Abstract
Progressive multifocal leukoencephalopathy (PML) is a deadly demyelinating disease due to central nervous system replication of the human polyomavirus JC virus (JCV) in immunosuppressed patients. The only effective therapeutic approach is to restore anti-JCV T-cell responses. In this study, we describe a case of rapidly fatal PML with JCV T-cell anergy in a renal transplant patient treated with CTLA4-Ig (belatacept, a CD28-B7 costimulation blocker and T-cell anergy inducer). T-cell anergy could not be reversed despite several therapeutic approaches. Progressive multifocal leukoencephalopathy secondary to biotherapy-induced T-cell anergy may thus represent a subset of PML with major resistance to anti-JCV immune recovery.
Keywords: JCV-specific T cells, progressive multifocal leukoencephalopathy, transplantation
Progressive multifocal leukoencephalopathy (PML) is a rare but deadly demyelinating disease of the central nervous system. The causative agent is the human polyomavirus JC virus (JCV), which infects oligodendrocytes and astrocytes [1]. Progressive multifocal leukoencephalopathy usually occurs in patients with profound cellular immunodeficiencies, due to human immunodeficiency virus (HIV) infection or to immunosuppressive biotherapies such as natalizumab or rituximab for autoimmune diseases [1, 2]. Progressive multifocal leukoencephalopathy is also a rare complication of solid organ transplantation. After renal transplantation, the estimated incidence of PML is 0.027%, the mortality rate is approximately 80%, and the median survival time is 6 months [3]. The only therapeutic approach with proven clinical efficacy is the restoration of JCV T-cell responses [1] through early withdrawal of immunosuppressive drugs. In this study, we describe a case of anti-JCV T-cell anergy that could not be reversed despite several therapeutic approaches and was associated with rapidly fatal PML in a renal transplant recipient on belatacept (a CD28-B7 costimulation blocker and T-cell anergy inducer).
CASE REPORT
Preprogressive Multifocal Leukoencephalopathy History
A 67-year-old white man who had been on hemodialysis since July 2012 for immunoglobulin (Ig)A nephropathy received a deceased-donor kidney transplant in October 2013. The immunosuppressive regimen included basiliximab induction and belatacept/mycophenolate mofetil (MMF)/corticosteroid maintenance. Belatacept was used because the kidney came from an extended-criteria donor [4]. Belatacept was administered intravenously, as recommended for the less intensive regimen (10 mg/kg on day 1 (D1), D5, week 2 (W2), W4, W8, W12; then beyond the third month, 5 mg/kg per month) [4]. During the posttransplantation period, the patient experienced multiple complications of viral reactivation, including cytomegalovirus disease at 7 months treated with valganciclovir; BK virus (BKV) reactivation at month 9 without polyomavirus-associated nephropathy on biopsy leading to a reduction in the MMF dosage (500 mg twice a day); and Epstein-Barr virus viremia at month 16 with no signs of a posttransplant lymphoproliferative disorder. Fourteen months after transplantation, he received pulsed intravenous corticosteroids for cellular rejection (grade Ib). The estimated glomerular filtration rate stabilized at 53 mL/min per 1.73 m2 (Modification of Diet in Renal Disease formula). He had persistent lymphopenia, with median CD4 and CD8 T-cell counts of 183/µL and 115/µL, respectively.
Progressive Multifocal Leukoencephalopathy
In June 2015, 20 months after renal transplantation, he was admitted to Bicêtre hospital for left upper-limb weakness and visual disturbances. His family described mood disorders and confusion, which had started the month before. He developed progressive right-sided hemiparesis with temporospatial disorientation. Magnetic resonance imaging (MRI) revealed left frontoparietal subcortical white-matter lesions, without gadolinium enhancement, suggesting no vascular disease occurrence. These lesions were consistent with demyelination and suggested PML (Figure 1A). Progressive multifocal leukoencephalopathy was confirmed by the presence of JCV deoxyribonucleic acid in cerebrospinal fluid ([CSF] 7.15 × 103 copies/mL) ( Figure 1A). At PML diagnosis, he had lymphopenia (421 CD3 cells/µL), with 276 CD4 cells/µL and 145 CD8 cells/µL (Figure 1A). He was HIV seronegative. Mycophenolate mofetil was stopped when PML was confirmed. The last injection of belatacept was given 2 days before his admission. Because of the 2-week half-life of belatacept, we performed 2 plasma exchanges to hasten belatacept elimination, on D6 and D11 after PML diagnosis (Figure 1A). An intravenous perfusion of polyclonal Igs (0.5 g/kg per day for 2 days) was given for mild hypogammaglobulinemia (IgG level: 6 g/L). He rapidly developed complete right hemiplegia with aphasia, cortical blindness, and no understanding of simple orders. The PML worsened and the patient became bedridden. JCV viral load in CSF increased (1.67 × 104 copies/mL on D23), whereas on D33, his brain lesions deteriorated on MRI (Fig.1A). The CD4 T-cell count increased 12 days after PML diagnosis, but CD8 lymphopenia (180 CD8 cells/µL) persisted (Figure 1A). Interleukin (IL)-7 treatment was then started, with approval from the French National Agency of Medicine and Health Products Safety (ANSM). A single cycle of recombinant human IL-7 (r-hIL-7) was administered, comprising 3 subcutaneous injections on D28, D35, and D42 after PML diagnosis (Figure 1A). Each injection delivered 20 µg/kg, for a total dose of 1200 µg per injection. His clinical neurological status worsened, with onset of coma on D41. JC virus viral load in CSF remained stable (1.45 × 104 copies/mL on D56) (Figure 1A). He died of respiratory failure 69 days after PML diagnosis.
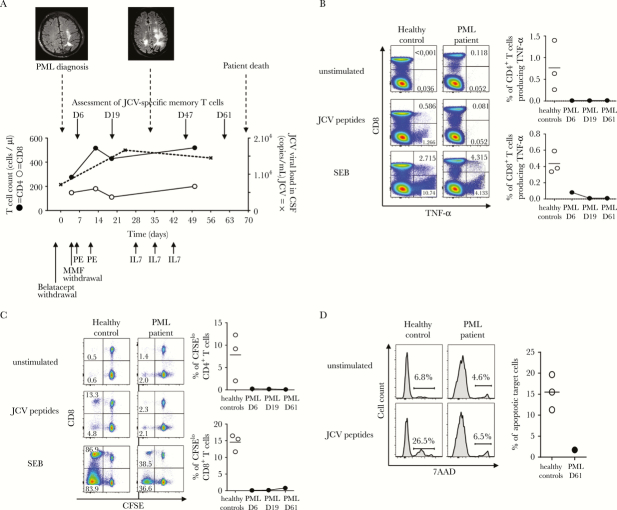
Figure 1.
Clinical, neuroradiological, and immunobiological monitoring of progressive multifocal leukoencephalopathy (PML). (A) shows the time course of PML, with fluid attenuation inversion recovery-sequence magnetic resonance imaging aspect at PML onset and 33 days later, the different therapeutic approaches, as well as changes in the CD4 T-cell count (closed circles), the CD8 T-cell count (open circles), and JC virus (JCV) viral load in cerebrospinal fluid (dashed line). (B–D) illustrate anergy of anti-JCV T-cell responses. Peripheral blood mononuclear cells were incubated in Roswell Park Memorial Institute medium alone (unstimulated cells) or with overlapping JCV peptides or Staphylococcal enterotoxin B (SEB). T-cell functionality was evaluated in the PML patient and in representative healthy donors responsive to JCV. (B) Tumor necrosis factor (TNF)-α production in response to JCV peptides was evaluated by intracellular staining. Numbers indicate the percentages of CD8+ or CD8− (CD4+) T cells expressing TNF-α. Similar results were found for interferon-γ (data not shown). (C) Proliferation in response to JCV peptides was measured by carboxyfluorescein diacetate succinimidyl ester (CFSE) dilution. Numbers indicate the percentage of CFSElow cells among CD8+ or CD8− (CD4+) T cells. (D) Cytotoxicity for JCV-coated target cells was measured in terms of 7-amino-actinomycin D (AAD) uptake. Results are expressed as the percentage of 7-AAD-positive target cells. (B–D) show the lack of improvement in JCV-specific T-cell functions during the course of PML (from D6 to D61). The frequencies of responsive JCV-specific T cells were calculated by subtracting the frequency of cells detected in the absence of stimulation, to correct for background staining. The horizontal bar indicates the median value obtained with cells from 3 healthy donors responsive to JCV. Abbreviations: CSF, cerebrospinal fluid; IL, interleukin; PE, plasma exchanges.
Immunovirological Monitoring
On D6, D19, D47, and D61 after PML diagnosis, we analyzed the phenotype of lymphocytes and their ability to functionally respond to JCV antigens. As controls, we used 3 healthy donors who were responsive to JCV. Peripheral blood mononuclear cells (PBMCs) were incubated overnight with a pool of JCV peptides spanning the entire VP1 and VP2 regions that activate both CD4 and CD8 T-cells [5]. Cells were stained for surface markers and then fixed and permeabilized before being incubated with anti-tumor necrosis factor (TNF)-α. As shown in Figure 1B, TNF-α secretion was severely impaired at PML onset.
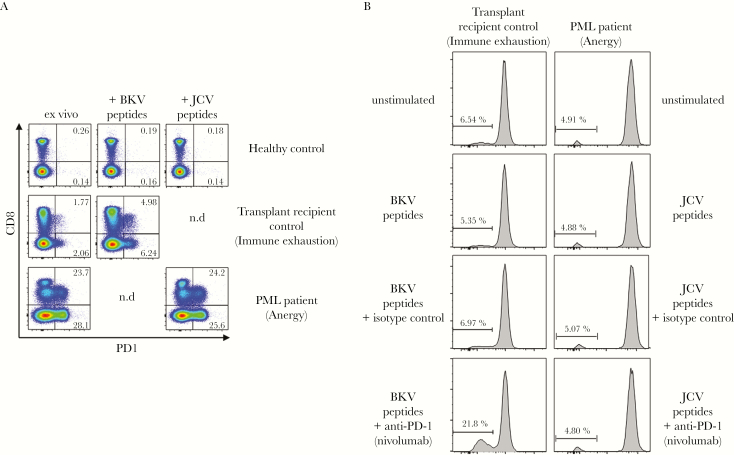
We tested the ability of CD4 and CD8 T cells to proliferate in response to JCV peptides. Peripheral blood mononuclear cells were stained with 0.5 µM carboxyfluorescein diacetate succinimidyl ester and then incubated for 5 days with JCV peptides. Specific T-cell proliferation was very weak (Figure 1C). To measure JCV-specific cytotoxicity, PBMCs were coincubated overnight with JCV peptide-coated autologous target cells. As control, cells were coincubated with uncoated autologous target cells. The mortality rate of target cells was evaluated by 7-amino-actinomycin D staining. No specific cytotoxicity was observed in the presence of JCV-specific peptides (Figure 1D). Despite the patient’s treatment, his T-cell responses to JCV remained weak throughout follow-up with no significant change (Figure 1B–D). We also assessed the expression of the inhibitory receptor programmed death-1 (PD-1). We found that the patient’s T cells expressed high levels of PD-1 up to day 61 (1 week before death) both ex vivo and after in vitro activation (Figure 2A). We then examined the effects of a neutralizing anti-PD-1 antibody (nivolumab) on T-cell proliferation in vitro. Programmed death-1 blockade failed to reverse this T-cell anergy (Figure 2B). Of interest, we tested in parallel a transplant recipient patient who had been receiving tacrolimus, MMF, and corticoids since November 2014. This patient developed BKV nephropathy along with BKV viremia (8.4 × 106 copies/mL) and was treated by replacing tacrolimus with everolimus (an inhibitor of mammalian target of rapamycin) in April 2015. T lymphocytes from this patient were nonresponsive to BKV in vitro (Figure 2B) and expressed significant levels of PD-1 after BKV peptide activation (Figure 2A). In contrast to the PML patient on belatacept, who was refractory to PD-1 blockade, nivolumab significantly restored anti-BKV T-cell responsiveness in this second patient (Figure 2B).
Figure 2.
Inhibitory receptor programmed death-1 (PD1) expression in the progressive multifocal leukoencephalopathy (PML) patient. (A) shows ex vivo PD-1 expression on T cells from the PML patient, compared with a healthy donor responsive to JC virus (JCV) and a kidney transplant recipient with BK virus (BKV) reactivation and possible immune exhaustion (control transplant recipient). Numbers indicate the percentages of CD8+ or CD8− (CD4+) T cells expressing PD-1. (B) shows the lack of improvement in T-cell functionality after PD-1 blockade with nivolumab in the anergic PML patient compared with the control transplant recipient.
Expression of PD-1 and restoration of functionality after PD-1 neutralization is compatible with immune exhaustion [6]. Together, our findings suggested that chronic blockade of the CD28-B7 costimulatory pathway led to refractory anti-JCV T-cell anergy that persisted until the patient’s death, 2 months after belatacept withdrawal.
DISCUSSION
The first treatment aim in this patient was to reverse lymphopenia by withdrawing MMF (an inhibitor of T-cell proliferation) and by adding r-hIL-7. Interleukin-7 induces widespread T-cell proliferation, increased T-cell numbers, and a more diverse T-cell receptor repertoire. We recently reported the case of a PML patient with profound lymphopenia after allogenic bone marrow transplantation, in whom r-hIL-7 led to the recovery of effective polyfunctional JCV-specific T-cell responses, virus clearance from CSF, and a favorable clinical outcome [7]. In this study, r-hIL-7 had no significant effect on the CD4 and CD8 T-cell counts. The subsequent CD4 cell recovery would be due more to MMF withdrawal. However, the CD8 T-cell count remained low. The patient had been on belatacept for 20 months, resulting in a long-term lack of CD28-mediated costimulation. Belatacept, which can provide significantly better renal function and both patient and renal graft survival than calcineurin inhibitor-based immunosuppression [8], provokes T-cell anergy in vivo [9]. The CD4 T-cell count recovery observed in our patient was not associated with improved functionality in terms of cytokine secretion, proliferation, or cytotoxicity. These functional abnormalities persisted for 60 days after PML diagnosis (the patient died 69 days after PML diagnosis) and were associated with strong expression of the inhibitory receptor PD-1. However, treatment with a therapeutic anti-PD1 receptor antibody (nivolumab) ex vivo failed to improve T-cell function.
Anergy is defined as a long-term state of hyporesponsiveness that occurs in T cells in response to suboptimal activation, including a lack of costimulation through CD28-B7 blockade [10, 11]. T-cell anergy is distinct from T-cell exhaustion, although both states may be associated with expression of inhibitory receptors, including PD-1. T-cell exhaustion can be reversed through PD-1 blockade [12], contrary to T-cell anergy [11]. The PML case on belatacept described here is the first for which analysis of JCV T-cell response was performed, although 2 other rapidly fatal cases of PML (death within 1 month) occurred during the phase II and III of a belatacept trial in kidney [4] and liver transplant patients [13]. In 2011, the US Food and Drug Administration along with the manufacturer BMS instituted a Risk Evaluation and Mitigation Strategy program [14]. Treatment of PML, based mainly on immune restoration, should start as rapidly as possible to limit the extension of brain lesions and thereby to improve survival and functional outcome [15].
CONCLUSIONS
Several mechanisms of immunosuppression may lead to JCV replication in the brain (and consequently to PML), including HIV infection, hematological malignancies, and treatment with natalizumab or rituximab [1, 2]. Each cause of immunosuppression may require a specific therapeutic approach to achieve anti-JCV immune recovery. Treatment of PML related to immunological mechanisms of T-cell anergy could be very challenging, because there is currently no way of reversing T-cell anergy [11]. Thus, the use of belatacept and future biotherapies that induce T-cell anergy may lead to the emergence of a subset of PML that is extremely difficult to manage.
Acknowledgments
We thank Anne Marie Roque and the Department of Virology, Hôpitaux Universitaires Paris-Sud.
Financial support. This work was funded by Institut National de la Santé et de la Recherche Médicale.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Brew BJ, Davies NW, Cinque P et al. . Progressive multifocal leukoencephalopathy and other forms of JC virus disease. Nat Rev Neurol 2010; 6:667–79. [DOI] [PubMed] [Google Scholar]
- 2. Tan CS, Koralnik IJ. Progressive multifocal leukoencephalopathy and other disorders caused by JC virus: clinical features and pathogenesis. Lancet Neurol 2010; 9:425–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mateen FJ, Muralidharan R, Carone M et al. . Progressive multifocal leukoencephalopathy in transplant recipients. Ann Neurol 2011; 70:305–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Durrbach A, Pestana JM, Pearson T et al. . A phase III study of belatacept versus cyclosporine in kidney transplants from extended criteria donors (BENEFIT-EXT study). Am J Transplant 2010; 10:547–57. [DOI] [PubMed] [Google Scholar]
- 5. Hendel-Chavez H, de Goër de Herve MG, Giannesini C et al. . Immunological hallmarks of JC virus replication in multiple sclerosis patients on long-term natalizumab therapy. J Virol 2013; 87:6055–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol 2015; 15:486–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gasnault J, de Goër de Herve MG, Michot JM et al. . Efficacy of recombinant human interleukin 7 in a patient with severe lymphopenia-related progressive multifocal leukoencephalopathy. Open Forum Infect Dis 2014; 1:ofu074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vincenti F, Rostaing L, Grinyo J et al. . Belatacept and long-term outcomes in kidney transplantation. N Engl J Med 2016; 374:333–43. [DOI] [PubMed] [Google Scholar]
- 9. Rochman Y, Yukawa M, Kartashov AV, Barski A. Functional characterization of human T cell hyporesponsiveness induced by CTLA4-Ig. PLoS One 2015; 10:e0122198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fathman CG, Lineberry NB. Molecular mechanisms of CD4+ T-cell anergy. Nat Rev Immunol 2007; 7:599–609. [DOI] [PubMed] [Google Scholar]
- 11. Valdor R, Macian F. Induction and stability of the anergic phenotype in T cells. Semin Immunol 2013; 25:313–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang C, Thudium KB, Han M et al. . In vitro characterization of the anti-PD-1 antibody nivolumab, BMS-936558, and in vivo toxicology in non-human primates. Cancer Immunol Res 2014; 2:846–56. [DOI] [PubMed] [Google Scholar]
- 13. Klintmalm GB, Feng S, Lake JR et al. . Belatacept-based immunosuppression in de novo liver transplant recipients: 1-year experience from a phase II randomized study. Am J Transplant 2014; 14:1817–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bristol-Myers Squibb Company. Risk Evaluation and Mitigation Strategy (REMS). NULOJIXTM (belatacept) Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/125288Orig1s000remsNulojix.pdf. Accessed 8 August 2011.
- 15. Gasnault J, Costagliola D, Hendel-Chavez H et al. . Improved survival of HIV-1-infected patients with progressive multifocal leukoencephalopathy receiving early 5-drug combination antiretroviral therapy. PLoS One 2011; 6:e20967. [DOI] [PMC free article] [PubMed] [Google Scholar]