Abstract
OBJECTIVES
American Congress of Obstetricians and Gynecologists recommends a single dose of antibiotic prophylaxis before all cesarean sections (C/S). This recommendation is based on pharmacokinetic studies that include only non-obese patients. We sought to evaluate 1) cefazolin plasma concentrations among obese and non-obese patients after administration of a 2-g cefazolin dose for prevention of surgical wound infections, and 2) whether cefazolin concentration in fetal circulation may be protective against pathogens that cause early onset neonatal sepsis.
METHODS
Maternal and fetal cefazolin plasma concentrations were compared between obese (body mass index [BMI] ≥ 30 kg/m2) and non-obese (BMI < 25 kg/m2) healthy, term pregnant women undergoing scheduled C/S. Liquid chromatographic–tandem mass spectrometric (LC-MS/MS) methods were used for quantification of total and free cefazolin concentrations in maternal blood (MB) and umbilical cord blood (UCB).
RESULTS
Eight women were screened and consented. There was no difference between groups in MB total and free cefazolin concentrations. All MB samples had total and free cefazolin concentrations greater than the minimum inhibitory concentration 90 (MIC90) for Group B Streptococcus (GBS), Staphylococcus aureus, and Escherichia coli. All UCB samples had total and free cefazolin concentrations greater than MIC90 for GBS and S aureus, even when administered as briefly as 18 minutes before delivery. A lower concentration of total cefazolin was detected in UCB of neonates of obese women compared to non-obese women (p > 0.05).
CONCLUSIONS
Administration of 2 g of cefazolin to women undergoing scheduled C/S might be an adequate prophylactic dose for surgical wound infection in both non-obese and obese patients; and cefazolin concentration in fetal circulation may be protective against GBS and S aureus.
Keywords: antibiotic prophylaxis, cefazolin, obesity, pregnancy, surgical wound infection
Introduction
Antibiotics are used in cesarean sections (C/S) for prevention of both surgical wound infections and neonatal sepsis. Surgical wound infection is one of the most common postoperative complications, affecting up to 20% of all intra-abdominal surgeries.1,2 Approximately 2.8% to 26.6% of women delivering via C/S develop a surgical wound infection in the United States.3,4 The incidence of postoperative surgical wound infection decreases for elective C/S with the use of antibiotic prophylaxis to 4.6%, while rates continue to remain high for C/S with labor and ruptured membranes at a current rate of 20% to 85%.3,5 As the occurrence of cesarean deliveries continues to increase in the United States,3 it is essential to determine an adequate prophylactic antibiotic for prevention of surgical wound infections.
Cefazolin is a first-generation cephalosporin that is effective against both Gram-positive bacteria and Escherichia coli.6–8 This antibiotic is routinely administered intravenously to pregnant women undergoing C/S to decrease the risk of surgical wound infection.6–8 Substantial evidence currently exists to validate the safety of this antibiotic in pregnant women and to verify the effectiveness of cefazolin in reducing the rate of postoperative surgical wound infections.7,9
The American Congress of Obstetricians and Gynecologists currently recommends antimicrobial prophylaxis with a single 1-g dose of cefazolin within 60 minutes before the start of cesarean delivery.10 Current recommendations regarding clinical dose adjustments for patients with variable body types range from 2 to 3 g per dose.10 The current guidelines are based on pharmacokinetic studies including only non-obese patients10; therefore, a larger dose of cefazolin may be required for obese women for surgical wound prophylaxis. As optimal dose standards in relation to weight have not yet been developed and the demographics of pregnant women in the United States have significantly changed in recent years, with 18.5% to 38.8% of women categorized as obese or morbidly obese,11 the need for revised and accurate cefazolin dosage guidelines is present.
Cefazolin is also administered to pregnant women as an alternative to penicillin or ampicillin to prevent early onset Group B Streptococcus (GBS) disease among infants born to penicillin-allergic mothers.12 Despite its routine use in clinical care, data on intrapartum cefazolin plasma concentrations are limited and conflicting.7,13,14
In this study, we sought to evaluate maternal blood (MB) and umbilical cord blood (UCB) cefazolin plasma concentrations among obese and non-obese patients after administration of a single 2-g dose for prevention of surgical wound infections. As our secondary outcome, we evaluated whether fetal circulation concentration of cefazolin administered within 40 minutes before delivery may reach a level that is protective against common pathogens that cause early onset neonatal sepsis, including GBS disease. We hypothesized that cefazolin plasma concentrations would be lower among obese women and their infants, which would impact its effectiveness in this population. The effectiveness of antibiotic prophylaxis, in terms of both surgical wound infection and neonatal sepsis, is determined by the maintenance of free cefazolin concentrations above its minimum inhibitory concentration (MIC) against targeted bacteria.13
Materials and Methods
Subjects. This was a prospective cohort study of healthy, full-term pregnant women undergoing scheduled C/S at an urban hospital (Johns Hopkins Hospital) between November 2013 and March 2014. Patients were screened and consented before enrollment and classified into 2 groups: obese (BMI ≥ 30 kg/m2) and non-obese (BMI < 25 kg/m2). Maternal body mass index (BMI) was based on height and weight measured at the time of delivery. Women who were 18 years of age or older, greater than 37 weeks' gestation, and scheduled to deliver via C/S before onset of labor or rupture of membranes were consented and included in this study. Women with multiple gestation pregnancy, allergy to penicillin or other cephalosporins, exposure to other antibiotics within 24 hours before the scheduled C/S, history of illicit substance use, diagnosis of polyhydramnios, fetal or chromosomal defects or major anomalies, signs of infection at the time of delivery, or diagnosis of any of the following disorders including maternal hypertensive disorders, maternal diabetes, renal insufficiency (serum creatinine > 1 mg/dL), and chronic infections—including hepatitis B, hepatitis C, or HIV—were excluded from this study. This study was reviewed and approved by the Institutional Review Board at Johns Hopkins University. Appropriate informed consent was obtained for all patients enrolled in the study.
Samples. MB and UCB samples were collected during the scheduled C/S for cefazolin concentration measurements. All patients in this study received 2 g of cefazolin administered intravenously by bolus injection over an average of 2 minutes and 30 seconds before scheduled C/S per routine clinical care. The average time between cefazolin administrations to delivery was 30 minutes (18–37 minutes). Time points were recorded for cefazolin administration, delivery, and research-related blood draws. MB was drawn within 10 minutes of cefazolin administration (first MB) and again within 10 minutes after delivery (second MB) by venipuncture performed by the anesthesiologist. UCB was collected within 10 minutes of delivery by trained research personnel using an ex utero aseptic funnel technique.15 All samples were collected within heparin anticoagulant tubes and transferred to the laboratory for further processing.
Blood plasma isolation procedures were performed on the MB and UCB samples. Samples were processed in the microcentrifuge at 13,200 rpm for 5 minutes. Plasma was removed, placed in cryovials, and frozen at −80°C for storage.
Liquid Chromatographic–Tandem Mass Spectrometric Analysis. Total and free cefazolin concentrations were determined in maternal and umbilical cord plasma by using a previously described liquid chromatographic–tandem mass spectrometric (LC-MS/MS) assay.16 The method was validated as per US Food and Drug Administration Bioanalytical Method Validation guidelines. Cefazolin-spiked plasma calibrators, quality control samples, and unknown specimens were prepared identically. Briefly, 0.020 mL of sample was required for analysis of total or free drug. For total cefazolin drug quantification, samples were subjected to protein precipitation. Ultrafiltration was used for isolation of the free drug. Chromatographic separation was achieved on a Phenomenex Kinetix C8, 50 × 2.1-mm UPLC column (Torrance, CA). The eluent was analyzed by using a Thermo Vantage mass analyzer (Thermo Fisher Scientific, San Jose, CA) operated in selected reaction-monitoring mode. Peak area ratios were determined by using the structural analog cloxacillin. Transitions monitored were as follows: cefazolin transition m/z 455.0 - > 156.0; cloxacillin transition m/z 437.0 -> 278.0. The analytic measuring ranges of the assay were 0.48 to 480 mg/L and 0.048 to 48 mg/L for total and free cefazolin, respectively. Calibration curves were generated by using weighted 1/χ2 linear regression. The average matrix effects across 3 quality control levels were 95.8% and 94.8% for cefazolin and cloxacillin, respectively. The appropriateness of UCB was proven by spiking cefazolin into remnant UCB specimens and assessing recovery from a blood plasma calibration curve.
Statistical Analysis. Statistical analyses were performed with STATA 13.0 statistical software (Stata Corporation, College Station, TX). MB and UCB concentrations were plotted against maternal BMI for both total and free concentrations of cefazolin to assess for a difference between the 2 groups of women within the study. Linear regression analysis was performed to determine the relationship between BMI and cefazolin concentration. Free cefazolin concentrations in MB and UCB were compared against the MIC required to inhibit 90% of growth for several pathogens. This evaluation was necessary, as free drug concentrations are considered active drug for pharmacologic effects6 and therefore necessary to assess the level of protection that cefazolin provides against surgical wound infections and neonatal sepsis. The pathogens assessed included GBS andEscherichia coli, common pathogens causing neonatal sepsis, and Staphylococcus aureus, associated with surgical wound infections.17
Results
Eight women were enrolled and completed the study. These 8 patients represented 2 groups: 4 obese (BMI ≥ 30 kg/m2) and 4 non-obese (BMI < 25 kg/m2). Maternal characteristics and timing of blood draws are summarized in the Table.
Table.
Characteristics of Study Patients *

Time intervals varied between the 3 sample collection times. The first MB collection was performed within 10 minutes after cefazolin administration for all patients in the study. The second MB collection occurred within 10 minutes after delivery. The timing for collection of UCB ranged between 3 and 19 minutes after delivery. The variation in timing for UCB collection is attributed to the time required for the trained research personnel to transfer the placenta to the designated collection room and perform the collection.
The total drug concentration in the MB and UCB was comparable among obese and non-obese women. As expected, the drug concentration decreased in the second MB and UCB specimens compared to the first MB specimen (Figure 1). The total and free cefazolin concentration measured in maternal plasma after delivery (second MB) exceeded 47 and 17 mg/L, respectively, for all women evaluated in the study. There was no correlation between maternal total and free cefazolin concentration after delivery and maternal BMI (Figure 2).
Figure 1.

Total drug concentrations among obese as compared to non-obese women and their fetus at different time points.
Figure 2.

Maternal blood (post-delivery) total and free cefazolin concentration.
The total cefazolin concentrations measured in UCB plasma exceeded 23.0 mg/L. A direct correlation was seen between maternal BMI and total cefazolin concentration in UCB (r2 = 0.66; p = 0.014), and a lower concentration of total cefazolin was detected in the UCB of infants born to obese women (BMI ≥ 30 kg/m2) compared to non-obese women (Figure 3). Free cefazolin concentrations measured in MB and UCB plasma exceeded 17.3 and 5.9 mg/L, respectively, for all patients in the study (Figure 4). While the free cefazolin concentrations detected in MB and UCB were greater than the MIC90 for GBS (0.5 mg/L) and S aureus (0.12 mg/L), the UCB plasma-free cefazolin concentrations did not exceed the MIC90 for E coli (16 mg/L).18
Figure 3.
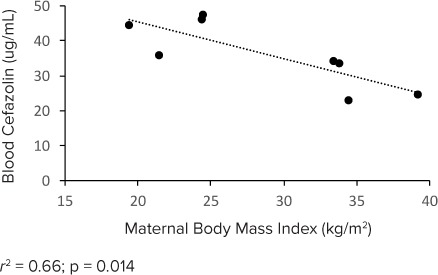
Umbilical cord blood total cefazolin concentration.
Figure 4.

Maternal blood (post-delivery) and umbilical cord blood free cefazolin concentrations.
Discussion
The prophylactic and transplacental properties of cefazolin have been previously described,19,20 although the impact of maternal habitus on maternal and fetal drug concentrations has not been fully elucidated.12,19 However, cefazolin dose standards among obese pregnant women and cefazolin's protective effect against neonatal sepsis are still poorly understood. This is one of the first studies examining cefazolin's coverage against common bacteria that cause post-C/S surgical wound infections and possible pathogens causing early onset neonatal sepsis.
Our results do not suggest a correlation between maternal BMI and total and free cefazolin concentrations; therefore, the results of this pilot study do not support an altered dosage guideline for obese women undergoing cesarean delivery; however, further studies with a larger sample size are required to confirm these results. Free cefazolin concentration in MB was greater than the MIC90s for GBS, S aureus, and E coli. Our results suggest that routine administration of a 2-g dose of cefazolin to obese pregnant women undergoing scheduled C/S for prophylaxis is adequate and provides further support for the current recommendations of The American Congress of Obstetricians and Gynecologists to administer a 2- to 3-g cefazolin dose for patients with variable body types.10
Other studies have demonstrated inconsistencies for cefazolin dose standards between obese and non-obese patients. Stitely et al9 assessed antibiotic tissue concentrations for prophylactic cefazolin, which was administered as 2- and 4-g doses to pregnant women undergoing cesarean delivery. Although the authors speculated that 2 g might be insufficient to exceed MIC breakpoints for several pathogens related to surgical wound infections for obese patients, their report, similar to our current study, suggests that 2 g of cefazolin is an adequate prophylactic dose for C/S surgical wound infections.9 In contrast to our results, a study performed by Pevzner et al2 demonstrated that 2 g of cefazolin administered to obese patients was an inadequate prophylactic dose for surgical wound infections after C/S according to concentration levels in adipose tissue. A potential variable that may have contributed to this discrepancy is the time between antibiotic administration and incision.9 Pevzner et al2 waited at least 30 minutes between the antibiotic administration and incision time, while Stitely et al9 waited an average of 12 minutes. Another contributing factor to this discrepancy is the infusion time of antibiotics. Stitely et al9 administered cefazolin with an intravenous push taking approximately 30 to 60 seconds; however, Pevzner et al2 did not report the antibiotic administration procedures. This variation in results demonstrates the need for further research focused on establishing the dose standards of cefazolin for obese and non-obese pregnant women.
We identified a direct correlation between total cefazolin concentration in UCB plasma and maternal BMI, with lower total cefazolin concentrations detected in infants born to obese women (BMI ≥ 30 kg/m2). Antimicrobial levels and transplacental crossover are influenced by volume of distribution.21 Several studies have demonstrated a correlation between an increase in body weight measures and volume of distribution.22,23 As volume of distribution increases, total and free cefazolin concentrations can decrease among obese patients.24 The decrease of fetal cefazolin concentrations in infants born to obese women could be representative of this trend, although we were unable to detect a decrease in maternal drug concentrations. The results of our study indicate the need to further evaluate the impact of maternal obesity on antimicrobial drug regimens for prevention of neonatal GBS disease.8
Free cefazolin is considered an active drug as compared to protein-bound drugs and has been shown to better correlate with tissue drug concentration compared to total cefazolin concentrations.25 In pregnancy, concentration of albumin decreases significantly, which can further impact the activity level of a drug that is highly bound to albumin (80%) in vivo.26,27 We found that free cefazolin concentrations in UCB were greater than the MIC90 for GBS and S aureus even when administered as briefly as 18 minutes before delivery; however, it did not exceed the MIC90 for E coli. The use of preoperative cefazolin might provide adequate prophylactic coverage for S aureus and GBS bacteria even when administered immediately before delivery. These results can have important clinical implications, as GBS is the most common cause for early onset neonatal sepsis,12 a significant cause of mortality and morbidity in both developed and developing countries.28 To our knowledge, our study is the first to examine the effect of maternal habitus on UCB cefazolin concentration and its potential impact on prevention of early onset neonatal sepsis.
Our results are consistent with recent studies examining the impact of cefazolin dose alteration among obese women29–31; our study is the first to examine free cefazolin concentrations in addition to total concentrations and to provide important preliminary data regarding implications of current dose regimen for prevention of GBS disease among infants born to obese women.
There are several limitations to this study, including the sample size, which was limited owing to the pilot nature of this study. Risk factors for infection were controlled for in this study, since we aimed to examine prophylactic antibiotic concentrations. Pregnant women who were scheduled for C/S at our institution were carefully screened to ensure there were no confounding variables that could alter the drug concentrations or risk of wound infection or sepsis. As such, our results are not generalizable to women who deliver preterm or may have concurrent medical conditions including diabetes. Although this study provides important preliminary data for neonatal drug concentrations after maternal administration of cefazolin, we did not include women with rupture of membranes or those in labor whose infants are at greater risk for GBS disease. The second limitation is that we did not enroll any patient with a BMI > 40 kg/m2. Patients with a larger BMI could be at greater risk for receiving an inadequate dose of prophylactic antibiotics, and additional studies are needed to better elucidate the drug pharmacokinetics in this population. In this study, cefazolin concentration measurements were obtained by using plasma values. Concentration measurements taken directly from tissue would provide a better assessment of efficacy, since the site of action is targeted.2,29–31 To overcome this limitation, both total and free drug concentrations are reported for cefazolin concentration in this study.6 Another important strength of this pilot study is to provide preliminary data for future randomized controlled trials to further investigate cefazolin dose standards among obese and non-obese patients and to evaluate prevention of surgical wound infection and neonatal sepsis. Future studies might also include overweight women (BMI ≥ 25 kg/m2) who might not meet criteria for having a diagnosis of obesity, yet might be at risk for similar complications.
In summary, routine administration of 2 g of cefazolin to pregnant women undergoing planned C/S initially proves to be an adequate dose for prophylaxis in both non-obese and obese patients. Future studies are necessary to further evaluate the prophylactic dose standards. Prophylactic cefazolin administered before scheduled C/S might provide adequate protection against neonatal GBS disease.
Acknowledgments
The study was supported by the Gerber Foundation, the Thrasher Research Fund, Thomas Wilson Sanitarium for Children of Baltimore City, The Johns Hopkins (KL2) Mentored Career Development Award, National Institute of Child Health and Human Development (NICHD; K08 HD 073315), The Dr. Sydney H. Kane, Emma B. Kane, David M. Kane and Family Endowment Fund, and The Sheila S. and Lawrence C. Pakula, M.D., Endowment for Neonatal Research. A portion of this manuscript was presented at the Society for Maternal-Fetal Medicine (SMFM) 2015 Annual Meeting in San Diego, California.
Abbreviations
- BMI
body mass index
- C/S
cesarean section
- E coli
Escherichia coli
- GBS
Group B streptococcus
- MB
maternal blood
- MIC
minimum inhibitory concentration
- S aureus
Staphylococcus aureus
- UCB
umbilical cord blood
Footnotes
Disclosure The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria. The authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Copyright Published by the Pediatric Pharmacy Advocacy Group. All rights reserved. For permissions, email: matthew.helms@ppag.org
REFERENCES
- 1. Barie PS. . Surgical site infections: epidemiology and prevention. Surg Infect (Larchmt). 2002; 3( suppl 1): S9– S21. [DOI] [PubMed] [Google Scholar]
- 2. Pevzner L, Swank M, Krepel C, . et al. Effects of maternal obesity on tissue concentrations of prophylactic cefazolin during cesarean delivery. Obstet Gynecol. 2011; 117( 4): 877– 882. [DOI] [PubMed] [Google Scholar]
- 3. Mivumbi VN, Little SE, Rulisa S, . et al. Prophylactic ampicillin versus cefazolin for the prevention of post-cesarean infectious morbidity in Rwanda. Int J Gynaecol Obstet. 2014; 124( 3): 244– 247. [DOI] [PubMed] [Google Scholar]
- 4. Sarsam SE, Elliott JP, Lam GK. . Management of wound complications from cesarean delivery. Obstet Gynecol Surv. 2005; 60( 7): 462– 473. [DOI] [PubMed] [Google Scholar]
- 5. Smaill FM, Grivell RM. . Antibiotic prophylaxis versus no prophylaxis for preventing infection after cesarean section. Cochrane Database Syst Rev. 2014; 10: CD007482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van Hasselt JG, Allegaert K, van Calsteren K, . et al. Semiphysiological versus empirical modelling of the population pharmacokinetics of free and total cefazolin during pregnancy. Biomed Res Int. 2014; 2014: 897216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Allegaert K, van Mieghem T, Verbesselt R, . et al. Cefazolin pharmacokinetics in maternal plasma and amniotic fluid during pregnancy. Am J Obstet Gynecol. 2009; 200( 2): 170.e171– 177. [DOI] [PubMed] [Google Scholar]
- 8. Elkomy MH, Sultan P, Drover DR, . et al. Pharmacokinetics of prophylactic cefazolin in parturients undergoing cesarean delivery. Antimicrob Agents Chemother. 2014; 58( 6): 3504– 3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stitely M, Sweet M, Slain D, . et al. Plasma and tissue cefazolin concentrations in obese patients undergoing cesarean delivery and receiving differing pre-operative doses of drug. Surg Infect (Larchmt). 2013; 14( 5): 455– 459. [DOI] [PubMed] [Google Scholar]
- 10. American College of Obstetrics, Gynecologists. . ACOG Practice Bulletin No. 120: use of prophylactic antibiotics in labor and delivery. Obstet Gynecol. 2011; 117( 6): 1472– 1483. [DOI] [PubMed] [Google Scholar]
- 11. Yogev Y, Catalano PM. . Pregnancy and obesity. Obstet Gynecol Clin North Am. 2009; 36( 2): 285– 300, viii. [DOI] [PubMed] [Google Scholar]
- 12. Verani JR, Schrag SJ. . Group B streptococcal disease in infants: progress in prevention and continued challenges. Clin Perinatol. 2010; 37( 2): 375– 392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Popovic J, Grujic Z, Sabo A. . Influence of pregnancy on ceftriaxone, cefazolin and gentamicin pharmacokinetics in caesarean vs. non-pregnant sectioned women. J Clin Pharm Ther. 2007; 32( 6): 595– 602. [DOI] [PubMed] [Google Scholar]
- 14. Mitchell TF. . Cefazolin: maternal and transplacental pharmacokinetics. Inpharma 1321 ( 2002): 19. [Google Scholar]
- 15. Kurtzberg J, Lyerly AD, Sugarman J. . Untying the Gordian knot: policies, practices, and ethical issues related to banking of umbilical cord blood. J Clin Invest. 2005; 115( 10): 2592– 2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Crutchfield CA, Marzinke MA. . Bioanalytical development and validation of liquid chromatographic–tandem mass spectrometric methods for the quantification of total and free cefazolin in human plasma and cord blood. Practical Lab Med. 2015; 1: 12– 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weston EJ, Pondo T, Lewis MM, . et al. The burden of invasive early-onset neonatal sepsis in the United States, 2005–2008. Pediatr Infect Dis J. 2011; 30( 11): 937– 941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Infectious Diseases Society of America. . Alert: antimicrobial susceptibility testing. 2016. http://www.idsociety.org/Topics_of_Interest/Antimicrobial_Resistance/Professionals/Antimicrobial_Susceptibility_Testing/. Accessed February 19, 2017.
- 19. Brown CE, Christmas JT, Bawdon RE. . Placental transfer of cefazolin and piperacillin in pregnancies remote from term complicated by Rh isoimmunization. Am J Obstet Gynecol. 1990; 163( 3): 938– 943. [DOI] [PubMed] [Google Scholar]
- 20. Fiore Mitchell T, Pearlman MD, Chapman RL, . et al. Maternal and transplacental pharmacokinetics of cefazolin. Obstet Gynecol. 2001; 98( 6): 1075– 1079. [DOI] [PubMed] [Google Scholar]
- 21. Loebstein R, Lalkin A, Koren G. . Pharmacokinetic changes during pregnancy and their clinical relevance. Clin Pharmacokinet. 1997; 33( 5): 328– 343. [DOI] [PubMed] [Google Scholar]
- 22. Chumlea WC, Schubert CM, Sun SS, . et al. A review of body water status and the effects of age and body fatness in children and adults. J Nutr Health Aging. 2007; 11( 2): 111– 118. [PubMed] [Google Scholar]
- 23. Alexander JK, Dennis EW, Smith WG, . et al. Blood volume, cardiac output, and distribution of systemic blood flow in extreme obesity. Cardiovasc Res Cent Bull. 1962; 1: 39– 44. [PubMed] [Google Scholar]
- 24. an Kralingen S, Taks M, Diepstraten J, . et al. Pharmacokinetics and protein binding of cefazolin in morbidly obese patients. Eur J Clin Pharmacol. 2011; 67( 10): 985– 992. [DOI] [PubMed] [Google Scholar]
- 25. Howard GW, Begg EJ, Chambers ST, . et al. Free and total cefazolin plasma and interstitial fluid concentrations at steady state during continuous infusion. J Antimicrob Chemother. 2002; 50( 3): 429– 432. [DOI] [PubMed] [Google Scholar]
- 26. Marshall WF, Blair JE. . The cephalosporins. Mayo Clin Proc. 1999; 74( 2): 187– 195. [DOI] [PubMed] [Google Scholar]
- 27. Feghali MN, Mattison DR. . Clinical therapeutics in pregnancy. J Biomed Biotechnol. 2011; 2011: 783528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Camacho-Gonzalez A, Spearman PW, Stoll BJ. . Neonatal infectious diseases: evaluation of neonatal sepsis. Pediatr Clin North Am. 2013; 60( 2): 367– 389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Maggio L, Nicolau DP, DaCosta M, . et al. Cefazolin prophylaxis in obese women undergoing cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2015; 125( 5): 1205– 1210. [DOI] [PubMed] [Google Scholar]
- 30. Swank ML, Wing DA, Nicolau DP, . et al. Increased 3-gram cefazolin dosing for cesarean delivery prophylaxis in obese women. Am J Obstet Gynecol. 2015; 213( 3): 415.e411– 418. [DOI] [PubMed] [Google Scholar]
- 31. Young OM, Shaik IH, Twedt R, . et al. Pharmacokinetics of cefazolin prophylaxis in obese gravidae at time of cesarean delivery. Am J Obstet Gynecol. 2015; 213( 4): 541.e1– 7. [DOI] [PMC free article] [PubMed] [Google Scholar]


