Abstract
The in vitro activities of penicillin, erythromycin, clindamycin, and telithromycin were determined against 155 viridans group streptococci (VGS) and 18 Streptococcus bovis blood isolates. Heterogeneity in the susceptibility patterns and macrolide resistance phenotypes and genotypes in the different groups of VGS was detected. We found seven telithromycin-resistant S. bovis isolates all harboring the erm(B) gene.
Viridans group streptococci (VGS) are the main commensals of the oropharyngeal cavity in humans. They are the leading cause of subacute bacterial endocarditis and have also emerged as important pathogens causing sepsis in neutropenic cancer patients. Streptococcus bovis is frequently found as part of the commensal bowel flora. These organisms are also agents of endocarditis. Bacteremia caused by S. bovis isolates is associated with malignancies of the gastrointestinal tract.
Recent studies have shown that VGS are becoming increasingly resistant to many antibiotics, and it seems that antimicrobial resistance is not homogeneous among the different members of the group (1, 7, 8, 20). Most studies of VGS report that Streptococcus mitis, in addition to being the most frequently isolated group, is also the group that shows the highest rates of penicillin resistance (1, 7, 8, 10, 17). However, reports about the susceptibility of other VGS and S. bovis are scarce. The aim of our study was to determine overall rates of VGS susceptibility to penicillin, macrolides, and telithromycin and also to determine whether there are any differences in the susceptibility patterns and erythromycin resistance mechanisms of the different species that make up this group.
A total of 155 VGS and 18 S. bovis strains isolated from blood were collected between 1998 and 2003. Of the 173 isolates, 111 were isolated from 1998 to 2002 and were previously characterized in terms of their macrolide resistance (15). All strains were identified by standard methods (16) and by the Rapid ID32 Strep system (bioMérieux, Marcy l'Étoile, France) and then classified according to the review of Coykendall (4).
Susceptibility to penicillin, erythromycin, clindamycin, and telithromycin was determined by the agar dilution method. MICs were interpreted using NCCLS criteria for Streptococcus other than Streptococcus pneumoniae. For telithromycin, since no NCCLS breakpoints have been established, we used those defined for S. pneumoniae (12). Macrolide resistance phenotypes were determined using a double-disk diffusion method (18). The presence of erm and mef genes was determined by PCR amplification with previously described primers specific for erm(A), erm(B), erm(C), and mef(A) (18, 19).
Of the 155 VGS, 72 isolates belonged to the S. mitis group; other groups included Streptococcus anginosus (49 isolates), Streptococcus sanguis (21 isolates), Streptococcus salivarius (11 isolates), and Streptococcus mutans (2 isolates).
The in vitro activities of the antibiotics tested for the different streptococci, except for the two S. mutans group isolates, which were susceptible to all antibiotics tested, are shown in Table 1.
TABLE 1.
MIC50, MIC90, ranges, and susceptibility percentages of the antibiotics tested for different groups of speciesa
| Antibiotic | MIC | All strains |
Streptococcus group
|
||||
|---|---|---|---|---|---|---|---|
| S. mitis (n = 72) | S. anginosus (n = 49) | S. sanguis (n = 21) | S. salivarius (n = 11) | S. bovis (n = 18) | |||
| Penicillin | Range | ≤0.03-64 | ≤0.03-64 | ≤0.03-2 | ≤0.03-2 | 0.06-8 | 0.03-2 |
| MIC50 | 0.06 | 0.12 | 0.06 | 0.12 | 0.25 | 0.06 | |
| MIC90 | 2 | 4 | 0.12 | 0.25 | 1 | 0.25 | |
| % S | 71.1 | 55 | 94 | 57 | 64 | 89 | |
| % I | 21.3 | 28 | 6 | 43 | 27 | 11 | |
| % R | 7.6 | 17 | 0 | 0 | 9 | 0 | |
| Erythromycin | Range | ≤0.03-256 | 0.06-256 | ≤0.03-256 | 0.06-256 | 0.06-256 | 0.06-256 |
| MIC50 | 0.12 | 0.12 | 0.12 | 1 | 0.06 | 256 | |
| MIC90 | 256 | 128 | 256 | 64 | 4 | 256 | |
| % S | 52.6 | 50 | 63 | 48 | 73 | 22 | |
| % I | 1.8 | 4 | 0 | 0 | 0 | 0 | |
| % R | 45.6 | 46 | 37 | 52 | 27 | 78 | |
| Clindamycin | Range | ≤0.03-256 | ≤0.03-256 | ≤0.03-128 | ≤0.03-64 | ≤0.03-0.12 | ≤0.03-256 |
| MIC50 | 0.06 | 0.06 | 0.06 | 0.06 | 0.06 | 64 | |
| MIC90 | 256 | 128 | 128 | 32 | 0.12 | 256 | |
| % S | 72.3 | 78 | 69 | 81 | 100 | 28 | |
| % I | 0 | 0 | 0 | 0 | 0 | 0 | |
| % R | 27.7 | 22 | 31 | 19 | 0 | 72 | |
| Telithromycin | Range | ≤0.015-128 | ≤0.015-2 | ≤0.015-1 | ≤0.015-0.25 | ≤0.015-0.06 | ≤0.015-128 |
| MIC50 | ≤0.015 | ≤0.015 | ≤0.015 | ≤0.015 | ≤0.015 | 0.12 | |
| MIC90 | 0.25 | 0.12 | 0.12 | 0.12 | 0.03 | 64 | |
| % S | 95.5 | 99 | 100 | 100 | 100 | 61 | |
| % I | 0.5 | 1 | 0 | 0 | 0 | 0 | |
| % R | 4 | 0 | 0 | 0 | 0 | 39 | |
MIC50, MIC at which 50% of the isolates tested are inhibited; MIC90, MIC at which 90% of the isolates tested are inhibited; % S, percent susceptible; % I, percent intermediate; % R, percent resistant. All MICs are in micrograms per milliliter.
Overall, the rate of nonsusceptibility to penicillin was 29%. Of the 50 penicillin-nonsusceptible strains, 13 (8%) were highly resistant to penicillin and 37 (21%) showed intermediate resistance. S. mitis showed the highest rate of penicillin resistance (45%). Moreover, of the 13 highly penicillin-resistant strains, 12 belonged to the S. mitis group and one belonged to the S. salivarius group. These results agree with other surveys which report that penicillin resistance is more common in S. mitis and S. salivarius than in S. anginosus (1, 7, 8).
The resistance percentages for erythromycin and clindamycin were 45.6 and 27.7%, respectively. High percentages (30 to 50%) of erythromycin resistance have been reported in recent studies of VGS isolated from blood cultures (1, 5-7). Resistance to erythromycin varied by species, and Streptococcus bovis group isolates showed the highest rates of resistance to erythromycin and clindamycin. S. bovis isolates were found to be more resistant to these antibiotics than other streptococcal species in Denmark were (14). The lowest percentages of erythromycin and clindamycin resistance were found in S. salivarius isolates.
Overall the ketolide telithromycin was the most active antimicrobial agent tested, with 96% susceptibility. However, we found seven telithromycin-resistant strains. These belonged to the S. bovis group, and the MICs for them ranged from 4 to 128 μg/ml. Telithromycin-resistant VGS have been recently reported in Belgium (11), but, to our knowledge, ours is the first report that describes telithromycin-resistant S. bovis strains and the first that shows MICs as high as 128 μg/ml.
Of the 79 erythromycin-resistant strains, 61% displayed constitutive macrolide-lincosamide-streptogramin B (cMLSB) resistance, 35% displayed the M phenotype, and 4% displayed the inducible MLSB (iMLSB) phenotype (Table 2). The predominance of the cMLSB phenotype in VGS isolated from blood has been reported from Korea (61.1%), France (77%), and Holland (73%) (3, 9, 22). However, in the United States, Canada, and Latin America (6, 8), the M phenotype was the most common. In VGS isolated from normal flora, there are also differences between phenotypes in the different countries (2, 11, 17)
TABLE 2.
Distribution of erythromycin resistance genes according to erythromycin resistance phenotypes and to the different streptococcus groups
| Streptococcus group | No. of ER strainsa | Pheno- type | No. (%) | No. with genotype
|
||
|---|---|---|---|---|---|---|
| erm(B) | mef(A) | erm(B) plus mef(A) | ||||
| S. mitis | 33 | M | 17 (51) | 0 | 16 | 0 |
| iMLSB | 0 | 0 | 0 | 0 | ||
| cMLSB | 16 (49) | 12 | 0 | 4 | ||
| S. anginosus | 18 | M | 2 (11) | 0 | 2 | 0 |
| iMLSB | 1 (6) | 1 | 0 | 0 | ||
| cMLSB | 15 (83) | 12 | 0 | 3 | ||
| S. sanguis | 11 | M | 7 (64) | 0 | 7 | 0 |
| iMLSB | 0 | 0 | 0 | 0 | ||
| cMLSB | 4 (36) | 4 | 0 | 0 | ||
| S. salivarius | 3 | M | 2 (67) | 0 | 2 | 0 |
| iMLSB | 1 (33) | 1 | 0 | 0 | ||
| cMLSB | 0 | 0 | 0 | 0 | ||
| S. bovis | 14 | M | 0 | 0 | 0 | 0 |
| iMLSB | 1 (7) | 1 | 0 | 0 | ||
| cMLSB | 13 (93) | 12 | 0 | 1 | ||
ER, erythromycin resistant.
In our study, the cMLSB phenotype was the most predominant in S. anginosus and S. bovis, while in S. sanguis and S. salivarius the most frequent was the M phenotype. Jacobs et al. (9) reported that 73% of S. anginosus strains studied had the cMLSB phenotype strains, and for S. bovis, Rennenberg et al. (14) reported a high percentage of strains with the cMLSB phenotype. However, in the study by Teng et al. (21) in Taiwan, iMLSB was the most common phenotype in 38 erythromycin-resistant S. bovis isolates.
Therefore, the proportions of the different phenotypes may vary according to country and source of strains and may even depend on the groups of VGS that are included in the studies.
The erm(B) gene was amplified in all strains with the cMLSB and iMLSB phenotypes and was negative for all isolates with the M phenotype. mef(A) was detected in most isolates with the M phenotype as well as in eight isolates with a cMLSB phenotype. These strains were S. mitis (four), S. anginosus (three), and S. bovis (one). As in previous studies of VGS (8, 11, 13), we did not find any differences in MICs between the strains harboring the erm(B) and mef(A) genes and those harboring erm(B) alone. Therefore, the MIC makes it impossible to know which genes are present in each strain and whether it is necessary to perform a genotypic analysis. No erm(B), erm(A), erm(C), or mef(A) genes were detected in one S. mitis isolate with the M phenotype.
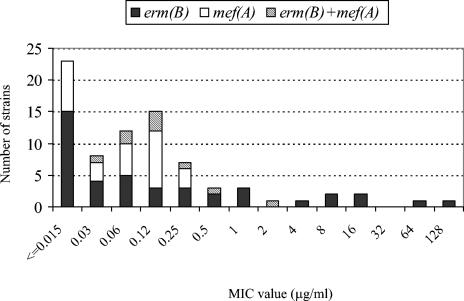
Figure 1 shows the distribution of macrolide resistance genes according to telithromycin MICs. The strains harboring the mef(A) gene showed a range of ≤0.015 to 0.25 μg/ml, while all strains for which the MIC was ≥0.5 μg/ml harbored the erm(B) gene, either alone or with the mef(A) gene. All telithromycin-resistant S. bovis isolates harbored the erm(B) gene, although not all erm(B)-positive S. bovis isolates were resistant to telithromycin.
FIG. 1.
Distribution of macrolide resistance genes according to the telithromycin MICs.
Walsh et al. (23) found an L22 riboprotein mutation and a 210-bp erm(B) attenuator deletion in an S. pneumoniae telithromycin-resistant strain which was generated in vitro. Quite recently, there have been reports of erm(B)-positive S. pneumoniae telithromycin-resistant clinical isolates which also exhibited erm(B) attenuator mutations (D. J. Farrell, I. Morrissey, S. Bakker, and D. Felmingham, Abstr. 14th Eur. Cong. Clin. Microbiol. Infect. Dis., abstr. 1465, 2004).
Thus, telithromycin resistance in streptococci might depend either on the species, at the level of erm(B) gene expression, as occurs in S. pneumoniae, or on an additional mechanism.
The results of the present investigation and the data from the literature suggest that the clinical microbiology laboratory should carry out an accurate identification of VGS at species level, as differences in the patterns of susceptibility to the antimicrobial agents and in the mechanisms responsible for this resistance are observed. The finding of S. bovis group isolates showing telithromycin resistance implies the necessity of further studies to determine the mechanisms responsible for this resistance.
Acknowledgments
This work was supported by grant FIS PI02/0037 from the Fondo de Investigación Sanitaria, Madrid, Spain.
REFERENCES
- 1.Alcaide, F., M. A. Benítez, J. Carratalá, F. Gudiol, J. Liñares, and R. Martín. 2001. In vitro activities of the new ketolide HMR3647 (telithromycin) in comparison with those of eight other antibiotics against viridans group streptococci isolated from blood of neutropenic patients with cancer. Antimicrob. Agents Chemother. 45:624-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aracil, B., M. Miñambres, J. Oteo, C. Torres, J. L. Gómez-Garcés, and J. I. Alós. 2001. High prevalence of erythromycin-resistant and clindamycin-susceptible (M phenotype) viridans streptococci from pharyngeal samples: a reservoir of mef genes in commensal bacteria. J. Antimicrob. Chemother. 48:587-595. [DOI] [PubMed] [Google Scholar]
- 3.Arpin, C., M.-H. Canron, J. Maugein, and C. Quentin. 1999. Incidence of mefA and mefE genes in viridans group streptococci. Antimicrob. Agents Chemother. 43:2335-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coykendall, A. L. 1989. Classification and identification of the viridans streptococci. Clin. Microbiol. Rev. 2:315-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Azavedo, J. C. S., L. Trpeski, S. Pong-Porter, S. Matsumura, the Canadian Bacterial Surveillance Network, and D. E. Low. 1999. In vitro activities of fluoroquinolones against antibiotic-resistant blood culture isolates of viridans group streptococci from across Canada. Antimicrob. Agents Chemother. 43:2299-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diekema, D. J., M. L. Beach, M. A. Pfaller, R. N. Jones, and the SENTRY Participants Group. 2001. Antimicrobial resistance in viridans group streptococci among patients with and without the diagnosis of cancer in the USA, Canada and Latin America. Clin. Microb. Infect. 7:152-157. [DOI] [PubMed] [Google Scholar]
- 7.Doern, G. V., M. J. Ferraro, A. B. Brueggemann, and K. L. Ruoff. 1996. Emergence of high rates of antimicrobial resistance among viridans group streptococci in the United States. Antimicrob. Agents Chemother. 40:891-894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gershon, A. S., J. C. S. de Azavedo, A. McGeer, K. J. Ostrowska, D. Church, D. J. Hoban, G. K. M. Harding, K. Weiss, L. Abbott, F. Smaill, M. Gourdeau, G. Murray, and D. E. Low. 2002. Activities of new fluoroquinolones, ketolides, and other antimicrobials against blood culture isolates of viridans group streptococci from across Canada. Antimicrob. Agents Chemother. 46:1553-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobs, J. A., G. J. van Baar, N. H. H. J. London, J. H. T. Tjhie, L. M. Schouls, and E. E. Stobberingh. 2001. Prevalence of macrolide resistance genes in clinical isolates of the Streptococcus anginosus (“S. milleri”) group. Antimicrob. Agents Chemother. 45:2375-2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lyytikäinen, O., M. Rautio, P. Carlson, V.-J. Anttila, R. Vuento, H. Sarkkinen, A. Kostiala, M. L. Vaisanen, A. Kanervo, and P. Ruutu. 2004. Nosocomial bloodstream infections due to viridans streptococci in haematological and non-haematological patients: species distribution and antimicrobial resistance. J. Antimicrob. Chemother. 53:631-634. [DOI] [PubMed] [Google Scholar]
- 11.Malhotra-Kumar, S., C. Lammens, A. Martel, C. Mallentjer, S. Chapelle, J. Verhoeven, M. Wijdooghe, F. Haesebrouck, and H. Goossens. 2004. Oropharyngeal carriage of macrolide-resistant viridans group streptococci: a prevalence study among healthy adults in Belgium. J. Antimicrob. Chemother. 53:271-276. [DOI] [PubMed] [Google Scholar]
- 12.National Committee for Clinical Laboratory Standards. 2004. Performance standards for antimicrobial susceptibility testing; 14th informational supplement, M100-S14. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 13.Perez-Trallero, E., D. Vicente, M. Montes, J. M. Marimon, and L. Piñeiro. 2001. High proportion of pharyngeal carriers of commensal streptococci resistant to erythromycin in Spanish adults. J. Antimicrob. Chemother. 48:225-229. [DOI] [PubMed] [Google Scholar]
- 14.Rennenberg, J., L. L. Niemann, and E. Gustschik. 1997. Antimicrobial susceptibility of 278 streptococcal blood isolates to seven antimicrobial agents. J. Antimicrob. Chemother. 39:135-140. [DOI] [PubMed] [Google Scholar]
- 15.Rodríguez-Avial, I., C. Rodríguez-Avial, E. Culebras, and J. J. Picazo. 2003. Distribution of tetracycline resistance genes tet(M), tet(O), tet(L) and tet(K) in blood isolates of viridans group streptococci harbouring erm(B) and mef(A) genes. Susceptibility to quinupristin/dalfopristin and linezolid. Int. J. Antimicrob. Agents 21:536-541. [DOI] [PubMed] [Google Scholar]
- 16.Ruoff, K., R. A. Whiley, and D. Beighton. 2003. Streptococcus, p. 405-421. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. A. Pfaller, and R. H. Yolken (ed.), Manual of clinical microbiology, 8th ed. American Society for Microbiology, Washington, D.C.
- 17.Seppälä, H., M. Haanperä, M. Al-Juhaish, H. Järvinen, J. Jalava, and P. Houvinen. 2003. Antimicrobial susceptibility patterns and macrolide resistance genes of viridans group streptococci from normal flora. J. Antimicrob. Chemother. 52:636-644. [DOI] [PubMed] [Google Scholar]
- 18.Seppälä, H., M. Skurnik, H. Soini, M. C. Roberts, and P. Huovinen. 1998. A novel erythromycin resistance methylase gene (ermTR) in Streptococcus pyogenes. Antimicrob. Agents Chemother. 42:257-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sutcliffe, J., T. Grebe, A. Tait-Kamradt, and L. Wondrack. 1996. Detection of erythromycin-resistant determinants by PCR. Antimicrob. Agents Chemother. 40:2562-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teng, L. J., P. R. Hsueh, Y. C. Cheng, S. W. Ho, and K. T. Luh. 1998. Antimicrobial susceptibility of viridans group streptococci in Taiwan with an emphasis on the high rates of resistance to penicillin and macrolides in Streptococcus oralis. J. Antimicrob. Chemother. 41:621-627. [DOI] [PubMed] [Google Scholar]
- 21.Teng, L. J., P. R. Hsueh, S. W. Ho, and K. T. Luh. 2001. High prevalence of inducible erythromycin resistance among Streptococcus bovis isolates in Taiwan. Antimicrob. Agents Chemother. 45:3362-3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uh, Y., D. H. Shin, I. H. Jang, G. Y. Hwang, M. K. Lee, K. J. Yoon, and H. Y. Kim. 2004. Antimicrobial susceptibility patterns and macrolide resistance genes of viridans streptococci from blood cultures in Korea. J. Antimicrob. Chemother. 53:1095-1097. [DOI] [PubMed] [Google Scholar]
- 23.Walsh, F., J. Willcock, and S. Amyes. 2003. High-level telithromycin resistance in laboratory-generated mutants of Streptococcus pneumoniae. J. Antimicrob. Chemother. 52:345-353. [DOI] [PubMed] [Google Scholar]