SUMMARY
Objective
To determine the association of varus thrust during walking to incident and worsening medial tibiofemoral cartilage damage and bone marrow lesions (BMLs) over 2 years in older adults with or at risk for osteoarthritis (OA).
Method
Subjects from the Multicenter Osteoarthritis Study (MOST) were studied. Varus thrust was visually assessed from high-speed videos of forward walking trials. Baseline and two-year MRIs were acquired from one knee per subject and read for cartilage loss and BMLs. Logistic regression with generalized estimating equations was used to estimate the odds of incident and worsening cartilage loss and BMLs, adjusting for age, sex, race, body mass index (BMI), and clinic site. The analysis was repeated stratified by varus, neutral, and valgus alignment.
Results
1 007 participants contributed one knee each. Varus thrust was observed in 29.9% of knees. Knees with thrust had 2.17 [95% CI: 1.51, 3.11] times the odds of incident medial BML, 2.51 [1.85, 3.40] times the odds of worsening medial BML, and 1.85 [1.35, 2.55] times the odds of worsening medial cartilage loss. When stratified by alignment, varus knees also had significantly increased odds of these outcomes.
Conclusion
Varus thrust observed during walking is associated with increased odds of incident and worsening medial BMLs and worsening medial cartilage loss. Increased odds of these outcomes persist in varus-aligned knees.
Keywords: Osteoarthritis, Varus knee thrust, Gait, Bone marrow lesions, Cartilage loss, MRI
Introduction
The medial compartment of the tibiofemoral joint is the most commonly affected area in knee osteoarthritis (OA). Potentially-modifiable risk factors for medial knee OA are related to increased or abnormal loads to the medial joint compartment. One such risk factor is varus knee thrust, a visible manifestation of excessive varus frontal-plane tibiofemoral motion during the weight-acceptance phase of gait with a return to neutral or less varus alignment in the late-stance phase1. As thrust is potentially modifiable by non-invasive methods such as bracing, muscle strengthening, and gait retraining2, its relation to OA-related structural damage is of interest. Varus thrust has been associated with a four-fold increase in the odds of medial radiographic OA disease progression3. Knees with varus thrust have also been reported to be at least four times more likely to have pain during weight-bearing than those without a varus thrust4.
To date, the relationship of knee thrust to OA risk has only been assessed through radiography1,3. Radiographic osteophytes and joint space narrowing are likely to provide only a coarse measure of the structural damage sustained in the presence of knee thrust. Magnetic resonance imaging (MRI) is a more sensitive measure of structural damage: cartilage damage can be directly visualized on MRI and damage to the bone can also be ascertained through examination of bone marrow lesions (BMLs), which represent traumatic lesions to subcortical bone. These lesions can appear prior to the development of features characteristic of radiographic OA5,6, and therefore detecting these lesions presents an opportunity for early detection and prevention of knee OA.
Varus thrust represents a dynamic malalignment of the knee. Cartilage damage and BML have been previously shown to increase in both frequency and size in response to altered static knee alignment7–10. Varus thrust has also been associated with the external knee adduction moment (KAM), an indicator of medial tibiofemoral load derived from gait analysis3. Prior studies have shown an association between the KAM and the presence of medial BML11,12 and cartilage loss12–14. It is therefore likely that varus thrust will have a similar effect on the development of these lesions. Unlike static alignment or the KAM, thrust can be assessed without the aid of radiographic or gait laboratory equipment; therefore detecting an association between thrust and MRI lesions justifies the use of thrust assessment as an inexpensive alternative to other methods of assessing OA risk.
Our objective was to determine the relation of varus knee thrust observed during walking to MRI-detected incident and worsening medial knee cartilage damage and BMLs in older adults with or at risk for knee OA. We hypothesized that knees with thrust would have higher odds of those outcomes compared to knees without thrust.
Methods
Sample
The Multicenter Osteoarthritis Study (MOST) is a prospective, observational cohort study of knee OA in older Americans that have OA or are at an increased risk of developing it. Factors considered to contribute to an increased risk of knee OA included being overweight; having knee symptoms without radiographic OA; and having a prior knee injury or previous knee surgery. Subjects were recruited from two communities: Birmingham, Alabama, and Iowa City, Iowa. The MOST protocol was approved by the Institutional Review Boards at the University of Iowa; University of Alabama, Birmingham; University of California, San Francisco; and Boston University. Details of the MOST sample, including exclusion criteria, are described elsewhere15.
Gait data were collected from eligible participants who completed the MOST 60-month clinic visit. Participants were instructed to walk across a 4.9-meter pressure-sensitive gait carpet, during repeated trials at a self-selected normal pace. A high-speed (60 Hz) video camera positioned at a fixed distance from the end of the walkway recorded each subject’s gait pattern. The camera was mounted to the wall and its position relative to the walkway was standardized at both clinic sites. GAITRite resident software (GAI-TRite Inc., Clifton, NJ, http://www.gaitrite.com) was used to compute spatiotemporal gait parameters such as walking velocity and step length.
MOST participants in the 60-month gait exam had to be able to walk independently over short indoor distances without the use of a walking aid or orthotic knee brace. Participants with recent (<6 weeks) lower limb injury resulting in restricted weight bearing for over 1 week, recent hospitalization for a cardiovascular or respiratory disorder, lower limb amputation proximal to the toes, or difficulty walking because of a neurological condition were excluded.
Assessment of varus knee thrust
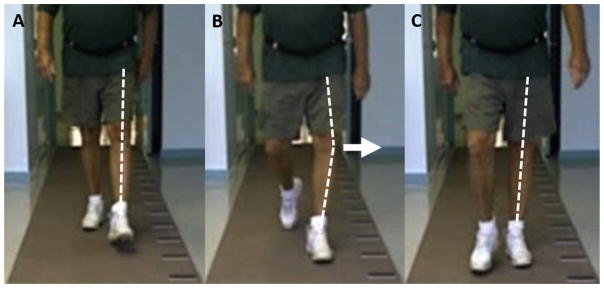
A single trained observer (AW), blinded to knee disease and MRI status, assessed thrust from high-speed videos of participants in the MOST 60-month gait exam during two self-paced walking trials. Participants dressed in short pants and their customary shoes. Skin markers were placed over the centers of the patellae and tibial tuberosities to facilitate visualization of the knee. Knees were excluded from the thrust assessment if a clear view of either marker was obscured by clothing. Thrust was defined as the dynamic worsening or abrupt onset of varus alignment during the weight acceptance phase of gait, with a return to more neutral alignment during the lift-off and swing phases1 (Fig. 1). Thrust presence was graded on a Likert-type scale of “definitely present,” “probably present,” “probably absent,” or “definitely absent.” Further, for knees with thrust “definitely present” or “probably present,” the proportion of steps exhibiting definite or probable thrust was noted as thrust during “all steps,” “greater than half (but not all) of steps,” or “fewer than half of steps.” For the purposes of the current study, a simplified dichotomous variable was defined, wherein thrust was considered present when thrust was graded “definitely present” during any (≥1) steps or “probably present” during “all steps.” A randomly-selected subsample of 150 knees (with balanced representation of the two clinic sites) underwent blinded reassessment, revealing substantial intra-rater reliability for the dichotomous variable of varus thrust (κ = 0.73; 95% CI 0.63, 0.84).
Fig. 1.
Assessment of varus thrust from high-speed video. The subject’s left knee is in a neutral position during early stance (A), abruptly thrusts into varus during mid-stance (B), and then returns to neutral during late stance (C). Dotted lines are for illustrative purposes only and do not represent actual joint angles.
MRI acquisition
Subjects in the MOST study underwent MRI of bilateral knees with a 1.0T extremity magnetic resonance system (OrthOne; ONI Medical Systems, Wilmington, MA) at 60 and 84 months. All MRIs were acquired using fat suppressed, fast spin-echo, proton density-weighted sequences in the sagittal plane (repetition time [TR] 4 800 ms, time to echo [TE] 35 ms, slice thickness 3 mm, interslice gap 0 mm, field of view [FOV] 14 cm × 14 cm, matrix 288 × 192 pixels, number of excitations [NEX] 2) and the axial plane (TR 4 700 ms, TE 13.2 ms, slice thickness 3 mm, interslice gap 0 mm, FOV 14 cm × 14 cm, matrix 288 × 192 pixels, NEX 2) and a short-tau inversion recovery (STIR) sequence in the coronal plane (TR 7 820 ms, TE 14 ms, inversion time 100 ms, slice thickness 3 mm, interslice gap 0 mm, FOV 14 cm × 14 cm, matrix 256 × 256 pixels, NEX 2)16.
Assessment of cartilage loss and BMLs
To assess cartilage loss, two musculoskeletal radiologists (AG and FWR) with 15 and 13 years of experience in semiquantitative MRI analysis, respectively, scored one knee per subject using the Whole-Organ MRI Score (WORMS) for knee OA17. Where high quality MR images were available from both the 60 and 84 month exams for both knees of a subject, the one knee to be read was selected at random. Inter-reader weighted kappa values for WORMS scoring ranged from 0.62 (95% CI 0.57, 0.68) for BML to 0.78 (95% CI 0.76, 0.81) for cartilage16. For each knee, five medial tibiofemoral sub-regions were scored. We assessed incident cartilage loss for sub-regions with a WORMS score of 0 (normal thickness) or 1 (normal thickness but increased signal) at baseline, and defined incident cartilage damage as a WORMS score ≥ 2 at 2 years. Worsening cartilage damage was defined as any increase in WORMS score over 2 years, including incidence and within-grade worsening. Sub-regions were excluded from analysis if they had the maximum WORMS score at 60 months, as there could theoretically be no progression. To investigate more definitive changes in cartilage damage, we repeated this analysis using a stricter definition of progression: a full-grade or greater increase in WORMS score.
Subchondral BMLs were scored from 0 to 3 based on the extent of involvement for each of five medial tibiofemoral sub-regions (0 = none; 1 ≤ 25% of the sub-region; 2 = 25–50%; 3 ≥ 50%). A within-grade change of BML was also recorded, which designated definite change that did not fulfil criteria for a full-grade change in BML score18. For sub-regions with a score of 0 at baseline, BML incidence was defined as an increase in score over 2 years. Among knees with a sub-maximal BML score at 60 months, BML enlargement (worsening) was defined as any increase in score over 2 years, including incidence.
Assessment of static knee alignment
Mechanical hip–knee–ankle (HKA) alignment was assessed at the MOST 60-month visit from full-view, fully-extended, weight-bearing anterior–posterior radiographs of the lower extremity. The HKA angle was defined as the angle formed by the intersection of a line from the center of the head of a femur to the center of the tibial spines and a second line from the center of the talus to the center of the tibial spines. Varus alignment was defined as a mechanical HKA angle less than 179°; knees with HKA angles between 179 and 181° were considered neutral; and knees with HKA angles greater than 181° were considered valgus.
Statistical analysis
We evaluated the odds of incident and worsening tibiofemoral joint damage (i.e., cartilage loss and BML) in the presence of varus thrust using logistic regression with an adjustment for age, sex, race, body mass index (BMI), and clinic site. Generalized estimating equations were used to account for correlation between multiple sub-regions within a single knee. In sensitivity analysis to determine the whether the relationship of varus thrust to risk of MRI outcomes was modified by the presence of static varus malalignment, the main analysis was repeated separately within varus and non-varus alignment strata, and a multiplicative interaction term was added to the model. Results from each logistic regression model are reported as odds ratios (ORs) with associated 95% confidence intervals (CIs). Statistical analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC).
Results
Of 2 768 participants in the MOST 60-month clinic visit, 2 049 met eligibility criteria for completion of the gait exam. Of these, 1 007 subjects had readable videos for assessment of knee thrust along with readable MRIs at 60-month (baseline) and 84-month follow-up visits. These 1 007 subjects contributed one knee each (Fig. 2), with a total of 5 035 knee sub-regions available for the subregion-based analysis. At baseline, 85 sub-regions had maximal WORMS scores for cartilage damage while 44 sub-regions had maximal scores for BML; these sub-regions were excluded from analyses of worsening damage. Varus thrust was observed in 29.9% of eligible knees. Of the 301 knees with thrust, 161 (53.5%) were graded as thrust “definitely present on any steps” and 140 (46.5%) were graded as thrust “probably present on all steps.” Characteristics of the study sample are presented in Table I. Subjects with varus thrust were slightly older than subjects without thrust (P = 0.046), and the proportion of males to females was higher in the group with thrust than in the group without thrust (P < 0.000 1). A larger proportion of knees with thrust had radiographic tibiofemoral OA (defined as Kellgren–Lawrence grade ≥ 2) compared to knees without thrust (P = 0.000 7), and the mean HKA angle in knees with thrust was more varus than in knees without thrust (P < 0.000 1).
Fig. 2.
Study subject selection flowchart.
Table I.
Demographics of study participants (n = 1 007 subjects contributing 1 knee each)
| With varus thrust (n = 301) | Without varus thrust (n = 706) | |
|---|---|---|
| Age, years (mean ± S.D.) | 67.4 ± 7.6 | 66.4 ± 7.5 |
| Sex (% female) | 49.8 | 67.8 |
| Racial background | ||
| - White/caucasian (%) | 93.7 | 90.9 |
| - Black/African American (%) | 5.3 | 8.1 |
| - Other (%) | 1.0 | 1.0 |
| BMI, kg/m2 (mean ± S.D.) | 29.8 ± 4.9 | 29.3 ± 4.7 |
| Site (% Alabama) | 32.7 | 41.2 |
| Radiographic tibiofemoral OA* (%) | 45.5 | 34.2 |
| - KL = 2 (%) | 16.6 | 18.8 |
| - KL = 3 (%) | 22.9 | 14.5 |
| - KL = 4 (%) | 6.0 | 1.0 |
| HKA Angle, degrees (mean ± S.D.) | 177.5 ± 3.2 | 179.0 ± 3.0 |
Defined as Kellgren–Lawrence (KL) grade ≥ 2.
As shown in Table II, knees with varus thrust had 2.17 times the odds of medial compartment BML incidence at 2 years (95% CI: 1.51, 3.11) after adjusting for covariates. However, there was no statistically significant association between varus thrust and incident medial cartilage damage. Knees with varus thrust had 2.51 times the odds of medial BML worsening (95% CI: 1.85, 3.40) and 1.85 times the odds of worsening medial cartilage damage (95% CI: 1.35, 2.55) after adjusting for covariates. Further adjustment for baseline Kellgren–Lawrence grade attenuated these results somewhat, but did not alter either their direction or statistical significance. Results were similar when a stricter definition of worsening (at least a full grade WORMS increase) was applied (results not shown).
Table II.
Odds of incident and worsening MRI lesions in the presence of varus knee thrust
| Outcome | n/N* | Crude OR (95% CI) | P | Adjusted OR† (95% CI) | P | Adjusted OR‡ (95% CI) | P |
|---|---|---|---|---|---|---|---|
| Incident medial BML | 173/4 399 | 2.10 (1.48, 2.97) | <0.000 1 | 2.17 (1.51, 3.11) | <0.000 1 | 2.01 (1.42, 2.84) | <0.000 1 |
| Incident medial cartilage loss | 118/3 395 | 0.78 (0.47, 1.30) | 0.35 | 0.77 (0.45, 1.29) | 0.32 | 0.75 (0.45, 1.26) | 0.28 |
| Worsening medial BML | 346/4 987 | 2.43 (1.82, 3.24) | <0.000 1 | 2.51 (1.85, 3.40) | <0.000 1 | 2.01 (1.52, 2.66) | <0.000 1 |
| Worsening medial cartilage loss | 379/4 950 | 1.77 (1.30, 2.41) | 0.000 3 | 1.85 (1.35, 2.55) | 0.000 2 | 1.60 (1.17, 2.19) | 0.003 |
Bold indicates statistical significance, either Odds Ratio that excludes 1.00 or P < 0.05.
Sub-regions with outcome/total number of sub-regions analyzed.
Adjusted for age, sex, race, BMI, and clinic site.
Adjusted for age, sex, race, BMI, clinic site, and baseline KL grade.
To determine whether the relationship between thrust and MRI outcomes was modified in the presence of static varus alignment, we repeated each of the main analyses within separate strata of varus and non-varus HKA alignment and introduced an interaction term into our multivariable regression model. Of 1 007 knees, 576 were varus aligned, and 431 were non-varus (236 were neutral and 195 were valgus). After adjusting for covariates, we found statistically-significant increased odds of incident medial BMLs (OR 2.62; 95% CI: 1.67, 4.10), worsening medial BMLs (OR 2.44; 95% CI: 1.74, 3.42) and worsening medial cartilage loss (OR 1.89; 95% CI: 1.30, 2.75) in varus knees with thrust compared to varus knees without thrust. Among non-varus knees, however, while relationships were in a similar direction, point estimates of the increased odds associated with thrust were of a smaller magnitude and failed to achieve statistical significance (see Table III). When examined separately, neutral- and valgus-aligned knees showed no significant relationships with these MRI outcomes (results not shown). The interaction test results were not statistically significant for incident medial cartilage loss (P = 0.87), worsening medial BML (P = 0.12), and worsening medial cartilage loss (P = 0.40), though the results neared significance for incident medial BML (P = 0.07).
Table III.
Adjusted odds of incident and worsening MRI lesions in the presence of varus knee thrust in varus and non-varus knees with a test for interaction
| Outcome | Varus knees (HKA <179°) with varus thrust
|
Non-varus knees (HKA ≥ 179°) with varus thrust
|
Test for interaction | ||||
|---|---|---|---|---|---|---|---|
| n/N* | OR† (95% CI) | P | n/N | OR† (95% CI) | P | ||
| Incident medial BML | 108/2 232 | 2.62 (1.67, 4.10) | 0.000 1 | 62/2 021 | 1.33 (0.73, 2.40) | 0.35 | P = 0.07 |
| Incident medial cartilage loss | 50/1 584 | 0.82 (0.39, 1.72) | 0.60 | 63/1 695 | 0.76 (0.36, 1.59) | 0.46 | P = 0.87 |
| Worsening medial bml | 248/2 664 | 2.44 (1.74, 3.42) | 0.000 1 | 89/2 148 | 1.42 (0.77, 2.60) | 0.26 | P = 0.12 |
| Worsening medial cartilage loss | 222/2 628 | 1.89 (1.30, 2.75) | 0.000 8 | 140/2 149 | 1.40 (0.75, 2.61) | 0.29 | P = 0.40 |
Bold indicates statistical significance, either Odds Ratio that excludes 1.00 or P < 0.05.
Sub-regions with outcome/total number of sub-regions analyzed.
Adjusted for age, sex, race, BMI, and clinic site.
Discussion
Varus knee thrust presence visualized during walking was associated with increased odds of incident and worsening BMLs and with increased odds of worsening cartilage damage after adjusting for age, sex, race, BMI, and clinic site. There was no statistically significant association found between varus thrust and incident cartilage damage. It is important to note that the assessment of thrust and our definition of “baseline” took place 60 months (5 years) into the MOST study, and therefore these results are perhaps evidence of a “depletion-of-susceptibles” effect, wherein knee sub-regions that had not developed cartilage damage by that point in the MOST study were perhaps not likely to develop it at all. This same effect would have had less influence on results pertaining to the risk that existing damage might worsen in the presence of thrust.
In sensitivity analysis, we found that varus-aligned knees with thrust had increased odds of incident and worsening BML and worsening cartilage damage compared to varus-aligned knees without thrust. In contrast, the effect of thrust on risk of these outcomes was not statistically significant among non varus-aligned knees. These results are similar to the findings of Chang et al.3 who saw a three-fold increase in the odds of radiographic OA progression in varus-aligned knees with varus thrust compared to varus-aligned knees without thrust. Furthermore, Lo et al.4 and Iijima et al.19 reported that varus thrust was more strongly correlated with knee pain than was static varus malalignment alone. Considered together, these findings suggest that dynamic malalignment (i.e., thrust) has potential to compound the trauma placed on the tibiofemoral joint by static malalignment. Unfortunately, our test for statistical interaction may have been under-powered (evident by wide CIs in the stratified analysis compared to the main analysis), and we could not confirm that the effects of varus thrust on risk of structural damage are modified by the presence of static knee malalignment.
Previous authors have found significant associations between varus knee thrust and knee pain4,19. Our findings of an association between thrust and incident and worsening BMLs suggest a potential mechanism for the relation of thrust to knee pain. BMLs are correlated with knee pain in OA20,21, and BMLs are hypothesized to be the source of this pain due to the presence of nociceptive fibers in the bone marrow. BMLs are thought to be the result of ongoing local bone trauma associated with malalignment7,22. The repetitive loading created by thrust could cause such an injury and elicit a pain response in bone. Further investigation into the role of thrust in the development of knee pain is required.
Among knees without radiographic OA, Guermazi et al.5 found a high prevalence of MRI detected features, and Sharma et al.6 found that worsening MRI lesions were associated with incident radiographic OA over 3 years. In knees with OA, knees with medial BMLs had over six times the odds of medial disease progression compared to knees without BMLs7. The association of thrust with MRI lesions presents an opportunity to identify those without, but at risk for, or at the early stages of radiographic knee OA.
Hunt and Bennell23 identified factors correlated with the peak KAM and therefore indicative of increased knee joint loading that could be easily identified in the clinic. These factors included body mass, tibial alignment, and walking speed. Visually-observed varus knee thrust has been shown to be correlated with several quantitatively-derived gait variables including external KAM, peak knee varus angular velocity, and peak knee varus angle during stance2,3,24,25. Visual detection of varus thrust is another reliable alternative to expensive gait analysis to detect increased loading to the medial knee joint.
OA risk factors resulting from increased mechanical loading are potentially modifiable using noninvasive and inexpensive therapies26,27. Hunt et al.2 employed various gait-related interventions (increased toe-out, ipsilateral trunk lean, custom-made orthotics, and lateral-wedge insoles) known to reduce medial joint load in a single subject with varus thrust. While thrust was still evident following these modifications, the magnitude of the thrust as well as the peak KAM was reduced in response to increased toe-out and trunk lean. Bennell et al.28 found that a neuromuscular exercise regime focusing on trunk and lower extremity position and movement quality improved pain and physical function in those with thrust, though thrust during the course of the exercise intervention was not assessed. As an alternative to these methods that require the patient to adopt a new gait pattern or exercise regime, valgus bracing of the knee has also been shown to reduce medial knee loads and the moments of force associated with varus thrust during walking29. Further research regarding the specific causes of knee thrust is necessary to better develop strategies to mitigate thrust.
This study’s strengths include the large sample size as well as its longitudinal design. Limitations are those inherent to studies relying on visual assessment of gait. While visual assessment of thrust from high-speed videos yielded high intra-rater reliability, conditions in the clinic setting (e.g., lighting, camera angle relative to subject) as well as conditions of MOST participants (e.g., body mass, walking a non-straight path) could have interfered with our ability to accurately detect the presence of varus thrust. Chang et al.25 found that thrust was not only related to peak knee varus angle, but also to peak knee varus angular velocity. While the varus position of the knee can be visualized, it is not possible to accurately estimate the varus angle or assess the angular velocity visually. The non-quantitative nature of the thrust assessment also limits our ability to make conclusions about altered joint loading. For these reasons, this method may not be ideal for precise assessment of the effects of thrust-reducing interventions in clinical trials; however, our method for detecting thrust (and subsequent OA risk) is likely similar to what might be employed in a clinic setting where quantitative testing methods are not available. A second limitation is that thrust was assessed by only one observer, and therefore this study lacks inter-rater reliability data. Using a similar protocol to ours, Iijima et al.19 reported good inter-rater reliability (κ = 0.73) for visual assessment of thrust. While we report strong intra-rater reliability, having multiple readers with varying levels of experience would strengthen our findings. A third limitation is that our static alignment subgroup analysis (i.e., test for interaction) may have been underpowered. To increase power in our analysis, we combined neutral- and valgus-aligned knees into one “non-varus” category; however, it would be of interest to examine the relationships of thrust to MRI outcomes in these separate strata in a larger sample.
In summary, our results indicate that varus thrust is a risk factor for worsening cartilage loss and BMLs, as well as for BML incidence. The odds of these outcomes persist in knees that are already statically-varus aligned, suggesting that interventions to mitigate varus thrust (and subsequent OA risk) should target these individuals.
Acknowledgments
Role of the funding source
MOST is funded by the NIH and NIA (Felson – AG18820, AR47785; Torner – AG18832; Lewis – AG18947; Nevitt – AG19069). This research was supported in part by the Rheumatology Research Foundation Health Professional Research Preceptorship and the Boston University Department of Anatomy and Neurobiology.
The authors would like to acknowledge all participants in the MOST study for their contributions.
Footnotes
Supported by NIH AR47785 and MOST U01-AG18820, AG18832, AG18947, and AG19069.
Author contributions
All authors participated in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. All authors can take responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design: Wink, Gross, Felson.
Acquisition of data: Wink, Guermazi, Roemer, Torner, Lewis, Nevitt, Tolstykh, Felson.
Analysis and Interpretation of data: All authors.
Competing interests
Ali Guermazi has received consultancies, speaking fees, and/or honoraria from Sanofi-Aventis, Merck Serono, OrthoTrophix, AstraZeneca, and TissuGene and is President and shareholder of Boston Imaging Core Lab (BICL), LLC.
Frank W. Roemer is Chief Medical Officer and shareholder of BICL, LLC.
References
- 1.Chang A, Hochberg M, Song J, Dunlop D, Chmiel JS, Nevitt M, et al. Frequency of varus and valgus thrust and factors associated with thrust presence in persons with or at higher risk of developing knee osteoarthritis. Arthritis Rheum. 2010;62:1403–41. doi: 10.1002/art.27377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hunt MA, Schache AG, Hinman RS, Crossley KM. Varus thrust in medial knee osteoarthritis: quantification and effects of different gait-related interventions using a single case study. Arthritis Care Res. 2011;63:293–7. doi: 10.1002/acr.20341. [DOI] [PubMed] [Google Scholar]
- 3.Chang A, Hayes K, Dunlop D, Hurwitz D, Song J, Cahue S, et al. Thrust during ambulation and the progression of knee osteoarthritis. Arthritis Rheum. 2004;50:3897–903. doi: 10.1002/art.20657. [DOI] [PubMed] [Google Scholar]
- 4.Lo GH, Harvey WF, McAlindon TE. Associations of varus thrust and alignment with pain in knee osteoarthritis. Arthritis Rheum. 2012;64:2252–9. doi: 10.1002/art.34422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guermazi A, Niu J, Hayashi D, Roemer FW, Englund M, Neogi T, et al. Prevalence of abnormalities in knees detected by MRI in adults without knee osteoarthritis: population based observational study (Framingham Osteoarthritis Study) BMJ. 2012;345:e5339. doi: 10.1136/bmj.e5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma L, Nevitt M, Hochberg M, Guermazi A, Roemer FW, Crema M, et al. Clinical significance of worsening versus stable preradiographic MRI lesions in a cohort study of persons at higher risk for knee osteoarthritis. Ann Rheumatic Dis. 2015;0:1–7. doi: 10.1136/annrheumdis-2015-208129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Felson DT, McLaughlin S, Goggins J, LaValley MP, Gale E, Totterman S, et al. Bone marrow edema and its relation to progression of knee osteoarthritis. Ann Intern Med. 2003;139:330–6. doi: 10.7326/0003-4819-139-5_part_1-200309020-00008. [DOI] [PubMed] [Google Scholar]
- 8.Hunter DJ, Zhang Y, Niu J, Goggins J, Amin S, LaValley MP, et al. Increase in bone marrow lesions associated with cartilage loss: a longitudinal magnetic resonance imaging study of knee osteoarthritis. Arthritis Rheum. 2006;54:1529–35. doi: 10.1002/art.21789. [DOI] [PubMed] [Google Scholar]
- 9.Sharma L, Eckstein F, Song J, Guermazi A, Prasad P, Kapoor D, et al. Relationship of meniscal damage, meniscal extrusion, malalignment, and joint laxity to subsequent cartilage loss in osteoarthritic knees. Arthritis Rheum. 2008;58:1716–26. doi: 10.1002/art.23462. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi D, Englund M, Roemer FW, Niu J, Sharma L, Felson DT, et al. Knee malalignment is associated with an increased risk for incident and enlarging bone marrow lesions in the more loaded compartments: the MOST study. Osteoarthritis Cartilage. 2012;20:1227–33. doi: 10.1016/j.joca.2012.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bennell KL, Creaby MW, Wrigley TV, Bowles K-A, Hinman RS, Cicuttini F, et al. Bone marrow lesions are related to dynamic knee loading in medial knee osteoarthritis. Ann Rheumatic Dis. 2010;69:1151–4. doi: 10.1136/ard.2009.118182. [DOI] [PubMed] [Google Scholar]
- 12.Chang AH, Moisio KC, Chmiel JS, Eckstein F, Guermazi A, Prasad PV, et al. External knee adduction and flexion moments during gait and medial tibiofemoral disease progression in knee osteoarthritis. Osteoarthritis Cartilage. 2015;23:1099–106. doi: 10.1016/j.joca.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Creaby MW, Wang Y, Bennell KL, Hinman RS, Metcalf BR, Bowles K-A, et al. Dynamic knee loading is related to cartilage defects and tibial plateau bone area in medial knee osteoarthritis. Osteoarthritis Cartilage. 2010;18:1380–5. doi: 10.1016/j.joca.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 14.Bennell KL, Bowles K-A, Wang Y, Cicuttini F, Davies-Tuck M, Hinman RS. Higher dynamic medial knee load predicts greater cartilage loss over 12 months in medial knee osteoarthritis. Ann Rheumatic Dis. 2011;70:1770–4. doi: 10.1136/ard.2010.147082. [DOI] [PubMed] [Google Scholar]
- 15.Segal NA, Nevitt MC, Gross KD, Hietpas J, Glass NA, Lewis CE, et al. The Multicenter Osteoarthritis Study: opportunities for rehabilitation research. Phys Med Rehabilitation. 2013;5:647–54. doi: 10.1016/j.pmrj.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Javaid MK, Lynch JA, Tolstykh I, Guermazi A, Roemer F, Aliabadi P, et al. Pre-radiographic MRI findings are associated with onset of knee symptoms: the MOST study. Osteoarthritis Cartilage. 2010;18:323–8. doi: 10.1016/j.joca.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peterfy CG, Guermazi A, Zaim S, Tirman PFJ, Miaux Y, White D, et al. Whole-organ magnetic resonance imaging score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12:177–90. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Roemer FW, Nevitt MC, Felson DT, Niu J, Lynch JA, Crema MD, et al. Predictive validity of within-grade scoring of longitudinal changes of MRI-based cartilage morphology and bone marrow lesion assessment in the tibio-femoral joint–the MOST study. Osteoarthritis Cartilage. 2012;20:1391–8. doi: 10.1016/j.joca.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iijima H, Fukutani N, Aoyama T, Fukumoto T, Uritani D, Kaneda E, et al. Clinical phenotype classifications based on static varus alignment and varus thrust in Japanese patients with medial knee osteoarthritis. Arthritis Rheum. 2015;67:2354–62. doi: 10.1002/art.39224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Felson DT, Chalsson CE, Hill CL, Totterman SMS, Gale E, Skinner KM, et al. The association of bone marrow lesions with pain in knee osteoarthritis. Ann Intern Med. 2001;134:541–9. doi: 10.7326/0003-4819-134-7-200104030-00007. [DOI] [PubMed] [Google Scholar]
- 21.Felson DT, Niu J, Guermazi A, Roemer F, Aliabadi P, Clancy M, et al. Correlation of the development of knee pain with enlarging bone marrow lesions on magnetic resonance imaging. Arthritis Rheum. 2007;56:2986–92. doi: 10.1002/art.22851. [DOI] [PubMed] [Google Scholar]
- 22.Lo GH, Hunter DJ, Zhang Y, McLennan CE, LaValley MP, Kiel DP, et al. Bone marrow lesions in the knee are associated with increased local bone density. Arthritis Rheuma. 2005;52:2814–21. doi: 10.1002/art.21290. [DOI] [PubMed] [Google Scholar]
- 23.Hunt MA, Bennell KL. Predicting dynamic knee joint load with clinical measures in people with medial knee osteoarthritis. Knee. 2011;18:231–4. doi: 10.1016/j.knee.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 24.Kuroyanagi Y, Nagura T, Kiriyama Y, Matsumoto H, Otani T, Toyama Y, et al. A quantitative assessment of varus thrust in patients with medial knee osteoarthritis. Knee. 2012;19:130–4. doi: 10.1016/j.knee.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 25.Chang AH, Chmiel JS, Moisio KC, Almagor O, Zhang Y, Cahue S, et al. Varus thrust and knee frontal plane dynamic motion in persons with knee osteoarthritis. Osteoarthritis Cartilage. 2013;21:1668–73. doi: 10.1016/j.joca.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fregly BJ, D’Lima DD, Colwell CW. Effective gait patterns for offloading the medial compartment of the knee. J Orthop Res. 2009;27:1016–21. doi: 10.1002/jor.20843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barrios JA, Krossley KM, Davis IS. Gait retraining to reduce the knee adduction moment through real-time visual feedback of dynamic knee alignment. J Biomech. 2010;43:2208–13. doi: 10.1016/j.jbiomech.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bennell KL, Dobson F, Roos WM, Skou ST, Hodges P, Wrigley TV, et al. Influence of biomechanical characteristics on pain and function outcomes from exercise in medial knee osteoarthritis and varus malalignment: exploratory analyses from a randomized controlled trial. Arthritis Care Res. 2015;67:1281–8. doi: 10.1002/acr.22558. [DOI] [PubMed] [Google Scholar]
- 29.Pollo FE, Otis JC, Backus SI, Warren RF, Wickiewicz TL. Reduction of medial compartment loads with valgus bracing of the osteoarthritic knee. Am J Sports Med. 2002;30:414–21. doi: 10.1177/03635465020300031801. [DOI] [PubMed] [Google Scholar]