Abstract
Novel pharmacotherapies introduce additional options to providers and patients in how to best treat chronic obstructive pulmonary disease (COPD). Emerging data question the role of inhaled corticosteroids in COPD treatment, particularly as combination dual bronchodilator pharmacotherapies demonstrate robust results. For those maximized on pharmacotherapy with continued dyspnea or exacerbations or both, emerging bronchoscopic procedures may offer additional therapy in select patients. This review focuses on data supporting the use of novel ultra bronchodilators, particularly in combination, and on the role for inhaled corticosteroid withdrawal and new bronchoscopic procedures.
Keywords: COPD, LAMA, LABA, ICS
Introduction
Chronic obstructive pulmonary disease (COPD) is projected to become the third most common cause of death worldwide by 2030 1– 3. Acute exacerbations of COPD are associated with worsening symptoms, including breathlessness, decreased quality of life (QOL) 4, and an accelerated loss of lung function 5. Those hospitalized for acute exacerbations of COPD are at an increased risk of one-year mortality of at least 18% 6. The majority of an estimated $50 billion cost associated with COPD care in the United States is spent treating acute exacerbations 7. An array of emerging pharmacotherapies challenges the traditional way COPD has been managed. This review will focus on the current evidence for use of combined long-acting muscarinic antagonists (LAMAs) with long-acting beta-2 agonists (LABAs), withdrawal of inhaled corticosteroids (ICSs), and emerging data on bronchoscopic interventions in COPD.
Ultra long-acting beta-2 agonists
Long-acting bronchodilators improve lung function, thereby improving symptoms and exercise performance, and prevent exacerbations 8– 10. Long-acting bronchodilators show similar efficacy in patients with moderate compared with more severe COPD 10, 11, indicating that forced expiratory volume in one second (FEV 1) does not predict bronchodilator treatment response. Several once-daily LABAs have become available over the past several years, and indacaterol, olodaterol, and vilanterol are the newest. The existing drug classes (beta-2 agonists and muscarinic receptor antagonists) work by relaxing airway smooth muscle tone, leading to reduced respiratory muscle activity and subsequent reduction in airway resistance and making it easier for patients to breathe. Bronchodilation aims at alleviating bronchial obstruction and airflow limitation, reducing hyperinflation, improving emptying of the lung and exercise performance 12, 13, thus improving dyspnea. This explains why all current COPD practice recommendations highlight that inhaled bronchodilators are the mainstay of current management regardless of disease severity 14– 16.
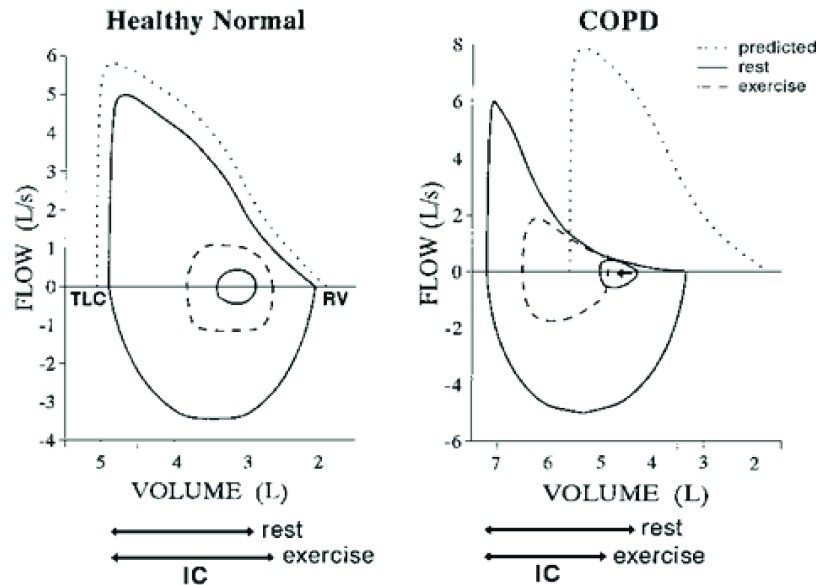
Hyperinflation is a common occurrence leading to breathlessness in COPD. Lung volumes are stable when the tidal volume is completely exhaled prior to the next breath. As the tidal volume increases with exercise, expiratory muscles are recruited to increase pleural and alveolar pressures and increase expiratory flow to ensure that the increased tidal volume is completely exhaled. Hyperinflation occurs when the end-expiratory volume is increased, typically because of airflow limitation, such as in COPD. Compared with healthy patients, patients with COPD have decreased elastic recoil pressure such that the elastic recoil pressure falls to zero at a larger end-expiratory volume. Hyperinflation may also occur as the airways in patients with COPD collapse when the pleural pressure is positive, preventing increased expiratory flow 17, 18, and therefore exhalation may not be completed prior to the onset of the next breath 19, 20 ( Figure 1).
Figure 1. Schematic representation of a normal subject (left) and dynamic hyperinflation in a chronic obstructive pulmonary disease (COPD) subject (right) at rest and during exercise.
IC, inspiratory capacity; RV, right ventricle; TLC, total lung capacity.
The efficacy of ultra LABAs is well established. Among others, two randomized, double-blind, placebo-controlled, parallel-group phase 3 studies have shown the long-term efficacy and safety of once-daily olodaterol 5 and 10 μg in patients with moderate to severe COPD continuing usual-care maintenance therapy 21. Lung function effects of indacaterol are significantly greater than those of the traditional (twice-daily dosing) LABAs formoterol, salmeterol, and arformoterol and are similar to those of the LAMA tiotropium 22– 26.
The debate as to which class of inhaled bronchodilator should be the first-line agent in COPD continues. Guidelines do not distinguish which long-acting bronchodilator agent, LABA or LAMA, should be considered first line, but rather only that the use of a long-acting bronchodilator agent is advised 14, 15. Although a randomized, placebo-controlled 6-month trial of tiotropium versus salmeterol was conducted 27, it is debatable whether such a direct LABA-versus-LAMA comparison will be performed, particularly given the increasing number of options available 28.
Cautious indirect comparisons may be made from the existing clinical trial database about how each class of drug impacts COPD outcomes, although the limitations of this approach are obvious. Nonetheless, limitations aside, LABAs are more effective than LAMAs if we consider symptoms or health-related quality of life (HRQOL) as the primary outcome 29, although LAMAs also impact favorably on both outcomes 28. By contrast, LAMAs appear to be more effective than LABAs if exacerbations are the expected primary outcome, regardless of whether LABAs are administered on a twice-daily 30 or once-daily 31 basis. The outcome of interest may largely determine which bronchodilator to start with in a patient with COPD 32. In the symptomatic patient, there is no substantial difference between LABAs or LAMAs, whereas in frequent exacerbators it seems preferable to use a LAMA.
Long-acting muscarinic antagonists
Until recently, tiotropium was the only globally available ultra LAMA, and it has a rich database of efficacy outcomes in COPD 3, 9, 33. Over the past few years, data have emerged documenting the efficacy of other drugs in the LAMA class ( Table 1). The ACCLAIM trials documented an improvement in FEV 1 and delayed time to first exacerbation with once-daily aclidinium treatment 34. Further study with twice-daily aclidinium in the ATTAIN trial showed significant increase in trough and peak FEV 1, dyspnea, and improvement in quality of life (QOL) scores 35. Umeclidinium significantly improved trough FEV 1, dyspnea, and QOL scores 36. The GEM (Glycopyrrolate Effect on Symptoms and Lung Function) 1 and 2 studies of glycopyrronium versus placebo show improvements in FEV 1, dyspnea, QOL scores, and rescue medication use in patients with moderate to severe airflow limitation 37. In recent studies of a novel soluble glycopyrrolate solution delivered via the investigational eFlow ® nebulizer, the nebulized LAMA formulation was reported to be safe and well tolerated, and there were no significant changes in cardiovascular signs and electrocardiography parameters 38. There was a dose-related and clinically significant improvement in FEV 1 following nebulized glycopyrrolate, providing support for its development as a convenient nebulized LAMA bronchodilator for patients with COPD 38. US Food and Drug Administration (FDA) approval would bring forth a novel nebulized ultra LAMA option available to patients with COPD. Availability of a nebulized LAMA would greatly complement the currently available nebulized LABA medications (formoterol and arformoterol). Of 400 caregivers and patients with COPD randomly surveyed via phone, the overwhelming majority were satisfied with traditional nebulization therapy, reporting benefits in symptom relief, ease of use, and improved QOL 39.
Table 1. Key findings of recent LAMA and dual-agent LAMA/LABA trials reviewed.
| Trial | Pharmacotherapy | Results |
|---|---|---|
| Jones
et al.
34 (2011)
Jones et al. 35 (2012) |
Aclidinium | Improved FEV
1, delay to first
exacerbation |
| Trivedi et al. 36 (2014) | Umeclidinium | Improved FEV 1, dyspnea, QOL |
| LaForce et al. 37 (2016) | Glycopyrronium | Improved dyspnea, QOL |
| Wedzicha et al. 32 (2016) | Glycopyrronium/indacaterol
versus salmeterol/fluticasone |
Decreased exacerbations |
| Singh
et al.
47 (2014)
D’Urzo et al. 48 (2014) Bateman et al. 49 (2015) |
Aclidinium/formoterol | Improved dyspnea and
exacerbations, delay to first exacerbation |
| Buhl et al. 51 (2015) | Tiotropium/olodaterol | Improved FEV 1, QOL |
| Donohue et al. 45 (2014) | Umeclidinium/vilaterol | Decreased exacerbations |
FEV 1, forced expiratory volume in one second; LABA, long-acting beta-2 agonist; LAMA, long-acting muscarinic antagonist; QOL, quality of life.
Dual-agent long-acting bronchodilators
For patients with COPD whose disease is not well controlled—whether in terms of symptoms or exacerbation frequency as recommended by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) COPD statement—with a single long-acting bronchodilator, the most recent guidelines depart from prior use of ICSs and recommend the use of dual long-acting bronchodilators, unless a recurrent or severe exacerbator 14. Studies showing benefit of combination LABA and LAMA in separate devices with both short- and long-acting components 40– 42 prompted the development of a single component with multiple long-acting bronchodilators. The first of the ultra LAMA/LABA combination inhalers approved was for umeclidinium/vilanterol. Umeclidinium/vilanterol appears to be safe, produces greater improvements in lung function compared with monocomponents, and in some studies reduces the risk of exacerbations 43– 46. However, umeclidinium/vilanterol combination, compared with tiotropium or the monocomponents umeclidinium or vilanterol, has not shown dramatic improvements in dyspnea or HRQOL. The bulk of the data for the aclidinium/formoterol combination is from two 24-week randomized, placebo-controlled studies—AUGMENT and ACLIFORM studies—showing improved lung function, dyspnea, and HRQOL 47, 48. Combining data from these two studies showed reduced exacerbations 49 and similar cardiovascular events over 6 months for the twice-daily-administered LAMA/LABA compared with placebo 50.
The FDA approved tiotropium/olodaterol in a soft mist inhaler in 2015. In a 6-week crossover study, it improved lung function, and a combined analysis of the TOnado 1 and 2 studies documented improved dyspnea and HRQOL 51. These studies were not powered for exacerbation reduction, but other LAMA/LABA combinations have been shown to reproducibly reduce exacerbations. In a multicenter trial, glycopyrronium/indacaterol once daily was compared with fluticasone/salmeterol twice daily in 3,362 patients with moderate to severe COPD with a history of at least one moderate to severe exacerbation in the previous year 52. Glycopyrronium/indacaterol reduced the rate of mild to severe COPD exacerbations by 11% compared with fluticasone/salmeterol over the 52-week trial 52. Patients with a history of two or more moderate exacerbations or one hospitalization in the previous year had similar exacerbation rates between the two treatment arms. Of note, glycopyrronium/indacaterol was associated with slightly fewer episodes of pneumonia (3.2%) compared with fluticasone/salmeterol (4.8%). The combination of a long-acting anticholinergic plus an inhaled glucocorticoid has not been compared with a long-acting anticholinergic alone.
Patients with dyspnea despite the use of either a LAMA or LABA may have improvement in HRQOL, dyspnea, and reduced rescue medication use with LAMA/LABA combination, and some agents have reported improvement in exacerbations. However, the degree of symptom improvement along with lung function improvement remains a clinical question of extreme importance. Early studies of LAMA/LABA combination describe transition dyspnea index and Saint George’s Respiratory Questionnaire meeting the minimal clinically important difference (MCID) for breathlessness versus placebo whereas individual monocomponents did not 43, 47, 53, 54. Of note, the primary outcome of these studies was lung function, not patient-reported outcomes (PROs). Statistically significant differences have been shown for subsequent studies with PROs as the primary endpoint 55, 56 as well as a pooled analysis 49. They provide a signal but are below the MCID thresholds. An associated reduction in reliever medication use suggests clinical relevance 54, 55, although overall the clinical impact of LAMA/LABA combination versus its monocomponents is unclear. Evidence of excessive ICS/LABA prescribing, coupled with emerging data (discussed below) of safe steroid withdrawal, makes LAMA/LABA combination more reasonable, particularly with the aim of maximizing bronchodilation in those with persistent dyspnea.
A role for steroid withdrawal
As new clinical trial data emerge, a debate regarding the appropriate role of ICS withdrawal in COPD has formed. Several earlier studies reported that an abrupt withdrawal of ICS precipitates exacerbations and results in a deterioration in lung function and symptoms 57– 59. There has remained equipoise around this issue, however, and a meta-analysis of three of these older trials, the only trials deemed to be acceptable in terms of quality and level of bias, determined that withdrawal of ICS was not actually associated with any statistically significant increase in the exacerbation rate and that the effects on other outcomes, such as lung function and health status, were inconclusive 60. Methodological limitations marred these studies, and the contradictory findings may be due to differences in heterogeneity in patient characteristics, disease severity, and outcome definitions among other factors 57– 60.
Recently, two randomized controlled trials and a prospective study revealed that ICS can be safely withdrawn in certain patients. Those with COPD and a low risk of exacerbations should not be prescribed ICS-containing regimens, according to the latest (2017) GOLD guidelines 14. However, a large proportion of patients are already initiated on ICS-containing regimens 5, 61– 63. Recent studies evaluating GOLD groups A and B (individuals with relatively preserved lung function and not at risk for exacerbations but perhaps for high burden of symptoms) have shown no consequences associated with ICS withdrawal. It was prospectively demonstrated that withdrawal of ICS in patients with symptomatic, moderate COPD with fewer than two exacerbations a year was not associated with any deterioration in lung function, symptoms, and exacerbation rate over a 6-month observation period 64.
A subsequent randomized controlled trial of those with moderate COPD and no prior exacerbation history found that switching from a fixed-dose combination of ICS/LABA to a LABA was not associated with any differences in lung function, symptoms, health status, and exacerbations 65. These studies support the current GOLD recommendations that groups A and B do not benefit from ICS-containing regimens. Furthermore, they suggest that ICS therapy can be safely withdrawn from patients with moderate COPD and a low risk of exacerbations who continue taking long-acting bronchodilators. A growing armamentarium of novel, ultra LABA, ultra LAMA, and LABA/LAMA combinations can be considered to optimize bronchodilation and permit ICS withdrawal.
The WISDOM trial was the largest and first to examine stepwise withdrawal of ICS in patients with COPD receiving maintenance therapy of long-acting bronchodilators, including those at risk for exacerbations. The stepwise withdrawal of glucocorticoids was non-inferior to the continuation of such therapy with respect to the risk of moderate or severe exacerbations 66. The WISDOM trial findings indicate that not all patients benefit from including ICS in their treatment regimen despite current guidelines. Whether a subset of patients will benefit from continuing an ICS-containing regimen and how to identify such a population has not been studied, although subgroup analyses of the WISDOM trial did not show differences in exacerbation occurrence with respect to age, sex, smoking status, body mass index, ICS or beta-blocker therapy at screening, chronic bronchitis, GOLD stage and group, and prior therapy with antibiotics or systemic glucocorticoids 66. This has led to our practice of withdrawing ICS in patients with stable COPD. This practice is further supported by a recent landmark study where a combination LAMA/LABA was superior to a combination ICS/LABA in preventing exacerbations 52.
Emerging bronchoscopic therapies
Lung volume reduction surgery is the only surgical procedure to prolong life in COPD 67. However, only a particular subset of patients, those with known upper lobe predominant emphysema and low post-rehabilitation exercise capacity, derive a mortality benefit. The opportunity to expand the population with severe emphysema who may benefit from intervention by less invasive means has driven the development of potential bronchoscopic interventions for severe emphysema. A recent trial evaluated the efficacy, safety, cost, and cost-effectiveness of nitinol coils versus usual care in patients with severe emphysema 68. The trial randomly assigned patients to a usual-care arm consisting of rehabilitation and bronchodilators with or without ICS and oxygen or to the treatment arm where patients received usual care plus additional therapy of approximately 10 coils per lobe placed in two bilateral lobes in two procedures. The study resulted in improved exercise capacity with high short-term costs. A second study of lung volume reduction coils showed a wide range of clinical outcomes among study participants, and some experienced important improvements in exercise tolerance and lung function whereas others had a less robust result. Although the primary endpoint of 6-minute walk distance between the treatment and control groups was statistically significant, it did not appear to be clinically meaningful 69.
Bronchoscopic lung volume reduction with the use of one-way endobronchial valves is another potential treatment for patients with severe emphysema. To date, the benefits have been modest but have been hypothesized to be much larger in patients without interlobar collateral ventilation than in those with collateral ventilation. A single-center, double-blind, sham-controlled trial in patients with severe COPD, significant hyperinflation, and restricted exercise tolerance with a target lobe with intact interlobar fissures on chest computed tomography showed significant improvement in lung function at 3 months 70. A subsequent study of 64 patients randomly assigned to the endobronchial valve group or the control group and intention-to-treat analyses showed greater improvements in the pulmonary function and exercise capacity in those treated with endobronchial valves 71. Further investigation is warranted to determine whether this will be a potential approved therapy in patients with severe COPD and intact fissures.
A characteristic of COPD is a disproportionately high prevalence of common comorbidities such as cardiovascular disease, diabetes, lung cancer, depression, metabolic syndrome, skeletal muscle dysfunction, and osteoporosis 14. These comorbidities are so common that they are now part of the GOLD definition 14 and can occur in patients with mild, moderate, or severe airflow limitation 72. Comorbidities in COPD influence mortality and hospitalizations independently 73. COPD itself has significant systemic effects, including skeletal muscle dysfunction which may be characterized by loss of muscle cells or abnormal function of remaining cells or both 74. Although skeletal muscle dysfunction may be caused by inactivity, poor diet, inflammation, and hypoxia, it is a remediable source of exercise intolerance 75. Bronchoscopic therapies aim to widen the population to whom non-pharmacologic therapies are available. However, comorbidities may present challenges of candidacy and tolerance of procedures in addition to impacting the optimization of pulmonary rehabilitation after lung volume reduction procedures have been performed.
Conclusions
Emerging evidence that withdrawal of ICS is safe in some patients makes combination LAMA/LABA pharmacotherapy a reasonable option for many, particularly those with persistent dyspnea on a single long-acting bronchodilator. The growth of novel dual-agent long-acting bronchodilator inhalers may decrease the excessive over-prescription of combination ICS/LABAs. Further evidence is needed to better understand the role of bronchoscopic lung volume reduction.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Roberto Rodriguez-Roisin, Department of Medicine, Universitat de Barcelona, Barcelona, Spain
Jill Ohar, Wake Forest University, Winston-Salem, NC, USA
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 1; referees: 2 approved]
References
- 1. Mathers CD, Loncar D: Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442. 10.1371/journal.pmed.0030442 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 2. Diaz-Guzman E, Mannino DM: Epidemiology and prevalence of chronic obstructive pulmonary disease. Clin Chest Med. 2014;35(1):7–16. 10.1016/j.ccm.2013.10.002 [DOI] [PubMed] [Google Scholar]
- 3. Cheyne L, Irvin-Sellers MJ, White J: Tiotropium versus ipratropium bromide for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015: (9):CD009552. 10.1002/14651858.CD009552.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 4. Seemungal TA, Donaldson GC, Paul EA, et al. : Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157(5 Pt 1):1418–22. 10.1164/ajrccm.157.5.9709032 [DOI] [PubMed] [Google Scholar]
- 5. Vestbo J, Vogelmeier C, Small M, et al. : Understanding the GOLD 2011 Strategy as applied to a real-world COPD population. Respir Med. 2014;108(5):729–36. 10.1016/j.rmed.2014.03.002 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 6. Singanayagam A, Schembri S, Chalmers JD: Predictors of mortality in hospitalized adults with acute exacerbation of chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2013;10(2):81–9. 10.1513/AnnalsATS.201208-043OC [DOI] [PubMed] [Google Scholar]
- 7. Marchetti N, Criner GJ, Albert RK: Preventing acute exacerbations and hospital admissions in COPD. Chest. 2013;143(5):1444–54. 10.1378/chest.12-1801 [DOI] [PubMed] [Google Scholar]
- 8. Calverley PM, Anderson JA, Celli B, et al. : Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775–89. 10.1056/NEJMoa063070 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 9. Tashkin DP, Celli B, Senn S, et al. : A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359(15):1543–54. 10.1056/NEJMoa0805800 [DOI] [PubMed] [Google Scholar]
- 10. Decramer M, Celli B, Kesten S, et al. : Effect of tiotropium on outcomes in patients with moderate chronic obstructive pulmonary disease (UPLIFT): a prespecified subgroup analysis of a randomised controlled trial. Lancet. 2009;374(9696):1171–8. 10.1016/S0140-6736(09)61298-8 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 11. Decramer M, Dahl R, Kornmann O, et al. : Effects of long-acting bronchodilators in COPD patients according to COPD severity and ICS use. Respir Med. 2013;107(2):223–32. 10.1016/j.rmed.2012.10.022 [DOI] [PubMed] [Google Scholar]
- 12. Cazzola M, Page CP, Calzetta L, et al. : Pharmacology and therapeutics of bronchodilators. Pharmacol Rev. 2012;64(3):450–504. 10.1124/pr.111.004580 [DOI] [PubMed] [Google Scholar]
- 13. Cazzola M, Matera MG: Bronchodilators: current and future. Clin Chest Med. 2014;35(1):191–201. 10.1016/j.ccm.2013.10.005 [DOI] [PubMed] [Google Scholar]
- 14. Global Initiative for Chronic Obstructive Lung Disease: Global Strategy for the Diagnosis, Management and Prevention of COPD 2017 Report.2017. Reference Source [Google Scholar]
- 15. Qaseem A, Wilt TJ, Weinberger SE, et al. : Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155(3):179–91. 10.7326/0003-4819-155-3-201108020-00008 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 16. National Clinical Guideline Centre: Chronic Obstructive Pulmonary Disease: Management of Chronic Obstructive Pulmonary Disease in Adults in Primary and Secondary Care [Internet]. National Clinical Guideline Centre, London,2010. [Google Scholar]
- 17. MacNee W: Pathophysiology of acute exacerbations of chronic obstructive pulmonary disease. In: Acute exacerbations of chronic obstructive pulmonary disease Siafakas NM, Anthonisen NR, Georgopoulos D (Eds), Siafakas NM AN, Georgopoulos D, editor.2004;183:29 Reference Source [Google Scholar]
- 18. Barnes PJ: Chronic obstructive pulmonary disease. N Engl J Med. 2000;343(4):269–80. 10.1056/NEJM200007273430407 [DOI] [PubMed] [Google Scholar]
- 19. Milic-Emili J: Dynamic pulmonary hyperinflation and intrinsic PEEP: consequences and management in patients with chronic obstructive pulmonary disease. Recenti Prog Med. 1990;81(11):733–7. [PubMed] [Google Scholar]
- 20. Johnson BD, Beck KC, Zeballos RJ, et al. : Advances in pulmonary laboratory testing. Chest. 1999;116(5):1377–87. 10.1378/chest.116.5.1377 [DOI] [PubMed] [Google Scholar]
- 21. Ferguson GT, Feldman GJ, Hofbauer P, et al. : Efficacy and safety of olodaterol once daily delivered via Respimat ® in patients with GOLD 2-4 COPD: results from two replicate 48-week studies. Int J Chron Obstruct Pulmon Dis. 2014;9(1):629–45. 10.2147/COPD.S61717 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 22. Kornmann O, Dahl R, Centanni S, et al. : Once-daily indacaterol versus twice-daily salmeterol for COPD: a placebo-controlled comparison. Eur Respir J. 2011;37(2):273–9. 10.1183/09031936.00045810 [DOI] [PubMed] [Google Scholar]
- 23. Korn S, Kerwin E, Atis S, et al. : Indacaterol once-daily provides superior efficacy to salmeterol twice-daily in COPD: a 12-week study. Respir Med. 2011;105(5):719–26. 10.1016/j.rmed.2011.02.008 [DOI] [PubMed] [Google Scholar]
- 24. Donohue JF, Fogarty C, Lötvall J, et al. : Once-daily bronchodilators for chronic obstructive pulmonary disease: indacaterol versus tiotropium. Am J Respir Crit Care Med. 2010;182(2):155–62. 10.1164/rccm.200910-1500OC [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 25. Dahl R, Chung KF, Buhl R, et al. : Efficacy of a new once-daily long-acting inhaled beta 2-agonist indacaterol versus twice-daily formoterol in COPD. Thorax. 2010;65(6):473–9. 10.1136/thx.2009.125435 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 26. Buhl R, Dunn LJ, Disdier C, et al. : Blinded 12-week comparison of once-daily indacaterol and tiotropium in COPD. Eur Respir J. 2011;38(4):797–803. 10.1183/09031936.00191810 [DOI] [PubMed] [Google Scholar]
- 27. Donohue JF, van Noord JA, Bateman ED, et al. : A 6-month, placebo-controlled study comparing lung function and health status changes in COPD patients treated with tiotropium or salmeterol. Chest. 2002;122(1):47–55. 10.1378/chest.122.1.47 [DOI] [PubMed] [Google Scholar]
- 28. Cope S, Donohue JF, Jansen JP, et al. : Comparative efficacy of long-acting bronchodilators for COPD: a network meta-analysis. Respir Res. 2013;14:100. 10.1186/1465-9921-14-100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rodrigo GJ, Neffen H: Comparison of indacaterol with tiotropium or twice-daily long-acting β -agonists for stable COPD: a systematic review. Chest. 2012;142(5):1104–10. 10.1378/chest.11-2252 [DOI] [PubMed] [Google Scholar]
- 30. Vogelmeier C, Magnussen H, LaForce C, et al. : Profiling the bronchodilator effects of the novel ultra-long-acting β 2-agonist indacaterol against established treatments in chronic obstructive pulmonary disease. Ther Adv Respir Dis. 2011;5(5):345–57. 10.1177/1753465811410100 [DOI] [PubMed] [Google Scholar]
- 31. Decramer ML, Chapman KR, Dahl R, et al. : Once-daily indacaterol versus tiotropium for patients with severe chronic obstructive pulmonary disease (INVIGORATE): a randomised, blinded, parallel-group study. Lancet Respir Med. 2013;1(7):524–33. 10.1016/S2213-2600(13)70158-9 [DOI] [PubMed] [Google Scholar]
- 32. Singh D, Roche N, Halpin D, et al. : Current Controversies in the Pharmacological Treatment of Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2016;194(5):541–9. 10.1164/rccm.201606-1179PP [DOI] [PubMed] [Google Scholar]
- 33. Lee TA, Wilke C, Joo M, et al. : Outcomes associated with tiotropium use in patients with chronic obstructive pulmonary disease. Arch Intern Med. 2009;169(15):1403–10. 10.1001/archinternmed.2009.233 [DOI] [PubMed] [Google Scholar]
- 34. Jones PW, Rennard SI, Agusti A, et al. : Efficacy and safety of once-daily aclidinium in chronic obstructive pulmonary disease. Respir Res. 2011;12(1):55. 10.1186/1465-9921-12-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jones PW, Singh D, Bateman ED, et al. : Efficacy and safety of twice-daily aclidinium bromide in COPD patients: the ATTAIN study. Eur Respir J. 2012;40(4):830–6. 10.1183/09031936.00225511 [DOI] [PubMed] [Google Scholar]
- 36. Trivedi R, Richard N, Mehta R, et al. : Umeclidinium in patients with COPD: a randomised, placebo-controlled study. Eur Respir J. 2014;43(1):72–81. 10.1183/09031936.00033213 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 37. LaForce C, Feldman G, Spangenthal S, et al. : Efficacy and safety of twice-daily glycopyrrolate in patients with stable, symptomatic COPD with moderate-to-severe airflow limitation: the GEM1 study. Int J Chron Obstruct Pulmon Dis. 2016;11(1):1233–43. 10.2147/COPD.S100445 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 38. Leaker BR, Barnes PJ, Jones CR, et al. : Efficacy and safety of nebulized glycopyrrolate for administration using a high efficiency nebulizer in patients with chronic obstructive pulmonary disease. Br J Clin Pharmacol. 2015;79(3):492–500. 10.1111/bcp.12517 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 39. Sharafkhaneh A, Wolf RA, Goodnight S, et al. : Perceptions and attitudes toward the use of nebulized therapy for COPD: patient and caregiver perspectives. COPD. 2013;10(4):482–92. 10.3109/15412555.2013.773302 [DOI] [PubMed] [Google Scholar]
- 40. In chronic obstructive pulmonary disease, a combination of ipratropium and albuterol is more effective than either agent alone. An 85-day multicenter trial. COMBIVENT Inhalation Aerosol Study Group. Chest. 1994;105(5):1411–9. 10.1378/chest.105.5.1411 [DOI] [PubMed] [Google Scholar]
- 41. Routine nebulized ipratropium and albuterol together are better than either alone in COPD. The COMBIVENT Inhalation Solution Study Group. Chest. 1997;112(6):1514–21. 10.1378/chest.112.6.1514 [DOI] [PubMed] [Google Scholar]
- 42. van Noord JA, Aumann J, Janssens E, et al. : Combining tiotropium and salmeterol in COPD: Effects on airflow obstruction and symptoms. Respir Med. 2010;104(7):995–1004. 10.1016/j.rmed.2010.02.017 [DOI] [PubMed] [Google Scholar]
- 43. Donohue JF, Maleki-Yazdi MR, Kilbride S, et al. : Efficacy and safety of once-daily umeclidinium/vilanterol 62.5/25 mcg in COPD. Respir Med. 2013;107(10):1538–46. 10.1016/j.rmed.2013.06.001 [DOI] [PubMed] [Google Scholar]
- 44. Decramer M, Anzueto A, Kerwin E, et al. : Efficacy and safety of umeclidinium plus vilanterol versus tiotropium, vilanterol, or umeclidinium monotherapies over 24 weeks in patients with chronic obstructive pulmonary disease: results from two multicentre, blinded, randomised controlled trials. Lancet Respir Med. 2014;2(6):472–86. 10.1016/S2213-2600(14)70065-7 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 45. Donohue JF, Niewoehner D, Brooks J, et al. : Safety and tolerability of once-daily umeclidinium/vilanterol 125/25 mcg and umeclidinium 125 mcg in patients with chronic obstructive pulmonary disease: results from a 52-week, randomized, double-blind, placebo-controlled study. Respir Res. 2014;15(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 46. Celli B, Crater G, Kilbride S, et al. : Once-daily umeclidinium/vilanterol 125/25 mcg in COPD: a randomized, controlled study. Chest. 2014;145(5):981–91. 10.1378/chest.13-1579 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 47. Singh D, Jones PW, Bateman ED, et al. : Efficacy and safety of aclidinium bromide/formoterol fumarate fixed-dose combinations compared with individual components and placebo in patients with COPD (ACLIFORM-COPD): a multicentre, randomised study. BMC Pulm Med. 2014;14:178. 10.1186/1471-2466-14-178 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 48. D'Urzo AD, Rennard SI, Kerwin EM, et al. : Efficacy and safety of fixed-dose combinations of aclidinium bromide/formoterol fumarate: the 24-week, randomized, placebo-controlled AUGMENT COPD study. Respir Res. 2014;15:123. 10.1186/s12931-014-0123-0 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 49. Bateman ED, Chapman KR, Singh D, et al. : Aclidinium bromide and formoterol fumarate as a fixed-dose combination in COPD: pooled analysis of symptoms and exacerbations from two six-month, multicentre, randomised studies (ACLIFORM and AUGMENT). Respir Res. 2015;16:92. 10.1186/s12931-015-0250-2 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 50. Donohue JF, D'Urzo AD, Singh D, et al. : Cardiovascular safety of fixed-dose combination aclidinium bromide/formoterol fumarate: results of two 6-month studies in patients with moderate to severe COPD. Am J Respir Crit Care Med. 2014;189:A6011 Reference Source [Google Scholar]
- 51. Buhl R, Maltais F, Abrahams R, et al. : Tiotropium and olodaterol fixed-dose combination versus mono-components in COPD (GOLD 2-4). Eur Respir J. 2015;45(4):969–79. 10.1183/09031936.00136014 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 52. Wedzicha JA, Banerji D, Chapman KR, et al. : Indacaterol-Glycopyrronium versus Salmeterol-Fluticasone for COPD. N Engl J Med. 2016;374(23):2222–34. 10.1056/NEJMoa1516385 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 53. Bateman ED, Ferguson GT, Barnes N, et al. : Dual bronchodilation with QVA149 versus single bronchodilator therapy: the SHINE study. Eur Respir J. 2013;42(6):1484–94. 10.1183/09031936.00200212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jones PW, Beeh KM, Chapman KR, et al. : Minimal clinically important differences in pharmacological trials. Am J Respir Crit Care Med. 2014;189(3):250–5. 10.1164/rccm.201310-1863PP [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 55. Singh D, Ferguson GT, Bolitschek J, et al. : Tiotropium + olodaterol shows clinically meaningful improvements in quality of life. Respir Med. 2015;109(10):1312–9. 10.1016/j.rmed.2015.08.002 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 56. Mahler DA, Decramer M, D'Urzo A, et al. : Dual bronchodilation with QVA149 reduces patient-reported dyspnoea in COPD: the BLAZE study. Eur Respir J. 2014;43(6):1599–609. 10.1183/09031936.00124013 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 57. van der Valk P, Monninkhof E, van der Palen J, et al. : Effect of discontinuation of inhaled corticosteroids in patients with chronic obstructive pulmonary disease: the COPE study. Am J Respir Crit Care Med. 2002;166(10):1358–63. 10.1164/rccm.200206-512OC [DOI] [PubMed] [Google Scholar]
- 58. Wouters EF, Postma DS, Fokkens B, et al. : Withdrawal of fluticasone propionate from combined salmeterol/fluticasone treatment in patients with COPD causes immediate and sustained disease deterioration: a randomised controlled trial. Thorax. 2005;60(6):480–7. 10.1136/thx.2004.034280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Choudhury AB, Dawson CM, Kilvington HE, et al. : Withdrawal of inhaled corticosteroids in people with COPD in primary care: a randomised controlled trial. Respir Res. 2007;8(1):93. 10.1186/1465-9921-8-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nadeem NJ, Taylor SJ, Eldridge SM: Withdrawal of inhaled corticosteroids in individuals with COPD--a systematic review and comment on trial methodology. Respir Res. 2011;12(1):107. 10.1186/1465-9921-12-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Price D, West D, Brusselle G, et al. : Management of COPD in the UK primary-care setting: an analysis of real-life prescribing patterns. Int J Chron Obstruct Pulmon Dis. 2014;9(1):889–904. 10.2147/COPD.S62750 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 62. Suissa S, Barnes PJ: Inhaled corticosteroids in COPD: the case against. Eur Respir J. 2009;34(1):13–6. 10.1183/09031936.00190908 [DOI] [PubMed] [Google Scholar]
- 63. Vogelmeier CF, Bateman ED, Pallante J, et al. : Efficacy and safety of once-daily QVA149 compared with twice-daily salmeterol-fluticasone in patients with chronic obstructive pulmonary disease (ILLUMINATE): a randomised, double-blind, parallel group study. Lancet Respir Med. 2013;1(1):51–60. 10.1016/S2213-2600(12)70052-8 [DOI] [PubMed] [Google Scholar]
- 64. Rossi A, Guerriero M, Corrado A: Withdrawal of inhaled corticosteroids can be safe in COPD patients at low risk of exacerbation: a real-life study on the appropriateness of treatment in moderate COPD patients (OPTIMO). Respir Res. 2014;15(1):77. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 65. Rossi A, van der Molen T, del Olmo R, et al. : INSTEAD: a randomised switch trial of indacaterol versus salmeterol/fluticasone in moderate COPD. Eur Respir J. 2014;44(6):1548–56. 10.1183/09031936.00126814 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 66. Magnussen H, Disse B, Rodriguez-Roisin R, et al. : Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N Engl J Med. 2014;371(14):1285–94. 10.1056/NEJMoa1407154 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 67. Fishman A, Martinez F, Naunheim K, et al. : A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003;348(21):2059–73. 10.1056/NEJMoa030287 [DOI] [PubMed] [Google Scholar]
- 68. Deslée G, Mal H, Dutau H, et al. : Lung Volume Reduction Coil Treatment vs Usual Care in Patients With Severe Emphysema: The REVOLENS Randomized Clinical Trial. JAMA. 2016;315(2):175–84. 10.1001/jama.2015.17821 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 69. Sciurba FC, Criner GJ, Strange C, et al. : Effect of Endobronchial Coils vs Usual Care on Exercise Tolerance in Patients With Severe Emphysema: The RENEW Randomized Clinical Trial. JAMA. 2016;315(20):2178–89. 10.1001/jama.2016.6261 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 70. Davey C, Zoumot Z, Jordan S, et al. : Bronchoscopic lung volume reduction with endobronchial valves for patients with heterogeneous emphysema and intact interlobar fissures (the BeLieVeR-HIFi study): a randomised controlled trial. Lancet. 2015;386(9998):1066–73. 10.1016/S0140-6736(15)60001-0 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 71. Klooster K, ten Hacken NH, Hartman JE, et al. : Endobronchial Valves for Emphysema without Interlobar Collateral Ventilation. N Engl J Med. 2015;373(24):2325–35. 10.1056/NEJMoa1507807 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 72. Agusti A, Calverley PM, Celli B, et al. : Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res. 2010;11:122. 10.1186/1465-9921-11-122 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 73. Mannino DM, Thorn D, Swensen A, et al. : Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J. 2008;32(4):962–9. 10.1183/09031936.00012408 [DOI] [PubMed] [Google Scholar]
- 74. Wagner PD: Possible mechanisms underlying the development of cachexia in COPD. Eur Respir J. 2008;31(3):492–501. 10.1183/09031936.00074807 [DOI] [PubMed] [Google Scholar]
- 75. Skeletal muscle dysfunction in chronic obstructive pulmonary disease. A statement of the American Thoracic Society and European Respiratory Society. Am J Respir Crit Care Med. 1999;159(4 Pt 2):S1–40. 10.1164/ajrccm.159.supplement_1.99titlepage [DOI] [PubMed] [Google Scholar]