Abstract
Background. Absent adaptive, individualized dose-finding in early-phase oncology trials, subsequent ‘confirmatory’ Phase III trials risk suboptimal dosing, with resulting loss of statistical power and reduced probability of technical success for the investigational drug. While progress has been made toward explicitly adaptive dose-finding and quantitative modeling of dose-response relationships, most such work continues to be organized around a concept of ‘the’ maximum tolerated dose (MTD). The purpose of this paper is to demonstrate concretely how the aim of early-phase trials might be conceived of, not as ‘dose-finding’, but as dosing algorithm-finding. Methods. A Phase I dosing study is simulated, for a notional cytotoxic chemotherapy drug, with neutropenia constituting the critical dose-limiting toxicity. The drug’s population pharmacokinetics and myelosuppression dynamics are simulated using published parameter estimates for docetaxel. The amenability of this model to linearization is explored empirically. The properties of a simple dose titration algorithm targeting neutrophil nadir of 500 cells/mm 3 using a Newton-Raphson heuristic are explored through simulation in 25 simulated study subjects. Results. Individual-level myelosuppression dynamics in the simulation model approximately linearize under simple transformations of neutrophil concentration and drug dose. The simulated dose titration exhibits largely satisfactory convergence, with great variance in individualized optimal dosing. Some titration courses exhibit overshooting. Conclusions. The large inter-individual variability in simulated optimal dosing underscores the need to replace ‘the’ MTD with an individualized concept of MTD i . To illustrate this principle, the simplest possible dose titration algorithm capable of realizing such a concept is demonstrated. Qualitative phenomena observed in this demonstration support discussion of the notion of tuning such algorithms. The individual-level linearization of myelosuppression dynamics demonstrated for the simulation model used here suggest that a titration algorithm specified in the more general terms of the linear Kalman filter will be worth exploring.
Keywords: Dose-finding studies, oncology, Phase I clinical trial, individualized dose-finding, precision medicine
Introduction
Despite advances in Bayesian adaptive designs 1, 2 and model-based dose-finding 3, oncology dose-finding studies remain conceptually in the thrall of the maximum tolerated dose (MTD). This concept stands opposed to the long-recognized heterogeneity of cancer patients’ pharmacokinetics and pharmacodynamics (PK/PD), and to the diversity of their individual values and goals of care. Under this conceptual yoke, these dose-finding studies constitute a significant choke-point in drug development, where a severe discount may be applied to the potential value in new molecules through the hobbling of subsequent ‘efficacy’ trials by inadequate individual-level dosing 4.
Strangely, Bayesian innovation in dose-finding studies has proceeded apace without issuing a meaningful challenge to the inherently frequentist conception of an MTD as determined by whole-cohort frequencies of dose-limiting toxicities (DLTs). Thus, even as Bayesianism has made progress toward the ethical imperative of efficient use of data 5 in such studies, it has neglected to confront the distinct ethical dimension of individualism 6. This seems a great irony, as the dynamic learning model of Bayesianism is equally suited, and indeed equally essential, to solving the latter problem.
This paper demonstrates individualized dose-finding in a simulated Phase I study of a cytotoxic chemotherapy drug for which neutropenia constitutes the critical dose-limiting toxicity. Importantly, myelosuppression is interpreted also as a monotone index of therapeutic efficacy, without a dose-response ‘plateau’ 7 of the type postulated for molecularly targeted agents (MTAs). This creates a problem setting where simple heuristics apply, simplifying the demonstration undertaken here. The aim of this exercise is to elaborate a concrete setting in which ‘dose-finding study’ may be seen as a misnomer. Under the view advanced here, early-phase studies of this kind should be conceived as dose titration algorithm tuning (DTAT) studies.
The idea that ‘dose finding studies’ should yield dose titration algorithms is not new. More than a quarter-century ago, Sheiner and colleagues 8 advocated a learn-as-you-go concept for “dose-ranging studies”, addressing concerns about “parallel-dose designs” that are not far removed from the motivations for the present paper. As in the advocacy of Sheiner et al., parametric models play an important role in this paper, although in keeping with a spirit of pragmatism this dependence is relaxed to some extent by means of a semiparametric dynamic on-line learning heuristic.
Methods
A hypothetical cytotoxic drug is considered, modeled notionally after docetaxel, to be infused in multiple 3-week cycles. The pharmacokinetics are taken to follow a 2-compartment model with parameters as estimated for docetaxel in a recent population pharmacokinetic study 9. Chemotherapy-induced neutropenia (CIN) is taken to follow a myelosuppression model due to Friberg et al. 10. Together, these models form a population pharmacokinetic/pharmacodynamic (PK/PD) model within which dose titration algorithms may be simulated and tuned for optimality. For simulation purposes, and anticipating the future value of ready access to a variety of inference procedures in follow-on work, this PK/PD model is implemented in R package pomp 11. R version 3.3.2 was used 12.
Basic behaviors of the models are illustrated by simulation graphics generated for 25 individuals randomly generated from the population PK/PD model. Properties with specific relevance to absolute neutrophil count nadir ( ANC nadir)-targeted dose titration are then investigated, with an eye to demonstrating the predictability of nadir timing. In particular, an approximate linearization of neutrophil nadir level and timing is demonstrated, achieved through suitable power-law transformation of infusion doses and logarithmic transformation of neutrophil concentration. Within this transformed parameter space, a simple recursive dose titration algorithm is defined on the basic heuristic of the Newton-Raphson method for root-finding. For simplicity, monitoring of CIN is not modeled endogenously to this algorithm, but is treated as exogenous such that nadir timing and level are known precisely. A ‘DTAT’ study is simulated and visualized for 25 patients, with the tuning parameters of the recursive titration algorithm held fixed. The visualization supports a discussion of how these parameters might be tuned over the course of a Phase I study. All simulations and figures in this paper were generated by a single R script, archived on OSF 13.
Pharmacokinetic model
We take the population pharmacokinetics of our cytotoxic drug to obey a 2-compartment model, with parameters drawn notionally from estimates published for docetaxel [ 9, Table 2]; see Table 1.
Table 1. Two-compartment pharmacokinetics of docetaxel, from Onoue et al. (2016) [ 9, Table 2].
CL: clearance; Q: intercompartmental clearance; V c: volume of central compartment; V p volume of peripheral compartment; CV: coefficient of variation. (*) A CV for V c was unavailable in 9 and has been set arbitrarily to 0.1.
| Param | Units | Mean | CV |
|---|---|---|---|
| CL | L/hour | 32.6 | 0.295 |
| Q | L/hour | 5.34 | 0.551 |
| V c | L | 5.77 | 0.1* |
| V p | L | 11.0 | 0.598 |
Figure 1 shows illustrative pharmacokinetic profiles for 25 randomly-generated individuals from this population, administered a 100 mg dose.
Figure 1. Two-compartment pharmacokinetics of a 1-hour infusion of 100 mg of the modeled drug, for 25 randomly generated individuals in our population pharmacokinetic model.
C c and C p are drug concentrations in the central and peripheral compartments, respectively.
Myelosuppression model
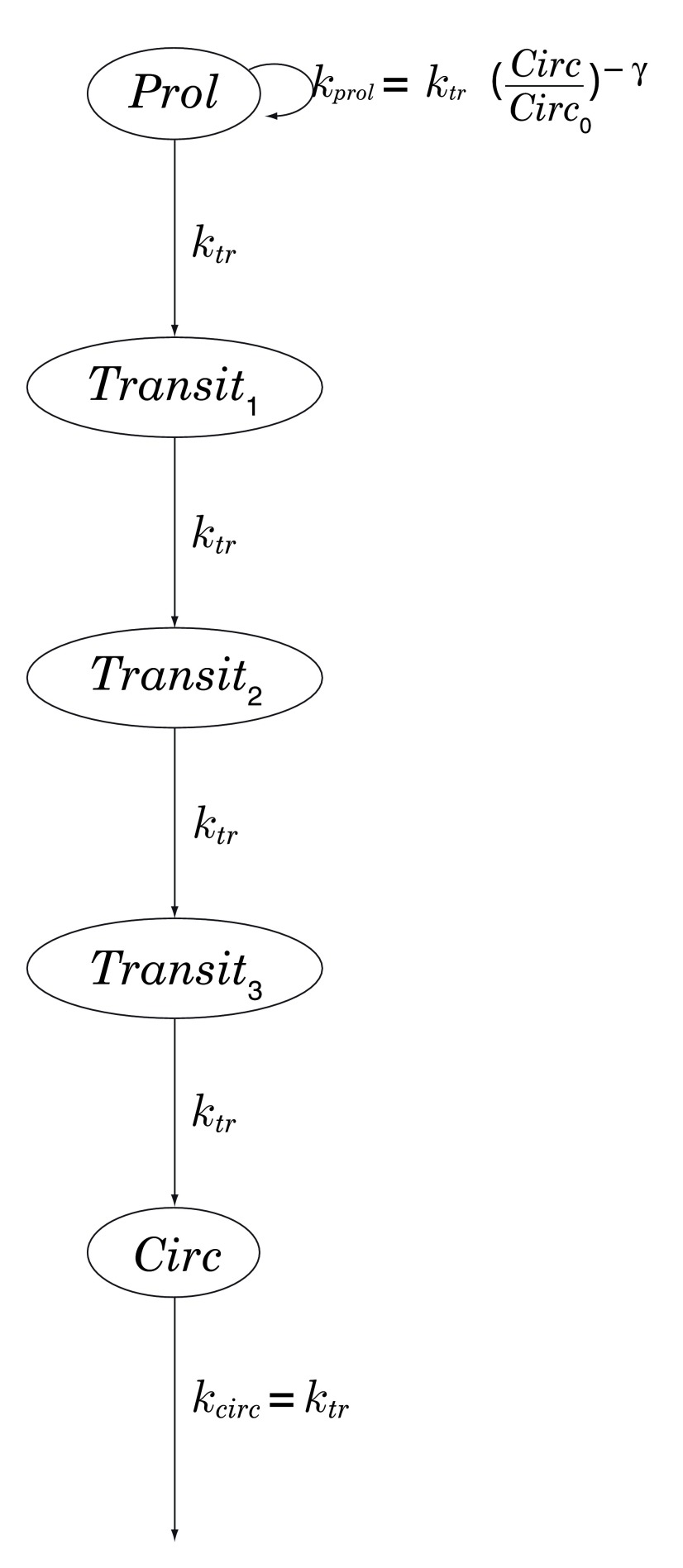
Chemotherapy-induced neutropenia is simulated using the 5-compartment model of Friberg et al. [ 10, Table 4], in which myelocytes (here, neutrophils) arise from progenitor cells in a proliferative compartment, mature through a series of 3 transitional states, and emerge into the systemic circulation; see Figure 2. Transit between successive compartments in this model is a Poisson process with time constant k tr, total mean transit time being therefore given by MTT = 4 /k tr. See Table 2.
Figure 2. Chemotherapy-induced myelosuppression model of Friberg et al. [ 10, Table 4].
Prol: proliferative compartment; Transit n: maturation compartments; Circ: systemic circulation; k tr: transition rate; k prol: rate of proliferation of progenitor cells, regulated by a negative-feedback loop parametrized by γ > 0.
Table 2. Parameters of the chemotherapy-induced myelosuppression model of Friberg et al. [ 10, Table 4].
Circ 0: baseline neutrophil concentration; MTT : mean transit time between the 5 model compartments; γ: exponent of feedback loop; EC 50, E max: parameters of a model (of the standard E max type) governing docetaxel-induced depletion in the proliferative compartment.
| Param | Units | Mean | CV |
|---|---|---|---|
| Circ 0 | cells/mm 3 | 5050 | 0.42 |
| MTT | hours | 89.3 | 0.16 |
| γ | - | 0.163 | 0.039 |
| E max | µM | 83.9 | 0.33 |
| EC 50 | µM | 7.17 | 0.50 |
Figure 3 shows illustrative myelosuppression profiles for 25 randomly-generated individuals from this population, administered a 100 mg dose.
Figure 3. Myelosuppression profiles for the same 25 randomly generated individuals as in Figure 1.
Note how a chemotherapeutic ‘shock’ to the proliferative compartment Prol propagates through the maturation compartments Tx 1,2,3 and thence to the systemic circulation Circ. ( ANC: absolute neutrophil count.)
Linearizing CIN dynamics by dose rescaling
When parametrized by , individuals’ trajectories in (log( ANC nadir) ×time nadir)-space may be approximately linearized, as shown in Figure 4. A consequence of this rough linearity is that we may hold out some hope that a linear predictive model could be a suitable basis for an adaptive dose titration scheme.
Figure 4. Trajectories of ANC nadirs during dose escalation in 25 randomly-generated individuals.
The 10 doses plotted are evenly spaced on a fourth-root scale. Not only are the trajectories themselves nearly linear in (log( ANC) × time)-space, but each one is traversed at roughly ‘constant velocity’ with respect to .
Dose titration
Recursive nonlinear filtering, as implemented in the extended Kalman filter (EKF) or its more modern adaptations 14, constitutes a powerful conceptual framework for approaching model-based dose titration 15. Under a suitable linearization of the dynamics, as for example suggested by Figure 4, even the linear Kalman filter 16 might succeed admirably. The ‘tuning’ in ‘DTAT’ has itself been suggested by the practice of tuning a Kalman filter for optimal performance.
For present purposes, however, it suffices to implement a model-free recursive titration algorithm built on the Newton-Raphson method, with a numerically-estimated derivative based on most recent infusion doses and their corresponding ANC nadirs. In this algorithm, a relaxation factor ω = 0.75 is applied to any proposed dose increase, with safety in mind. Whereas the slope of log( ANC nadir) with respect to is expected to be strictly negative at steady state, hysteresis effects arising during initial steps of dose titration do sometimes yield positive numerical estimates for this slope; so the slope estimates are constrained to be ≤ 0. The infusion dose for cycle 1 is 50 mg, and the cycle-2 dose is calculated conservatively using a slope −2.0, which is larger (in absolute terms) than for any of our simulated patients except id1 and id13; see Figure 4. For reference, these starting values for the tuning parameters of the titration algorithm are collected in Table 3.
Table 3. Values of the tuning parameters of the dose titration algorithm simulated in Figure 5.
| Param | Description | Value |
|---|---|---|
| ω | Relaxation factor | 0.75 |
| slope 1 | Slope for cycle-2 dosing | −2.0 |
| dose 1 | Initial (cycle-1) dose | 50 mg |
Figure 5. Titration profiles in 25 simulated patients over ten 3-week cycles of chemotherapy.
C c and C p are drug concentrations in the central and peripheral compartments, respectively.
With the illustrative purpose of this article again in mind, we treat neutropenia monitoring as an exogenous process yielding precise nadir timing and levels. This enables a demonstration of the main point without the encumbrance of additional modeling infrastructure peripheral to the main point.
On ‘tuning’
If one considers Figure 5 as a sequence of titration outcomes emerging in serially enrolled study subjects, it becomes clear that even quite early in the study it will seem desirable to ‘retune’ the titration algorithm. For example, provided that course-1 CIN monitoring is implemented with sufficient intensity to deliver advance warning of an impending severely neutropenic nadir, so that timely colony-stimulating factor may be administered prophylactically 17, then upon review of the titration courses in the first 10 subjects it may well appear desirable to increase dose 1 from 50mg to 100mg. Likewise, given the third-dose overshooting that occurs in 4 of the first 10 subjects, it may seem desirable to adjust the relaxation factor ω downward. Of note, at any given time any such proposed retuning may readily be subjected to a ‘dry run’ using retrospective data from all convergent titration courses theretofore collected. (Hysteresis effects would however be inaccessible to a strictly data-driven dry run absent formal modeling.) Furthermore, the ‘tuning’ idea readily generalizes to the fundamental modification or even wholesale replacement of a dose titration algorithm; the overshooting seen for subjects id10, id12 and id23, for example, inspires further thought about refining (or replacing) the admittedly very naive Newton-Raphson method employed herein.
A further dimension of ‘tuning’ that must be discussed is the potential for driving the tuning parameters using statistical models built on baseline covariates. Surely, to the extent that the great heterogeneity in final dosing evident in Figure 5 could be predicted based on age, sex, weight or indeed on pharmacogenomic testing, then dose 1 should be made a function of these covariates. The recalibration of such models as data accumulate from successive study subjects is very much a part of the full concept of ‘tuning’ I wish to advance.
Finally, whereas I have discussed ‘tuning’ here largely in terms of reflective, organic decision-making such as occurs in the creative refinement of algorithms or in data-driven statistical model development, I do not mean to exclude more formal approaches to algorithm tuning. A decision-theoretic framing of the tuning problem should enable formal algorithm tuning to be specified and carried out meaningfully. Such framing would also have the salutary effect of bringing into view objectively the important matter of patients’ heterogeneity with respect to values and goals of care. It seems quite likely that the balance of benefits from aggressive titration versus harms of toxicities will generally differ from one patient to another. Dose titration algorithms should most emphatically be tuned to these factors as well.
Discussion
It is where pharmacometrics meets the field of optimal control that the current literature seems to make its closest point of contact with the DTAT concept I am advancing here. In optimal-control investigations of chemotherapy 18– 23, as in DTAT, relatively large decision spaces are explored. Indeed, the infinite-dimensional spaces of control functions posited for exploration in optimal control applications dwarf the finite-dimensional spaces of tuning parameters in DTAT as dramatically as the latter dwarf the finite sets of discrete doses trialed in now-standard Phase I studies. This intermediate ‘cardinality’ of DTAT reflects an important advantage in an era when, to almost universal chagrin, the detested 3+3 dose-finding design retains its hegemony due partly to widespread resistance to modeling 24. In such an era, optimal control applications that involve detailed mathematical modeling of tumor biology and dynamics sadly seem consigned to the fringes of practice. Acceptance of such ambitious problem formulations, expressing as they do the spirit of a future age, must await deep cultural changes in the medical sciences and clinical practice.
As easy as it is, however, to disparage ‘resistance to modeling’ as some kind of antediluvian attitude, this resistance does rightfully assert the importance of unmodeled complexities that necessitate application of organic forms of clinical judgment 25. It should be clear from the above discussion of ‘tuning’ that DTAT readily accommodates and veritably invites scrutiny, supervision and modification by clinical judgment. For example, if during the course of a DTAT study adverse effects other than neutropenia were to emerge as occasional dose-limiting toxicities, then the full concept of ‘tuning’ advanced above would invite dynamic, ‘learn-as-you-go’ modifications of the titration algorithm. Such modifications may begin with decreasing the relaxation factor ω, but might also involve efforts to classify and predict these new DLTs, and to incorporate such new understanding explicitly into the dose titration algorithm yielded by the study. Indeed, whatever philosophical challenge DTAT embodies is likely to take the form of requiring an intensified commitment to clinical judgment, in a learn-as-you-go world where the always-provisional nature of medical knowledge must frankly be acknowledged 6, 26.
Conclusions
I have advanced a concept of dose titration algorithm tuning (DTAT), drawing connections with recursive filtering and optimal control. I have illustrated key elements of DTAT by simulating neutrophil-nadir-targeted titration of a hypothetical cytotoxic chemotherapy drug with pharmacokinetics and myelosuppressive dynamics patterned on previously estimated population models for docetaxel. I believe DTAT presents a prima facie case for discarding the outmoded concept of ‘the’ maximum tolerated dose (MTD) of a chemotherapy drug. This argument should be of interest to a wide range of stakeholders, from cancer patients with a stake in receiving optimal individualized ‘MTD i’ dosing, to shareholders in pharmaceutical innovation with a stake in efficient dose-finding before Phase III trials.
Data availability
The data referenced by this article are under copyright with the following copyright statement: Copyright: © 2017 Norris DC
Open Science Framework: Code and Figures for v1 of F1000Research submission: Dose Titration Algorithm Tuning (DTAT) should supersede the Maximum Tolerated Dose (MTD) concept in oncology dose-finding trials, doi 10.17605/osf.io/vwnqz 13
Endorsement
Daniela Conrado (Associate Director, Quantitative Medicine at Critical Path Institute) confirms that the author has an appropriate level of expertise to conduct this research, and confirms that the submission is of an acceptable scientific standard. Daniela Conrado declares she has no competing interests.
Funding Statement
The author declared that no grants were involved in supporting this work.
[version 1; referees: 1 approved
References
- 1. Lunn D, Best N, Spiegelhalter D, et al. : Combining MCMC with 'sequential' PKPD modelling. J Pharmacokinet Pharmacodyn. 2009;36(1):19–38. 10.1007/s10928-008-9109-1 [DOI] [PubMed] [Google Scholar]
- 2. Bailey S, Neuenschwander B, Laird G, et al. : A Bayesian case study in oncology Phase I combination dose-finding using logistic regression with covariates. J Biopharm Stat. 2009;19(3):469–484. 10.1080/10543400902802409 [DOI] [PubMed] [Google Scholar]
- 3. Pinheiro J, Bornkamp B, Glimm E, et al. : Model-based dose finding under model uncertainty using general parametric models. Stat Med. 2014;33(10):1646–1661. 10.1002/sim.6052 [DOI] [PubMed] [Google Scholar]
- 4. Lisovskaja V, Burman CF: On the choice of doses for phase III clinical trials. Stat Med. 2013;32(10):1661–1676. 10.1002/sim.5632 [DOI] [PubMed] [Google Scholar]
- 5. Berry DA: Bayesian Statistics and the Efficiency and Ethics of Clinical Trials. Statist Sci. 2004;19(1):175–187. 10.1214/088342304000000044 [DOI] [Google Scholar]
- 6. Palmer CR: Ethics, data-dependent designs, and the strategy of clinical trials: time to start learning-as-we-go? Stat Methods Med Res. 2002;11(5):381–402. 10.1191/0962280202sm298ra [DOI] [PubMed] [Google Scholar]
- 7. Zang Y, Lee JJ, Yuan Y: Adaptive designs for identifying optimal biological dose for molecularly targeted agents. Clin Trials. 2014;11(3):319–327. 10.1177/1740774514529848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sheiner LB, Beal SL, Sambol NC: Study designs for dose-ranging. Clin Pharmacol Ther. 1989;46(1):63–77. 10.1038/clpt.1989.108 [DOI] [PubMed] [Google Scholar]
- 9. Onoue H, Yano I, Tanaka A, et al. : Significant effect of age on docetaxel pharmacokinetics in Japanese female breast cancer patients by using the population modeling approach. Eur J Clin Pharmacol. 2016;72(6):703–710. 10.1007/s00228-016-2031-3 [DOI] [PubMed] [Google Scholar]
- 10. Friberg LE, Henningsson A, Maas H, et al. : Model of chemotherapy-induced myelosuppression with parameter consistency across drugs. J Clin Oncol. 2002;20(24):4713–4721. 10.1200/JCO.2002.02.140 [DOI] [PubMed] [Google Scholar]
- 11. King MD, Grech-Sollars M: A Bayesian spatial random effects model characterisation of tumour heterogeneity implemented using Markov chain Monte Carlo (MCMC) simulation [version 1; referees: 1 approved]. F1000Res. 2016;5:2082 10.12688/f1000research.9355.1 [DOI] [Google Scholar]
- 12. R Core Team: R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria,2016. Reference Source [Google Scholar]
- 13. Norris DC: Code and Figures for v1 of F1000Research submission: Dose Titration Algorithm Tuning (DTAT) should supersede the Maximum Tolerated Dose (MTD) concept in oncology dose-finding trials. Open Science Foundation. 2017. Data Source [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Julier SJ, Uhlmann JK: New extension of the Kalman filter to nonlinear systems.1997;3068:182–193. 10.1117/12.280797 [DOI] [Google Scholar]
- 15. Norris DC, Gohh RY, Akhlaghi F, et al. : Kalman filtering for tacrolimus dose titration in the early hospital course after kidney transplant. F1000Res. 2017;6 10.7490/f1000research.1113595.1 [DOI] [Google Scholar]
- 16. Kalman RE: A New Approach to Linear Filtering and Prediction Problems. J Basic Eng. 1960;82(1):35–45. 10.1115/1.3662552 [DOI] [Google Scholar]
- 17. Weycker D, Li X, Edelsberg J, et al. : Risk and Consequences of Chemotherapy-Induced Febrile Neutropenia in Patients With Metastatic Solid Tumors. J Oncol Pract. 2015;11(1):47–54. 10.1200/JOP.2014.001492 [DOI] [PubMed] [Google Scholar]
- 18. De Pillis LG, Fister KR, Gu W, et al. : Optimal control of mixed immunotherapy and chemotherapy of tumors. J Biol Syst. 2008;16(01):51–80. 10.1142/S0218339008002435 [DOI] [Google Scholar]
- 19. Dua P, Dua V, Pistikopoulos EN: Optimal delivery of chemotherapeutic agents in cancer. Comput Chem Eng. 2008;32(1–2):99–107. 10.1016/j.compchemeng.2007.07.001 [DOI] [Google Scholar]
- 20. d’Onofrio A, Ledzewicz U, Maurer H, et al. : On optimal delivery of combination therapy for tumors. Math Biosci. 2009;222(1):13–26. 10.1016/j.mbs.2009.08.004 [DOI] [PubMed] [Google Scholar]
- 21. Krabs W, Pickl S: An optimal control problem in cancer chemotherapy. Appl Math Comput. 2010;217(3):1117–1124. 10.1016/j.amc.2010.05.008 [DOI] [Google Scholar]
- 22. Engelhart M, Lebiedz D, Sager S: Optimal control for selected cancer chemotherapy ODE models: a view on the potential of optimal schedules and choice of objective function. Math Biosci. 2011;229(1):123–134. 10.1016/j.mbs.2010.11.007 [DOI] [PubMed] [Google Scholar]
- 23. Ledzewicz U, Schättler H, Gahrooi MR, et al. : On the MTD paradigm and optimal control for multi-drug cancer chemotherapy. Math Biosci Eng. 2013;10(3):803–819. 10.3934/mbe.2013.10.803 [DOI] [PubMed] [Google Scholar]
- 24. Petroni GR, Wages NA, Paux G, et al. : Implementation of adaptive methods in early-phase clinical trials. Stat Med. 2017:36(2):215–224. 10.1002/sim.6910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Feinstein AR: " Clinical Judgment" revisited: the distraction of quantitative models. Ann Intern Med. 1994;120(9):799–805. 10.7326/0003-4819-120-9-199405010-00012 [DOI] [PubMed] [Google Scholar]
- 26. Norris DC: Casting a realist’s eye on the real world of medicine: Against Anjum’s ontological relativism. J Eval Clin Pract. 2017. 10.1111/jep.12689 [DOI] [PubMed] [Google Scholar]