Version Changes
Revised. Amendments from Version 1
Description of main differences between the two versions The following revisions have been made in the revised version in response to the reviewer’s comments We have clarified in the inclusion/exclusion criteria that the eligible studies were not filtered by study settings. We have noted in the discussion section that the different methods of assessment of neurological and mental manifestations in individual eligible studies may have contributed to the heterogeneity of pooled estimates observed during the analysis which informed our decision to apply the random effect model rather than the fixed effect model. The random effect model allows the prevalence of mental and neurological manifestations varies from one study to the next. We have noted in the limitation section that a quality check on all eligible studies was performed which may have excluded a number of children studies. We already described in the methods section of first version that the quality check was based on the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system for experimental studies and The Joanna Briggs Institute Prevalence Critical Appraisal Tool for observation studies.. We have now noted in the discussion session that the very high between study heterogeneity observed may be partly explained by factors not reported in the included studies such as levels of endemicity of malaria in the study sites and individual factors such as resistance to malaria, as suggested by the reviewer.
Abstract
Background: Antimalarial drugs affect the central nervous system, but it is difficult to differentiate the effect of these drugs from that of the malaria illness. We conducted a systematic review to determine the association between anti-malarial drugs and mental and neurological impairment in humans. Methods: We systematically searched online databases, including Medline/PubMed, PsychoInfo, and Embase, for articles published up to 14th July 2016. Pooled prevalence, heterogeneity and factors associated with prevalence of mental and neurological manifestations were determined using meta-analytic techniques. Results: Of the 2,349 records identified in the initial search, 51 human studies met the eligibility criteria. The median pooled prevalence range of mental and neurological manifestations associated with antimalarial drugs ranged from 0.7% (dapsone) to 48.3% (minocycline) across all studies, while it ranged from 0.6% (pyrimethamine) to 42.7% (amodiaquine) during treatment of acute malaria, and 0.7% (primaquine/dapsone) to 55.0% (sulfadoxine) during prophylaxis. Pooled prevalence of mental and neurological manifestations across all studies was associated with an increased number of antimalarial drugs (prevalence ratio= 5.51 (95%CI, 1.05-29.04); P=0.045) in a meta-regression analysis. Headaches (15%) and dizziness (14%) were the most common mental and neurological manifestations across all studies. Of individual antimalarial drugs still on the market, mental and neurological manifestations were most common with the use of sulphadoxine (55%) for prophylaxis studies and amodiaquine (42.7%) for acute malaria studies. Mefloquine affected more domains of mental and neurological manifestations than any other antimalarial drug. Conclusions: Antimalarial drugs, particularly those used for prophylaxis, may be associated with mental and neurological manifestations, and the number of antimalarial drugs taken determines the association. Mental and neurological manifestations should be assessed following the use of antimalarial drugs.
Keywords: Antimalarial drugs, Mental and neurological manifestations, Toxicity, Systematic review, Meta-Analysis
Introduction
Over 3.2 billion people in the world are at risk of malaria (Malaria fact sheet, World Health Organization) and a wide range of antimalarial drugs is used to prevent and treat malaria. Malaria continues to be a major cause of morbidity and mortality, but both have declined with the introduction of effective anti-malarial drugs malaria (Malaria fact sheet, World Health Organization). Mental and neurological manifestations are common in patients with malaria, particularly children admitted to hospital with falciparum malaria in Africa 1, 2. Survivors of severe malaria develop a wide range of neuro-cognitive sequelae, including epilepsy, language deficits, motor and sensory deficits, and other neurobehavioral difficulties 3, 4. Antimalarial drugs are thought to have significant mental and neurological manifestations 5– 7. Antimalarials prescribed to prevent malaria are associated with mental and neurological manifestations, some of which are similar to the manifestations seen in acute malaria 8– 10. Therefore, the neuro-cognitive and behavioural sequelae observed after malaria may be related to the underlying malarial illness or the antimalarial drugs. Teratogenicity is reported after the use of antimalarial drugs in pregnancy, but mental and neurological damage is not extensively studied 11.
Mental and neurological manifestations of antimalarial drugs are observed in both animals and humans 8, 12– 18. Animal studies identify potential mechanisms of mental and neurological manifestations and the parts of the central nervous system (CNS) affected. To date, there have been no human studies attempting to explore the role that drugs play in causing mental and neurological manifestations after accounting for the malarial illness, although some studies have acknowledged that malaria illness alone cannot explain neuro-cognitive and behavioural sequelae observed after treatment 4. Reports have highlighted severe neuropsychiatric reactions after use of mefloquine for prophylaxis 19, 20, but properly designed studies are required to quantify and clarify the extent of these manifestations. Studying the use of antimalarial drugs for prophylaxis can help to estimate the prevalence of mental and neurological manifestations in non-infected subjects, and compare with the prevalence observed in patients with malaria to understand if antimalarial drugs add to mental and neurological manifestations.
We conducted a systematic review and meta-analysis of the published literature on mental and neurological manifestations associated with antimalarial drugs, and reported the findings according to the PRISMA guidelines 21. We estimated the pooled overall prevalence of mental and neurological outcomes among the human studies identified and examined if prevalence differed by type and number of antimalarial drugs used. We also investigated and quantified the sources of heterogeneity between the studies and attempted to identify the factors explaining the variation in prevalence of mental and neurological outcomes.
Methods
Information sources
We searched the following online databases systematically: MEDLINE, EMBASE, CINHL, PsycINFO, Central Registration for Clinical Trials, Open Grey Database, Canadian Agency for Drugs and Technologies in Health, Directory of Open-Access Repository, World Cat database, and Web of Science. Reference lists of identified articles were also searched for relevant titles and these were in turn searched online. All authors contributed to the search strategy. Consensus was used to set the selection criteria according to recommendations 22.
Search strategies
An initial limited search of MEDLINE, COCHRANE LIBRARY and EMBASE was undertaken followed by analysis of the text words contained in the title and abstract, and of the index terms used to describe articles. Combined text words and Medical Subject Headings (MeSH) terminology were used in addition to the two main search terms/facets [Mental and neurological and Antimalarial Drugs] ( Supplementary Table 1). Boolean operators, such as “AND” and “OR”, were used to combine search terms as necessary. Truncation, wildcard, adjacent searching, and floating subheadings were also used to increase the sensitivity of the results in unpublished data, where necessary. The construction of search terms followed the recommendations by the National Health Service Centre for Reviews and Disseminations.
Inclusion and exclusion criteria
We included studies that met the following criteria: (i) use of an antimalarial agent ( Table 1) (either as a prophylactic drug or as treatment for malaria or another illness); and (ii) report of mental and neurological symptoms, including psychiatric disorders, cognitive impairments, sensory problems, and seizures (during or after using the antimalarial) ( Table 2). We also included studies reporting foetal teratogenicity following use of antimalarial drug in pregnancy. Only empirical studies were considered for the main analysis, while case series/reports studies were excluded because their findings cannot be generalized. There was no restriction on age of the participants or on the study settings.
Table 1. Classification of antimalarial drugs.
| Class | Drugs |
|---|---|
| 4-Aminoquinolines | Chloroquine,
amodiaquine, hydroxychloroquine |
| 8-Aminoquinoline | Primaquine, pamaquine, pentaquine,
isopentaquine |
| 4-quinolinemethanols | Quinine, quinidine, mefloquine |
| Phenanthrene methanol | Halofantrine |
| Artemisinin derivatives | Artemisinin, artemether, artesunate,
arteether |
| Antimetabolites | Proguanil, pyrimethamine, atovaquone,
dapsone |
| Antibiotics | Tetracycline, doxycycline, minocycline |
| Diaminopyridines | Pyrimethamine |
Table 2. Classification of neurological manifestations.
| Category | Specific symptoms |
|---|---|
| Psychiatric disorders | Suicidality, violence, hallucinations,
delusions, psychosis, depression, phobias, anxiety, anorexia |
| Mild neurological
perturbations |
Stupor, dizziness, fainting, confusion |
| Motor impairment | Motor impairments, ataxia |
| Sleep disturbances | Nightmares, vivid dreams, insomnia,
sleep pattern disturbance |
| Personality changes | Mood changes, altered esteem,
personality changes |
| Sensory impairments | Peripheral neuropathy, anorexia,
paraesthesia |
| Seizures | Convulsions, seizures |
| Headache | Headache |
| Hearing and balance | Hearing loss, tinnitus, vertigo |
| Visual | Blurred vision, diplopia, loss of vision |
| Cognition | Altered memory, concentration
problems, speech problems |
Eligible English articles were considered and articles in French, Dutch, Chinese, Hebrew, Spanish, and German were also retrieved, translated and reviewed for eligibility. Unpublished work and proceedings from scientific conferences were included in the review if they fulfilled the criteria above. There were no restrictions on dates of earliest possible publications, but articles published up to 14 th July 2016, which was the last search date, were included. Excluded from the analysis were commentaries and conference abstracts without full length and duplicate publications, as were studies of special duplicate populations 21.
Data extraction
Data was extracted into a Microsoft Excel spreadsheet with a list of variables ( Supplementary Table 2) determined a priori by the authors. The template was piloted on ten randomly selected studies that satisfied the inclusion criteria. The extraction was performed manually included a two-stage process: first, a determination of eligibility based on titles and abstracts; second, determination of eligibility after reviewing the full texts.
Eligibility assessment was performed independently in a standardized manner by MAB under the guidance of SMK and CRN. All articles were reviewed by at least two authors. Disagreements between reviewers were resolved by consensus. Follow-up time was defined as the number of days between administration of the antimalarial agent and the appearance of the mental and neurological symptoms.
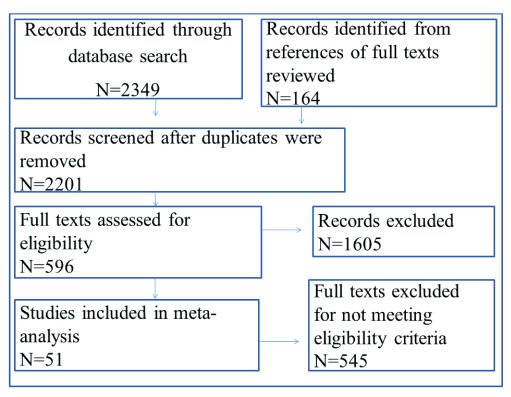
Where overall prevalence was not reported, but an n (number of people reporting a specific symptom) was assigned for each mental and neurological symptom, we took the symptom for which n had the highest value and calculated the overall prevalence using the formula ( n/N)*100; where N= total sample size of the study. The assumption was that the symptoms were not mutually exclusive. The extracted information contained Population, Interventions/treatment, Comparison groups, and Outcomes (PICO) 21. Of the 120 studies in languages other than English, retrieval and translation was only possible for 12 full articles. Figure 1 illustrates the selection process 23.
Figure 1. Study selection process.
Classification of mental and neurological symptoms
Anti-malarial drug effects of the eight classes of antimalarial drugs were studied under ( Table 1). Specific mental and neurological symptoms were classified into 12 categories of related symptoms ( Table 2).
Critical appraisal of studies included in meta-analysis
The quality of all observational studies that met the inclusion criteria was investigated using the The Joanna Briggs Institute Prevalence Critical Appraisal Tool 24. The tool is a ten question questionnaire with four possible responses: yes, no, unclear or not applicable. Scores of quality were calculated as a percentage, with ten as the denominator unless a section was marked as ‘not applicable’, in which case we excluded that section from the total quality score. This was done to avoid downgrading the total score of quality by a domain that does not apply to that study. Each positive (yes) response to a domain was equal to one point, whereas a negative response (no/unclear) attracted no point. For experimental studies, the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system was used 25. Studies which fulfilled >80% of the criteria for quality were included in this review.
Statistical analysis
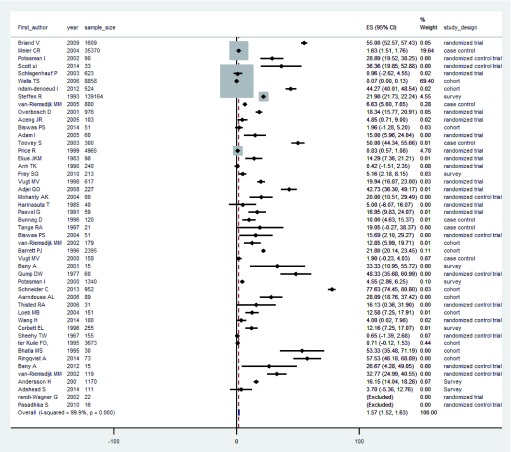
We computed crude median prevalence of mental and neurological manifestations expressed per 100 subjects or as a percentage and the corresponding interquartile ranges (IQR). Prevalence of mental and neurological manifestations was determined for human studies only. The 95% confidence interval (95%CI) for each study’s prevalence of mental and neurological outcomes were calculated using the formula: ; where p is the prevalence as a percentage and N is the sample size 26. For unweighted pooled median prevalence of mental and neurological manifestations, we fitted a random effect model to the individual study prevalence estimates and their corresponding 95%CI using STATA version 13.1 (Stata Corp, Texas, USA). Random effect models allow effect estimates to vary across the studies. The pooled estimates from this model and their corresponding 95%CIs were obtained on the original prevalence scale. These estimates were also summarised in a forest plot ( Figure 2). Comparison of the spectrum of mental and neurological manifestations and/or their severities across treatment groups was done with Persons Chi-square test or Fishers exact test, where appropriate. Comparison of prevalence between those on antimalarial drugs and controls not on antimalarial drugs was done using Pearson’s Chi-square test.
Figure 2. Forest plot of the prevalence of mental and neurological manifestations in studies included in the meta-analysis.
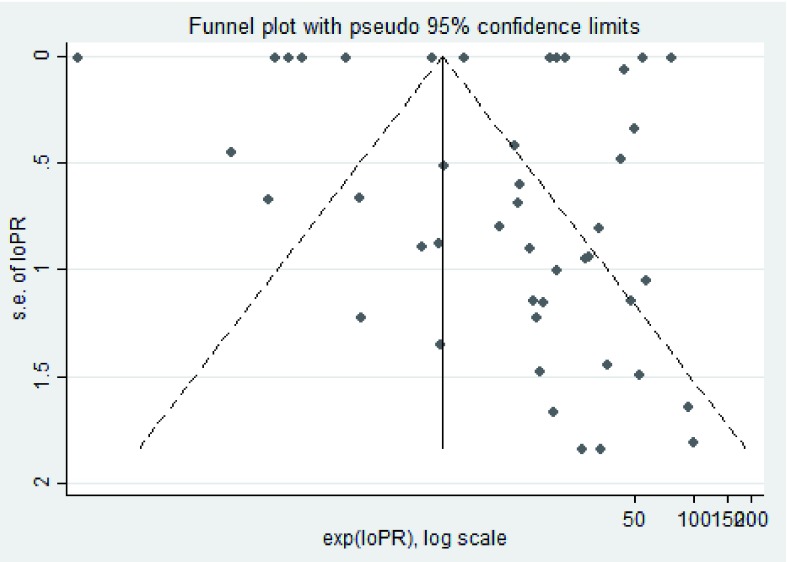
The Cochrane Q-statistic was used to test the null hypothesis that the prevalence of mental and neurological manifestations was uniform across the studies. The degree of heterogeneity (I 2) of the pooled estimates was derived from the random effect models as a function of the Q statistics and degrees of freedom, expressed as a percentage ([Q-df/Q] × 100). We further investigated the contribution of factors, such as age, study design and malaria status, to the variation in the documented prevalence of mental and neurological outcomes. This was implemented by fitting two random effect meta-regression models, one as a null model without the covariates of interest and another with the variables of interest, both models with the documented prevalence as the dependent variable and the associated standard errors specified. The proportion of variation explained by the covariates studied was determined by dividing the difference in components of variance between the two models (τ0 2 − τ 2) by the variance in the null model (τ0 2). Where two variables showed strong multi-collinearity, one was picked at random for inclusion in the multivariable model. Reporting and publication ( Figure 3) bias were examined in STATA using funnel plots.
Figure 3. A funnel plot of bias of the selected studies.
Results
Search results and study characteristics
The initial search yielded 2349 results, of which 596 were retained for full review based on title and abstract examination ( Figure 1). After full text review, we excluded 545 articles in the quantitative analysis: articles in foreign languages for which translation could not be obtained (N=108); articles that did not mention neurotoxic outcomes (N=341); reviews (N=16) and case reports (N=80). A total of 51 studies with a reporting on 205,175 subjects were retained. The study characteristics are defined in Table 3.
Table 3. Description of study characteristics.
| First author (Ref) | Year | Country | Design | Sample
size |
Malaria
study |
Region | Children/
Adult |
Sex (%
female) |
|---|---|---|---|---|---|---|---|---|
| Sjowall J 27 | 2012 | Sweden | Randomized
control trial |
15 | No | High
income |
Adult | 40.0 |
| Scott UI 28 | 2014 | USA | Randomized
control trial |
33 | No | High
income |
Adult | 36.4 |
| Frey SG 9 | 2010 | Cameroon | Survey | 213 | Yes | Low
income |
Child | 51.2 |
| Wells TS 29 | 2006 | Japan | Cohort | 8858 | No | High
income |
Adult | 0.0 |
| Pasadhika S 10 | 2010 | USA | Randomized
control trial |
16 | No | High
income |
Adult | 100.0 |
| Van-riemsdijk MM 30 | 2004 | Netherlands | Cohort | 151 | No | High
income |
Adult | 57.1 |
| Bhatia MS 31 | 1995 | India | Cohort | 30 | No | Low
income |
Adult | Absent |
| Potasman I 32 | 2000 | Israel | Survey | 1340 | No | High
income |
Adult | 54.7 |
| Ter Kuile FO 33 | 1995 | Thailand | Cohort | 3673 | No | High
income |
Adult | 37.6 |
| Corbett El 34 | 1996 | Britain | Survey | 255 | No | High
income |
Adult | Absent |
| Barrett PJ 35 | 1996 | Britain | Cohort | 2395 | No | High
income |
Adult | 58.1 |
| Beny A 5 | 2001 | Israel | Survey | 15 | No | High
income |
Adult | 33.3 |
| Gump DW 36 | 1977 | USA | Randomized
control trial |
60 | No | High
income |
Adult | 100.0 |
| Wang H 37 | 2014 | China | Randomized
control trial |
100 | No | High
income |
Adult | 53.0 |
| Schneider C 38 | 2013 | Switzerland | Cohort | 952 | No | High
income |
Adult | 66.5 |
| Biswas PS 39 | 2014 | India | Cohort | 51 | No | Low
income |
Adult | 50.0 |
| Sheehy TW 40 | 1967 | Vietnam | Randomized
control trial |
155 | No | Low
income |
Adult | 0.0 |
| Thisted RA 6 | 2006 | USA | Randomized
control trial |
31 | No | High
income |
Adult | 17.0 |
| Aarnoudse Al 41 | 2006 | Absent | Cohort | 89 | No | Absent | Adult | 46.1 |
| Van-riemsdijk MM 42 | 2002 | Netherlands | Cohort | 179 | No | High
income |
Adult | 46.9 |
| Bhatia MS 31 | 1994 | India | Randomized
control trial |
30 | No | High
income |
Adult | Absent |
| Ringqvist A 43 | 2014 | Denmark | Cohort | 73 | No | High
income |
Adult | 54.8 |
| Loeb MB 44 | 2004 | Canada | Randomized
control trial |
51 | No | High
income |
Adult | 58.8 |
| Held TH 45 | 1991 | Germany | Randomized
control trial |
20 | No | High
income |
Adult | Absent |
| Adjei GO 46 | 2008 | Ghana | Randomized
trial |
227 | Yes | High
income |
Child | 47.6 |
| Briand V 47 | 2009 | Benin | Randomized
trial |
1609 | No | Low
income |
Adult | 100 |
| Vugt MV 48 | 2000 | Thailand | Case control | 158 | No | High
income |
Mixed | 29.1 |
| Aceng JR 49 | 2005 | Uganda | Randomized
trial |
103 | Yes | Low
income |
Child | Absent |
| Adam I 50 | 2005 | Sudan | Randomized
trial |
60 | Yes | Low
income |
Adult | 56.7 |
| Toovey S 51 | 2003 | Mozambique | Case control | 300 | Yes | Low
income |
Adult | 1.3 |
| Tange RA 52 | 1997 | Netherlands | Case control | 21 | Yes | High
income |
Adult | 38.1 |
| Pasvol G 53 | 1991 | Kenya | Randomized
trial |
59 | Yes | Low
income |
Child | Absent |
| Schlagenhauf P 54 | 2003 | Multiple | Randomized
trial |
623 | No | High
income |
Adult | Absent |
| Vugt MV 55 | 1998 | Thailand | Randomized
trial |
617 | Yes | High
income |
Mixed | 31.8 |
| Ekue JMK 56 | 1983 | Zambia | Randomized
trial |
98 | Yes | Low
income |
Mixed | 0 |
| Price R 57 | 1999 | Thailand | Randomized
trial |
4965 | Yes | High
income |
Mixed | 43.7 |
| Overbosch D 58 | 2001 | Multiple | Randomized
trial |
976 | No | Low
income |
Mixed | 45 |
| Anh TK 59 | 1990 | Vietnam | Randomized
trial |
240 | Yes | Low
income |
Mixed | 0 |
| Bunnag D 60 | 1996 | Thailand | Comparative
study |
120 | Yes | High
income |
Adult | Absent |
| Ndam-Denoeud L 61 | 2012 | Benin | Cohort | 524 | No | Low
income |
Adult | 0 |
| Harinasuta T 62 | 1985 | Thailand | Randomized
trial |
40 | Yes | High
income |
Mixed | 40 |
| Mohanty AK 63 | 2004 | India | Randomized
control trial |
80 | Yes | Low
income |
Child | 42 |
| Potasman I 64 | 2002 | Israel | Randomized
control trial |
90 | No | High
income |
Adult | 54.6 |
| Rendi-Wagner P 65 | 2002 | Austria | Randomized
trial |
22 | No | High
income |
Adult | 52.5 |
| Steffen R 66 | 1993 | Europe | Survey | 139164 | No | High
income |
Mixed | 70 |
| Van-riemsdijk MM 67 | 2005 | Netherlands | Case control
study |
800 | No | High
income |
Adult | Absent |
| Meier CR 68 | 2004 | UK | Case control
study |
35370 | No | High
income |
Adult | 37.8 |
| Van Riemsdijk MM 7 | 2002 | Netherlands | Randomized
control trial |
119 | No | High
income |
Adult | Absent |
| Andersson H 69 | 2008 | Sweden | Survey | 1170 | No | High
income |
Adult | 4.8 |
| Adshead S 70 | 2014 | UK | Survey | 111 | No | High
income |
Adult | 87.5 |
There were 5 studies on children (<18 years) with a total sample size of 682 (3.4%). The remaining studies were either studies on adults or mixed populations with a population of 205,175. The male and female sex ratio in the studies was well balanced (P=0.357). The median follow up time was 8 days (IQR, 3–28) for the studies that reported follow up data.
Estimates of overall prevalence and heterogeneity
Of the 51 eligible studies, 48 (94.9%) reported a prevalence of at least one category of mental and neurological outcomes. The estimated range of pooled prevalence of mental and neurological manifestation following antimalarial drug use from the random effect models of all the studies was between 0.7% (95%CI 0.62–1.91) for primaquine and dapsone users to 48.3% (95%CI 35.7–61.0) for minocycline users. The random effect model of the pooled prevalence across all human studies was associated with a very high between-study heterogeneity (Q=10.94, I 2=97.8%). There were only two studies (N=229) in which controls were persons who were not taking any antimalarial drugs 48, 51. In these two studies, the average prevalence of mental and neurological manifestations was 3.0%.
Factors explaining the variation in documented overall prevalence
Several factors were assessed in the univariable analysis of human studies, and five appeared to explain the highest variation in the documented median prevalence, but none reached statistical significance level of P<0.05 ( Table 4). In the multivariable meta-regression analysis, the number of drugs used was independently associated with the prevalence of mental and neurological outcomes (Prevalence ratio=5.51 [95% CI, 1.05–29.04], P=0.045). Other factors, such as being a child and having an acute malarial illness, were not associated with variation in prevalence of mental and neurological outcomes. The factors investigated in the multivariable analysis explained 14.1% variability of the prevalence across all human studies. In the multivariable linear regression model, there was no evidence for interaction between malaria illness and the number of drugs in explaining the variation in prevalence of symptoms (interaction parameter: beta co-efficient=2.38 [95%CI, 0.15–37.32; P=0.503]).
Table 4. Heterogeneity and associated factors.
| Factor | Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|---|
| Prevalence ratio
(95%CI) |
P value | Heterogeneity
(%) |
Prevalence ratio
(95%CI) |
P value | |
| Year of publication | 1.04 (0.99–1.1) | 0.125 | 4.3 | 0.74 (0.39–1.41) | 0.789 |
| Study design | 0.91 (0.71–1.15) | 0.407 | 1.2 | 0.84 (0.45–1.56) | 0.372 |
| Malaria
vs prophylaxis
study |
0.99 (0.21–4.71) | 0.988 | 23.4 | 2.38 (0.15–37.32) | 0.663 |
| Paediatric study | 1.05 (0.18–6.02) | 0.955 | 2.9 | 0.87 (0.04–19.73) | 0.945 |
| Number of drugs | 1.11 (0.62–2.00) | 0.719 | 0.9 | 5.51 (1.05–29.04) | 0.045 |
Pooled prevalence of mental and neurological manifestations of individual antimalarial drugs
The highest pooled prevalence of mental and neurological manifestations in the prophylaxis group was reported in those on sulphadoxine (55.0%; 95%CI 52.6–57.4) followed by minocycline (48.3%; 95%CI 35.7–61.0). In the treatment groups, patients receiving amodiaquine reported the highest prevalence (42.7%; 95%CI 36.3–49.2) followed by those on lumefantrine (29.5%; 95%CI 27.0–32.0) ( Table 5). The lowest overall prevalence was reported by dapsone and primaquine users (0.7%; 95%CI 0.6–1.9), while for prophylaxis it was 0.6% (95%CI 0.2–1.4; pyrimethamine) and for malaria studies it was 0.7% (95%CI 0.6–1.9; quinine, primaquine and dapsone).
Table 5. Overall pooled prevalence of mental and neurological manifestations for individual drugs.
| Antimalarial
drug |
Estimated
percentage pooled median prevalence with corresponding 95%CI |
Median overall
prevalence in prophylaxis studies (95%CI) |
Median overall
prevalence in malaria studies c(95%CI) |
|---|---|---|---|
| Amodiaquine | 42.7 (36.3–49.2) | - | 42.7 (36.3–49.2) |
| Artemether | 1.1 (0.9–1.4) | 2.4 (0.5–4.3) | 1.1 (0.9–1.4) |
| Artesunate | 1.1 (0.9–1.3) | 1.9 (0.2–4.0) | 1.1 (0.8–1.3) |
| Atovaquone | 8.0 (7.3–8.7) | 8.7 (8.0–9.4) | 8.0 (7.3–8.7) |
| Chloroquine | 7.1 (7.0–7.2) | 7.1 (7.0–7.2) | 4.9 (1.9–7.8) |
| Dapsone | 0.7 (0.6–1.9) | 0.7 (0.6–1.9) | - |
| Doxycycline | 1.6 (1.5–1.8) | 1.6 (1.5–1.8) | - |
| Lumefantrine | 29.5 (27.0–32.0) | - | 29.5 (27.0–32.0) |
| Mefloquine | 1.9 (1.6–2.1) | 25.0 (23.8–26.3) | 1.0 (0.7–1.2) |
| Minocycline | 48.3 (35.7–61.0) | 48.3 (35.7–61.0) | - |
| Primaquine | 0.7 (0.6–1.9) | 0.6 (0.6–1.9) | - |
| Proguanil | 7.3 (7.2–7.4) | 7.3 (7.2–7.4) | - |
| Pyrimethamine | 20.8 (20.6–21.0) | 22.3 (22.0–22.5) | 0.6 (0.2–1.4) |
| Quinidine | 16.1 (3.2–29.1) | 16.1 (3.2–29.1) | - |
| Quinine | 1.7 (0.5–2.9) | 0.7 (0.6–1.9) | 9.3 (5.9–12.7) |
| Sulfadoxine | 6.0 (5.2–6.8) | 55.0 (52.6–57.4) | 0.6 (0.1–1.4) |
- No observations
Spectrum of mental and neurological effects
For all the studies that reported the number of subjects with specific mental and neurological symptoms (N=205,120), headache was the most frequent symptom (N=30,726; 15.0%), followed by dizziness (N=28,626; 14.0%); neither being mutually exclusive of other mental and neurological outcomes. For studies of acute treatment of malaria, the commonest mental and neurological manifestations were hearing and balance problems (N=184; 2.6%). For prophylactic studies, the commonest manifestations were headaches (N=30,709; 15.5%) and dizziness (N=28,472; 14.4%).
Individual drugs and domains of mental and neurological outcomes
(a) Malaria treatment studies. Mefloquine and quinine were associated with mental and neurological manifestations in more of the domains (6 out of 10) investigated than any other antimalarial drug. Mild neurological perturbations were the most commonly reported symptom in all individual drugs studied, except amodiaquine, with the highest prevalence being in lumefantrine (10.8%). Cognition problems were infrequently reported in malaria treatment studies ( Table 6).
Table 6. Mental and neurological outcomes for individual drugs by malaria treatment studies.
-No observations.
| Drug | Psychiatric/
behavioural |
Mild
neurological perturbations |
Motor
problems |
Sleep pattern
disturbances |
Personality
changes |
Seizures | Headache | Hearing &
balance problems |
Visual
problems |
Cognition
problems |
|---|---|---|---|---|---|---|---|---|---|---|
|
Mefloquine,
N=6293 (%) |
2 (0.03) | 136 (2.16) | 40 (0.64) | 114 (1.81) | 1 (0.02) | 1 (0.02) | - | - | - | - |
|
Chloroquine,
N=189 (%) |
2 (1.06) | 14 (7.41) | - | - | 1 (0.53) | 1 (0.53) | - | - | - | - |
|
Sulfadoxine,
N=340 (%) |
2 (0.59) | 9 (2.65) | - | - | - | - | 1 (0.29) | - | - | - |
|
Pyrimethamine,
N=340 (%) |
2 (0.59) | 9 (2.65) | - | - | - | - | 1 (0.29) | - | - | - |
|
Artemether,
N=6332 (%) |
- | 123 (1.94) | 40 (0.63) | 114 (1.80) | - | - | - | 150 (2.37) | - | - |
|
Artesunate,
N=6282 (%) |
- | 136 (2.16) | 40 (0.64) | 114 (1.81) | - | - | 16 (0.25) | 8 (0.13) | 1 (0.02) | - |
|
Quinine, N=263
(%) |
- | 8 (3.04) | 10 (3.80) | - | - | 5 (1.90) | 16 (6.08) | 34 (12.93) | 1 (0.38) | - |
|
Lumefantrine,
N=1144 (%) |
- | 123 (10.75) | 40 (3.50) | 114 (9.97) | - | - | - | 150
(13.11) |
- | - |
|
Amodiaquine,
N=227 (%) |
- | - | - | - | - | - | - | - | - | - |
(b) Prophylaxis studies. Mefloquine was associated with mental and neurological manifestations in 8 out of 10 domains investigated, lacking prevalence reports in only motor impairments and seizures. Chloroquine and proguanil reported 6 out of the 10 domains each ( Table 6). Psychiatric/behavioural problems were reported in all the drugs examined except in quinidine and minocycline, with the highest prevalence being in atovaquone (30.7%) followed by pyrimethamine (4.7%). The lowest prevalence of psychiatric symptoms was in sulfadoxine users (0.1%). There were no reports of seizures and/or motor impairments in groups using antimalarial drugs for prophylaxis. The prevalence of mental and neurological manifestations did not differ across categories of dosage (Χ 2=4.65, P=0.460). Table 7 summarizes these findings.
Table 7. Mental and neurological outcomes for individual drugs for prophylaxis studies.
- No observations.
| Drug | Psychiatric/
behavioural |
Mild
neurological perturbations |
Motor
problems |
Sleep
pattern disturbances |
Personality
changes |
Seizures | Headache | Hearing &
balance problems |
Visual
problems |
Cognition
problems |
|---|---|---|---|---|---|---|---|---|---|---|
|
Atovaquone,
N=2670 (%) |
819 (30.67) | 54 (2.02) | - | 179 (6.70) | - | - | 51 (1.91) | - | 24 (0.90) | - |
|
Mefloquine,
N=197959 (%) |
8146 (4.11) | 28699 (14.50) | - | 23630 (11.94) | 9 (0.00) | - | 30697 (15.51) | 4 (0.00) | 14321 (7.235) | 53 (0.03) |
|
Primaquine,
N=155 (%) |
1 (0.65) | - | - | - | - | - | - | - | - | |
|
Pyrimethamine,
N=140773 (%) |
6600 (4.69) | 28094 (19.96) | - | 23280 (16.54) | - | - | 30593
(21.73) |
- | 14297
(10.16) |
- |
|
Chloroquine,
N=180314 (%) |
7985 (4.43) | 28140 (15.61) | - | 22456 (12.45) | - | - | 30593
(16.97) |
- | 14297
(7.93) |
25 (0.01) |
|
Proguanil,
N=180571 (%) |
8060 (4.46) | 28194 (15.61) | - | 22635 (12.54) | - | - | 30644
(16.97) |
- | 14321
(7.93) |
25 (0.01) |
|
Sulfadoxine,
N=1609 (%) |
1 (0.06) | - | - | 885 (55.00) | - | - | - | - | - | - |
|
Quinine, N=160
(%) |
1(0.63) | - | - | - | - | - | - | - | - | - |
|
Dapsone,
N=155 (%) |
1 (0.65) | - | - | - | - | - | - | - | - | - |
|
Doxycycline,
N=36077 (%) |
578 (1.60) | - | - | 8 (0.02) | - | - | - | - | - | - |
|
Quinidine,
N=31(%) |
- | 5 (16.13) | - | 3 (9.68) | - | - | 4 (12.90) | 1 (3.23) | - | - |
|
Minocycline,
N=120 (%) |
- | - | - | - | 58 (48.33) | - | 44 (36.67) | 6 (5.00) | - | 48 (40.00) |
Discussion
The pooled estimates from this study show that the prevalence of mental and neurological manifestations differ with antimalarial drugs, as well as malaria status, of individuals using these drugs. The range of overall prevalence is higher in the absence of acute malaria (0.6–42.7% vs 0.7–55.0%). Similar to previous reports 71, minocycline had the highest prevalence of mental and neurological outcomes (48.3%) and artesunate had the lowest (1.1%). Headaches and dizziness are the most frequent manifestations, and symptoms for psychiatric disorders and cognitive impairment were common with malaria prophylaxis. The number of antimalarial drugs used independently explained the variation in documented overall prevalence. While sulphadoxine and minocycline contributed the highest prevalence of mental and neurological manifestations, mefloquine affected the most domains assessed. These results are based on few human studies (N=51) out of 2,349 abstracts initially identified, suggesting a significant research gap with regards to evaluation of antimalarial drugs for mental and neurological outcomes in humans.
Prevalence and heterogeneity
The pooled prevalence of mental and neurological manifestations is robust and accounts for heterogeneity between studies, unlike descriptive median estimates, which would otherwise underestimate the true prevalence. The pooled estimates compare favourably with those of some randomised studies, although these studies focused on fewer drugs 37, 49. Headaches and dizziness were the most common symptoms, and particularly those on prophylactic treatment, and are often asked for or assessed during studies. Mental and neurological manifestations of the drugs were evaluated in the short-term for most studies (median follow-up, 10 days; IQR, 5–21 days), so this may underestimate prevalence of conditions, such as epilepsy, which take time to develop following neurotoxicity 65, 72, 73. This may explain why some domains, such as seizures and motor impairments, were infrequently documented following prophylaxis. There were no reports of cognition problems in malaria studies probably because neurocognition data during the acute phase of malaria may go undocumented due to misattribution of poor cognition to malaria disease, rather than the drugs used to treat the disease, or perhaps because neuropsychological tests are performed following recovery from the episode of acute malaria. The heterogeneity was greater than 70%, usually considered as the proportion attributable to between studies heterogeneity. The excess heterogeneity may be related to bias from publication, reporting and selection, as supported by some studies plotting outside the funnel outline in the meta-funnel analysis as shown in Figure 3.
Most negative studies may be unpublished, since majority of the eligible studies reported at least one domain of mental and neurological manifestations. There was between study variations in the methods of assessment of mental and neurological manifestations which may have contributed to the heterogeneity of pooled estimates observed during the analysis. This informed our decision to apply the random effect model rather than the fixed effect model during analysis.
Factors explaining variation in prevalence of mental and neurological manifestations
We investigated the contribution of five factors (decided by the authors a priori) to the variation in prevalence of mental and neurological manifestations, and found that an increasing number of drug combinations were associated with mental and neurological manifestations. The five factors investigated only explained 14% of the variability in prevalence, and it is possible the prevalence is in part dependent upon other factors unreported in the included studies. These factors may include levels of endemicity of malaria in the study sites and individual factors such as resistance to malaria, which may vary across the eligible studies. The random effects model that we applied for the meta-analysis allowed effect estimates of mental and neurological manifestations to vary across the sites. While the World Health Organization (WHO) recommends a maximum of two drugs in combination therapies aimed at reducing development of resistance to newer drugs, such as artemesinin derivatives (WHO guidelines for malaria treatment), three drug combinations were common (30%) in many studies identified by this review. Although combinations of antimalarials are used to increase efficacy, prevent transmission and reduce resistance, such combinations may increase levels of mental and neurological manifestations, probably from cumulative toxicity of individual drugs. The number of drugs may also be a surrogate marker of possible neurotoxcity, since the two were highly correlated. There was no evidence of interaction between the number of drugs and malaria status/illness, with regard to prevalence of mental and neurological manifestations. Age group was not associated with prevalence of mental and neurological manifestations, although there were significantly fewer children compared to adults in this analysis, yet children bear the brunt for malaria morbidity and mortality in Africa. Ethical considerations may in part explain fewer children participants in the studies, as early-phase clinical trials usually exclude children. The lack of association with year of study may highlight lack of new studies in recent years compared to earlier years, justifying the need for recognition of the problem through conduct of more future studies.
Mental and neurological manifestations and individual antimalarial drugs
For prophylactic studies, sulfadoxine and minocycline were associated with the highest prevalence of mental and neurological manifestations. For acute malaria studies, hydroxychloroquine was associated with the highest prevalence of mental neurological manifestations. This finding is important as some of these drugs are routinely being used for the treatment of malaria according to WHO recommendations in the guidelines for the treatment of malaria 72, 74, 75. Antibiotics, such as doxycycline and tetracycline, which were associated with some mental and neurological manifestations, are often combined with other drugs in 2 nd line regimens for uncomplicated malaria 76, 77, while clindamycin is used during pregnancy 78, 79. However, it is important to note that data provided on minocycline are based on one study only and, as Dr Grabias and Dr. Remington noted in their reviews on psychiatric effects of malaria and antimalarials, psychiatric reports following tetracycline use in malaria is almost non-existent 74, 75. Artemisinin derivatives are the drugs of choice for malarial treatment when combined with other therapies. Similarly, diaminopyridines, such as pyrimethamine, are combined with artesunate for first line treatment, and are used in pregnancy and prophylaxis (Guidelines for the treatment of malaria, WHO). There is however lack of sufficient studies on their safety to the foetal brain. Some drugs, such as hydroxychloroquine, are no longer routinely recommended by the WHO for use in the management of malaria 80, although they are still widely used in the rheumatology community 81. Although drugs, such as minocycline, are not in routinely used as an antimalarial, they are still used for conditions, such as acne, for which patients on the drug may benefit from evaluation of mental and neurological status. The low prevalence of mental and neurological manifestations with artemether and artesunate are reassuring, since these are the mainstay drugs for the management of falciparum malaria. Also no prospective studies have examined mental and neurological outcomes of artemisinin derivate use alone 75. Reassuringly, very low frequencies were observed for specific domains of neurological manifestation supporting the safety profile of artemether and artesunate. It is however worth noting that this conclusion may be biased since artemether and artesunate are recent drugs for which adverse events are yet to be exhaustively studied. Mefloquine toxicity has been the subject of many case reports 20, 82– 85. Our review however found a relatively low prevalence of mefloquine toxicity. Also, unlike a study published by Weinke and colleagues 86, our study observed higher prevalence in prophylactic use than in studies of acute treatment of malaria. However, the low prevalence from mefloquine may have been caused by the inclusion of the MALPRO observational study 66 in our meta-analysis, which contained a large sample size, although, as with artemisinin derivatives, no large randomized studies exist on psychiatric effects of mefloquine on healthy subjects.
The commonest domain affected by most drugs was mild neurological perturbations (e.g. dizziness) for malaria studies, and psychiatric or behavioural problems for prophylaxis studies. Mefloquine was associated with impairments in more domains investigated than any other antimalarial drug, probably explaining why it has been commonly mentioned in previous reviews 87– 89. Psychiatric manifestations were the most reported outcomes following use of mefloquine from our present study, which is in agreement with previous findings 75. Mild neurological perturbations, such as stupor, were largely contributed by use of pyrimethamine (20%), which is still used for prophylaxis of malaria. Given that pyrimethamine is usually combined with sulphadoxine, which had the highest frequency of manifestations in an individual drug category, individuals on these drugs should be monitored closely and patients counselled appropriately. These findings highlight the importance of focusing on assessment of specific domains of mental and neurological manifestations, rather than the overall prevalence with regards to their association with antimalarial drugs.
Hypotheses of mechanisms of mental and neurological manifestations of antimalarial drugs
Animal studies have indicated that antimalarial drugs commonly affect the hind brain, which contains the reticular formation (controls transitions from sleep to consciousness), and brain stem (has cranial nerves, some innervating the head), perhaps explaining the high prevalence of dizziness and headaches in humans. For instance, mefloquine causes mental and neurological manifestations by disrupting calcium homeostasis of neuronal cells, inhibition of enzymes such as acetylcholinesterase, and blockade of intercellular channels, particularly connexion Cx36, which is a gap junction protein thought to be involved in synchronizing rhythmic activity of neurons in several brain regions 87. Chloroquine may interact with multiple neurotransmitter systems: prostaglandin-E antagonism, acetylcholine imbalance and excess dopamine are among the postulated mechanisms 31. It is possible that the mechanism of mental and neurological manifestations of antimalarials involves a complex interaction of multiple systems and more studies are required to determine the precise mechanisms by which these deleterious effects occur. The process through which anti-malarial drugs cause damage to the brain is not yet clear, but several hypotheses are proposed for specific drugs, as summarized in Table 8.
Table 8. Proposed mechanisms of neurotoxicity.
| Drug | Proposed mechanisms of neurotoxicity |
|---|---|
| Chloroquine | • Sensitize cell-killing effects
90
• Cerebrocortical stimulant that increases EEG frequencies 91 • Prostaglandin E antagonism 92 • Acetylcholinesterase inhibition 93 • Depression of cortical activity 94 • Inhibition of membrane calcium channels 95 • Glucose 6 phosphate dehydrogenase deficiency 92 • Alteration of dopamine levels 93 • Induction of cholinergic imbalance 94 |
| Mefloquine | • Disruption of gap junction communication and GABAergic
interneuron dysfunction 82 • Inhibition of cellular transport 87 • Disrupts direct intercellular electrical communication 96 • Acetylcholinesterase inhibition 42 • Primary hepatocellular injury 97 • Depression of cortical activity 94 |
| Minocycline | • Disrupts microglia distribution in the developing somatosensory cortex
98
• Modifies electrophysiological properties of layer 5 microglia 98 |
| Quinine | • Inhibits cytochrome P450-3A4 99 |
Strengths and limitations
The study focused on all types of antimalarial drugs, which provides empirical basis for evaluating new vs older drugs and safety profiles of antimalarial drugs recommended by the WHO. We have used robust statistical approaches to estimate the overall prevalence, while accounting for potential heterogeneity between studies. Most of the studies are based on hospital data, which may bias results towards severity, especially for low-income countries where rate of hospital use is often low. We may have underestimated prevalence for specific domains, since some studies did not report multiple domains of mental and neurological manifestations. Our results are based on short-term evaluation of mental and neurological manifestations after antimalarial drugs, so it is unclear what the long-term effects are. Some prevalence estimates are based on a small denominator, thus studies with larger sample sizes are required in future. It is difficult to separate the mental and neurological manifestation of malarial disease from that of the drugs used to treat malaria in settings where drugs were not used for prophylaxis. Some prevalence estimates are based on observational studies rather than randomised controlled studies. As a result, it is difficult to appraise the methodology of observational studies. Additionally our meta-analysis found high between study heterogeneity, which we could not account for in our analysis. Further studies need to be done to explain the differences observed between studies. We applied a stringent quality check on all studies based on GRADE for experimental studies and The Joanna Briggs Institute Prevalence Critical Appraisal Tool for observation studies. This quality check may have excluded a number of children studies.
Conclusions
This review suggests that mental and neurological manifestations may occur following antimalarial drug use. Potential adverse effects should be assessed and addressed after use of an antimalarial drugs, particularly following prophylactic use. In addition, the mental and neurological effect of antimalarials is poorly researched; few human studies were identified and most of these were not recent. Efforts to develop new effective and safer antimalarial drugs should be accelerated by scientists and development partners. Pharmacovigilance (Phase IV) studies should be set up to document the long-term effects of antimalarials.
Data availability
The data referenced by this article are under copyright with the following copyright statement: Copyright: © 2017 Bitta MA et al.
The dataset used for this analysis is available on the Open Science Framework: DOI, 10.17605/OSF.IO/2RMCN ( https://osf.io/2rmcn/) 100.
Acknowledgements
I wish to acknowledge Mr. Alex Maina for his assistance in retrieving hard copy articles from the Oxford Library in the UK. This study is published with permission from the director of KEMRI.
Funding Statement
MB, SK and CRJCN are supported by the Wellcome Trust [IDeAL, 107769], [099782] and [083744], respectively.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 2; referees: 2 approved]
Supplementary materials
Supplementary File 1: PRISMA checklist.
Supplementary Table 1: Search strategy. We provided the search strategy used in the Pubmed database.
Supplementary Table 2: Variables for which data were extracted.
References
- 1. Kariuki SM, Ikumi M, Ojal J, et al. : Acute seizures attributable to falciparum malaria in an endemic area on the Kenyan coast. Brain. 2011;134(Pt 5):1519–28. 10.1093/brain/awr051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Carter JA, Ross AJ, Neville BG, et al. : Developmental impairments following severe falciparum malaria in children. Trop Med Int Health. 2005;10(1):3–10. 10.1111/j.1365-3156.2004.01345.x [DOI] [PubMed] [Google Scholar]
- 3. Birbeck GL, Molyneux ME, Kaplan PW, et al. : Blantyre Malaria Project Epilepsy Study (BMPES) of neurological outcomes in retinopathy-positive paediatric cerebral malaria survivors: a prospective cohort study. Lancet Neurol. 2010;9(12):1173–81. 10.1016/S1474-4422(10)70270-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Newton CR, Hien TT, White N: Cerebral malaria. J Neurol Neurosurg Psychiatry. 2000;69(4):433–41. 10.1136/jnnp.69.4.433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beny A, Paz A, Potasman I: Psychiatric problems in returning travelers: features and associations. J Travel Med. 2001;8(5):243–6. 10.2310/7060.2001.24019 [DOI] [PubMed] [Google Scholar]
- 6. Thisted RA, Klaff L, Schwartz SL, et al. : Dextromethorphan and quinidine in adult patients with uncontrolled painful diabetic peripheral neuropathy: a 29-day, multicenter, open-label, dose-escalation study. Clin Ther. 2006;28(10):1607–18. 10.1016/j.clinthera.2006.10.005 [DOI] [PubMed] [Google Scholar]
- 7. van Riemsdijk MM, Sturkenboom MC, Ditters JM, et al. : Atovaquone plus chloroguanide versus mefloquine for malaria prophylaxis: a focus on neuropsychiatric adverse events. Clin Pharmacol Ther. 2002;72(3):294–301. 10.1067/mcp.2002.127113 [DOI] [PubMed] [Google Scholar]
- 8. Atigari OV, Hogan C, Healy D: Doxycycline and suicidality. BMJ Case Rep. 2013;2013: pii: bcr2013200723. 10.1136/bcr-2013-200723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Frey SG, Chelo D, Kinkela MN, et al. : Artesunate-mefloquine combination therapy in acute Plasmodium falciparum malaria in young children: a field study regarding neurological and neuropsychiatric safety. Malar J. 2010;9:291. 10.1186/1475-2875-9-291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pasadhika S, Fishman GA, Choi D, et al. : Selective thinning of the perifoveal inner retina as an early sign of hydroxychloroquine retinal toxicity. Eye (Lond). 2010;24(5):756–62; quiz 63. 10.1038/eye.2010.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nosten F, McGready R, d'Alessandro U, et al. : Antimalarial drugs in pregnancy: a review. Curr Drug Saf. 2006;1(1):1–15. 10.2174/157488606775252584 [DOI] [PubMed] [Google Scholar]
- 12. Brewer TG, Grate SJ, Peggins JO, et al. : Fatal neurotoxicity of arteether and artemether. Am J Trop Med Hyg. 1994;51(3):251–9. [DOI] [PubMed] [Google Scholar]
- 13. Brewer TG, Peggins JO, Grate SJ, et al. : Neurotoxicity in animals due to arteether and artemether. Trans R Soc Trop Med Hyg. 1994;88(Suppl 1):S33–6. 10.1016/0035-9203(94)90469-3 [DOI] [PubMed] [Google Scholar]
- 14. Genovese RF, Newman DB, Petras JM, et al. : Behavioral and neural toxicity of arteether in rats. Pharmacol Biochem Behav. 1998;60(2):449–58. 10.1016/S0091-3057(98)00019-7 [DOI] [PubMed] [Google Scholar]
- 15. Tran TM, Browning J, Dell ML: Psychosis with paranoid delusions after a therapeutic dose of mefloquine: a case report. Malar J. 2006;5:74. 10.1186/1475-2875-5-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Markeljević J, Sarac H, Rados M: Tremor, seizures and psychosis as presenting symptoms in a patient with chronic lyme neuroborreliosis (LNB). Coll Antropol. 2011;35(Suppl 1):313–8. [PubMed] [Google Scholar]
- 17. Rockwell DA: Psychiatric complications with chloroquine and quinacrine. Am J Psychiatry. 1968;124(9):1257–60. 10.1176/ajp.124.9.1257 [DOI] [PubMed] [Google Scholar]
- 18. Panossian LA, Garga NI, Pelletier D: Toxic brainstem encephalopathy after artemisinin treatment for breast cancer. Ann Neurol. 2005;58(5):812–3. 10.1002/ana.20620 [DOI] [PubMed] [Google Scholar]
- 19. Nevin RL: Idiosyncratic quinoline central nervous system toxicity: Historical insights into the chronic neurological sequelae of mefloquine. Int J Parasitol Drugs Drug Resist. 2014;4(2):118–25. 10.1016/j.ijpddr.2014.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Peterson AL, Seegmiller RA, Schindler LS: Severe neuropsychiatric reaction in a deployed military member after prophylactic mefloquine. Case Rep Psychiatry. 2011;2011: 350417. 10.1155/2011/350417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liberati A, Altman DG, Tetzlaff J, et al. : The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sampson M, McGowan J, Cogo E, et al. : An evidence-based practice guideline for the peer review of electronic search strategies. J Clin Epidemiol. 2009;62(9):944–52. 10.1016/j.jclinepi.2008.10.012 [DOI] [PubMed] [Google Scholar]
- 23. Shamseer L, Moher D, Clarke M, et al. : Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349:g7647. 10.1136/bmj.g7647 [DOI] [PubMed] [Google Scholar]
- 24. Munn Z, Moola S, Riitano D, et al. : The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. Int J Health Policy Manag. 2014;3(3):123–8. 10.15171/ijhpm.2014.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guyatt GH, Oxman AD, Schünemann HJ, et al. : GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64(4):380–2. 10.1016/j.jclinepi.2010.09.011 [DOI] [PubMed] [Google Scholar]
- 26. Newcombe RG: Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998;17(8):857–72. [DOI] [PubMed] [Google Scholar]
- 27. Sjöwall J, Ledel A, Ernerudh J, et al. : Doxycycline-mediated effects on persistent symptoms and systemic cytokine responses post-neuroborreliosis: a randomized, prospective, cross-over study. BMC Infect Dis. 2012;12:186. 10.1186/1471-2334-12-186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Scott IU, Jackson GR, Quillen DA, et al. : Effect of doxycycline vs placebo on retinal function and diabetic retinopathy progression in mild to moderate nonproliferative diabetic retinopathy: a randomized proof-of-concept clinical trial. JAMA Ophthalmol. 2014;132(9):1137–42. 10.1001/jamaophthalmol.2014.1422 [DOI] [PubMed] [Google Scholar]
- 29. Wells TS, Smith TC, Smith B, et al. : Mefloquine use and hospitalizations among US service members, 2002–2004. Am J Trop Med Hyg. 2006;74(5):744–9. [PubMed] [Google Scholar]
- 30. van Riemsdijk MM, Sturkenboom MC, Ditters JM, et al. : Low body mass index is associated with an increased risk of neuropsychiatric adverse events and concentration impairment in women on mefloquine. Br J Clin Pharmacol. 2004;57(4):506–12. 10.1046/j.1365-2125.2003.02035.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bhatia MS, Malik SC: Psychiatric complications of chloroquine. Indian J Psychiatry. 1994;36(2):85–7. [PMC free article] [PubMed] [Google Scholar]
- 32. Potasman I, Beny A, Seligmann H: Neuropsychiatric problems in 2,500 long-term young travelers to the tropics. J Travel Med. 2000;7(1):5–9. 10.2310/7060.2000.00002 [DOI] [PubMed] [Google Scholar]
- 33. ter Kuile FO, Nosten F, Luxemburger C, et al. : Mefloquine treatment of acute falciparum malaria: a prospective study of non-serious adverse effects in 3673 patients. Bull World Health Organ. 1995;73(5):631–42. [PMC free article] [PubMed] [Google Scholar]
- 34. Corbett EL, Doherty JP, Behrens RH: Adverse events associated with mefloquine. Study in returned travellers confirms authors' findings. BMJ. 1996;313(7071):1552. 10.1136/bmj.313.7071.1552b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Barrett PJ, Emmins PD, Clarke PD, et al. : Comparison of adverse events associated with use of mefloquine and combination of chloroquine and proguanil as antimalarial prophylaxis: postal and telephone survey of travellers. BMJ. 1996;313(7056):525–8. 10.1136/bmj.313.7056.525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gump DW, Ashikaga T, Fink TJ, et al. : Side effects of minocycline: different dosage regimens. Antimicrob Agents Chemother. 1977;12(5):642–6. 10.1128/AAC.12.5.642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang HL, Xiang YT, Li QY, et al. : The effect of artemether on psychotic symptoms and cognitive impairment in first-episode, antipsychotic drug-naive persons with schizophrenia seropositive to Toxoplasma gondii. J Psychiatr Res. 2014;53:119–24. 10.1016/j.jpsychires.2014.02.016 [DOI] [PubMed] [Google Scholar]
- 38. Schneider C, Adamcova M, Jick SS, et al. : Antimalarial chemoprophylaxis and the risk of neuropsychiatric disorders. Travel Med Infect Dis. 2013;11(2):71–80. 10.1016/j.tmaid.2013.02.008 [DOI] [PubMed] [Google Scholar]
- 39. Biswas PS, Sen D, Majumdar R: Psychosis following chloroquine ingestion: a 10-year comparative study from a malaria-hyperendemic district of India. Gen Hosp Psychiatry. 2014;36(2):181–6. 10.1016/j.genhosppsych.2013.07.012 [DOI] [PubMed] [Google Scholar]
- 40. Sheehy TW, Reba RC, Neff TA, et al. : Supplemental sulfone (dapsone) therapy. Use in treatment of chloroquine-resistant falciparum malaria. Arch Intern Med. 1967;119(6):561–6. 10.1001/archinte.1967.00290240083003 [DOI] [PubMed] [Google Scholar]
- 41. Aarnoudse AL, van Schaik RH, Dieleman J, et al. : MDR1 gene polymorphisms are associated with neuropsychiatric adverse effects of mefloquine. Clin Pharmacol Ther. 2006;80(4):367–74. 10.1016/j.clpt.2006.07.003 [DOI] [PubMed] [Google Scholar]
- 42. van Riemsdijk MM, Ditters JM, Sturkenboom MC, et al. : Neuropsychiatric events during prophylactic use of mefloquine before travelling. Eur J Clin Pharmacol. 2002;58(6):441–5. 10.1007/s00228-002-0492-z [DOI] [PubMed] [Google Scholar]
- 43. Ringqvist A, Bech P, Glenthoj B, et al. : Acute and long-term psychiatric side effects of mefloquine: a follow-up on Danish adverse event reports. Travel Med Infect Dis. 2015;13(1):80–8. 10.1016/j.tmaid.2014.10.021 [DOI] [PubMed] [Google Scholar]
- 44. Loeb MB, Molloy DW, Smieja M, et al. : A randomized, controlled trial of doxycycline and rifampin for patients with Alzheimer's disease. J Am Geriatr Soc. 2004;52(3):381–7. 10.1111/j.1532-5415.2004.52109.x [DOI] [PubMed] [Google Scholar]
- 45. Held T, Trautmann M, Weinke T, et al. : A prospective clinical trial of the treatment of falciparum malaria with mefloquine, with special reference to neuro-psychiatric side effects. Trans R Soc Trop Med Hyg. 1991;85(4):444–5. 10.1016/0035-9203(91)90209-H [DOI] [PubMed] [Google Scholar]
- 46. Adjei GO, Kurtzhals JA, Rodrigues OP, et al. : Amodiaquine-artesunate vs artemether-lumefantrine for uncomplicated malaria in Ghanaian children: a randomized efficacy and safety trial with one year follow-up. Malar J. 2008;7:127. 10.1186/1475-2875-7-127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Briand V, Bottero J, Noël H, et al. : Intermittent treatment for the prevention of malaria during pregnancy in Benin: a randomized, open-label equivalence trial comparing sulfadoxine-pyrimethamine with mefloquine. J Infect Dis. 2009;200(6):991–1001. 10.1086/605474 [DOI] [PubMed] [Google Scholar]
- 48. Van Vugt M, Angus BJ, Price RN, et al. : A case-control auditory evaluation of patients treated with artemisinin derivatives for multidrug-resistant Plasmodium falciparum malaria. Am J Trop Med Hyg. 2000;62(1):65–9. [DOI] [PubMed] [Google Scholar]
- 49. Aceng JR, Byarugaba JS, Tumwine JK: Rectal artemether versus intravenous quinine for the treatment of cerebral malaria in children in Uganda: randomised clinical trial. BMJ. 2005;330(7487):334. 10.1136/bmj.330.7487.334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Adam I, A-Elbasit IE, Idris SM, et al. : A comparison of the efficacy of artesunate plus sulfadoxine-pyrimethamine with that of sulfadoxine-pyrimethamine alone, in the treatment of uncomplicated, Plasmodium falciparum malaria in eastern Sudan. Ann Trop Med Parasitol. 2005;99(5):449–55. 10.1179/136485905X36299 [DOI] [PubMed] [Google Scholar]
- 51. Toovey S, Jamieson A: Audiometric changes associated with the treatment of uncomplicated falciparum malaria with co-artemether. Trans R Soc Trop Med Hyg. 2004;98(5):261–7; discussion 268–9. 10.1016/j.trstmh.2003.11.001 [DOI] [PubMed] [Google Scholar]
- 52. Tange RA, Dreschler WA, Claessen FA, et al. : Ototoxic reactions of quinine in healthy persons and patients with Plasmodium falciparum infection. Auris Nasus Larynx. 1997;24(2):131–6. 10.1016/S0385-8146(96)00031-4 [DOI] [PubMed] [Google Scholar]
- 53. Pasvol G, Newton CR, Winstanley PA, et al. : Quinine treatment of severe falciparum malaria in African children: a randomized comparison of three regimens. Am J Trop Med Hyg. 1991;45(6):702–13. [DOI] [PubMed] [Google Scholar]
- 54. Schlagenhauf P, Tschopp A, Johnson R, et al. : Tolerability of malaria chemoprophylaxis in non-immune travellers to sub-Saharan Africa: multicentre, randomised, double blind, four arm study. BMJ. 2003;327(7423):1078. 10.1136/bmj.327.7423.1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. van Vugt M, Brockman A, Gemperli B, et al. : Randomized comparison of artemether-benflumetol and artesunate-mefloquine in treatment of multidrug-resistant falciparum malaria. Antimicrob Agents Chemother. 1998;42(1):135–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kofi Ekue JM, Ulrich AM, Rwabwogo-Atenyi J, et al. : A double-blind comparative clinical trial of mefloquine and chloroquine in symptomatic falciparum malaria. Bull World Health Organ. 1983;61(4):713–8. [PMC free article] [PubMed] [Google Scholar]
- 57. Price R, van Vugt M, Phaipun L, et al. : Adverse effects in patients with acute falciparum malaria treated with artemisinin derivatives. Am J Trop Med Hyg. 1999;60(4):547–55. [DOI] [PubMed] [Google Scholar]
- 58. Overbosch D, Schilthuis H, Bienzle U, et al. : Atovaquone-proguanil versus mefloquine for malaria prophylaxis in nonimmune travelers: results from a randomized, double-blind study. Clin Infect Dis. 2001;33(7):1015–21. 10.1086/322694 [DOI] [PubMed] [Google Scholar]
- 59. Trinh KA, Nguyen VK, Arnold K, et al. : Double-blind studies with mefloquine alone and in combination with sulfadoxine-pyrimethamine in 120 adults and 120 children with falciparum malaria in Vietnam. Trans R Soc Trop Med Hyg. 1990;84(1):50–3. 10.1016/0035-9203(90)90377-Q [DOI] [PubMed] [Google Scholar]
- 60. Bunnag D, Kanda T, Karbwang J, et al. : Artemether or artesunate followed by mefloquine as a possible treatment for multidrug resistant falciparum malaria. Trans R Soc Trop Med Hyg. 1996;90(4):415–7. 10.1016/S0035-9203(96)90529-5 [DOI] [PubMed] [Google Scholar]
- 61. Denoeud-Ndam L, Clément MC, Briand V, et al. : Tolerability of mefloquine intermittent preventive treatment for malaria in HIV-infected pregnant women in Benin. J Acquir Immune Defic Syndr. 2012;61(1):64–72. 10.1097/QAI.0b013e3182615a58 [DOI] [PubMed] [Google Scholar]
- 62. Harinasuta T, Bunnag D, Lasserre R, et al. : Trials of mefloquine in vivax and of mefloquine plus 'fansidar' in falciparum malaria. Lancet. 1985;1(8434):885–8. 10.1016/S0140-6736(85)91670-8 [DOI] [PubMed] [Google Scholar]
- 63. Mohanty AK, Rath BK, Mohanty R, et al. : Randomized control trial of quinine and artesunate in complicated malaria. Indian J Pediatr. 2004;71(4):291–5. 10.1007/BF02724090 [DOI] [PubMed] [Google Scholar]
- 64. Potasman I, Juven Y, Weller B, et al. : Does mefloquine prophylaxis affect electroencephalographic patterns? Am J Med. 2002;112(2):147–9. 10.1016/S0002-9343(01)01065-8 [DOI] [PubMed] [Google Scholar]
- 65. Rendi-Wagner P, Noedl H, Wernsdorfer WH, et al. : Unexpected frequency, duration and spectrum of adverse events after therapeutic dose of mefloquine in healthy adults. Acta Trop. 2002;81(2):167–73. 10.1016/S0001-706X(01)00210-8 [DOI] [PubMed] [Google Scholar]
- 66. Steffen R, Fuchs E, Schildknecht J, et al. : Mefloquine compared with other malaria chemoprophylactic regimens in tourists visiting east Africa. Lancet. 1993;341(8856):1299–303. 10.1016/0140-6736(93)90814-W [DOI] [PubMed] [Google Scholar]
- 67. van Riemsdijk MM, Sturkenboom MC, Pepplinkhuizen L, et al. : Mefloquine increases the risk of serious psychiatric events during travel abroad: a nationwide case-control study in the Netherlands. J Clin Psychiatry. 2005;66(2):199–204. [DOI] [PubMed] [Google Scholar]
- 68. Meier CR, Wilcock K, Jick SS: The risk of severe depression, psychosis or panic attacks with prophylactic antimalarials. Drug Saf. 2004;27(3):203–13. 10.2165/00002018-200427030-00005 [DOI] [PubMed] [Google Scholar]
- 69. Andersson H, Askling HH, Falck B, et al. : Well-tolerated chemoprophylaxis uniformly prevented Swedish soldiers from Plasmodium falciparum malaria in Liberia, 2004–2006. Mil Med. 2008;173(12):1194–8. 10.7205/MILMED.173.12.1194 [DOI] [PubMed] [Google Scholar]
- 70. Adshead S: The adverse effects of mefloquine in deployed military personnel. J R Nav Med Serv. 2014;100(3):232–7. [PubMed] [Google Scholar]
- 71. Tran TM, Browning J, Dell ML: Psychosis with paranoid delusions after a therapeutic dose of mefloquine: a case report. Malar J. 2006;5(1):74. 10.1186/1475-2875-5-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. WHO: Guidelines for the treatment of malaria.2015. Reference Source [Google Scholar]
- 73. Carter JA, Mung'ala-Odera V, Neville BG, et al. : Persistent neurocognitive impairments associated with severe falciparum malaria in Kenyan children. J Neurol Neurosurg Psychiatry. 2005;76(4):476–81. 10.1136/jnnp.2004.043893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Grabias B, Kumar S: Adverse neuropsychiatric effects of antimalarial drugs. Expert Opin Drug Saf. 2016;15(7):903–10. 10.1080/14740338.2016.1175428 [DOI] [PubMed] [Google Scholar]
- 75. Nevin RL, Croft AM: Psychiatric effects of malaria and anti-malarial drugs: historical and modern perspectives. Malar J. 2016;15:332. 10.1186/s12936-016-1391-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ejaz A, Haqnawaz K, Hussain Z, et al. : Treatment of uncomplicated plasmodium falciparum malaria with quinine-doxycycline combination therapy. J Pak Med Assoc. 2007;57(10):502–5. [PubMed] [Google Scholar]
- 77. Tan KR, Magill AJ, Parise ME, et al. : Doxycycline for malaria chemoprophylaxis and treatment: report from the CDC expert meeting on malaria chemoprophylaxis. Am J Trop Med Hyg. 2011;84(4):517–31. 10.4269/ajtmh.2011.10-0285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Obonyo CO, Juma EA: Clindamycin plus quinine for treating uncomplicated falciparum malaria: a systematic review and meta-analysis. Malar J. 2012;11:2. 10.1186/1475-2875-11-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lell B, Kremsner PG: Clindamycin as an antimalarial drug: review of clinical trials. Antimicrob Agents Chemother. 2002;46(8):2315–20. 10.1128/AAC.46.8.2315-2320.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Guidelines for the Treatment of Malaria. WHO Guidelines Approved by the Guidelines Review Committee. 3rd ed. Geneva,2015. Reference Source [Google Scholar]
- 81. Smolen JS, Landewé R, Breedveld FC, et al. : EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. 2014;73(3):492–509. 10.1136/annrheumdis-2013-204573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Nevin RL: Limbic encephalopathy and central vestibulopathy caused by mefloquine: a case report. Travel Med Infect Dis. 2012;10(3):144–51. 10.1016/j.tmaid.2012.03.006 [DOI] [PubMed] [Google Scholar]
- 83. Nevin RL: Hallucinations and persecutory delusions in mefloquine-associated suicide. Am J Forensic Med Pathol. 2012;33(2):e8. 10.1097/PAF.0b013e31823a8caf [DOI] [PubMed] [Google Scholar]
- 84. Oueriagli Nabih F, Touhami M, Laffinti A, et al. : [Mood disorder after malaria prophylaxis with mefloquine (two case reports)]. Encephale. 2011;37(5):393–6. 10.1016/j.encep.2011.01.013 [DOI] [PubMed] [Google Scholar]
- 85. Thapa R, Biswas B: Childhood mefloquine-induced mania and psychosis: a case report. J Child Neurol. 2009;24(8):1008–9. 10.1177/0883073809332700 [DOI] [PubMed] [Google Scholar]
- 86. Weinke T, Trautmann M, Held T, et al. : Neuropsychiatric side effects after the use of mefloquine. Am J Trop Med Hyg. 1991;45(1):86–91. [DOI] [PubMed] [Google Scholar]
- 87. Schlagenhauf P, Adamcova M, Regep L, et al. : The position of mefloquine as a 21 st century malaria chemoprophylaxis. Malar J. 2010;9:357. 10.1186/1475-2875-9-357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Taylor WR, White NJ: Antimalarial drug toxicity: a review. Drug saf. 2004;27(1):25–61. 10.2165/00002018-200427010-00003 [DOI] [PubMed] [Google Scholar]
- 89. Toovey S: Mefloquine neurotoxicity: a literature review. Travel Med Infect Dis. 2009;7(1):2–6. 10.1016/j.tmaid.2008.12.004 [DOI] [PubMed] [Google Scholar]
- 90. Bogaczewicz J, Sobów T, Bogaczewicz A, et al. : Exacerbations of bipolar disorder triggered by chloroquine in systemic lupus erythematosus--a case report. Lupus. 2014;23(2):188–93. 10.1177/0961203313513818 [DOI] [PubMed] [Google Scholar]
- 91. Good MI, Shader RI: Behavioral toxicity and equivocal suicide associated with chloroquine and its derivatives. Am J Psychiatry. 1977;134(7):798–601. 10.1176/ajp.134.7.798 [DOI] [PubMed] [Google Scholar]
- 92. Garg P, Mody P, Lall KB: Toxic psychosis due to chloroquine--not uncommon in children. Clin Pediatr (Phila). 1990;29(8):448–50. 10.1177/000992289002900806 [DOI] [PubMed] [Google Scholar]
- 93. Mohan D, Mohandas E, Rajat R: Chloroquine psychosis: a chemical psychosis? J Natl Med Assoc. 1981;73(11):1073–6. [PMC free article] [PubMed] [Google Scholar]
- 94. Alisky JM, Chertkova EL, Iczkowski KA: Drug interactions and pharmacogenetic reactions are the basis for chloroquine and mefloquine-induced psychosis. Med Hypotheses. 2006;67(5):1090–4. 10.1016/j.mehy.2006.01.059 [DOI] [PubMed] [Google Scholar]
- 95. Telgt DS, van der Ven AJ, Schimmer B, et al. : Serious psychiatric symptoms after chloroquine treatment following experimental malaria infection. Ann Pharmacother. 2005;39(3):551–4. 10.1345/aph.1E409 [DOI] [PubMed] [Google Scholar]
- 96. Ritchie EC, Block J, Nevin RL: Psychiatric side effects of mefloquine: applications to forensic psychiatry. J Am Acad Psychiatry Law. 2013;41(2):224–35. [PubMed] [Google Scholar]
- 97. Fuller SJ, Naraqi S, Gilessi G: Paranoid psychosis related to mefloquine antimalarial prophylaxis. P N G Med J. 2002;45(3–4):219–21. [PubMed] [Google Scholar]
- 98. Arnoux I, Hoshiko M, Sanz Diez A, et al. : Paradoxical effects of minocycline in the developing mouse somatosensory cortex. Glia. 2014;62(3):399–410. 10.1002/glia.22612 [DOI] [PubMed] [Google Scholar]
- 99. Lim AK, Ho L, Levidiotis V: Quinine-induced renal failure as a result of rhabdomyolysis, haemolytic uraemic syndrome and disseminated intravascular coagulation. Intern Med J. 2006;36(7):465–7. 10.1111/j.1445-5994.2006.01104.x [DOI] [PubMed] [Google Scholar]
- 100. Bitta M: Dataset:Antimalarial drugs and the prevalence of mental and neurological manifestations: A systematic review and meta-analysis.2017. Data Source [DOI] [PMC free article] [PubMed] [Google Scholar]