Abstract
Background
Based on prior research finding the 5HTTLPR L allele associated with increased cardiovascular reactivity to laboratory stressors and increased risk of myocardial infarction, we hypothesized that the 5HTTLPR L allele will be associated with increased blood pressure (BP) and increased hypertension prevalence in 2 large nationally representative samples in the United States and Singapore.
Methods
Logistic regression and linear models tested associations between triallelic (L’S’, based on rs25531) 5HTTLPR genotypes and hypertension severity and mean systolic and diastolic blood pressure (SBP and DBP) collected during the Wave IV survey of the National Longitudinal Study of Adolescent to Adult Health (Add Health, N = 11,815) in 2008–09 and during 2004–07 in 4196 Singaporeans.
Results
In US Whites, L’ allele carriers had higher SBP (0.9 mm Hg, 95% CI = 0.26–1.56) and greater odds (OR = 1.23, 95% CI = 1.10–1.38) of more severe hypertension than those with S’S’ genotypes. In African Americans, L’ carriers had lower mean SBP (−1.27 mm Hg, 95% CI = −2.53 to −0.01) and lower odds (OR = 0.78, 95% CI = 0.65–0.94) of more severe hypertension than those with the S’S’ genotype. In African Americans, those with L’L’ genotypes had lower DBP (−1.13 mm Hg, 95% CI = −2.09 to −0.16) than S’ carriers. In Native Americans, L’ carriers had lower SBP (−6.05 mm Hg, 95% CI = −9.59 to −2.51) and lower odds of hypertension (OR = 0.34, 95% CI = 0.13–0.89) than those with the S’S’ genotype. In Asian/Pacific Islanders those carrying the L’ allele had lower DBP (−1.77 mm Hg, 95% CI = −3.16 to −0.38) and lower odds of hypertension (OR = 0.68, 95% CI = 0.48–0.96) than those with S’S’. In the Singapore sample S’ carriers had higher SBP (3.02 mm Hg, 95% CI = 0.54–5.51) and DBP (1.90 mm Hg, 95% CI = 0.49–3.31) than those with the L’L’ genotype.
Conclusions
These findings suggest that Whites carrying the L’ allele, African Americans and Native Americans with the S’S’ genotype, and Asians carrying the S’ allele will be found to be at higher risk of developing cardiovascular disease and may benefit from preventive measures.
Elevated blood pressure affects about one-third of adults, is a contributor to over 13 million deaths worldwide annually and accounts for about half the global risk for stroke and ischemic heart disease (IHD).1,2 The impact of blood pressure on stroke and IHD risk is not something that is only manifest once a certain threshold – eg, 140 mm Hg for SBP or 90 mm Hg for DBP – is reached; rather, it is proportional across all levels of usual blood pressure down to 115 mm Hg, and it has been estimated that for each 2 mm Hg increase in SBP there is a 10% increase in stroke mortality and a 7% increase in IHD mortality.3
With heritability estimates of 30% to 60%,4,5 and genes with large effects in familial forms of hypertension like salt-sensitivity explaining only a small proportion of hypertension in the general population,6 recent research efforts have been directed toward identifying new genetic variants associated with elevated blood pressure, to identify persons at high risk who may benefit from preventive measures. One approach has used genome-wide association studies (GWASs) to identify novel genetic variants that contribute to blood pressure regulation. Despite robust statistical associations found between single nucleotide polymorphisms (SNPs) and SBP and DBP, they explain only ≤0.3% to ≤1.2% of the variance in SBP,7–9 and it has been estimated that each copy of the risk allele of these SNPs is associated with an increase of about 1 mm Hg in SBP or 0.5 mm Hg in DBP.5 In contrast, candidate gene studies are based on hypotheses that target genes involved in regulation of biological endophenotypes that underlie blood pressure regulation. SNPs on the genes encoding for natriuretic peptide precursor A (NPPA)10 and the β1 adrenergic receptor (ADRB1),11 for example, have been found to be associated with increased SBP and DBP as well as increased hypertension prevalence.
In the present study we take a similar candidate gene approach that builds upon prior research on psychological stress and BP showing that increased BP reactivity to acute mental challenge is prospectively associated with more rapid progression of carotid atherosclerosis,12 especially among persons of low socioeconomic status13 or who work in stressful jobs.14 Larger BP responses to a video game challenge predicted both hypertension and coronary artery calcification in the CARDIA study.15,16 Increased BP reactivity to stress has also been found associated with increased stroke incidence in middle-aged men,17 increased insulin resistance,18 and increased blood lipid levels.19 Strong heritabilities for both BP and heart rate responses to various laboratory stressors20–22 document the role of genetic factors in cardiovascular reactivity (CVR) to stress, and candidate gene studies have found associations between CVR to stress and polymorphisms of genes encoding for angiotensin-converting enzyme,23 the angiotensin II Type I receptor,24 and the β2 adrenergic receptor.25–28
In addition to genes regulating peripheral mechanisms involved in BP control, there is reason to evaluate association of CVR to stress with genes that regulate central nervous system (CNS) serotonin function. The CNS serotonin 5HT1A receptor mediates decreased sympathetic nervous system (SNS) outflow, while the 5HT2C receptor mediates increased SNS outflow in animal models.29 Treatment with selective serotonin inhibitors (SSRIs) is associated with both decreased SNS outflow30 and decreased CVD risk.31 Treatment with the SSRI escitalopram did not affect BP in patients with psychiatric disorders32 or CHD.33 In contrast, acute depletion of CNS serotonin produces increased CVR to mental stress in recovered anxiety disorder patients.34
5HTTLPR is a 43 base pair insertion/deletion polymorphism in the promoter region of SLC6A4 on chromosome 17 that codes for the serotonin transporter. Two variants, long (L) and short (S), have been identified, with the short variant (S) being associated with neuroticism among Whites.35 5HTTLPR has also been associated with a number of negative psychological/biological phenotypes, including depression36 and other emotion-related disorders,37 as well as sleep quality.38
We have previously reported that the more functional 5HTTLPR long (L) allele is associated with both increased baseline BP and CVR to mental stress in both African Americans and Whites.39,40 Consistent with the hypothesis that increased CVR to mental stress in persons carrying the 5HTTLPR L allele will put them at higher risk for the development of CVD, 3 independent case-control studies – 2 in White samples41,42 and one in a Japanese sample43 – found increased risk of myocardial infarction in persons carrying the L allele, even with control for established IHD risk factors. The diallelic L allele has been found associated with increased incidence of a new cardiac event in depressed patients (87.5% white) following coronary bypass surgery44 and another study found the 5HTTLPR L allele is associated with both higher LDL cholesterol levels and a 2-fold higher history of myocardial infarction or stroke in a White sample.45 Based on these findings that the 5HTTLPR is associated with higher baseline BP and reactivity to stress and increased CVD events, we hypothesize that the 5HTTLPR L allele will be associated with increased BP and prevalence of hypertension among population subgroups in a nationally representative sample of 14,299 young adults in the United States46 and a nationally representative sample of 4218 Chinese, Indian and Malay residents of Singapore.47
Testing of this hypothesis will require that we take into account recent findings regarding function of the 5HTTLPR. Hu et al.48 identified an A → G SNP in the high activity L allele at location rs25531 that reduces the transcriptional efficiency of that allele to the level of the S allele, resulting in the triallelic version of 5HTTLPR with S and LG categorized together as S’ (low activity) and LA categorized as L’ (high activity). We have found population differences in the proportion of L alleles carrying the rs25531 G allele with lower levels in non-Hispanic whites (0.12) and Native Americans (0.08) compared to non-Hispanic blacks (0.28) and Asian groups (0.32–0.40).49 The triallelic 5HTTLPR genotype (L’/S’) has been less studied than the original diallelic (L/S) version but is receiving increasing attention as a more informative version of 5HTTLPR. Population differences in the associations of di- and triallelic 5HTTLPR genotype with psychological50 and biological51 phenotypes combine with the reduced transcriptional efficiency of the LG variant to suggest that the di-allelic 5HTTLPR overestimates the proportion of the high activity allele in a study population, resulting in potentially biased genotype–phenotype associations as well as reduced power to detect differences among groups. Using the triallelic 5HTTLPR genotype, we confirmed that the L’ allele is associated with increased CVR to mental stress in blacks and whites.40 Reanalysis of data from our earlier study39 using the triallelic 5HTTLPR genotype (Tables S11–S14 in supplement) confirmed that the L’ allele is significantly associated with higher resting SBP and DBP and SBP reactivity to stress in both blacks and whites. In contrast, however, there was a significant race × 5HTTLPR genotype interaction for DBP reactivity to stress. In whites the L’ allele is significantly associated with higher DBP reactivity, but in blacks DBP reactivity does not differ significantly across all 3 triallelic genotypes.
The foregoing indicates that using the triallelic instantiation of the 5HTTLPR genotypes is preferred for evaluating associations between 5HTTLPR and endophenotypes, as it represents the biological activity of the gene with greater precision compared to the original diallelic representation. In addition, because we have found that associations of 5HTTLPR genotype with an index of CNS serotonin turnover – cerebrospinal fluid levels of the major serotonin metabolite, 5-hydroxy-indoleacetic acid (5HIAA) – are moderated by both race and gender,52 it will also be necessary to test for such moderation in the association between 5HTTLPR genotype and blood pressure.
Methods
Participants
The current study tests the hypothesized associations of 5HTTLPR with blood pressure and hypertension prevalence using data from 2 population cohorts. The US sample is drawn from the National Longitudinal Study of Adolescent to Adult Health (Add Health), a nationally representative sample of ~15,000 young adults that was designed to assess the effects of health-related behaviors during adolescence and into young adulthood. The study was reviewed and approved by the institutional review board at the University of North Carolina-Chapel Hill. Written consent was obtained for all of the data collection. The participants were followed from grades 7 through 12 in 1995 (Wave I) through early adulthood in 2008–09 (Wave IV) in 4 waves of data collection.53 Participants without BP measures, BMI, medication, genotyping, or survey sample weights were excluded from the analyses. The full Add Health sample also includes a subsample of genetically related individuals. For the present analyses, BP was missing on 2.7% and we removed all but the case with the lowest study identification number from a given family cluster, leaving a final sample of N = 11,815, including 6407 Whites, 2562 African Americans, 755 Asian/Pacific Islanders, 1870 Hispanic/Latinos and 221 Native Americans.
The Singapore sample is drawn from 4 cohort studies begun between 1982 and 1998 at study inception.47 Briefly, all 4 studies were a random sample of participants from the Singapore population, with disproportionate sampling stratified by ethnicity to increase the numbers from the minority ethnic groups (Malays and Asian Indians) who participated in a follow-up examination between 2004 and 2007. The Ministry of Home Affairs provided current addresses and phone numbers of participants based on the unique National Registration Identity Card numbers. Participants were contacted either by mail or telephone to obtain an appointment for trained field interviewers to administer the questionnaires at the subjects’ homes. Three home visits were made at 3 different times of the day, including at least one weekend and one weekday, before a participant was deemed non-contactable. All interviewed participants were subsequently invited to attend a clinical examination for additional tests and collection of biological specimens, shortly after the home visit. This study included 4218 participants. Again, participants with missing genotype data, or BP, BMI, medication, age, or gender were excluded, yielding a final sample of 4196, including 2664 Chinese, 708 Indians, and 824 Malays aged 18 and over. Ethics approval was obtained from 2 institutional review boards (the National University of Singapore and the Singapore General Hospital) before study commencement. Informed consent was obtained from all participants before conducting the study.
This research uses data from the National Longitudinal Study of Adolescent to Adult Health, a program project directed by Kathleen Mullan Harris and designed by J. Richard Udry, Peter S. Bearman, and K.M.H. at the University of North Carolina at Chapel Hill, and funded by grant P01 HD31921 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, with cooperative funding from 23 other federal agencies and foundations. Special acknowledgment is due to Ronald R. Rindfuss and Barbara Entwisle for assistance in the original design. Information on how to obtain the National Longitudinal Study of Adolescent Health data files is available on the National Longitudinal Study of Adolescent to Adult Health Web site (http://www.cpc.unc.edu/addhealth). This research was also supported by grant P01 HL36587 from the National Heart, Lung and Blood Institute directed by Redford Williams and by the Duke Behavioral Medicine Research Center. This research was supported by the following grants from the National University of Singapore; R-581-000-117-101, R-581-000-099-101, R-581-000-090-101, R-581-000-083-101 and R-581-000-062-112. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents
SBP, DBP, and antihypertensive medication use
In the Add Health sample certified field interviewers measured respondents’ resting, seated systolic and diastolic blood pressures (in millimeters of mercury)54 during home visits that occurred during the morning, afternoon or evening hours. Time of visit did not differ as a function of 5HTTLPR di- or triallelic genotype in any of the Add Health subgroups After a 5-minute seated rest, 3 serial measurements were performed at 30-second intervals using a factory calibrated, MicroLife BP3MC1-PC-IB oscillometric blood pressure monitor (MicroLife USA, Inc., Dunedin, FL), and SBP and DBP were constructed as the average of measures 2 and 3 and are highly reliable.55 Antihypertensive and antidepressant medication status were assessed at the Wave IV in-home interview. In the Singapore sample, all BP measurements were done in the morning after an overnight fast, with 2 readings of blood pressure taken from participants after 5 minutes resting using an automated blood pressure monitor (Dinamap Pro100V2; Criticon, Norderstedt, Germany). A third reading was performed if the difference between 2 readings of systolic blood pressure was greater than 10 mm Hg or diastolic blood pressure was greater than 5 mm Hg. Mean values of the closest 2 readings were calculated. The inter- and intra-observer coefficients of variation for systolic blood pressure were 0.51–10.20% and 0–2.5% whilst it was 0.41–7.50% and 0–2.5% for diastolic blood pressure. Medications were assessed at the time of blood pressure measurement.
Alcohol, fast food, sweetened drinks intake, exercise, and smoking
Alcohol Consumption in Add Health was defined as follows: 0 = non-drinker; 1 = occasional drinker, drink 2 or fewer days of the week; 2 = light, drink 5–7 days per week and 2 or fewer drinks (1 or fewer if female); 3 = moderate, drink 5–7 days per week, 3 drinks for males, 2 drinks for females; 4 = heavy, drink 5–7 days per week, more than 3 drinks for males, more than 2 for females. Fast food intake was assessed with the item “How many times in the past 7 days did you eat food from a fast food restaurant, such as McDonald’s, Burger King, Wendy’s, Arby’s, Pizza Hut, Taco Bell, or Kentucky Fried Chicken or a local fast food restaurant? The number of times was truncated at 99. Similarly, sweetened drinks were measured with the item “In the past 7 days, how many regular (non-diet) sweetened drinks did you have? Include regular soda, juice drinks, sweetened tea or coffee, energy drinks, flavored water, or other sweetened drinks, again with the number truncated at 99. Exercise was represented by a yes/no variable that assessed regular (on a weekly basis) participation in any bouts of physical activity, such as walking or strenuous sports. Smoking was coded yes/no indicating current daily smoking. The above variables were assessed at Wave IV, concurrent with the SBP measurement.
BMI
Height and weight were measured in both Add Health and Singapore samples. BMI was calculated as BMI = weight (in kilograms)/height (in meters squared) measured at Wave IV.
Genotyping
The assay for 5HTTLPR and the SNP rs25531 are detailed in Haberstick et al.56 for the Add Health sample and in Wray et al.57 for the Singapore sample. The most common S and L alleles contain 14 or 16 repeat units, respectively.58 Extra-long alleles contain 18, 19, 20 and 22 repeat units. For the analysis of the “triallelic 5HTTLPR” we coded the S and LG alleles as S’ and the LA and extra-long alleles as L’ to denote their respective putative activity levels. In order to allow comparison with the triallelic analyses, we also present the results of parallel analyses using the “diallelic” version of 5HTTLPR in supplemental materials. The supplemental analysis of the diallelic 5HTTLPR used 14R alleles as “S” and alleles equal to or greater than 16R as “L”. Note that throughout the remainder of the text we refer to L’ and S’ as “alleles” for the sake of convenience, recognizing that these are actually activity bins and not individual alleles per se. Add Health African Americans were the only subgroup that departed significantly from Hardy–Weinberg equilibrium (HWE, evaluated using the chi-square goodness of fit test available in SAS PROC ALLELE), but only for the diallelic genotypes (P = .007). Overall, of the 15,250 DNA samples we failed to obtain triallelic genotypes on 187. The overall call rate was 15,063/15,250 = 98.77%.
Statistical analysis
Analyses of continuous variables (SBP, DBP) were accomplished utilizing a weighted linear mixed-model in SAS PROC MIXED (SAS Institute, Cary, NC). Initial models were fit simultaneously to the different population subgroups in both Add Health and Singapore samples with an interaction term for the transporter gene, with population subgroup and gender. Subsequent models were fit separately for population subgroups if the gene by subgroup interaction was significant, or with subgroups combined if the interaction was nonsignificant. In both Add Health and Singapore samples covariate control within the model included individual age, gender, BMI, and a dichotomous indicator of current antihypertensive medication use. For models in which population subgroups were combined, the subgroup was included as an additional covariate.
The Add Health sample was a probability sample of adolescents in 7–12th grade in 1995 and sampling weights are provided so that parameter estimates are representative of this national population. In addition, to adjust for the clustered sampling design of Add Health, the model was stratified by the major geographical regions comprising the sample, with school districts within regions specified as random sampling effects. The Singapore study consisted of a random sample and simpler linear models were fitted using SAS PROC GLM. Other than the weighting and stratification parameters, dependent variable modeling was the same in both studies. We performed additional analyses in which tobacco smoking, alcohol, fast food, and sweetened drink intake, exercise, and use of serotonergic specific reuptake inhibitors were included in the models as adjustment covariates; dietary data were not available. Additive (modeling the 3 genotypes in a graded, linear fashion) and dominant (modeling the presence of at least one risk allele versus none) analyses were conducted. Additive models were coded such that the parameter estimate reflected the effect of each additional copy of the L’ allele. The choice of dominant model was based on best model fit: for White, Black, Asian/Pacific Islander, Native America, and Malay L’ carriers were compared to S’ homozygotes; for Hispanic/Latino, Chinese, and Indians S’ carriers were compared to L’ homozygotes. The dominant Model for all Asians combined in the Singapore sample compares S’ Carriers to L’ homozygotes. Type 3 tests were used to evaluate significance of these fixed effects. The G information matrix and covariance parameters were calculated using residual (restricted) maximum likelihood (REML) estimation. SAS Institute’s MIXED procedure provided the software instrumentation. Models used to establish 5HTTLPR genotype relationships to the 4 discrete but ordinal hypertension categories – Normal (SBP <120 mm Hg and DBP <80 mm Hg), Prehypertension (SBP 120–139 mm Hg; or DBP 80–89 mm Hg, Stage 1 Hypertension (SBP 140–159 mm Hg or DBP 90–99 mm Hg) and Stage 2 Hypertension (SBP ≥ 160 mm Hg or DBP ≥ 100 mm Hg)59 – again using age, gender, BMI, and antihypertensive medication as covariates, fit as cumulative risk using SAS LOGISTIC. Both MIXED and LOGISTIC procedures in SAS implement the appropriate sampling weights by normalizing the weight to the size of the analytic sample. Finally, we performed internal model validation by estimating optimism for variance explained and regression slopes derived from 1000 bootstrap resamples using the validate procedure in the rms package in R,
Results
Table I shows the sample statistics for the Add Health and Singapore population subgroups. The Singapore sample was older and had lower BMI levels, higher SBP, hypertension prevalence and percent taking hypertension medications compared to the Add Health sample. Table II displays the frequencies of the triallelic genotypes for each population group in the Add Health and Singapore samples. As we have previously reported,49 the S’S’ genotype is far more frequent in Singapore subgroups and Add Health Asian/Pacific Islanders.
Table I.
Sample statistics by population group
| Add Health Sample | N | Age (SD), years | %Female | BMI (SD), Kg/M2 | SBP (SD), mm Hg | DBP (SD), mm Hg | %HTN | %HTN Medication |
|---|---|---|---|---|---|---|---|---|
| Group | ||||||||
| White | 6407 | 28.9 (1.7) | 53 | 28.5 (6.9) | 124.4 (13.2) | 79.1 (9.9) | 18.0 | 3.4 |
| African American | 2562 | 28.9 (1.8) | 57 | 30.6 (8.2) | 125.7 (14.3) | 79.7 (10.7) | 20.8 | 3.9 |
| Hispanic/Latino | 1870 | 29.3 (1.7) | 53 | 29.7 (7.2) | 123.7 (14.0) | 78.6 (10.3) | 17.5 | 2.1 |
| Asian/Pacific Islander | 755 | 29.4 (1.7) | 49 | 27.4 (6.9) | 124.1 (13.7) | 79.8 (10.5) | 20.3 | 3.2 |
| Native American | 221 | 28.8 (1.7) | 50 | 30.6 (8.7) | 126.0 (13.6) | 80.3 (10.3) | 22.6 | 3.6 |
| Total | 11,815 | 29.0 (1.7) | 53 | 29.1 (7.4) | 124.6 (13.6) | 79.2 (10.2) | 18.7 | 3.3 |
| Singapore Sample | ||||||||
| Chinese | 2664 | 49.4 (11.9) | 57 | 22.9 (3.7) | 130.3 (20.6) | 76.8 (10.8) | 31.8 | 17.0 |
| Indian | 708 | 53.4 (10.3) | 51 | 26.0 (4.8) | 134.9 (21.8) | 79.0 (10.5) | 38.4 | 22.5 |
| Malay | 824 | 49.5 (11.6) | 51 | 26.2 (4.8) | 137.1 (21.0) | 79.7 (10.7) | 41.5 | 7.8 |
| Total | 4196 | 50.1 (11.7) | 55 | 24.0 (4.4) | 132.4 (21.1) | 77.8 (10.8) | 34.8 | 18.1 |
BMI, Body Mass Index; SBP, Systolic Blood Pressure; DBP, Diastolic Blood Pressure; HTN, Met criteria for at least Stage I hypertension (SBP ≥ 140 or DBP ≥ 90); Reported taking antihypertensive medication.
Table II.
Triallelic 5HTTLPR genotype frequencies by population group
| Genotype
|
|||
|---|---|---|---|
| L’L’ | L’S’ | S’S’ | |
| Add Health Sample | |||
| White | 1607 (25.1) | 3191 (49.8) | 1609 (25.1) |
| African American | 705 (27.5) | 1280 (50.0) | 577 (22.5) |
| Hispanic/Latino | 355 (19.0) | 898 (48.0) | 617 (33.0) |
| Asian/Pacific Islander | 32 (4.2) | 248 (32.9) | 475 (62.9) |
| Native American | 50 (22.6) | 114 (51.6) | 57 (25.8) |
| Singapore Sample | |||
| Chinese | 79 (3.0) | 702 (26.4) | 1883 (70.7) |
| Indian | 55 (7.8) | 261 (36.9) | 392 (55.4) |
| Malay | 50 (6.1) | 312 (37.9) | 462 (56.1) |
Values are N (within group %).
The gender × genotype interaction was not significant in any of the initial multiple regression models predicting blood pressure levels or hypertension prevalence and was omitted in later models, though gender was retained as a covariate. In the model testing 5HTTLPR genotype in the Add Health sample controlling for age and gender, the race (African American, White, Hispanic/Latino, Asia/Pacific Islander, and Native American) × genotype interactions predicting SBP and DBP were statistically significant (P < .0001, .007, respectively), indicating that the 5HTTLPR genotype is associated with both SBP and DBP differently across the 5 Add Health population subgroups, which, therefore, need to be evaluated separately. In the Singapore sample, however, race (Chinese, Malay or Indian) × genotype interaction was nonsignificant for both SBP (P = .65) and DBP (P = .29), indicating that there is insufficient evidence to consider the relations between 5HTTLPR genotype and BP as different across the population subgroups. Therefore all 3 population subgroups were combined in analyses of associations of 5HTTLPR with SBP or DBP, including self-reported ethnicity as an additional adjustment variable. We report individual subgroup estimates for the sake of completeness.
Table III shows that in the Add Health Whites, 5HTTLPR genotype was associated with SBP. As we hypothesized, carriers of the L’ allele had mean SBP levels that were about 1 mm Hg higher than those with the S’S’ genotype. In contrast to Whites, among African Americans, L’ carriers had mean SBP levels that were 1.27 mm Hg lower than those with the S’S’ genotype. Among Native Americans the pattern of association between 5HTTLPR genotype and blood pressure was similar to that in African Americans: those carrying the L’ allele had SBP levels that were about 6 mm Hg lower than those with the S’S’ genotype. 5HTTLPR genotypes were not associated with SBP levels in Hispanics or Asian/Pacific Islanders. In the Singapore sample the L’ carriers tended to have lower SBP in all 3 races, though none of the differences within a given group reached statistical significance. In the combined Asian sample this association was significant, with SBP in S’ carriers about 3 mm Hg higher compared to those with the L’L’ genotypes.
Table III.
Systolic blood pressure and 5HTTLPR genotype by population group
| Group | Genotype
|
Additive model
|
Dominant model
|
||||
|---|---|---|---|---|---|---|---|
| L’L’ | L’S’ | S’S’ | Regression estimate (mm Hg) | P | Regression estimate (mm Hg) | P | |
| Add Health Sample | |||||||
| White | 126.11 (125.12,127.10) | 126.16 (125.27,127.05) | 125.23 (124.24,126.23) | 0.44 (0.04,0.84) | .032 | 0.91 (0.26,1.56) | .006 |
| African American | 129.51 (127.73,131.29) | 128.93 (127.25,130.61) | 130.40 (128.53,132.26) | −0.39 (−1.13,0.36) | .311 | −1.27 (−2.53, −0.01) | .048 |
| Hispanic/Latino | 125.77 (123.49,128.05) | 126.96 (124.90,129.01) | 126.69 (124.60,128.79) | −0.34 (−1.10, 0.42) | .379 | 1.07 (−0.34, 2.48) | .135 |
| Asian/Pacific Islander | 127.88 (122.46,133.29) | 129.65 (126.19,133.12) | 129.73 (126.34,133.11) | −0.38 (−1.84,1.09) | .614 | −0.23 (−1.91, 1.46) | .791 |
| Native American | 129.68 (124.22,135.15) | 126.90 (122.40,131.40) | 133.87 (128.60,139.13) | −2.16 (−4.40,0.75) | .058 | −6.05 (−9.59, −2.51) | .001 |
| Singapore sample | |||||||
| Chinese | 130.73 (127.13,134.33) | 134.21 (132.88,135.54) | 134.26 (133.32,135.21) | 0.67 (−0.49, 1.83) | .259 | 3.52 (−0.02, 7.12) | .055 |
| Indian | 134.93 (129.99,139.88) | 139.77 (137.34,142.20) | 138.55 (136.48,140.61) | 0.50 (−1.62, 2.62) | .646 | 4.10 (−0.94, 9.13) | .111 |
| Malay | 139.78 (134.90,144.66) | 139.75 (137.56,141.93) | 140.84 (138.95,142.73) | 0.82 (−1.10, 2.74) | .403 | −1.09 (−3.45, 1.27) | .364 |
| All Asians combined (Singapore sample only) | 133.38 (130.90,135.85) | 136.30 (135.23,137.37) | 136.41 (135.52,137.30) | 0.72 (−0.18, 1.62) | .117 | 2.99 (0.51, 5.48) | .018 |
Values in row with group name are point estimates. Values in parentheses in second row of each group are 95% confidence intervals.
For Add Health sample, estimates are derived from a mixed model adjusting for age, BMI, gender, and antihypertensive medication use, incorporating sample design parameters.
Estimates for the Singapore sample are derived from a general linear model with the same covariates used in the Add Health sample.
Additive models test for linear trend across 0, 1, or 2 copies of L’. Dominant model for White, Black, Asian/Pacific Islander, Native America, and Malay compares L’ carriers to S’ homozygotes. Dominant model for Hispanic/Latino, Chinese, and Indian compares S’ carriers to L’ homozygotes. Dominant Model for all Asians Combined in Singapore sample compares S’ Carriers to L’ homozygotes.
The pattern of associations between the 5HTTLPR genotype and DBP (Table IV) differed from that found for SBP. In Add Health Whites the 5HTTLPR genotype was not associated with DBP, but in the African Americans, L’ carriers had a little over 1 mm Hg lower DBP compared to S’ homozygotes. Similarly, among Asians/Pacific Islanders, each copy of the L’ allele was associated with about 1.6 mm Hg lower DBP. 5HTTLPR associations with DBP were weaker in the remaining Add Health subgroups. The pattern of 5HTTLPR associations with DBP in the Singapore subgroups and combined Asian sample was similar to that for SBP. Chinese S’ carriers had DBP that was about 2.5 mm Hg higher than that in those with the L’L’ genotype. Among the Malay subsample, an S’ dominant model was not statistically significant, but an L’ dominant model did show a statistically significant genetic effect, with S’ homozygotes exhibiting about 1.4 mm Hg higher DBP compared to L’ carriers. Finally, in the combined Asian sample S’ carriers had DBP that was 1.9 mm Hg higher than those with the L’L’ genotype. Internal model validation using bootstrap resampling suggested a range of very small to modest optimism bias in model variance explained and regression slopes, from about 0.05% bias in slope estimates for Whites to about 8% for Native Americans.
Table IV.
Diastolic blood pressure and 5HTTLPR genotype by population group
| Group | Genotype
|
Additive model
|
Dominant model
|
||||
|---|---|---|---|---|---|---|---|
| L’L’ | L’S’ | S’S’ | Regression estimate (95%CL) | P | Regression estimate (95%CL) | P | |
| Add Health sample | |||||||
| White | 80.22 (79.45,81.00) | 80.56 (79.87,81.26) | 80.32 (79.54,81.10) | −0.05 (−0.37, 0.27) | .771 | 0.13 (−0.39,0.69) | .615 |
| African American | 81.32 (79.91,82.72) | 82.36 (81.04,83.69) | 83.13 (81.67,84.60) | −0.92 (−1.49, −0.35) | .002 | −1.13 (−2.09, −0.16) | .022 |
| Hispanic/Latino | 79.88 (78.05,81.72) | 80.49 (78.83,82.14) | 80.84 (79.16,82.53) | −0.46 (−1.06, 0.15) | .138 | −0.52 (−1.42, 0.37) | .188 |
| Asian/Pacific Islander | 81.08 (76.71,85.46) | 82.59 (79.91,85.27) | 84.23 (81.61,86.86) | −1.62 (−2.83, −0.41) | .009 | −1.77 (−3.16, −0.38) | .012 |
| Native American | 84.33 (79.74,88.91) | 80.47 (76.69,84.25) | 84.61 (80.19,89.03) | −0.20 (−2.08, 1.67) | .831 | −2.86 (−5.86, 0.14) | .061 |
| Singapore Sample | |||||||
| Chinese | 76.04 (73.95,78.13) | 78.71 (77.94,79.48) | 78.41 (77.87,78.96) | 0.23 (−0.44, 0.90) | .498 | 2.46 (0.37, 4.54) | .021 |
| Indian | 78.95 (76.28,81.62) | 80.42 (79.11,81.73) | 80.39 (79.28,81.51) | 0.40 (−0.75, 1.54) | .496 | 1.45 (−1.26, 4.21) | .293 |
| Malay | 79.94 (77.12,82.76) | 80.18 (78.91,81.44) | 81.53 (80.44,82.63) | 1.08 (−0.03, 2.19) | .056 | −1.39 (−2.75, −0.03) | .046 |
| All Asians Combined (Singapore sample only) | 77.42 (76.01,78.84) | 79.26 (78.65,79.87) | 79.34 (78.83,79.85) | 0.46 (−0.06, 0.97) | .081 | 1.89 (0.47, 3.31) | .009 |
Values in row with group name are point estimates. Values in parentheses in second row of each group are 95% confidence intervals.
For Add Health sample, estimates are derived from a mixed model adjusting for age, BMI, gender, and antihypertensive medication use, incorporating sample design parameters.
Estimates for the Singapore sample are derived from a general linear model with the same covariates used in the Add Health sample.
Additive models test for linear trend across 0, 1, or 2 copies of L’. Dominant model for White, Black, Asian/Pacific Islander, Native America, and Malay compares L’ carriers to S’ homozygotes. Dominant model for Hispanic/Latino, Chinese, and Indian compares S’ carriers to L’ homozygotes. Dominant Model for all Asians Combined in Singapore sample compares S’ Carriers to L’ homozygotes.
Results using multiple logistic regression to assess the associations of 5HTTLPR genotypes with the 4 ordinal hypertension categories followed a pattern similar to those using continuous SBP and DBP. Table V displays the odds ratios and 95% confidence intervals for the additive and dominant model effects, adjusted for gender, age, BMI, and antihypertensive medications. Among Whites there was a 23% increase in the odds of Stage I or II hypertension in participants carrying the L’ allele compared to their S’S’ counterparts. In contrast, among African Americans, the odds of a more severe hypertension classification decreased about 15% for each copy of the L’ allele; African Americans carrying the L’ allele had a 22% decrease in odds of Stage I or II hypertension compared to those with the S’S’ genotype. Among Native Americans, those carrying the L’ allele had a 66% decrease in odds for Stage I or II hypertension compared to those with S’S’ genotype. Among Asian/Pacific Islanders, those carrying the L’ allele had 32% lower odds for Stage I or II hypertension than those with S’S’ genotype. Among the Singapore combined sample there is a nonsignificant 38% increase in the odds of Stage I or II hypertension in those carrying the S’ allele compared to those with the L’L’ genotype.
Table V.
Odds of increased severity of hypertension stage by triallelic 5HTTLPR genotype and population group
| Group | Odds ratio
|
P
|
||
|---|---|---|---|---|
| Additive | Dominant | Additive | Dominant | |
| Add Health sample | ||||
| White | 1.11a (1.04–1.19)b | 1.23 (1.10–1.38) | .003 | .0003 |
| African American | 0.85 (0.76–0.96) | 0.78 (0.65–0.94) | .006 | .010 |
| Hispanic | 1.06 (0.92–1.21) | 0.97 (0.75–1.26) | .429 | .838 |
| Asian/Pacific Islander | 0.71 (0.53–0.96) | 0.68 (0.48–0.96) | .024 | .029 |
| Native American | 0.73 (0.41–1.29) | 0.34 (0.13–0.89) | .276 | .028 |
| Singapore sample | ||||
| Chinese | 1.09 (0.95–1.25) | 1.51 (0.97–2.35) | .218 | .067 |
| Indian | 1.023 (0.829–1.275) | 1.17 (0.70–1.96) | .803 | .548 |
| Malay | 1.11 (0.90–1.37) | 0.87 (0.67–1.13) | .346 | .301 |
| All Asians Combined (Singapore sample only) | 1.09 (0.98–1.20) | 1.38 (0.98–1.72) | .052 | .068 |
Estimates are derived from a cumulative logistic regression model predicting hypertension status adjusting for age, BMI, gender, and antihypertensive medication, with sample design parameters for the Add Health sample as described earlier. Hypertension status was modeled as 4 ordered categories: Normal, Prehypertension, Stage I Hypertension, and Stage 2 Hypertension.
Additive models test for linear trend across 0, 1, or 2 copies of L’. Dominant model for White, Black, Asian/Pacific Islander, Native America, and Malay compares L’ carriers to S’ homozygotes. Dominant model for Hispanic/Latino, Chinese and Indian compares S’ carriers to L’ homozygotes. Dominant Model for All Asians Combined in Singapore sample compares S’ Carriers to L’ homozygotes.
Within-group mean.
95% Confidence Interval.
Additional, statistical adjustment for smoking status, alcohol, fast food, and sweetened drink intake, exercise, and use of serotonergic specific reuptake inhibitors made very little difference to the above findings with the following 2 exceptions, both in the Add Health sample. In the Native American group, the genotype effect in the additive model for SBP became statistically significant (P = .028), as did the dominant model for DBP (P = .041). These additional adjustment variables were not available for analysis in the Singapore sample. Finally, a parallel set of analyses is reported in the supplemental material using the diallelic genotype. Briefly, the pattern of associations was similar but generally weaker compared to the triallelic results, with associations detected for Whites and Native Americans for SBP (Table S2), and Whites and Asian/Pacific Islanders for hypertension stage (Table S4). As reported in the supplement, unadjusted triallelic and diallelic results are also generally weaker.
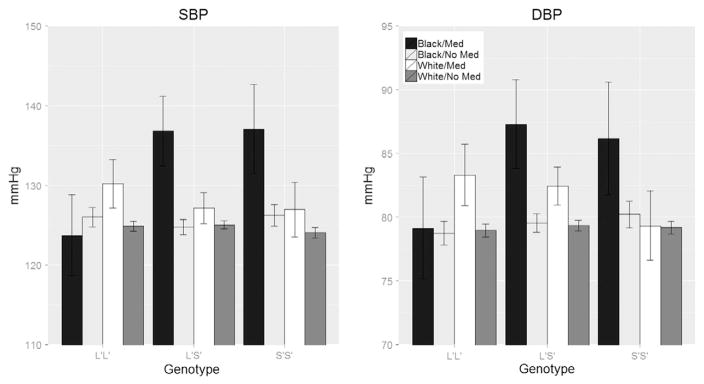
It is possible that differences between Whites and African Americans in blood pressure as a function of 5HTTLPR genotype might be due to racial differences in hypertension treatment and control.60,61 Therefore, in addition to adjusting for hypertension medication use, as was done in the original analyses, we also evaluated the frequency of antihypertensive medication use in Whites and African Americans in the Add Health sample. A higher proportion of African Americans reported using hypertensive medication compared to Whites (3.9% vs 3.4%). This difference, however, was not statistically significant (P = .19). We further examined whether the association between genotype and BP differed across African Americans and Whites as a function of hypertensive medication by testing a 3-way race by genotype by medication interaction. The 3-way interaction term was statistically significant for both SBP (P < .001) and DBP (P = .007). We further tested a 2-way medication by genotype interaction within each race. Among African Americans the 2-way interaction was significant for SBP (P < .001) and DBP (P = .012). Among Whites, however, the same 2-way interaction was not statistically significant for SBP (P = .244) or DBP (P = .091). The form of the interaction is depicted in Figure 1. Among those with the L’L’ genotype, Whites on hypertensive medications have a higher SBP (+6 mm Hg) and DBP (+4 mm Hg) than those not on medications; in African American L’L’s the differences in SBP and DBP between medicated and non-medicated participants were within 1–2 mm Hg. Among Whites carrying the S’ allele, medicated participants had higher SBP than those not taking medication (2 mm Hg among L’S’ and 3 mm Hg among S’S’), and higher DBP (3 mm Hg among L’S’ and 0.1 mm Hg among S’S’). Those difference were far larger among African Americans for both SBP (12 mm Hg among L’S’, 11 mm Hg among S’S’) and DBP (8 mm Hg among L’S’ and 6 mm Hg among S’S’). Similar to the results for the continuous BP models, internal validation using bootstrap resampling suggested a range of very small to modest optimism bias in model variance explained and regression slopes, from about 0.02% bias in slope estimates for Whites to about 7% for Native Americans.
Figure 1.
Blood pressure by triallelic HTTLPR genotype, race, and antihypertensive medication status in the Add Health sample. Values are predicted estimates from a mixed model adjusted for age, gender, and BMI. Error bars represent 95% confidence limits.
Discussion
These results support, in Add Health Whites only, our hypothesis that the 5HTTLPR L allele will be associated with increased SBP level and hypertension prevalence. This is consistent with prior research showing increased MI risk associated with L genotypes in 4 White samples41,42,44,45 and suggests – not surprisingly, given the low proportion of the rs25531 G allele in L alleles among Whites49 – that this increased risk will be present in Whites using either diallelic or triallelic 5HTTLPR genotypes. It is also consistent with recent research suggesting that “substantial overlap exists between genes that influence BP measured in the office, under laboratory stress and during real life.”62 We detected no association with DBP in Whites.
In African Americans and Native Americans, however, the pattern of associations between 5HTTLPR and both blood pressure and hypertension prevalence was quite different, with the L’ alleles being associated with lower SBP levels and hypertension prevalence. A similar pattern is seen for DBP in Singaporean Chinese and combined Asian samples, where S’ allele carriers have higher DBP. These opposite effects of 5HTTLPR genotypes on BP levels and hypertension prevalence could reflect a difference in effects of the rs25531 genotype on blood pressure in African Americans, Native Americans and Asians compared to Whites. Among Whites, in whom a small proportion convert from L to S’,49 diallelic and triallelic genotypes have virtually identical associations with SBP, DBP and hypertension prevalence (Tables II, III and IV). Among African Americans and Asians (but not Native Americans), a much larger proportion converts from L to S’.49 Among African Americans, S’S’ genotype was associated with an opposite and larger (roughly 1 mm Hg higher vs a little more than 1 mm Hg lower) effect on SBP than in Whites. A similar pattern is seen among the Singaporean sample, especially the Chinese, in whom the S’ allele carriers have higher SBP and DBP levels than those with the L’L’ genotype. Also noteworthy is the 4–6 mm Hg lower SBP in Native American L’ allele carriers compared to those with the S’S’ genotype – a pattern similar to that seen in African Americans and Asians, possibly reflecting the Asian origin of Native Americans. The higher SBP and DBP observed among S’ carriers in the Add Health Hispanic/Latinos could be a reflection of the substantial proportion of persons of Native American background in this subgroup.63
There are several biologically plausible mechanisms that could account for the differing patterns of 5HTTLPR associations with SBP and DBP and hypertension prevalence in Whites vs other population groups. The opposite direction of association we found between 5HTTLPR SS genotype and cerebrospinal fluid levels of the major serotonin metabolite 5-hydroxy-indoleacetic acid in Whites and African Americans52 suggests the possibility that opposite effects of 5HTTLPR SS genotype on brain serotonin function could be playing a role in the population differences in 5HTTLPR associations with BP and hypertension. The increased DBP in African Americans carrying S’ alleles could be a function of the increased role of sympathetic nervous system (SNS) effects on total peripheral resistance that have been reported in African Americans64–67 and is consistent with evidence suggesting that genetic pathways underlying blood pressure variation may “differentially influence SBP and DBP”.68 The associations of 5HTTLPR genotypes with SBP, DBP, and hypertension prevalence reported here were found even with control for BMI, increasing the likelihood that effects on SNS outflow are involved. Clearly further research will be required to identify the mechanisms underlying the current findings of population differences in the association of 5HTTLPR with BP and hypertension incidence.
Whatever mechanisms are eventually found to account for the remarkably different effects of 5HTTLPR genotype on SBP, DBP and hypertension prevalence in African Americans, Native Americans, Asians and Whites, there is considerable evidence documenting population differences in patterns of linkage disequilibrium (LD) between rs25531 and other sites on the serotonin transporter gene that are good candidates to be involved in those mechanisms. There is evidence, for example, that 5HTTLPR and rs25531 LD with other sites on the 5HTT gene differs in African Americans compared to Whites.50,51,69 Directly documenting potential clinical implications of such LD pattern differences, Gelernter et al.70 found that White Americans with one or 2 copies of the 5HTTLPR S allele have a higher neuroticism score than those homozygous for the L allele. In contrast, among African Americans S allele carriers had lower neuroticism scores than those homozygous for the L allele. Murdoch et al.71 found significant variation across populations in rs25531 distributions and caution that association studies of such serotonin transporter gene variants cannot be applied a priori across populations. Directly relevant to the current study’s findings, Gong and Hubner72 note that genetic variants found associated with hypertension in White samples have not been found associated with hypertension in African Americans, leading them to conclude, “The genetic factors that contribute to hypertension are likely to be different among different ethnic populations.” Our finding that the S’S’ genotype is associated with higher SBP in African Americans, Native Americans and Asians (S’ carriers) but lower SBP in Whites provides an example of 5HTTLPR genotypes having opposite effects on another endophenotype, SBP, in Whites compared to other population groups. Definitive identification of the mechanism(s) responsible for these opposite effects of the triallelic 5HTTLPR genotypes in Whites and other population groups will probably ultimately require sequencing of the 5HTT gene in the population groups we report here, documenting different LD of L’ (LA) with other sites in these groups and showing that L’ is in LD with other sites on the genes that are responsible for the population differences we found in 5HTTLPR genotype effects on blood pressure and hypertension prevalence.
The magnitude of 5HTTLPR associations with SBP (1 mm Hg in Whites, 1.3 mm Hg in African Americans, and 6 mm Hg in Native Americans) and DBP (2.0 mm Hg in African Americans and Asians) is comparable to, or larger than, those from GWASs that have found SNP risk alleles with genome-wide significance associated with increases of ~1 mm Hg in SBP and ~0.5 mm Hg in DBP.9 Given estimates that for each 2 mm Hg increase in SBP there is a 10% increase in stroke mortality and a 7% increase in IHD mortality,3 the potentially causal effects of 5HTTLPR genotype on SBP found in this study may have clinically significant implications – perhaps greater among African Americans, Native Americans and Asians than Whites – for public health. More specifically, these findings suggest that higher BP levels in Whites carrying the L or L’ allele, and African Americans, and Native Americans, with the S’S’ genotype and Asian S’ carriers will place them at increased risk of developing CVD and/ or suffering clinical events. This hypothesis is supported by the current study’s findings that the L’ allele is associated with a 23% increase in the odds of more severe hypertension among Whites but a 22% decrease in the odds of hypertension among Add Health black participants and a 64% decrease in Native Americans.
Based on prior research showing lower treatment success among African Americans than Whites,60,61 we explored whether the different patterns of association between 5HTTLPR and BP between Whites and African Americans might be explained by race differences in the use of and/or response to antihypertension medication. Given that the initial analyses adjusted for medication use and the relatively small and nonsignificant race difference in the proportion of participants using medication, medication use per se is not a likely explanation for the observed race differences in the association between the 5HTTLPR genotype and BP. In exploring this issue more deeply, however, we did find that the differences in BP between genotypes varied as a function of medication status, and that the pattern of this dependence differed across Whites and African Americans. Specifically, although medicated participants had higher SBP and DBP compared to non-medicated participants regardless of genotype, the differences were much larger among S’ carriers (Figure 1). In turn, these medication differences among S’ carriers were far greater among African Americans compared to Whites. Given that we cannot know what the BPs of the medicated participants would be when not on medication, we cannot ascribe this pattern to medication per se. However, this pattern does at least suggest that the positive association between the S’ allele and BP in medicated persons may be stronger among African Americans than Whites. More speculatively, this finding suggests that reduced responsiveness to antihypertensive medication among African American S’ carriers could be one mechanism accounting for previous observations60,61 of reduced treatment success among hypertensive African Americans compared to Whites. Further investigation will be necessary to clarify this issue.
A common concern for association studies among admixed populations is the unaccounted correlation between genetic variants and phenotypes. In the current study’s samples we observed genotype frequency differences among the racial groups,49 which could be suggestive of population stratification. In this study, by including the rs25531 data, we believe we have attenuated the potential for spurious correlation between 5HTTLPR and BP as suggested by equal genotype frequencies among groups in the adjusted data. Observed population differences in frequency of the rs25531 G allele49,51 combine with the current study’s finding that associations between 5HTTLPR genotypes and blood pressure levels and hypertension prevalence differ in both direction and effect size as a function of di- vs triallelic genotypes in these same population subgroups to make a strong case that studies evaluating associations of 5HTTLPR with various phenotypes need to include rs25531 in their assays of 5HTTLPR. To ignore this SNP is to run a serious risk of biasing associations obtained and quite likely missing important associations that are masked by inclusion of LG as high activity. The present findings suggest that the extent of bias is likely to be particularly important when assessing associations in non-White and, particularly, Asian populations, where the proportion of L alleles containing the rs25531 G allele is markedly higher. We also note that ancestry markers were not available in either dataset. We therefore cannot rule out the possibility of confounding due to unmeasured population stratification, or more broadly, other unobserved heterogeneity.
In conclusion, the present results suggest that the 5HTTLPR L and L’ alleles in Whites and the S’ allele in African Americans, Native Americans and Asians will be associated with increased BP levels and hence increased CVD risk. One implication of these findings is that persons carrying the high risk allele for their population group might benefit from interventions to reduce its impact on BP and associated increased CVD risk. The observation that SSRI treatment is associated with decreased SNS outflow31 suggests, for example, that SSRIs might reduce risk in Whites carrying the L or L’ allele and in African Americans, Native Americans and Asians carrying the S’ allele. Another approach that might be considered is training in stress management. Bishop et al.73 found, for example, that group training in cognitive behavioral stress management strategies among Chinese, Indian and Malay CHD patients in Singapore produced significant reductions in SBP at rest and during anger recall stress testing.
Appendix. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ahj.2016.12.013.
Footnotes
Author disclosures
Redford Williams holds a US patent on 5HTTLPR L allele as risk marker for CVD in persons exposed to chronic stress. Redford Williams is a founder and major stockholder in Williams LifeSkills, Inc, a company that develops, tests and markets behavioral products for stress and anger management.
References
- 1.Fields LE, Burt VL, Cutler JA, et al. The burden of adult hypertension in the United States 1999 to 2000: a rising tide. Hypertension. 2004;44:398–404. doi: 10.1161/01.HYP.0000142248.54761.56. [DOI] [PubMed] [Google Scholar]
- 2.Lawes CM, Vander Hoorn S, Rodgers A. Global burden of blood pressure-related disease, 2001. Lancet. 2008;371:1513–8. doi: 10.1016/S0140-6736(08)60655-8. [DOI] [PubMed] [Google Scholar]
- 3.Lewington S, Clarke R, Qizilbash N, et al. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Prospective Studies Collaboration. Lancet. 2002;360:1903–13. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 4.Levy D, DeStefano AL, Larson MG, et al. Evidence for a gene influencing blood pressure on chromosome 17. Genome scan linkage results for longitudinal blood pressure phenotypes in subjects from the Framingham heart study. Hypertension. 2000;36:477–83. doi: 10.1161/01.hyp.36.4.477. [DOI] [PubMed] [Google Scholar]
- 5.Ehret GB. Genome-wide association studies: contribution of genomics to understanding blood pressure and essential hypertension. Curr Hypertens Rep. 2010;12:17–25. doi: 10.1007/s11906-009-0086-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vehaskari VM. Heritable forms of hypertension. Pediatr Nephrol. 2009;24:1929–37. doi: 10.1007/s00467-007-0537-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levy D, Ehret GB, Rice K, et al. Genome-wide association study of blood pressure and hypertension. Nat Genet. 2009;41:677–87. doi: 10.1038/ng.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newton-Cheh C, Johnson T, Gateva V, et al. Genome-wide association study identifies eight loci associated with blood pressure. Nat Genet. 2009;41:666–76. doi: 10.1038/ng.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taal HR, Verwoert GC, Demirkan A, et al. Genome-wide profiling of blood pressure in adults and children. Hypertension. 2011;49:241–7. doi: 10.1161/HYPERTENSIONAHA.111.179481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newton-Cheh C, Larson MG, Vasan RS, et al. Association of common variants in NPPA and NPPB with circulating natriuretic peptides and blood pressure. Nat Genet. 2009;41:348–53. doi: 10.1038/ng.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson AD, Newton-Cheh C, Chasman DI, et al. Cohorts for Heart and Aging Research in Genomic Epidemiology Consortium; Global BPgen Consortium; Women’s Genome Health Study. Association of hypertension drug target genes with blood pressure and hypertension in 86,588 individuals. Hypertension. 2011;57:903–10. doi: 10.1161/HYPERTENSIONAHA.110.158667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnett PA, Spence JD, Manuck SB, et al. Psychological stress and the progression of carotid artery disease. J Hypertens. 1997;15:49–55. doi: 10.1097/00004872-199715010-00004. [DOI] [PubMed] [Google Scholar]
- 13.Lynch JW, Everson SA, Kaplan GA, et al. Does low socioeconomic status potentiate the effects of heightened cardiovascular response to stress on the progression of carotid atherosclerosis? Am J Public Health. 1998;88:389–94. doi: 10.2105/ajph.88.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Everson SA, Lynch JW, Chesney MA, et al. Interaction of workplace demands and cardiovascular reactivity in progression of carotid atherosclerosis: a population based study. BMJ. 1997;314:553–61. doi: 10.1136/bmj.314.7080.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matthews KA, Katholi CR, McCreath H, et al. Blood pressure reactivity to psychological stress predicts hypertension in the CARDIA study. Circulation. 2004;110:74–8. doi: 10.1161/01.CIR.0000133415.37578.E4. [DOI] [PubMed] [Google Scholar]
- 16.Matthews KA, Zhu S, Tucker DC, et al. Blood pressure reactivity to psychological stress and coronary calcification in the coronary artery risk development in young adults study. Hypertension. 2006;47:391–5. doi: 10.1161/01.HYP.0000200713.44895.38. [DOI] [PubMed] [Google Scholar]
- 17.Everson SA, Lynch JW, Kaplan GA, et al. Stress-induced blood pressure reactivity and incident stroke in middle-aged men. Stroke. 2001;32:1263–70. doi: 10.1161/01.str.32.6.1263. [DOI] [PubMed] [Google Scholar]
- 18.Moan A, Nordby G, Rostrup M, et al. Insulin sensitivity, sympathetic activity, and cardiovascular reactivity in young men. Am J Hypertens. 1995;8:268–75. doi: 10.1016/0895-7061(94)00206-Q. [DOI] [PubMed] [Google Scholar]
- 19.Burker EJ, Fredrikson M, Rifai N, et al. Serum lipids, neuroendocrine, and cardiovascular responses to stress in men and women with mild hypertension. Behav Med. 1994;19:155–61. doi: 10.1080/08964289.1994.9935186. [DOI] [PubMed] [Google Scholar]
- 20.Boomsma DI, Snieder H, de Geus EJ, et al. Heritability of blood pressure increases during mental stress. Twin Res. 1998;1:15–24. doi: 10.1375/136905298320566447. [DOI] [PubMed] [Google Scholar]
- 21.Cheng LS-C, Carmelli D, Hunt SC, et al. Segregation analysis of cardiovascular reactivity to laboratory stressors. Genet Epidemiol. 1997;14:35–49. doi: 10.1002/(SICI)1098-2272(1997)14:1<35::AID-GEPI3>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 22.Smith TW, Turner CW, Ford MH, et al. Blood pressure reactivity in adult male twins. Health Psychol. 1987;6:209–20. doi: 10.1037//0278-6133.6.3.209. [DOI] [PubMed] [Google Scholar]
- 23.Uemura K, Kohara K, Nakura J, et al. Deletion polymorphism of the ACE gene is associated with higher blood pressure after hospitalization in normotensive subjects. Hypertens Res. 2000;23:201–5. doi: 10.1291/hypres.23.201. [DOI] [PubMed] [Google Scholar]
- 24.Henrion D, Amant C, Benessiano J, et al. Antiotensin II type I receptor gene polymorphism is associated with an increased vascular reactivity in the human mammary artery in vitro. J Vasc Res. 1998;35z:356–62. doi: 10.1159/000025605. [DOI] [PubMed] [Google Scholar]
- 25.Cockcroft JR, Gazis AG, Cross DJ, et al. Beta(2)-adrenoreceptor polymorphism determines vascular reactivity in humans. Hypertension. 2000;36:371–5. doi: 10.1161/01.hyp.36.3.371. [DOI] [PubMed] [Google Scholar]
- 26.Gratze G, Fortin J, Labugger R, et al. Beta-2 adrenergic receptor variants affect resting blood pressure and agonist-induced vasodilatation in young adult Whites. Hypertension. 1999;33:1425–30. doi: 10.1161/01.hyp.33.6.1425. [DOI] [PubMed] [Google Scholar]
- 27.Li GH, Faulhaber HD, Rosenthal M, et al. Beta-2 adrenergic receptor gene variations and blood pressure under stress in normal twins. Psychophysiology. 2001;38:485–9. [PubMed] [Google Scholar]
- 28.Wu T, Snieder H, deGues E. Genetic influences on cardiovascular stress reactivity. Neurosci Biobehav Rev. 2010;35:58–68. doi: 10.1016/j.neubiorev.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 29.Ramage AG. Central cardiovascular regulation and 5-hydroxytryptamine receptors. Brain Res Bull. 2001;56:425–39. doi: 10.1016/s0361-9230(01)00612-8. [DOI] [PubMed] [Google Scholar]
- 30.Shores MM, Pasculaly M, Lewis NL, et al. Short-term sertraline treatment suppresses sympathetic nervous system activity in healthy human subjects. Psychoneuroendocrinology. 2001;26:433–9. doi: 10.1016/s0306-4530(01)00002-6. [DOI] [PubMed] [Google Scholar]
- 31.Sauer WH, Berlin JA, Kimmel SE. Selective serotonin reuptake inhibitors and myocardial infarction. Circulation. 2001;104:1894–8. doi: 10.1161/hc4101.097519. [DOI] [PubMed] [Google Scholar]
- 32.Thase ME, Larsen KG, Reines E, et al. The cardiovascular safety profile of escitalopram. Eur Neuropsychopharmacol. 2013;23(11):1391–400. doi: 10.1016/j.euroneuro.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 33.Jiang W, Velazquez EJ, Kuchibhatla M, et al. Effect of escitalopram on mental stress-induced myocardial ischemia: results of the REMIT trial. JAMA. 2013;309(20):2139–49. doi: 10.1001/jama.2013.5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davies SJ, Hood SD, Argyropoulos SV, et al. Depleting serotonin enhances both cardiovascular and psychological stress reactivity in recovered patients with anxiety disorders. J Clin Psychopharmacol. 2006;26:414–8. doi: 10.1097/01.jcp.0000227704.79740.c0. [DOI] [PubMed] [Google Scholar]
- 35.Lesch KP, Bengel D, Heils A, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–31. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 36.Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 37.Murphy DL, Fox MA, Timpano KR, et al. How the serotonin story is being rewritten by new gene-based discoveries prinicipally related to SLC6A4, the serotonin transporter gene, which functions to influence all cellular serotonin systems. Neuropharmacology. 2008;55:932. doi: 10.1016/j.neuropharm.2008.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brummett BH, Krystal AD, Ashley-Koch A, et al. Sleep quality varies as a function of 5-HTTLPR genotype and stress. Psychosom Med. 2007;69:621. doi: 10.1097/PSY.0b013e31814b8de6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams RB, Marchuk DA, Siegler IC, et al. Childhood socioeconomic status and serotonin transporter gene polymorphism enhance cardiovascular reactivity to mental stress. Psychosom Med. 2008;70:32–9. doi: 10.1097/PSY.0b013e31815f66c3. [DOI] [PubMed] [Google Scholar]
- 40.Brummett BH, Siegler IC, Ashley-Koch A, et al. Effects of 5HTTLPR on cardiovascular response to an emotional stressor. Psychosom Med. 2011;73:318–22. doi: 10.1097/PSY.0b013e3182118c16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fumeron F, Betoulle D, Nicaud V, et al. Serotonin transporter gene polymorphism and myocardial infarction. Circulation. 2002;105:2943–5. doi: 10.1161/01.cir.0000022603.92986.99. [DOI] [PubMed] [Google Scholar]
- 42.Coto E, Reguero JR, Alvarez V, et al. 5-Hytroxytrptamine 5-HT2A receptor and 5-hydroxytryptamine transporter polymorphisms in acute myocardial infarction. Clin Sci. 2003;104:241–5. doi: 10.1042/CS20020246. [DOI] [PubMed] [Google Scholar]
- 43.Arinami T, Ohtsuki T, Yamakawa-Kobayashi K, et al. A synergistic effect of serotonin transporter gene polymorphism and smoking in association with CHD. Thromb Haemost. 1999;81:853–6. [PubMed] [Google Scholar]
- 44.Phillips-Bute B, Mathew JP, Blumenthal JA, et al. Relationship of genetic variability and depressive symptoms to adverse events after coronary artery bypass graft surgery. Psychosom Med. 2008;70(9):953–9. doi: 10.1097/PSY.0b013e318187aee6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fischer P, Gruenblatt E, Pietschmann P, et al. Serotonin transporter polymorphism and LDL-cholesterol. Mol Psychiatry. 2006;11(8):707–9. doi: 10.1038/sj.mp.4001837. [DOI] [PubMed] [Google Scholar]
- 46.Harris CM, Halpern CT, Hussey JM, et al. [Accessed June 21, 2011];The National Longitudinal Study of Adolescent Health: research design. http://www.cpc.unc.edu/projects/addhealth/design.
- 47.Wee H-L, Wu J, Thumboo J, et al. Association of body mass index with Short Form 36 physical and mental component summary scores in a multiethnic Asian population. Int J Obes. 2010;34:1034–43. doi: 10.1038/ijo.2010.24. [DOI] [PubMed] [Google Scholar]
- 48.Hu XZ, Lipsky RH, Zhu G, et al. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive–compulsive disorder. Am J Hum Genet. 2006;78:815–26. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haberstick BC, Smolen A, Williams RB, et al. Population frequencies of the triallelic 5HTTLPR in six ethnically diverse samples from North America, Southeast Asia and Africa. Behav Genet. 2015;45:255–61. doi: 10.1007/s10519-014-9703-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gelernter J, Cubells JF, Kidd JR, et al. Population studies of polymorphisms of the serotonin transporter protein gene. Am J Med Genet (Neuropsych Genet) 1999;88:61–6. [PubMed] [Google Scholar]
- 51.Kohen R, Jarrett ME, Cain KC, et al. The serotonin transporter polymorphism rs25531 is associated with irritable bowel syndrome. Dig Dis Sci. 2009;54:2663–70. doi: 10.1007/s10620-008-0666-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Williams RB, Marchuk DA, Gadde KM, et al. Serotonin-related gene polymorphisms and central nervous system serotonin function. Neuropsychopharmacology. 2003;28:533–41. doi: 10.1038/sj.npp.1300054. [DOI] [PubMed] [Google Scholar]
- 53.Harris KM. An integrative approach to health. Demography. 2010;47:1–22. doi: 10.1353/dem.0.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Entzel PP, Whitsel AE, Richardson A, et al. Add Health Data Guides: Wave IV Cardiovascular and Anthropometric Documentation. Chapel Hill, NC: Carolina Population Center, University of North Carolina at Chapel Hill; 2009. [Google Scholar]
- 55.Nguyen QC, Tabor JW, Entzel PP, et al. Discordance in national estimates of hypertension among young adults. Epidemiology. 2011;22:532–41. doi: 10.1097/EDE.0b013e31821c79d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haberstick BC, Smolen A, Stetler GL, et al. Simple sequence repeats in the National Longitudinal Study of Adolescent Health: an ethnically diverse resource for genetic analysis of health and behavior. Behav Genet. 2014;44:487–97. doi: 10.1007/s10519-014-9662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wray NR, James MR, Gordon SD, et al. Accurate, large-scale genotyping of 5HTTLPR and flanking single nucleotide polymorphisms in an association study of depression, anxiety, and personality measures. Biol Psychiatry. 2009;66:468–76. doi: 10.1016/j.biopsych.2009.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nakamura M, Ueno S, Sano A, et al. The human serotonin transporter gene linked polymorphism (5-HTTLPR) shows ten novel allelic variants. Mol Psychiatry. 2000;5:32–8. doi: 10.1038/sj.mp.4000698. [DOI] [PubMed] [Google Scholar]
- 59.The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure. National Institutes of Health, National Heart, Lung, and Blood Institute, National High Blood Pressure Education Program. 2004 Aug;2004 NIH Publication No. 04–5230. [PubMed] [Google Scholar]
- 60.Kramer H, Han C, Post W, et al. Racial/ethnic differences in hypertension and hypertension treatment and control in the multi-ethnic study of atherosclerosis (MESA) Am J Hypertens. 2004;17(10):963–70. doi: 10.1016/j.amjhyper.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 61.Cushman WC, Reda DJ, Perry HM, et al. Regional and racial differences in response to antihypertensive medication use in a randomized controlled trial of men with hypertension in the United States. Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. Arch Intern Med. 2000;160(6):825–31. doi: 10.1001/archinte.160.6.825. [DOI] [PubMed] [Google Scholar]
- 62.Wang X, Ding X, Su S, et al. Genetic influence on blood pressure measured in the office, under laboratory stress and during real life. Hypertens Res. 2011;34:239–44. doi: 10.1038/hr.2010.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McQueen MB, Boardman JD, Domingue BW, et al. The National Longitudinal Study of Adolescent to Adult Health (Add Health) Sibling Pairs Genome-Wide Data. Behav Genet. 2014 doi: 10.1007/s10519-014-9692-4. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Anderson NB, Lane JD, Muranaka M, et al. Racial differences in blood pressure and forearm vascular responses to the cold face stimulus. Psychosom Med. 1988;50:57–63. doi: 10.1097/00006842-198801000-00007. [DOI] [PubMed] [Google Scholar]
- 65.Taherzadeh Z, Brewster LM, van Montrfans GA, et al. Function and structure of resistance vessels in black and white people. J Clin Hypertens. 2010;12:431–8. doi: 10.1111/j.1751-7176.2010.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brewster LM, Taherzadeh Z, Volger S, et al. Ethnic differences in resistance artery contractility in normotensive pregnant women. Am J Physiol Heart Circ Physiol. 2010;299:H431–6. doi: 10.1152/ajpheart.00919.2009. [DOI] [PubMed] [Google Scholar]
- 67.Hinds K, Strachenfeld NS. Greater orthostatic tolerance in young black compared with white women. Hypertension. 2010;56:75–81. doi: 10.1161/HYPERTENSIONAHA.110.150011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.ICBP-GWAS PP MAP Working and Writing Sub-Group. Genome-wide association study identifies six new loci influencing pulse pressure and mean arterial pressure. Nat Genet. 2011;43:1005–11. doi: 10.1038/ng.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gelernter J, Kranzler H, Cubells JF. Serotonin transporter protein (SLC6A4) allele and haplotype frequencies and linkage disequilibria in African- and European-American and Japanese populations and in alcohol-dependent subjects. Hum Genet. 1997;10:243. doi: 10.1007/s004390050624. [DOI] [PubMed] [Google Scholar]
- 70.Gelernter J, Kranzler H, Coccaro EF, et al. Serotonin transporter protein gene polymorphism and personality measures in African American and European American subjects. Am J Psychiatry. 1998;155:1332–8. doi: 10.1176/ajp.155.10.1332. [DOI] [PubMed] [Google Scholar]
- 71.Murdoch JD, Speed WC, Pakstis AJ, et al. Worldwide population variation and haplotype analysis at the serotonin transporter gene SLC6A4 and implications for association studies. Biol Psychiatry. 2013;74:879–89. doi: 10.1016/j.biopsych.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 72.Gong M, Hubner N. Molecular genetics of human hypertension. Clin Sci (Lond) 2006;110:315–26. doi: 10.1042/CS20050208. [DOI] [PubMed] [Google Scholar]
- 73.Bishop GD, Kaur D, Tan VLM, et al. Effects of a psychosocial skills training workshop on psychophysiological and psychosocial risk in patients undergoing coronary artery bypass grafting. Am Heart J. 2005;150:602–9. doi: 10.1016/j.ahj.2004.10.015. [DOI] [PubMed] [Google Scholar]