Abstract
The methodologies of cognitive architectures and functional magnetic resonance imaging can mutually inform each other. For example, four modules of the ACT-R (adaptive control of thought – rational) cognitive architecture have been associated with four brain regions that are active in complex tasks. Activity in a lateral inferior prefrontal region reflects retrieval of information in a declarative module; activity in a posterior parietal region reflects changes to problem representations in an imaginal module; activity in the anterior cingulate cortex reflects the updates of control information in a goal module; and activity in the caudate nucleus reflects execution of productions in a procedural module. Differential patterns of activation in such central regions can reveal the time course of different components of complex cognition.
Introduction
This paper will describe a rather unexpected convergence of an empirical and a theoretical methodology. The empirical methodology involves functional magnetic resonance imaging (fMRI), which has become a major research tool in cognitive science. The theoretical methodology involves cognitive architectures, which are formalisms for modeling the mental interactions that occur in the performance of complex tasks. These two methodologies are rather distant members of the cognitive science field: one has strong ties to traditional neuroscience whereas the other has strong ties to traditional artificial intelligence. However, they can be brought together such that fMRI data provide converging evidence for architectural assumptions, and the architectural assumptions provide explanations for when certain brain regions will show correlations in their activation and when they will not. Our specific case will involve the ACT-R (adaptive control of thought – rational) cognitive architecture and its relationship to several brain regions that are often active in the performance of complex tasks.
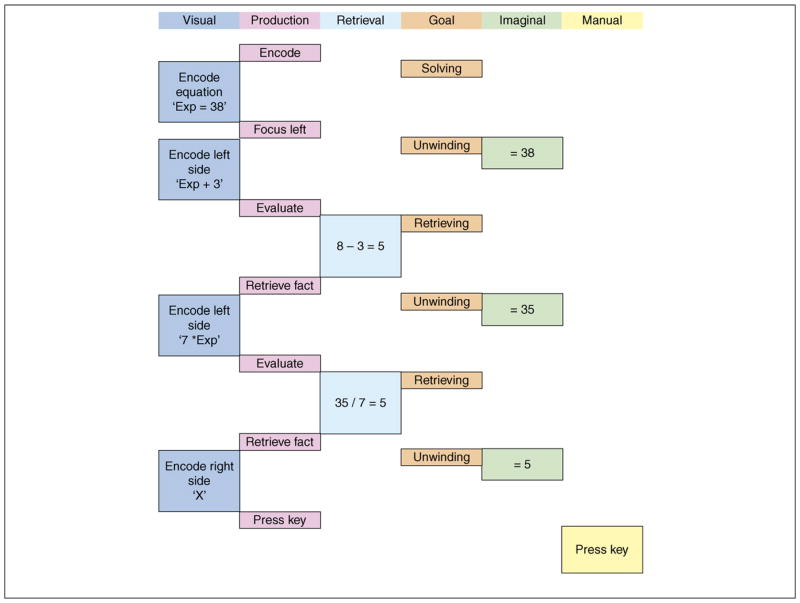
ACT-R [1,2] has been used to model people’s behaviors in a great variety of tasks including categorization, learning algebra and geometry, driving while talking on a cell phone and air traffic control (see ACT-R [http://act-r.psy.cmu.edu/] for the variety of tasks researchers have modeled). By specifying behavioral models within such a framework, one is forced to make the theory computationally explicit, thus allowing for true evaluation of the theory as well as allowing for predictions in novel circumstances. According to the ACT-R theory, cognition emerges through the interaction of several relatively independent modules. Figure 1 illustrates the modules in a model of solving equations such as 7x + 3 = 38. In Figure 1, the visual module is extracting information about the equation; the retrieval module is obtaining arithmetic facts relevant to this information; and the imaginal module is changing the representation of the solution to incorporate the retrieved information.
Figure 1.
A representation of the basic module operations in ACT-R to implement the unwind strategy in ACT-R to solve the equation 7*x + 3 = 38. (i) The visual module encodes pieces of the visual display such as fragments of an equation, for example ‘+ 3’. (ii) The retrieval module holds retrieval cues such as ‘8 – 3’ to drive the retrieval of task-relevant facts. (iii) The imaginal module creates and transforms problem representations, such as intermediate answers in the equation solution. (iv) The goal module sets control states to direct the path of information processing, such as whether information is to be retrieved or the equation is to be transformed. (v) The manual module programs the output such as the keying of 5 as the final answer. (vi) The procedural module executes productions that recognize patterns of activity in other modules, selects appropriate actions and relays information to the other modules. The height of the boxes in Figure 1 represents the time a module is active in doing things such as retrieval or constructing an internal problem representation. While a module is engaged during one of these activities, it might be performing a great many computations in parallel to achieve its objectives, such as the retrieval module matching a pattern against declarative memory. It places the results of its computation in its buffer associated for access by other modules. Multiple modules can work in parallel, but the need to pass information among modules imposes some seriality on the overall processing.
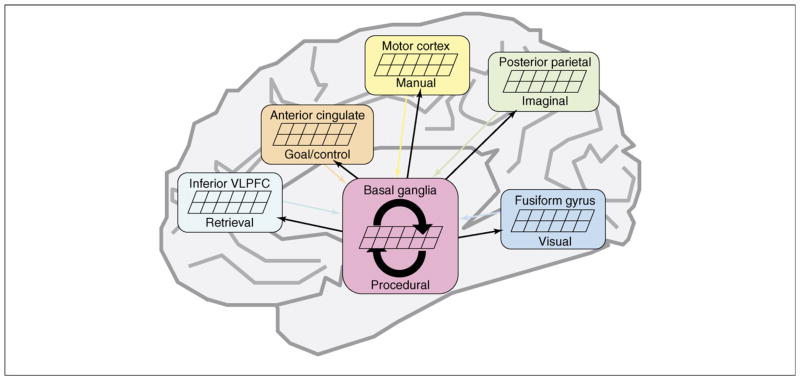
Before our work in brain imaging, ACT-R addressed only behavioral measures, such as the timing of keystrokes or patterns of eye movements. These behavioral data sources failed to test detailed assumptions about which modules were active in the performance of a task. We have recently been engaged in a process of using imaging data to provide converging data on module activity. Figure 2 illustrates the associations we have made between the six modules in Figure 1 and brain regions. Coordination among all of these components occurs through actions of the procedural module, which is mapped to the basal ganglia. Box 1 describes the methodology by which we are able to use the time course of the activity of the modules to make predictions about the blood-oxygen-level-dependent (BOLD) response obtained in fMRI.
Figure 2.
An illustration of how the various cortical modules of ACT-R are coordinated through the procedural module that is associated with the basal ganglia. VLPFC, ventrolateral prefrontal cortex.
Box 1. Predicting the BOLD response.
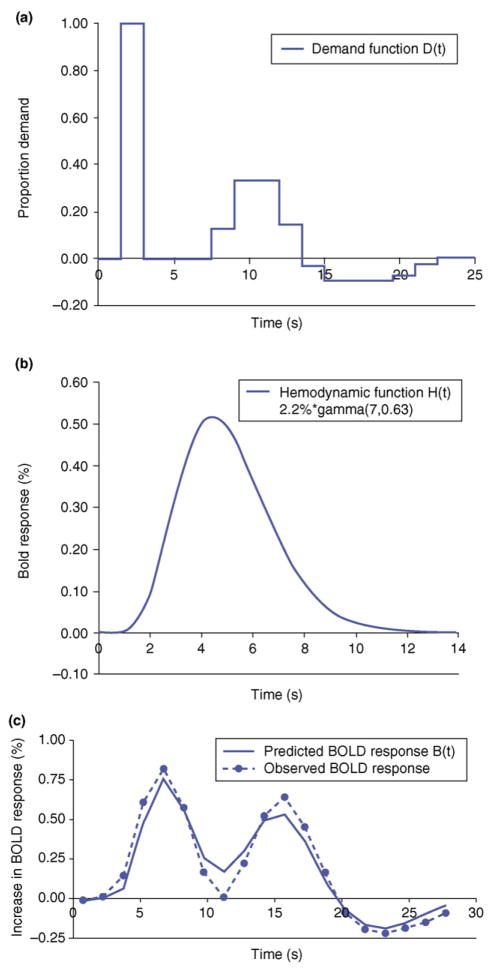
The BOLD response obtained in a brain region can be predicted from the time course of activity of modules in an ACT-R model. While a module is engaged, it will drive a metabolic demand in the corresponding region producing a hemodynamic response. Figure I illustrates the postulated activity of a module from the experiment reported in Ref. [3]. This figure represents the proportion of time the module should be engaged during each 1.5 s scan in a trial.
To model the hemodynamic function, we have adopted the standard gamma function that has been used (e.g. see Refs [60–63]) for the hemodynamic response. If the module is engaged, it will produce a BOLD response t time units later according to the function:
where m governs the magnitude, s scales the time and the exponent a determines the shape of the BOLD response such that with larger a the function rises and falls more steeply. Figure Ib illustrates the function assumed in [3]. As is typical of such functions, it shows a slow response that peaks 34–5 s after the actual activity. The peak of the function is at a*s. The parameter a is 7 for this function, and s is 0.63 s, and so the function peaks at a*s = 4.41 s.
The BOLD response accumulates whenever the region is engaged. Thus, if D (t) is a demand function giving the probability that the region is engaged at time t, the cumulative BOLD response can be obtained by convolving this function with the hemodynamic function:
This is the prediction for the BOLD response in the region associated with that demand function. Figure Ic shows the predicted BOLD response in this case. As can be seen, the predicted response preserves some of the structure of the demand function in Figure Ia, but the convolving with the BOLD response blurs some of the temporal structure and delays the peaks.
A similar convolution methodology is frequently used in analysis programs for fMRI data in which the condition structure of trials in an experiment is convolved with a hemodynamic response to produce a condition-sensitive pattern of activity. This pattern is regressed against brain activity to find which regions are sensitive to these conditions (e.g. see Ref. [64]). Our application is finer grained conceptually (using model behavior within a single trial) and is used for confirmatory rather than exploratory purposes.
Figure I.
Illustration of the prediction of a BOLD response for the auditory cortex for the experiment in Ref. [3]: (a) shows the proportion of time that the aural module in ACT-R will be engaged during each 1.5 s scan in a trial; (b) illustrates the hemodynamic function estimate for this region; (c) shows the predicted response obtained by convolving the demand function in (a) with the hemodynamic function in (b) and compares it with the observed BOLD response.
The basic information processing circuit
Although perceptual and motor modules can be very important to the performance of a task, this paper focuses on the four central modules and their associated areas, which we have shown to be independent of the modality of input or output [3]:
The module responsible for controlled retrieval from declarative memory is associated with a lateral inferior prefrontal region (Talairach coordinates x = +/−40, y = 21, z = 21) around the inferior frontal sulcus.
The module responsible for constructing imagined representations is associated with a parietal region centered at x = +/−23, y = −64, z = 34, on the border of the intraparietal sulcus.
The module associated with setting controlling goals is associated with the anterior cingulate cortex centered at x = +/−5, y = 10, z = 38 in the medial frontal cortex.
The module associated with procedural execution is associated with the head of the caudate nucleus, part of the basal ganglia, centered at x = +/−15, y = 9, z = 2.
Many researchers have noticed that these regions tend to activate together (e.g. see Refs [4–6]). However, there are systematic differences in the factors that these regions respond to, and these differences can be predicted from the properties of their associated modules. Below, we review evidence about each of these mappings and their regions. Box 2 illustrates an experiment in which specific regional responses were successfully predicted for each region by the computational properties of their associated modules.
Box 2. Learning to solve algebra equations.
The model described by Anderson [65] illustrates how one can use a cognitive architecture to understand the data from a complex task. The task [66] involved children (aged 11–14) learning to solve simple linear equations (e.g. 3x – 5 = 7). During the experiment, they practiced solving such problems for 1 h per day for 6 days. The first day (Day 0) they were given private tutoring on solving equations; on the remaining 5 days, they practiced solving three classes of equation on a computer:
0-step: e.g. 1x + 0 = 4
1-step: e.g. 3x + 0 = 12 or 1x + 8 = 12
2-step: e.g. 7x + 1 = 29
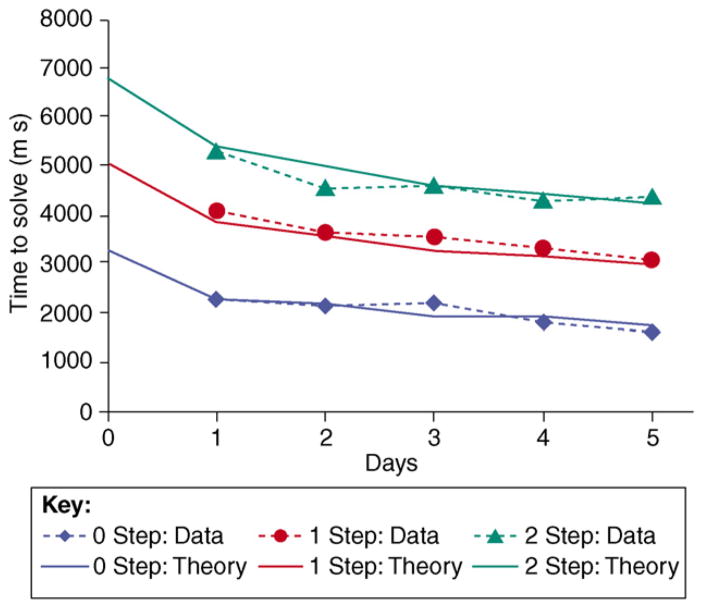
Figure I shows how the time required by the children to process these equations diminished over the course of the experiment. It also illustrates the predictions of a model implemented in the ACT-R architecture. The model, like the participants, took longer with more complex equations because it had to go through more cognitive steps. More interestingly, it improved gradually in task performance at the same rate as participants: the effect of the practice was to make a 2-step equation on Day 1 like a 1-step equation on Day 5 in terms of difficulty (as measured by solution time) and a 1-step equation like a 0-step equation. The learning in the ACT-R model involved both the acquisition of new procedures for solving equations and the speed up in the retrieval of arithmetic and algebraic facts from declarative memory.
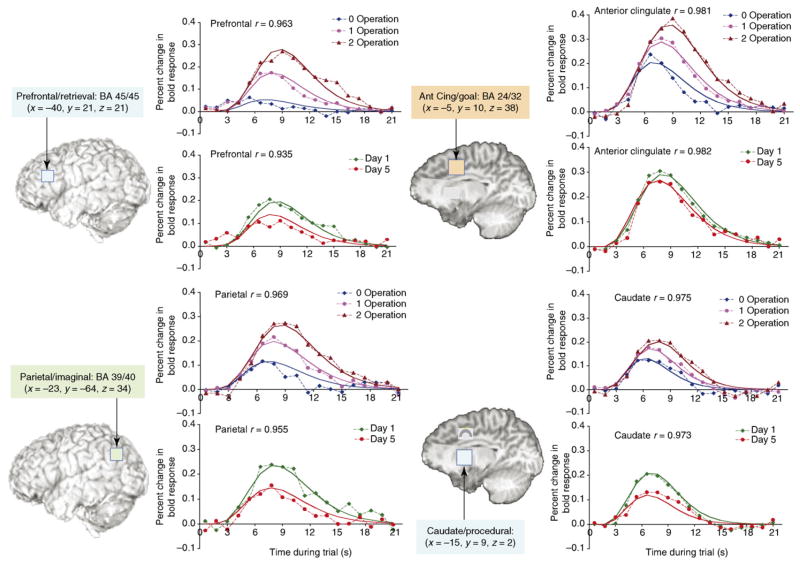
On days 1 and 5, the children whose behavioral data are reported in Figure I were solving the equations in an fMRI scanner. The activities of the modules in an ACT-R model for this task were used to make predictions about the activation that would be observed in the four regions of interest. Figure II (see next page) summarizes the predictions and the actual data. It shows separately the effects of problem complexity (averaging over days) and effects of practice (averaging over complexity). The responses in the different regions are similar in that they all show an effect of complexity and most show an effect of practice. Yet there are important differences. First, the prefrontal region shows almost no response in the 0-transformation condition. This is because virtually nothing has to be retrieved in that condition. Second, the ACC shows almost no effect of practice. This is because the underlying control structure of the problem-solving strategy remained unchanged. Although these are the two most distinguishing features there are other more subtle differences. In fact, the activity of the four ACT-R modules (retrieval, imaginal, goal and procedural) best fit their associated regions (prefrontal, parietal, ACC and caudate nucleus, respectively). The conclusion that this is the best-fitting mapping between modules and brain regions does not depend on parameter estimation (see Table 3a in Ref. [65]).
Figure I.
Mean solution times (and predictions of the ACT-R model) for the three types of equations as a function of delay. Although the data were not collected, the predicted times are presented for the practice session of the experiment (Day 0). Dashed lines connect the actual empirical points whereas the smooth lines show the predictions of an ACT-R model.
Figure II.
Effect of number of operations (collapsing over practice) and practice (collapsing over number of operations) on four regions in the central circuit. Dashed lines connect the actual empirical points whereas the solid lines show the predictions of an ACT-R mode.
When interpreting this research, it is important to understand the basic processing cycle that involves all of the modules associated with these regions in the ACT-R theory. At any point in time the state of the system is defined by the contents of the buffers of these modules. Because of the way computations are distributed across modules, four basic operations tend to repeat when moving to a new state:
Procedural: some mental action is selected (e.g. add a number to both sides of an equation) that is appropriate to this current state.
Goal: this might result in some change in the goal that is controlling the current step (e.g. get rid of the + 3 before the x).
Retrieval: frequently, it will be necessary to retrieve information (e.g. an addition fact) from declarative memory.
Imaginal: the problem representation is often updated to incorporate the retrieved information (e.g. ‘7x = 38 – 5’ to ‘7x = 35’)
It is difficult to come up with tasks that omit any of the activities 1–4 altogether. When showing the unique contribution of each brain region, it is necessary to find some manipulation that affects only its activity or affects all other regions apart from this region.
Lateral inferior prefrontal cortex reflects controlled retrieval
The human prefrontal cortex is a large structure that consists of many distinct areas, both in terms of structure and function (e.g. see Refs [7,8]). The region we have selected has been associated with retrieval factors in imaging studies (e.g. see Refs [9–12]). These imaging results are not particularly surprising given evidence about the memory deficits associated with prefrontal lesions [13,14]. This region is active in many tasks, particularly those involving language. As noted in Ref. [15], this involvement in such tasks can be understood in terms of accessing the information needed to perform the tasks.
In ACT-R, we conceive of this region as serving the role of maintaining the retrieval cues for accessing information stored elsewhere in the brain. The longer it takes to complete the retrieval successfully, the longer the cues will have to be maintained and the greater the activation. Focused studies that manipulate retrieval difficulty produce systematic differences in the activation of this region. In our own research, we have found that the lateral inferior prefrontal cortex (LIPFC) (and not the other three regions) tends to respond to manipulations of fan or associative interference [16,17], retention delay [18] and repetition [19]. All of these factors influence the duration of a single retrieval from declarative memory.
Perhaps the major competing interpretation of this prefrontal region is that it is activated in conditions that require difficult selections among retrieved information (e.g. see Refs [20,21]). However, it has been argued that these effects are due to greater retrieval demands in the more difficult conditions [12,22]. It has also been argued that a more anterior prefrontal region is sensitive to retrieval, whereas this region is sensitive to selection demands [23,24]. Recently, it has been suggested [25] that the distinction between retrieval and selection could be a false dichotomy, and certainly it is not a distinction that has any meaning in the ACT-R architecture. What drives the magnitude of response is the amount of time that this region has to hold the cues for retrieval, and this will increase when selection is harder.
The posterior parietal cortex reflects representational activities
The imaginal module in ACT-R is responsible for transforming problem representations, such as a change to an equation or a planned state in Tower of Hanoi task. Although it could be said that the whole cortex serves a representational role, what distinguishes the imaginal module of ACT-R is that it supports manipulations of representations that are not perceptually present. We assume that it is changes or updates to these states (not maintenance of the states) that drive activation in an fMRI study. There is considerable evidence to support the assumption that the parietal region plays this sort of role in visual–spatial and verbal representations. It is engaged in verbal encoding [26,27], mental rotation [28–30] and visual–spatial strategies in a variety of contexts [31–33]. Other authors (e.g. see Ref. [34]) have also proposed a representational role for the parietal region.
In our own laboratory, we have shown that this parietal region responds most strongly to manipulations of representational difficulty. This region responded more strongly than any other of our four regions in the Tower of Hanoi task [35], in which planning makes strong demands to represent future states of the problem. In a paired-associate memory study [18], we manipulated whether participants were simply shown a paired associate or had to construct one as part of a fragment-completion task. Despite the fact that the LIPFC is usually the region that responds in memory tasks, it responded equally in the two conditions; it was only the parietal region that differentiated between them, responding more strongly when participants had to construct the paired associate.
The anterior cingulate cortex reflects control activities
The ACT-R goal module is responsible for setting control states that enable different courses of information processing to be taken when conditions are otherwise equal. It thus enables internal control of cognition independent of external circumstances. The control states can be conceived of as determining which branch is taken at decision points in the information processing. This sense of ‘control’ is basically the same as in computer science, in which it indicates how the state transitions within a system are shaped and is similar to some theories of the anterior cingulated cortex (ACC) (e.g. see Refs [36–38]). Other theories relate ACC activity to error detection. There is the error-related negativity (ERN) in event-related potentials that has been observed when errors are made in speeded response tasks (e.g. see Refs [39,40]). However, the ACC responds more strongly in many tasks that do not involve errors. It has been argued [41–43] that the ACC activity reflects response conflict and that error trials are just a special case of this. For instance, the ACC responds more strongly on a conflict trial in the Stroop task even though the participant does not make an error.
As illustrated in Box 2, Figure II, in our experiments we have consistently found that the ACC responds to task difficulty when that difficulty is reflected in the number of mental steps. Because these effects occur in advance of any motor response and on error-free trials, they are not consistent with a theory that relates ACC activation to response competition or error detection. These effects can be viewed as consistent with the recent error-likelihood theory [44] that proposes that ACC activity reflects the learned probability of an error on a trial. However, in some of our research we have found within-trial fluctuations in ACC activation. For instance, in a logical reasoning task, ACC activity is greater when a participant is preparing for a more difficult logical judgment but does not yet know what the response will be [45]. Other research (J.R. Anderson et al., unpublished) has found that ACC activation rises when participants are selecting a strategy to solve an equation, then falls off while the equation is being solved, and finally rises again when the response is output. Such within-trial variations indicate that ACC activation reflects more than just the overall likelihood of an error in a trial but rather the within-trial variation in the need for control.
A frequent result in our laboratory is that the response of the ACC is not distinguished from the response of the other three regions. The one consistent difference, illustrated in Box 2, Figure II (see also Refs [46,47]), is that, unlike other regions, its response does not seem to be affected by practice. This is predicted because control states depend on how the various steps are articulated in a strategy, and they stay constant unless the strategy itself is modified. There have been reports of decreased ACC activation with practice, but these tend to be tasks in which learning changes the nature of the task. For instance, there is decreased activation in a task that involved repeated verb generation to the same noun [48]. With enough practice, the generation step can be bypassed, and the verb is just retrieved. In general, sufficient practice of simple, consistent mappings will enable some control states to be bypassed [49].
The caudate nucleus reflects procedural activities
The procedural module of the ACT-R has been mapped onto the group of cortical structures comprising the basal ganglia (caudate nucleus, putamen, pallidus and substantia nigra) and the thalamus. Most of the cortex sends projections to the caudate nucleus and putamen. The thalamic portions of this circuit project back to the cortex, mainly to prefrontal regions [50,51]. It has been proposed that these cortico–striatal–thalamic loops form the basis of a neural selection system (e.g. see Refs [52–54]) similar to the procedural module of ACT-R. Production rules are high-level specifications of how important patterns should be detected within the cortex (i.e. through afferent pathways to the striatum) and eventually routed to different locations. Although most of the thalamic projections in this loop are mainly to prefrontal regions, the posterior projections from the prefrontal cortex can influence regions such as the parietal cortex.
Although the basal ganglia have been implicated in many cognitive functions, they are most widely investigated for their involvement in procedural learning, skill acquisition and reinforcement learning (e.g. see Refs [55–57]). The ACT-R framework allows for a unification of these functions. Production rules implement the basic units of procedural knowledge, and their competition and selection is regulated by a reinforcement-like algorithm.
We have associated the procedural module with the head of the caudate nucleus and have had good success in using number of productions fired to predict its activity in relatively simple experiments, such as the one described in Box 2. However, in these experiments the caudate nucleus tends to give a pattern of response that is very similar to that of other regions. In more complex experiments (e.g. see Ref. [58]), the caudate nucleus is distinguished from other regions in that it has a spurt of activity at task boundaries. Unfortunately, we have not had success in predicting this boundary pattern activity in terms of number of productions. An alternative approach that seems to have some success is to base the predictions on the exact amount of information that these productions are relaying [59].
Concluding remarks
Understanding the human mind can be substantially guided by connections such as those reviewed between modules of a cognitive architecture and activation patterns in the brain. The activities of these brain regions provide converging data about the structure of task performance. The imaging data have had a major influence on the ACT-R theory at two levels. At the level of the architecture, they have helped us to articulate the current modular structure of ACT-R. For instance, as discussed in Ref. [2], imaging data helped indicate the need for a distinction between the goal and imaginal modules, which had been conflated into a single system in earlier versions of ACT-R. At the level of specific models within the architecture, imaging data have helped guide modeling decisions. As indicated in Box 3, many issues remain unresolved but their resolution might help to further guide the development of the ACT-R theory.
Box 3. Outstanding questions.
The picture sketched in this paper is very much a work in progress. Among the outstanding questions are the following:
Activity in the head of the caudate nucleus is more often related to response-contingent reinforcement learning (e.g. see Ref. [67]) than to amount of procedural activity as in ACT-R. Is there a way of uniting the ACT-R account with this body of literature? Could such unification offer an explanation for the early initial spike in the caudate nucleus observed in tasks that extend over tens of seconds (J.R. Anderson et al., unpublished)?
Although, as reviewed, there is evidence for a major role of the basal ganglia in coordinating cortical regions and action selection, it is by no means the only path of communication among cortical areas. In particular, how can the evidence for direct cortical-to-cortical connections be integrated into ACT-R?
The prefrontal cortex is the most expanded portion of human cortex. Only two small prefrontal regions have been related to ACT-R modules: a region of the ACC (goal) and a region in the LIPFC (retrieval). Many other regions have proven important in other fMRI studies. How is their activity to be understood?
Many of the tasks we study tend to involve mathematical problem solving. The ACT-R parietal region is distinct from other parietal regions that appear to serve a representational role in the performance of simpler mathematical tasks [68]. How are we to understand the relationship among these parietal regions?
Distinct periods of activity in a module can be distinguished in an fMRI signal only when they are many seconds apart (Box 1). This requires longer tasks than a task such as that described in Box 2. However, such tasks have high temporal variability that makes it hard to align the BOLD response from different trials. What would constitute an optimal solution remains an open question, but see Ref. [58].
In the other direction, a computationally explicit theory like ACT-R sheds light on the behavior of these four regions that have attracted considerable recent attention. ACT-R identifies the logical cycle of information processing that underlies the correlations among these regions. An understanding of the distinct functions of these regions serves to explain why the correlations vary across tasks and why some factors will only affect some regions.
Acknowledgments
This research was supported by NIMH award MH068243 and NSF award REC-0087396 to J.A. We would like to thank Jennifer Ferris for her comments on the paper.
References
- 1.Anderson JR, et al. An integrated theory of mind. Psychol Rev. 2004;111:1036–1060. doi: 10.1037/0033-295X.111.4.1036. [DOI] [PubMed] [Google Scholar]
- 2.Anderson JR. How Can the Human Mind Occur in the Physical Universe? Oxford University Press; 2007. [Google Scholar]
- 3.Anderson JR, et al. Information-processing modules and their relative modality specificity. Cognit Psychol. 2007;54:185–217. doi: 10.1016/j.cogpsych.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Cabeza R, et al. Attention-related activity during episodic memory retrieval: a crossfunction fMRI study. Neuropsychologia. 2003;41:390–399. doi: 10.1016/s0028-3932(02)00170-7. [DOI] [PubMed] [Google Scholar]
- 5.Dosenbach NUF, et al. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schneider W, Cole MW. The cognitive control network: Integrated cortical regions with dissociable functions. Neuroimage. 2007;16:343–360. doi: 10.1016/j.neuroimage.2007.03.071. [DOI] [PubMed] [Google Scholar]
- 7.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 8.Petrides M. The rostral–caudal axis of cognitive control within the lateral frontal cortex. In: Dehane S, et al., editors. From Monkey Brain to Human Brain. A Fyssen Foundation Symposium. MIT Press; 2005. pp. 293–314. [Google Scholar]
- 9.Buckner RL, et al. Frontal cortex contributes to human memory formation. Nat Neurosci. 1999;2:311–314. doi: 10.1038/7221. [DOI] [PubMed] [Google Scholar]
- 10.Cabeza R, et al. Similarities and differences in the neural correlates of episodic memory retrieval and working memory. Neuroimage. 2002;16:317–330. doi: 10.1006/nimg.2002.1063. [DOI] [PubMed] [Google Scholar]
- 11.Fletcher PC, Henson RNA. Frontal lobes and human memory: Insights from functional neuroimaging. Brain. 2001;124:849–881. doi: 10.1093/brain/124.5.849. [DOI] [PubMed] [Google Scholar]
- 12.Wagner AD, et al. Prefrontal contributions to executive control: fMRI evidence for functional distinctions within lateral prefrontal cortex. Neuroimage. 2001;14:1337–1347. doi: 10.1006/nimg.2001.0936. [DOI] [PubMed] [Google Scholar]
- 13.Shimamura AP. Memory and frontal lobe function. In: Gazzaniga MS, editor. The Cognitive Neurosciences. MIT Press; 1995. pp. 803–813. [Google Scholar]
- 14.Stuss DT, Benson DF. Neuropsychological studies of the frontal lobes. Psychol Bull. 1984;95:3–28. [PubMed] [Google Scholar]
- 15.Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 16.Sohn MH, et al. Competition and representation during memory retrieval: Roles of the prefrontal cortex and the posterior parietal cortex. Proc Natl Acad Sci U S A. 2003;100:7412–7417. doi: 10.1073/pnas.0832374100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sohn MH, et al. An information-processing model of three cortical regions: Evidence in episodic memory retrieval. Neuroimage. 2005;25:21–33. doi: 10.1016/j.neuroimage.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Anderson JR, et al. Role of prefrontal and parietal cortices in associative learning. Cereb Cortex. 2007 doi: 10.1093/cercor/bhm123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Danker JF, et al. A Bayesian model of the left prefrontal cortex accounts for both previous experience and current context during declarative retrieval. Cereb Cortex. in press. [Google Scholar]
- 20.Moss HE, et al. Selecting among competing alternatives: selection and controlled retrieval in the left prefrontal cortex. Cereb Cortex. 2005;15:1723–1735. doi: 10.1093/cercor/bhi049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson-Schill SL, et al. Role of left prefrontal cortex in retrieval of semantic knowledge: a re-evaluation. Proc Natl Acad Sci U S A. 1997;94:14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin RC, Cheng Y. Selection demands versus association strength in the verb generation task. Psychon Bull Rev. 2006;13:396–401. doi: 10.3758/bf03193859. [DOI] [PubMed] [Google Scholar]
- 23.Badre D, et al. Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron. 2005;47:907–918. doi: 10.1016/j.neuron.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 24.Gold BT, et al. Dissociation of automatic and strategic lexical semantics: fMRI evidence for differing roles of multiple frontotemporal regions. J Neurosci. 2006;26:6523–6532. doi: 10.1523/JNEUROSCI.0808-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thompson-Schill SL, Botvinick MM. Resolving conflict: a response to Martin and Cheng (2006) Psychon Bull Rev. 2006;13:402–408. doi: 10.3758/bf03193860. [DOI] [PubMed] [Google Scholar]
- 26.Clark D, Wagner AD. Assembling and encoding word representations: fMRI subsequent memory effects implicate a role for phonological control. Neuropsychologia. 2003;41:304–317. doi: 10.1016/s0028-3932(02)00163-x. [DOI] [PubMed] [Google Scholar]
- 27.Davachi L, et al. When keeping in mind supports later bringing to mind: neural markers of phonological rehearsal predict subsequent remembering. J Cogn Neurosci. 2001;13:1059–1070. doi: 10.1162/089892901753294356. [DOI] [PubMed] [Google Scholar]
- 28.Alivisatos B, Petrides M. Functional activation of the human brain during mental rotation. Neuropsychologia. 1997;35:111–118. doi: 10.1016/s0028-3932(96)00083-8. [DOI] [PubMed] [Google Scholar]
- 29.Carpenter PA, et al. Graded function activation in the visuospatial system with the amount of task demand. J Cogn Neurosci. 1999;11:9–24. doi: 10.1162/089892999563210. [DOI] [PubMed] [Google Scholar]
- 30.Zacks JM, et al. A parametric study of mental spatial transformation of bodies. Neuroimage. 2002;16:857–872. doi: 10.1006/nimg.2002.1129. [DOI] [PubMed] [Google Scholar]
- 31.Dehaene S, et al. Sources of mathematical thinking: behavioral and brain-imaging evidence. Science. 1999;284:970–974. doi: 10.1126/science.284.5416.970. [DOI] [PubMed] [Google Scholar]
- 32.Reichle ED, et al. The neural basis of strategy and skill in sentence-picture verification. Cognit Psychol. 2000;40:261–295. doi: 10.1006/cogp.2000.0733. [DOI] [PubMed] [Google Scholar]
- 33.Sohn MH, et al. Behavioral equivalence does not necessarily imply neural equivalence: Evidence in mathematical problem solving. Nat Neurosci. 2004;7:1193–1194. doi: 10.1038/nn1337. [DOI] [PubMed] [Google Scholar]
- 34.Bunge SA, et al. Dissociable contributions of prefrontal and parietal cortices to response selection. Neuroimage. 2002;17:1562–1571. doi: 10.1006/nimg.2002.1252. [DOI] [PubMed] [Google Scholar]
- 35.Anderson JR, et al. Tracing problem solving in real time: fMRI analysis of the subject-paced Tower of Hanoi. J Cogn Neurosci. 2005;17:1261–1274. doi: 10.1162/0898929055002427. [DOI] [PubMed] [Google Scholar]
- 36.Posner MI, Dehaene S. Attentional networks. Trends Neurosci. 1994;17:75–79. doi: 10.1016/0166-2236(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 37.D’Esposito M, et al. The neural basis of the central executive of working memory. Nature. 1995;378:279–281. doi: 10.1038/378279a0. [DOI] [PubMed] [Google Scholar]
- 38.Posner MI, DiGirolamo GJ. Executive attention: conflict, target detection and cognitive control. In: Parasuraman R, editor. The Attentive Brain. MIT Press; 1998. pp. 401–423. [Google Scholar]
- 39.Falkenstein M, et al. Event related potential correlates of errors in reaction tasks. In: Karmos G, et al., editors. Perspectives of Event-Related Potentials Research. Elsevier; 1995. pp. 287–296. [PubMed] [Google Scholar]
- 40.Gehring WJ, et al. A neural system for error detection and compensation. Psychol Sci. 1993;4:385–390. [Google Scholar]
- 41.Botvinick MM, et al. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- 42.Carter CS, et al. Parsing executive processes: strategic versus evaluative functions of the anterior cingulate cortex. Proc Natl Acad Sci U S A. 2000;97:1944–1948. doi: 10.1073/pnas.97.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yeung N, et al. The neural basis of error detection: conflict monitoring and the error-related negativity. Psychol Rev. 2004;111:931–959. doi: 10.1037/0033-295x.111.4.939. [DOI] [PubMed] [Google Scholar]
- 44.Brown JW, Braver TS. Learned predictions of error likelihood in the anterior cingulate cortex. Science. 2005;307:1118–1121. doi: 10.1126/science.1105783. [DOI] [PubMed] [Google Scholar]
- 45.Sohn MH, et al. Anticipatory conflict monitoring in the anterior cingulate cortex and the prefrontal cortex. Proc Natl Acad Sci U S A. 2007;104:10330–10334. doi: 10.1073/pnas.0703225104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anderson JR. Using brain imaging to guide the development of a cognitive architecture. In: Gray WD, editor. Integrated Models of Cognitive Systems. Oxford University Press; 2007. pp. 49–62. [Google Scholar]
- 47.Fincham JM, Anderson JR. Distinct roles of the anterior cingulate and prefrontal cortex in the acquisition and performance of a cognitive skill. Proc Natl Acad Sci U S A. 2006;103:12941–12946. doi: 10.1073/pnas.0605493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Petersen SE, et al. The effects of practice on the functional anatomy of task performance. Proc Natl Acad Sci U S A. 1998;95:853–860. doi: 10.1073/pnas.95.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chein JM, Schneider W. Neuroimaging studies of practice-related change: fMRI and meta-analytic evidence of a domain-general control network for learning. Cogn Brain Res. 2005;25:607–623. doi: 10.1016/j.cogbrainres.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 50.Middleton FA, Strick PL. Basal ganglia and cerebellar loops: Motor and cognitive circuits. Brain Res Rev. 2000;31:236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- 51.Middleton FA, Strick PL. The temporal lobe is a target of output from the basal ganglia. Proc Natl Acad Sci U S A. 1996;93:8683–8687. doi: 10.1073/pnas.93.16.8683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frank MJ, et al. Interactions between the frontal cortex and basal ganglia in working memory: A computational model. Cogn Affect Behav Neurosci. 2001;1:137–160. doi: 10.3758/cabn.1.2.137. [DOI] [PubMed] [Google Scholar]
- 53.Houk JC, Wise SP. Distributed modular architectures linking basal ganglia, cerebellum, and cerebral cortex: Their role in planning and controlling action. Cereb Cortex. 1995;2:95–110. doi: 10.1093/cercor/5.2.95. [DOI] [PubMed] [Google Scholar]
- 54.Redgrave P, et al. The basal ganglia: A vertebrate solution to the selection problem? Neuroscience. 1999;89:1009–1023. doi: 10.1016/s0306-4522(98)00319-4. [DOI] [PubMed] [Google Scholar]
- 55.Graybiel AM. Building action repertoires: memory and learning functions of the basal ganglia. Curr Opin Neurobiol. 1995;5:733–741. doi: 10.1016/0959-4388(95)80100-6. [DOI] [PubMed] [Google Scholar]
- 56.Jog MS, et al. Building neural representations of habits. Science. 1999;286:1745–1749. doi: 10.1126/science.286.5445.1745. [DOI] [PubMed] [Google Scholar]
- 57.Packard MG, Knowlton BJ. Learning and memory functions of the basal ganglia. Annu Rev Neurosci. 2002;25:563–593. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- 58.Anderson JR, Qin Y. Using brain imaging to extract the structure of complex events at the rational time band. J Cogn Neurosci. doi: 10.1162/jocn.2008.20108. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stocco A, Anderson JR. Endogenous control and task representation: an fMRI study in algebraic problem solving. J Cogn Neurosci. doi: 10.1162/jocn.2008.20089. in press. [DOI] [PubMed] [Google Scholar]
- 60.Boyton GM, et al. Linear systems analysis of functional magnetic resonance imaging in human V1. J Neurosci. 1996;16:4207–4221. doi: 10.1523/JNEUROSCI.16-13-04207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cohen MS. Parametric analysis of fMRI data using linear systems methods. Neuroimage. 1997;6:93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- 62.Dale AM, Buckner RL. Selective averaging of rapidly presented individual trials using fMRI. Hum Brain Mapp. 1997;5:329–340. doi: 10.1002/(SICI)1097-0193(1997)5:5<329::AID-HBM1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 63.Glover GH. Deconvolution of impulse response in event-related BOLD fMRI. Neuroimage. 1999;9:416–429. doi: 10.1006/nimg.1998.0419. [DOI] [PubMed] [Google Scholar]
- 64.Friston KJ. Introduction: Experimental design and statistical parametric mapping. In: Frackowiak RSJ, et al., editors. Human Brain Function. 2. Academic Press; 2003. pp. 599–633. [Google Scholar]
- 65.Anderson JR. Human symbol manipulation within an integrated cognitive architecture. Cogn Sci. 2005;29:313–342. doi: 10.1207/s15516709cog0000_22. [DOI] [PubMed] [Google Scholar]
- 66.Qin Y, et al. The change of the brain activation patterns along with the children’s practice in algebra equation solving. Proc Natl Acad Sci U S A. 2004;101:5686–5691. doi: 10.1073/pnas.0401227101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Delgado MR, et al. Dorsal striatum responses to reward and punishment: effects of valence and magnitude manipulations. Cogn Affect Behav Neurosci. 2003;3:27–38. doi: 10.3758/cabn.3.1.27. [DOI] [PubMed] [Google Scholar]
- 68.Dehaene S, et al. Three parietal circuits for number processing. Cogn Neuropsychol. 2002;20:487–506. doi: 10.1080/02643290244000239. [DOI] [PubMed] [Google Scholar]