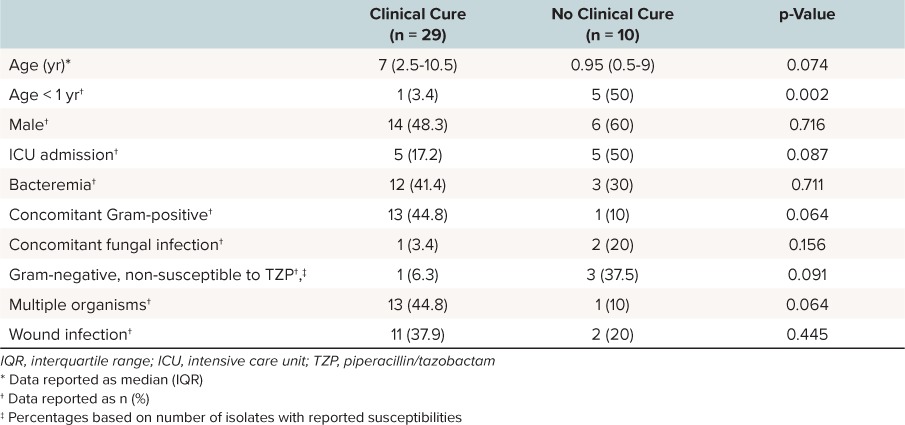
Overall, 74% (n = 29) of patients met the predefined criteria for 21-day clinical cure. In patients with follow-up cultures, 85.7% achieved clinical cure, while 68% of those without follow-up cultures met the definition for clinical cure. The reason for not meeting the clinical cure definition in all patients was ongoing infection-related symptoms. Though differences did not reach statistical significance, patients not meeting criteria for clinical cure were numerically younger (1 vs 7 years; p = 0.074) and had a higher severity of illness based on intensive care unit (ICU) admission (50% vs 17.2%; p = 0.087) and overall length of hospital stay (23 vs 11 days; p = 0.037). All 4 patients with bacteria isolated solely via bronchoscopy were admitted to the ICU, with only 1 of those meeting criteria for clinical cure. Characteristics of patients with and without 21-day clinical cure are displayed in Table 2. There were no deaths reported in this cohort, and 38.5% (n = 15) of patients had a 30-day readmission. Adverse effects related to extended-infusion TZP were not experienced in this cohort.
Table 2.
Clinical Cure Data
Discussion
Emergence of antimicrobial resistance is a growing concern in children. Antibiotic dose optimization, based on PK/PD parameters and desired targets, is a strategy to combat the emerging resistance while utilizing commonly available antibiotics with widely known efficacy and safety profiles. This strategy is particularly important in the pediatric population, as new or emerging anti-infective medications are typically studied in adults prior to market approval and because there is generally a delay from market approval to availability of pediatric data. This creates a need for pediatric practitioners to use currently available antibiotics in the most optimal manner. Though providing beta-lactams such as TZP via continuous infusion would certainly allow optimization of fT>MIC, continuous infusions present potential issues, such as decreased patient freedom and inability to co-infuse non-compatible IV medications. Extending infusions to 3 or 4 hours increases the fT>MIC for some organisms and leaves 12 to 15 hours of time to infuse non-compatible medications or for the patient to disconnect from the IV infusion.15 Extended-infusion TZP was used in this cohort of children, with 74% of patients achieving predefined criteria for clinical cure, and with microbiologic resolution in all instances of follow-up culturing.
Many studies conducted in adult patients have evaluated clinical outcomes associated with extended-infusion TZP, and some have compared it with standard infusion of TZP. Extended-infusion TZP has been associated with significant costs savings and has been suggested to be a safe, alternate dosing regimen to standard infusion TZP.20 Improved mortality in ICU patients receiving extended-infusion TZP compared to standard infusion TZP (19% vs 38%, p = 0.01) as well as a shorter duration of mechanical ventilation was observed in one small retrospective cohort of critically ill adults.21 Similar improvements in mortality were observed in adults with P. aeruginosa infection receiving extended-infusion TZP vs standard infusion TZP (12.2% vs 31.6%, p = 0.04).7 In adults with confirmed Gram-negative infections, multivariate regression analysis demonstrated that extended-infusion TZP improved survival by nearly 3 days and reduced overall mortality risk.9 Contrasting studies8,22 in septic adults and in adults with Gram-negative infection suggest no differences in mortality or clinical success outcomes, but they do suggest that extended-infusion TZP remains a safe alternate dosing regimen to standard infusion TZP. Demonstration of a difference between dosing regimens retrospectively can be challenging, as extended-infusion TZP may offer benefits only in certain subsets of individuals, and these studies included patients with lower severity of illness, lower MICs, and without demonstrated pathogens.
There is mounting evidence to support extended-infusion TZP in pediatric patients, but it remains significantly behind the amount of available adult data. Nichols and colleagues19 have commented on their experience with implementing extended-infusion TZP as a standard of care in their pediatric hospital and have suggested that standard use of extended-infusion TZP in children is feasible. Additional evidence has been provided by PK/PD studies. Monte Carlo simulations were created from pediatric population PK data derived from a single-dose traditional infusion TZP study.15,23 Piperacillin/tazobactam dosed at 300 mg/kg/day and administered every 8 hours over 3 hours resulted in a PTA of 83% against P. aeruginosa, with MIC values of 16 mg/L. Increasing the dose to 320 mg/kg/day every 6 hours administered over 3 hours increased the PTA to 100%. Traditional infusion times, whether utilized with every 8-hour or 6-hour dosing regimens, with doses ranging from 300 to 480 mg/kg/day, resulted in PTA ranges of 21% to 66%.15 Additional pediatric simulations suggest enhanced PTA with extended-infusion TZP. Cies and colleagues16,17 described simulations using population PK data of piperacillin in a pediatric oncology population and PT in critically ill children. A dose of 100 mg/kg every 8 hours administered over 4 hours in children with febrile neutropenia reached a PTA of approximately 90% for organisms with MIC values of 16 mg/L.17 Simulations in critically ill children were similar, with an approximate 90% PTA achieved with doses of 80 mg/kg every 8 hours over 4 hours at MIC values of 16 mg/L.16 The first pediatric PK/PD using data derived from children receiving extended-infusion TZP was recently published by Nichols and colleagues.18 Consistent with previous pediatric simulation data, TZP doses of 80 to 100 mg/kg every 6 to 8 hours and infused over 3 to 4 hours reached PTA of > 90% at MIC values of 16 mg/L when the target was ≥50% fT>MIC.18 Doses of 80 to 100 mg/kg every 6 to 8 hours administered over 30 minutes were less likely to achieve PD targets, especially in the setting of increasing MICs.18
The available body of pediatric evidence is an important driver of practice advancement and dose optimization, and there are a couple of important considerations. The first is that in the simulations from Cies and Courter, the population PK data utilized were derived from PT doses infused over 30 minutes.15–17 Courter et al15 used PK data from a single dose study, while Cies et al16,17 used PK data derived from febrile neutropenic or critically ill pediatric patients. Nichols' simulations used PK data from extended-infusion TZP, with findings consistent with those of previous studies.18 Another important consideration is that despite data suggesting extended-infusion TZP is feasible and that it enhances PTA in the presence of increasing MICs, the existing pediatric evidence is void of any outcomes data with extended-infusion TZP. A 2014 survey24 of children's hospitals reported that extended-infusion beta-lactams, and TZP specifically, is being used by nearly 25% of responding hospitals. Over half of the respondents not utilizing extended-infusion beta-lactams indicated the primary reason is due to lack of pediatric efficacy data.24
This study is the first pediatric study describing outcomes with extended-infusion TZP. Our cohort of children was being treated for a variety of Gram-negative infections, and extended-infusion TZP was associated with a favorable response. Nearly 75% of patients achieved clinical cure at 21 days. Though clinical cure at 21 days is not a well-established endpoint in the literature, identifying an optimal primary endpoint proved difficult, as mortality is so infrequently experienced. Clinical cure at 21 days was therefore chosen in order to allow for time for potential inclusion of patients with more complicated infection, such as meningitis. In patients with follow-up cultures, microbiologic clearance, as indicated by negative follow-up cultures, was 100%.
Patients who did not achieve clinical cure tended to be younger with a higher severity of illness but were categorized as not achieving clinical cure as a result of ongoing infection-associated symptoms. Anecdotally, the overall historic rate of cure with PT at our institution is 78%, which, though not statistically significant when compared with extended-infusion TZP, is clinically similar. While the sample size was small, our data suggest that multiple Gram-negative organisms from culture sites or concomitant fungal infection may be associated with negative outcomes. The 30-day readmission finding was of interest, but as reasons for readmission were not collected, it is unknown whether readmission was specifically infection-related. Most patients were cared for by the general surgery or oncology services, which could indicate complex disease or frequent admissions for multiple reasons, including chemotherapy.
Despite being the first report of outcomes with extended-infusion TZP, there are limitations to this study. Being a single-center retrospective cohort, generalizing our findings to pediatric hospitals with a different case-mix may be difficult. Relying on chart documentation and the variable availability of certain data could have affected our findings, as well as the generalizability of our findings. We were often unable to pinpoint reasons for changes in antibiotic regimens or for the seemingly persistent nature of patients' infections. Because patients only had to receive extended-infusion TZP for a minimum of 48 hours to be included, it is likely that other factors, including other antibiotic factors in some cases, had a more profound impact on outcomes than did extended-infusion TZP. It is also possible that some of the identified infections were not truly infections and that the persistent symptoms were secondary to non-infectious sources. Finally, many of the screened patients were ineligible for inclusion, decreasing the sample size and likely affecting the study power.
The majority of children in this cohort achieved 21-day clinical cure with extended-infusion TZP. Those without clinical cure tended to be younger and critically ill. Additional comparative studies evaluating traditional and extended-infusion TZP in pediatric populations are needed, particularly in those who are critically ill or in whom infecting pathogens possess elevated MICs to TZP.
Abbreviations
- IQR
interquartile range
- MIC
minimum inhibitory concentration
- PK/PD
pharmacokinetic and pharmacodynamic
- PTA
probability of target attainment
- TZP
piperacillin/tazobactam
Footnotes
Disclosure The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria. The authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Copyright Published by the Pediatric Pharmacy Advocacy Group. All rights reserved. For permissions, email: matthew.helms@ppag.org.
REFERENCES
- 1. El-Mahallawy HA, El-Wakil M, Moneer MM, Shalaby L. . Antibiotic resistance is associated with longer bacteremic episodes and worse outcome in febrile neutropenic children with cancer. Pediatr Blood Cancer. 2011; 57( 2): 283– 288. [DOI] [PubMed] [Google Scholar]
- 2. Qin X, Zerr DM, Weissman SJ, . et al. Prevalence and mechanisms of broad-spectrum beta-lactam resistance in Enterobacteriaceae: a children's hospital experience. Antimicrob Agents Chemother. 2008; 52( 11): 3909– 3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wisplinghoff H, Seifert H, Tallent SM, . et al. Nosocomial bloodstream infections in pediatric patients in United States hospitals: epidemiology, clinical features and susceptibilities. Pediatr Infect Dis J. 2003; 22( 8): 686– 691. [DOI] [PubMed] [Google Scholar]
- 4. Lee CY, Chen PY, Huang FL, Lin CF. . Microbiologic spectrum and susceptibility pattern of clinical isolates from the pediatric intensive care unit in a single medical center—6 years' experience. J Microbiol Immunol Infect. 2009; 42( 2): 160– 165. [PubMed] [Google Scholar]
- 5. Lodise TP, Lomaestro BM, Drusano GL. . Application of antimicrobial pharmacodynamic concepts into clinical practice: focus on β-lactam antibiotics. Pharmacotherapy. 2006; 26( 9): 1320– 1332. [DOI] [PubMed] [Google Scholar]
- 6. Craig WA. . Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1998; 26( 1): 1– 10. [DOI] [PubMed] [Google Scholar]
- 7. Lodise TP, Lomaesto B, Drusano GL.. Piperacillin-tazobactam for Pseudomonas aeruginosa infection: clinical implications of an extended-infusion dosing strategy. Clin Infect Dis. 2007; 44( 3): 357– 363. [DOI] [PubMed] [Google Scholar]
- 8. Patel GW, Patel N, Lat A, . et al. Outcomes of extended infusion piperacillin/tazobactam for documented Gram-negative infections. Diagn Microbiol Infect Dis. 2009; 64( 2): 236– 240. [DOI] [PubMed] [Google Scholar]
- 9. Yost RJ, Cappelletty DM. . The Retrospective Cohort of Extended-Infusion Piperacillin-Tazobactam (RECEIPT) Study: a multicenter study. Pharmacotherapy. 2011; 31( 8): 767– 775. [DOI] [PubMed] [Google Scholar]
- 10. Reese AM, Frei CR, Burgess DS. . Pharmacodynamics of intermittent and continuous infusion piperacillin/tazobactam and cefepime against extended-spectrum beta-lactamase producing organisms. Int J Antimicrob Agents. 2005; 26( 2): 114– 119. [DOI] [PubMed] [Google Scholar]
- 11. Shea KM, Cheatham SC, Smith DW, . et al. Comparative pharmacodynamics of intermittent and prolonged infusions of piperacillin/tazobactam using Monte Carlo simulations and steady-state pharmacokinetic data from hospitalized patients. Ann Pharmacother. 2009; 43( 11): 1747– 1754. [DOI] [PubMed] [Google Scholar]
- 12. Shea KM, Cheatham SC, Wack MF, . et al. Steady-state pharmacokinetics and pharmacodynamics of piperacillin/tazobactam administered by prolonged infusion in hospitalised patients. Int J Antimicrob Agents. 2009; 34( 5): 429– 433. [DOI] [PubMed] [Google Scholar]
- 13. Chung EK, Cheatham SC, Fleming MR, . et al. Population pharmacokinetics and pharmacodynamics of piperacillin and tazobactam administered by prolonged infusion in obese and nonobese adults. J Clin Pharmacol. 2015; 55( 8): 899– 908. [DOI] [PubMed] [Google Scholar]
- 14. Cheatham SC, Fleming MR, Healy DP, . et al. Steady-state pharmacokinetics and pharmacodynamics of piperacillin and tazobactam administered by prolonged infusion in obese patients. Int J Antimicrob Agents. 2013; 41( 1): 52– 56. [DOI] [PubMed] [Google Scholar]
- 15. Courter JD, Kuti JL, Girotto JE, . et al. Optimizing bactericidal exposure for beta-lactams using prolonged and continuous infusions in the pediatric population. Pediatr Blood Cancer. 2009: 53( 3): 379– 385. [DOI] [PubMed] [Google Scholar]
- 16. Cies JJ, Shankar V, Schlichting C, Kuti JL. . Population pharmacokinetics of piperacillin/tazobactam in critically ill young children. Pediatr Infect Dis J. 2014; 33( 2): 168– 173. [DOI] [PubMed] [Google Scholar]
- 17. Cies JJ, Jain J, Kuti JL. . Population pharmacokinetics of the piperacillin component of piperacillin/tazobactam in pediatric oncology patients with fever and neutropenia. Pediatr Blood Cancer. 2015; 62( 3): 477– 482. [DOI] [PubMed] [Google Scholar]
- 18. Nichols KR, Chung EK, Knoderer CA, . et al. Population pharmacokinetics and pharmacodynamics of extended-infusion piperacillin and tazobactam in critically ill children. Antimicrob Agents Chemother. 2015; 60( 1): 522– 531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nichols KR, Knoderer CA, Cox EG, . et al. System-wide implementation of the use of an extended-infusion piperacillin/tazobactam dosing strategy: feasibility of utilization from a children's hospital perspective. Clin Ther. 2012; 34( 6): 1459– 1465. [DOI] [PubMed] [Google Scholar]
- 20. Brunetti L, Poustchi S, Cunningham D, . et al. Clinical and economic impact of empirical extended-infusion piperacillin-tazobactam in a community medical center. Ann Pharmacother. 2015; 49( 7): 754– 760. [DOI] [PubMed] [Google Scholar]
- 21. Lee GC, Liou H, Yee R, . et al. Outcomes of extended-infusion piperacillin-tazobactam: a retrospective analysis of critically ill patients. Clin Ther. 2012; 34( 12): 2297– 2300. [DOI] [PubMed] [Google Scholar]
- 22. Cutro SR, Holzman R, Dubrovskaya Y, . et al. Extended-infusion versus standard-infusion piperacillin-tazobactam for sepsis syndromes at a tertiary medical center. Antimicrob Agents Chemother. 2014; 58( 8): 4470– 4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reed MD, Goldfarb J, Yamashita TS, . et al. Single-dose pharmacokinetics of piperacillin and tazobactam in infants and children. Antimicrob Agents Chemother. 1994; 38( 12): 2817– 2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Knoderer CA, Nichols KR, Cox EG. . Optimized antimicrobial dosing strategies—a survey of pediatric hospitals. Paediatr Drugs. 2014; 16( 6): 523– 529. [DOI] [PubMed] [Google Scholar]


