Summary
A number of reports including a recent publication in Osteoarthritis and Cartilage have suggested that smokers have a lower than expected prevalence of osteoarthritis (OA) than nonsmokers. We review the evidence linking smoking with OA, suggest approaches whereby the direct and indirect effects of smoking on OA might be distinguished, highlight two diseases, ulcerative colitis and Parkinson's disease, where smoking is protective, discuss mechanisms by which nicotine might act and lastly explore the association of smoking with enhanced musculoskeletal pain.
Keywords: Smoking, Nicotine, Osteoarthritis
In analyzing osteoarthritis (OA) data from a large US survey, we noted, even after adjustment for their thin body habitus, that smokers had a decreased prevalence of knee OA compared with non-smokers1. We corroborated this inverse relationship between smoking and OA in the Framingham Study in longitudinal analyses2. This led to a series of studies on smoking and OA.
Why study smoking and OA? Even if smokers were protected against OA, we would never advocate that persons smoke tobacco products given the multitude of negative effects of smoking on health. Rather, if we were to document favorable effects of tobacco on OA, this might provide an opportunity to identify constituents of tobacco smoke that have favorable effects on disease and to test these as treatments.
In this editorial we shall review the evidence that has accumulated since our original report, present new epidemiologic approaches to addressing this question and review associations of smoking with two other diseases where smoking has been found to be protective and with musculoskeletal pain.
Epidemiologic evidence linking smoking with osteoarthritis
A recent meta-analysis3 on smoking and hand, knee, hip and spine OA summarized data from 48 published studies. Overall, there was an inverse relationship between smoking and OA (odds ratio = 0.87; 95% CI, 0.80–0.94). While a protective association of smoking and OAwas seen in all subgroups of studies, it reached significance only in case control studies (where there were more studies) and failed to reach significance in cohort studies. The protective association was especially noteworthy for studies of knee OA where large numbers of subjects were evaluated. Among the case control studies, the association was stronger in hospital-based than community-based studies, with community based studies usually providing less biased findings, but the odds ratio examining the association of smoking with OA was protective in studies in which OA was defined radiographically. No publication bias was found. There was a stronger protective association for current smokers and no association of smoking with OA among past smokers.
Smoking has negative effects on disc disease, and spinal OA studies were included in this meta-analysis; these studies showed that smokers had an increased risk of spinal OA and this biased the meta-analysis toward the null (even though findings were not null). Some studies included adjusted for BMI and others did not. Smokers are thinner than non-smokers, and obesity is a major risk factor for OA. The authors showed that the protective effect was greater when BMI was not adjusted for, but even when BMI was included in analyses, smokers still had a lower risk of OA than non-smokers3. Since this meta-analysis, investigators from a large epidemiologic cohort study from Singapore using as cases persons with total knee replacement for OA have reported in Osteoarthritis and Cartilage also that smokers have a lower risk of OA than non-smokers4.
In our view, the preponderance of evidence suggests that smokers are modestly protected against developing radiographic OA in the knee and hip.
There is no clearcut relationship of current smoking and cartilage loss in those without OA as assessed by MRI. One study5 has suggested that among those from families with an OA history, current smoking accelerated cartilage loss, whereas it had no effect on such loss in others.
Methodologic issues that complicate the study of smoking and OA
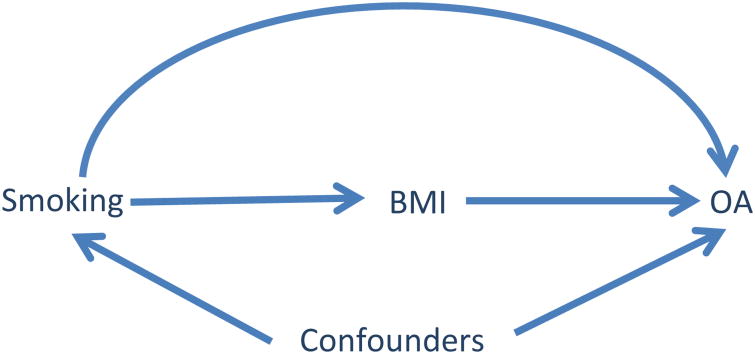
Most epidemiologic studies examining the association between smoking and the risk of OA have adjusted for BMI; this practice, however, may not be appropriate when the goal of the study is to assess the total effect of smoking on the risk of OA. In the causal diagram below [Fig. 1], we assume that there are two mechanisms through which smoking can affect the risk of knee OA: one is Through its effect on BMI (i.e., indirect effect: smoking → BMI → OA); another is smoking's effect on OA not through BMI (i.e., direct effect: smoking → OA). The total effect of smokingon risk of OA is the effect through all potential causal pathways (i.e., smoking → BMI → OA and smoking → OA). Thus, BMI is a mediator, not a confounder. This assumption is supported by empirical evidence which demonstrates that smokers are thinner than non-smokers6. On the other hand, to be a potential confounder, BMI would have to influence a person's smoking status and, to our knowledge, few, if any, studies have found this. Adjusting for BMI blocks one biological mechanism through which smoking affects the risk of OA (i.e., its indirect effect). Consequently, the effect one obtains from such an analysis is a direct effect of smoking (i.e., an effect of smoking on ROA not through its effect on BMI). Ultimately, examining the risk of OA in smokers vs non-smokers without adjusting for BMI provides a better estimate of the total effect of smoking on OA.
Fig. 1.
Causal Diagram depicting interrelationship of smoking, BMI, other covariates (i.e., confounders and other risk factors) and development of OA.
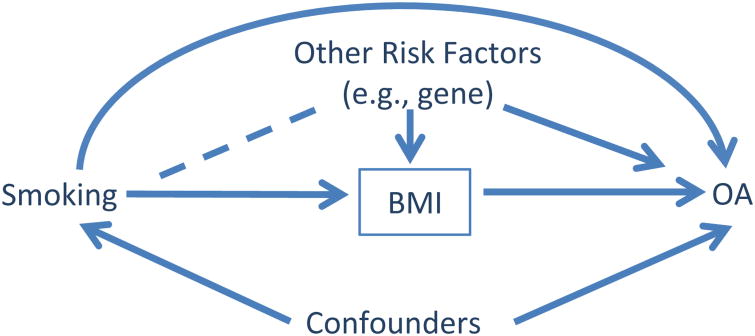
In addition, adjusting for BMI (indicated bya box around ‘BMI’ in Fig. 2) may introduce collider-stratification bias limiting the accuracy of the estimate of the direct effect of smoking on the risk of OA. Studies have shown that smokers tend to have a low BMI. If we found a thin non-smoker, we would expect that that person must have been exposed to other risk factor(s) for thinness (e.g., a gene). Adjusting for BMI at which two causal paths collide, i.e., smoking → low BMI and gene → low BMI, creates a non-causal path from smoking to OA (i.e., smoking — other risk factors → OA). Without appropriately adjusting for other risk factors (e.g., gene), the direct effect of smoking on the risk of OA is susceptible to potential collider-stratification bias7, 8 . Newly developed statistical models can be used to appropriately estimate the direct and indirect effect of a risk factor on OA7, 8 . Furthermore, assessment of the direct effect of smoking on OA may be more relevant from the point of view of treatment implications than evaluating the total effect which includes the indirect effect of smoking on thinness.
Fig. 2.
Causal diagram depicting evaluation of the effect of smoking on risk of ROA. Adjusting for BMI made previously independent variables of smoking and other risk factors correlated as depicted by the dashed line.
Favorable effects of smoking on ulcerative colitis and Parkinson's disease
Cigarette smoke has over 4,500 constituents and Identifying which one causes a given health effect is exceedingly challenging. Immune and inflammatory effects of smoking could be ascribed to a number of constituents, but nicotine's effects are similar to and perhaps stronger than the effects of tobacco smoke in general, suggesting that many of the immune inflammatory effects are nicotine related. Many of nicotine's complex biologic effects are mediated by nicotinic acetylcholine (Ach) receptors on both neuronal and non-neuronal cells9. Cells of the immune system, a major source of inflammatory cytokines, express the main nicotine sensitive acetylcholine receptor, the α7 receptor, and these cells are functionally responsive to nicotine. The net effect of cholinergic stimulation of these receptors is anti-inflammatory, a decreased production of cytokines that mediate the inflammatory response. However, effects may vary by cell type and tissue9.
Investigators examining risk factors for ulcerative colitis noted that smokers had a lower risk of disease than non-smokers10. This was in contrast to Crohn's disease where the opposite effect was seen. Smokers who stopped smoking experienced a flare in ulcerative colitis, and ex-smokers who recommenced smoking noted a reduction in disease activity. Nicotine was implicated as the cause of these changes. Some clinical trials testing nicotine as a therapeutic agent showed that those given nicotine patches experienced a reduction in disease activity and even evidence of histologic improvement in the gastrointestinal mucosa11. Even so, some trial results were not positive and nicotine's side effects limited therapeutic use. Studies are exploring the efficacy of nicotine administered directly to the gastrointestinal tract through enemas to minimize side effects.
Parkinson's disease is a degenerative nervous system motor disorder caused by depletion of dopamine from regions of the brain that control motor function. Non-smoking is among the strongest known disease risk factors. A recent meta-analysis showed a 66% reduction in Parkinson's disease risk among current compared to never-smokers12. While nicotine is a prime candidate as the constituent of smoke that protects against disease, other candidates include TMN which protects against neurodegeneration in mice13. The effect of nicotine appears to be mediated through its effect on neuronal acetylcholine receptors. In non-human primate models, nicotine administration reduces the onset of dyskinesia. Trials are underway in humans testing nicotine preparations.
Effects of smoking on musculoskeletal pain
Smokers more often have musculoskeletal (MSK) pain than non-smokers14 ,15 . Furthermore, among persons with MSK pain, smokers have more severe and persistent pain than non-smokers.
There are several explanations for the association between smoking and chronic pain. First, smoking can lead to downregulation of the hypothalamic pituitary axis which may increase pain sensitivity. Second, the dorsal root ganglion is richly innervated with nicotine sensitive acetylcholine receptors. Lastly, in the central nervous system, nicotine may act through its ability to stimulate the release of excitatory amino acids with pro-nociceptive effects16.
Thus, smoking may have contradictory effects on OA. On the one hand, effects mediated through the nicotine sensitive acetylcholine receptor may help prevent disease. On the other hand, similar receptors on neuronal cells may, when excited, induce musculoskeletal pain. The recent meta-analysis of smoking and mostly radiographic OA reported that smokers had less disease than non-smokers, but interestingly, in this meta-analysis, smokers had a modestly increased risk (OR = 1.25) of painful OA.
Could there be a therapeutic opportunity?
While smokers probably have a lower risk of radiographic OA than non-smokers, smokers also have a higher risk of musculoskeletal pain. The clinical relevance of any smoking effect in OA given the possible adverse effects on symptoms is unclear.
Much remains unresolved in the study of smoking and OA. First, the protective association with radiographic OA is not a strong one and may be altered by analytic approaches that assume that thinness plays a mediating role in the relationship between smoking and OA. Also unresolved is whether any favorable effect of smoking on OA might be mediated differently than an adverse effect of smoking on pain. If the effects of smoking on OA were, for example, not mediated by nicotine but nicotine caused heightened pain sensitivity, then a treatment opportunity might exist.
Acknowledgments
This work was supported by NIH AR47785. The funder had no role in the writing of this paper.
Footnotes
Author contributions: Both authors contributed to writing this paper and both approved the final version.
Conflict of interest: The authors are aware of no conflicts of interest.
Contributor Information
D.T. Felson, Clinical Epidemiology Research & Training Unit, Boston University School of Medicine, Boston, MA, USA NIHR Biomedical Research Unit, University of Manchester, Manchester, UK.
Y. Zhang, Clinical Epidemiology Research & Training Unit, Boston University School of Medicine, Boston, MA, USA
References
- 1.Anderson JJ, Felson DT. Factors associated with osteoarthritis of the knee in the first national Health and Nutrition Examination Survey (HANES I). Evidence for an association with overweight, race, and physical demands of work. Am J Epidemiol. 1988;128:179–89. doi: 10.1093/oxfordjournals.aje.a114939. [DOI] [PubMed] [Google Scholar]
- 2.Felson DT, Anderson JJ, Naimark A, Hannan MT, Kannel WB, Meenan RF. Does smoking protect against osteoarthritis? Arthritis Rheum. 1989;32:166–72. doi: 10.1002/anr.1780320209. [DOI] [PubMed] [Google Scholar]
- 3.Hui M, Doherty M, Zhang W. Does smoking protect against osteoarthritis? Meta-analysis of observational studies. Ann Rheum Dis. 2011;70:1231–7. doi: 10.1136/ard.2010.142323. [DOI] [PubMed] [Google Scholar]
- 4.Leung YY, Ang LW, Thumboo J, Wang R, Yuan JM, Koh WP. Cigarette smoking and risk of total knee replacement for severe osteoarthritis among Chinese in Singapore – the Singapore Chinese health study. Osteoarthr Cartil. 2014;22:764–70. doi: 10.1016/j.joca.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ding C, Cicuttini F, Blizzard L, Jones G. Smoking interacts with family history with regard to change in knee cartilage volume and cartilage defect development. Arthritis Rheum. 2007;56:1521–8. doi: 10.1002/art.22591. [DOI] [PubMed] [Google Scholar]
- 6.Flegal KM, Troiano RP, Pamuk ER, Kuczmarski RJ, Campbell SM. The influence of smoking cessation on the prevalence of overweight in the United States. N Engl J Med. 1995;333:1165–70. doi: 10.1056/NEJM199511023331801. [DOI] [PubMed] [Google Scholar]
- 7.Lange T, Vansteelandt S, Bekaert M. A simple unified approach for estimating natural direct and indirect effects. Am J Epidemiol. 2012;176:190–5. doi: 10.1093/aje/kwr525. [DOI] [PubMed] [Google Scholar]
- 8.Valeri L, Vanderweele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods. 2013;18:137–50. doi: 10.1037/a0031034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Filippini P, Cesario A, Fini M, Locatelli F, Rutella S. The Yin and Yang of non-neuronal alpha7-nicotinic receptors in inflammation and autoimmunity. Curr Drug Targets. 2012;13:644–55. doi: 10.2174/138945012800399008. [DOI] [PubMed] [Google Scholar]
- 10.Lunney PC, Leong RW. Review article: ulcerative colitis, smoking and nicotine therapy. Aliment Pharmacol Ther. 2012;36:997–1008. doi: 10.1111/apt.12086. [DOI] [PubMed] [Google Scholar]
- 11.McGrath J, McDonald JW, Macdonald JK. Transdermal nicotine for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2004:CD004722. doi: 10.1002/14651858.CD004722.pub2. [DOI] [PubMed] [Google Scholar]
- 12.Noyce AJ, Bestwick JP, Silveira-Moriyama L, et al. Meta-analysis of early nonmotor features and risk factors for Parkinson disease. Ann Neurol. 2012;72:893–901. doi: 10.1002/ana.23687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quik M, Perez XA, Bordia T. Nicotine as a potential neuropro-tective agent for Parkinson's disease. Mov Disord. 2012;27:947–57. doi: 10.1002/mds.25028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kvalheim S, Sandven I, Hagen K, Zwart JA. Smoking as a risk factor for chronic musculoskeletal complaints is influenced by age. The HUNT study. Pain. 2013;154:1073–9. doi: 10.1016/j.pain.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 15.Holley AL, Law EF, Tham SW, et al. Current smoking as a predictor of chronic musculoskeletal pain in young adult twins. J Pain. 2013;14:1131–9. doi: 10.1016/j.jpain.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miao FJ, Green PG, Benowitz N, Levine JD. Central terminals of nociceptors are targets for nicotine suppression of inflammation. Neuroscience. 2004;123:777–84. doi: 10.1016/j.neuroscience.2003.10.027. [DOI] [PubMed] [Google Scholar]