Abstract
Background
Rift Valley fever (RVF) is an acute viral anthropozoonosis that causes epizootics and epidemics among livestock population and humans. Multiple emergences and reemergences of the virus have occurred in Mauritania over the last decade. This article describes the outbreak that occurred in 2015 in Mauritania and reports the results of serological and molecular investigations of blood samples collected from suspected RVF patients.
Methods
An RVF outbreak was reported from 14 September to 26 November 2015 in Mauritania. Overall, 184 suspected cases from different localities were identified by 26 health facilities. Blood samples were collected and tested by enzyme-linked immunosorbent assay (ELISA) and real-time reverse-transcription polymerase chain reaction (RT-PCR) at the Institut Pasteur de Dakar (IPD). Sequencing of partial genomes and phylogenetic analyses were performed on RT-PCR–positive samples. As part of routine surveillance at IPD, samples were also screened for dengue, yellow fever, West Nile, Crimean Congo hemorrhagic fever, Zika, and Chikungunya viruses by ELISA and RT-PCR.
Results
Of the 184 suspected cases, there were 57 confirmed cases and 12 deaths. Phylogenetic analysis of the sequences indicated an emergence of a virus that originated from Northeastern Africa. Our results show co-circulation of other arboviruses in Mauritania—dengue, Crimean Congo hemorrhagic fever, and West Nile viruses.
Conclusion
The Northeastern Africa lineage of RVF was responsible for the outbreak in Mauritania in 2015. Co-circulation of multiples arboviruses was detected. This calls for systematic differential diagnosis and highlights the need to strengthen arbovirus surveillance in Africa.
Keywords: Mauritania, Northeastern African lineage, phylogeny, Rift Valley fever, 2015
Rift Valley fever virus (RVFV) is a mosquito-borne virus belonging to the Phlebovirus genus of the Bunyaviridae family. Rift Valley fever virus is a segmented virus with 3 single-stranded RNA genomes: L (large), M (medium), and S (small). The virus is primarily transmitted to animals and humans through infected mosquito bites. Other modes of transmission include exposure to body fluids, blood, and tissues of infected animals or contact with aerosols [1, 2]. In animals, Rift Valley fever (RVF) is characterized by abortions among pregnant females and high mortality rates for offspring [3]. In human, symptoms are generally mild but may evolve to severe disease, such as hemorrhagy, meningoencephalitis, and retinopathy with fatal outcomes [4]. Cases of miscarriage in pregnant women associated with RVFV infection have been shown recently in Sudan [5]. The disease causes a huge economic burden because of livestock-trade bans and restrictions, especially in the Arabian Peninsula.
Rift Valley fever virus is classified as a category A pathogen by the US Center for Diseases Control and Prevention and the Department of Agriculture. There is no commercially available vaccine for humans or animals, and effective antiviral drugs have not been identified. There is therefore an urgent need to develop countermeasures against propagation and introduction of RVFV into Europe and nonendemic countries. Rift Valley fever is one of the priority diseases identified by the World Health Organization (WHO) as a likely cause of a future outbreak [6]. Indeed, a new plan was developed after the Ebola epidemic for urgent research and development toward new diagnostic tools, vaccines, and medicines for RVFV.
In West Africa, the first major RVF outbreak occurred on October 1987 at the end of the rainy season in Mauritania. It was particularly notable in the Trarza region along the Senegal River after the impoundment of the Diama and flooding around the lowlands [7]. During that outbreak, 220 human deaths were recorded [8–10], and high abortion rates among small ruminants were observed [10, 11]. Subsequently, the country experienced several outbreaks, some of which had severe hemorrhagic manifestations [12–15]. In 1998, the Hodh El Gharbi region located in the southeast of Mauritania recorded >300 human RVF cases, including 6 deaths [10]. In 2003, several other regions of the south, southeast, and center experienced RVF outbreaks. Twenty-five confirmed human cases, including 16 with hemorrhagic signs and 4 deaths, were reported in the south (Trarza, Brakna, Gorgol), southeast (Assaba), and center (Tagant) [13]. Only the northern part was unaffected, until October 2010, when an RVF outbreak was identified in Adrar and Inchiri regions, with 70 human cases, including 13 deaths [14]. Two years later, another more extended RVF outbreak occurred in Assaba, Brakna, Hodh El Chargui, Hodh El Gharbi, Tagant, and Trarza regions and Nouakchott capital city of the country between September and October 2012. During this outbreak, 34 human cases, including 17 deaths, were reported [15]. The viruses isolated in 1989 formed a discrete West African lineage, whereas strains recovered in 2003 fall within the East/Central African lineage [13]. Indeed, the 2003 Mauritanian viruses were clustered into lineage C, together with strains isolated from Kenya in 2007, South Africa in 2008 and 2009, and Madagascar in 2008. Lineage C also includes strains isolated in Zimbabwe in 1976–1979 and 1998, suggesting that viruses originating from Zimbabwe might be responsible for outbreaks in Mauritania [16]. Phylogenetic analyses from the 2010 and 2012 outbreaks suggest a reemergence from local RVFV that clustered in the West African lineage [14, 15].
In the context of Ebola virus disease outbreak in West Africa, which was declared by WHO on August 8, 2014, to be a global public health emergency [17], the Mauritanian epidemiological surveillance office was alerted on September 14, 2015, of a suspected case of viral hemorrhagic fever. A sample was sent to the Institut Pasteur de Dakar (IPD) for Ebola diagnosis and arboviruses differential diagnosis in case of a negative Ebola result. Following this case, 10 more patients were hospitalized for hemorrhagic fever, and samples were also sent to IPD. Rift Valley fever virus was detected in 8 of these 11 samples. On October 7, 2015, the International Health Regulations were applied, the RVF outbreak was officially declared, and Mauritanian authorities reinforced the surveillance system.
We report herein the description of the outbreak and results of serological and molecular investigations of blood samples from suspected RVF patients.
PATIENTS AND METHODS
In Mauritania, the process for management of suspected cases of hemorrhagic fever in the context of the Ebola outbreak was to (1) isolate the patient, (2) complete a standard notification form, and (3) collect 2 venous samples in ethylene diamine tetra acetic acid and dry tube. For the first suspected case, the notification form and blood samples were sent to the WHO collaborating center for arboviruses and hemorrhagic fever viruses in IPD for Ebola diagnosis. The plasma and serums samples were tested for Ebola by real-time reverse-transcription polymerase chain reaction (RT-PCR) [18]. Because the sample was negative for Ebola, differential diagnosis for RVF, dengue, yellow fever, West Nile, Crimean Congo hemorrhagic fever (CCHF), and Chikungunya viruses was also performed by RT-PCR and enzyme-linked immunosorbent assay (ELISA).
Following this case, 10 more patients were hospitalized for hemorrhagic fever (Ebola was ruled out) in Mauritania and also tested for RVF, dengue, yellow fever, West Nile, CCHF, and Chikungunya viruses by RT-PCR and ELISA.
After the RVF outbreak declaration, investigations were performed, and the following case definition was used: patient consulting with an axillary temperature >37.5°C lasting for 48 hours, associated with at least 1 of the following signs: exhaustion, back pain, myalgia, headache, nausea/vomiting, diarrhea and/or cutaneous bleeding, bleeding to bite sites, epistaxis, gingival bleeding, or other bleeding. Blood samples were collected from a total of 173 patients.
All of the blood samples collected before the outbreak declaration (n = 10) and during outbreak investigation (n = 173) were sent to the Institut national de la recherche en santé publique (INRSP) in Nouakchott and tested by ELISA for detection of immunoglobulin M (IgM) against RVF, yellow fever, and CCHF viruses after sample inactivation by heating at 56oC for 30 minutes. Following INRSP diagnostics, samples were sent to IPD for confirmation of serological tests, RNA detection by RT-PCR, and differential diagnosis. ELISA for IgM of RVFV and other arboviruses was performed in the WHO collaborating center for arboviruses and hemorrhagic fever viruses in IPD using in-house methods with antigens and immune ascites produced in mice.
Regarding the RVFV RT-PCR, the primers (forward TGCCACGAGTYAGAGCCA, reverse TTGAACAGTGGGT CCGAGA) and probe 6FAM-TCCTTCTCCCAgTCAgCCCCA C-BHQ1 were used [19]. For other arboviruses, RT-PCR for Chikungunya [20], dengue [21], West Nile [22], yellow fever [23], Zika [24], and CCHF [25] viruses was performed as previously described. The RNA was amplified using ABI Prism 7000 SDS Real-Time apparatus (Applied Biosystems) with the QuantiTect kit (Qiagen).
Samples that were RT-PCR–positive for RVFV were used for sequencing of partial small (S), medium (M), and large (L) segments using specific primers (Supplementary Table 1). Sequences were analyzed using online tools revseq and merger emboss (http://www.bioinformatics.nl/cgi-bin/emboss/merger;http://emboss.bioinformatics.nl/cgi-bin/emboss/revseq), and phylogenetic studies were conducted using maximum likelihood method and MEGA software (MEGA 6. 06-mac) [26].
RESULTS
From September 14 to November 26, 2015, the IPD laboratory received in total 184 blood samples collected in 26 health facilities from patients with suspected hemorrhagic fever in Mauritania (Table 1). The mean age of patients was 25 years (range = 0.6–90), and the sex ratio (male/female) was 3.38. Among the 184 samples, 57 were positive for RVF by either RT-PCR and/or IgM (16 RT-PCR+/IgM+, 40 RT-PCR+/IgM−, and 1 RT-PCR−/IgM+). One sample was found coinfected with both RVF and CCHF viruses by RT-PCR, and 5 samples were positive only for CCHF IgM by ELISA. Twenty-seven samples were positive for dengue (8 RT-PCR+ /IgM−, 19 RT-PCR− /IgM+), with 8 samples positive for both RVF and dengue by RT-PCR. One sample was positive for West Nile IgM by ELISA and RVFV by RT-PCR (Table 1).
Table 1.
Seroepidemiologic, Virologic Results and Study Sites for Humans, Rift Valley Fever Outbreak, Mauritania, 2015
| Health Facilities (Region) |
Suspected Cases (M/F) | Confirmed Cases (RT-PCR and/or IgM) | ||
|---|---|---|---|---|
| Alive | Deaths (%) |
Total (PCR+/IgM+) |
||
| CH Aïoun (S-E) | 1 (0/1) | 1 | 0 | 1 (1/0) |
| CH Aleg (S) | 6 (5/1) | 2 | 1 (33.3) | 3 (3/0) |
| CH Amitié (Nktt) | 7 (6/1) | 2 | 0 | 2 (2/0) |
| CH Boulilmit (S) | 2 (2/0) | 1 | 0 | 1 (1/0) |
| CH Cheikh Zayed (Nktt) | 6 (5/1) | 3 | 0 | 3 (3/2) |
| CH Kaédi (S) | 3 (3/0) | 2 | 0 | 2 (2/0) |
| CH Kiffa (S-E) | 19 (13/6) | 12 | 4 (25) | 16 (16/10) |
| CH Mere et Enfant (Nktt) | 8 (6/2) | 2 | 0 | 2 (2/0) |
| CH Militaire (Nktt) | 3 (3/0) | 0 | 0 | 0 |
| CH National (Nktt) | 99 (73/26) | 17 | 3 (15) | 20 (19/4) |
| CH Néma (S-E) | 2 (2/0) | 0 | 0 | 0 |
| CH Nouadhibou (N) | 1 (1/0) | 0 | 0 | 0 |
| CH Tidjikja (C) | 3 (2/1) | 0 | 0 | 0 |
| CH Zoueratt (N) | 1 (1/0) | 0 | 0 | 0 |
| Clinique Ibn Sina (Nktt) | 1 (0/1) | 0 | 0 | 0 |
| Clinique Kissi (Nktt) | 3 (3/0) | 0 | 0 | 0 |
| CS Bababé (S) | 1 (0/1) | 1 | 0 | 1 (1/0) |
| CS Bassiknou (S-E) | 3 (3/0) | 1 | 0 | 1 (1/0) |
| CS Fassala (S-E) | 1 (1/0) | 0 | 0 | 0 |
| CS Guérou (S-E) | 1 (1/0) | 0 | 0 | 0 |
| CS Kankosa (S) | 1 (1/0) | 0 | 0 | 0 |
| CS Ksar (Nktt) | 1 (0/1) | 0 | 0 | 0 |
| CS Maghta Lahjar (S) | 8 (8/0) | 1 | 3 (75) | 4 (4/1) |
| CS Moudjeria (C) | 1 (1/0) | 0 | 1 (100) | 1 (1/0) |
| CS Waalata (S-E) | 1 (1/0) | 0 | 0 | 0 |
| PS Hassy Mhady (S-E) | 1 (1/0) | 0 | 0 | 0 |
| Total | 184 (142/42) | 45 | 12 (21.1) | 57 (56/17) |
| Health Facilities Regions | ||||
| Center | 4 (3/1) | 0 | 1 (100) | 1 (1/0) |
| Nouakchott | 128 (96/32) | 24 | 3 (11.1) | 27 (26/6) |
| North | 2 (2/0) | 0 | 0 | 0 |
| South | 21 (19/2) | 7 | 4 (36.3) | 11 (11/1) |
| Southeast | 29 (7/1) | 14 | 4 (22.2) | 18 (18/10) |
| Total | 184 (142/42) | 45 | 12 (21.1) | 57 (56/17) |
Abbreviations: C, center; CH, hospital center; Clinique, private health center; CS, health center; F, female; IgM, immunoglobulin M; M, male; N, north; NKTT, Nouakchott; PS, health post; RT-PCR, reverse-transcription polymerase chain reaction; S, south; S-E, southeast.
The overall infection rate by RVFV was 30.97% (n = 57/184), of which 78.94% were males (n = 45/57) and 21.05% were females (n = 12/57). The age groups 0–20 years and 21–40 years were the most affected with 38.59% (n = 22/57) and 45.61% (n = 26/57) of confirmed cases, respectively. With respect to age, the fatality rate was 33.3% (n = 1/3) in patients aged >61 years and 13.6% (n = 3/22) in patients aged 0–20 years (Table 2).
Table 2.
Rift Valley Fever Human Cases According to Age and Sex in Mauritania in 2015
| Sex and Age | Suspected Cases (M/F) | Confirmed Cases (PCR and/or IgM) | ||
|---|---|---|---|---|
| Alive | Deaths | Total (PCR/IgM) | ||
| Sex | ||||
| Male | 142 | 34 | 11 (24.4) | 45 (44/13) |
| Female | 42 | 11 | 1 (8.3) | 12 (12/4) |
| Age, y | ||||
| 0–20 | 92 (70/22) | 19 | 3 (13.6) | 22 (22/8) |
| 21–40 | 61 (47/14) | 19 | 7 (26.9) | 26 (25/9) |
| 41–60 | 20 (17/3) | 5 | 1 (16.1) | 6 (6/0) |
| >61 | 11 (8/3) | 2 | 1 (33.3) | 3 (3/0) |
| Total | 184 (142/42) | 45 | 12 (21.1) | 57 (56/17) |
Abbreviations: F, female; IgM, immunoglobulin M; M, male; PCR, polymerase chain reaction.
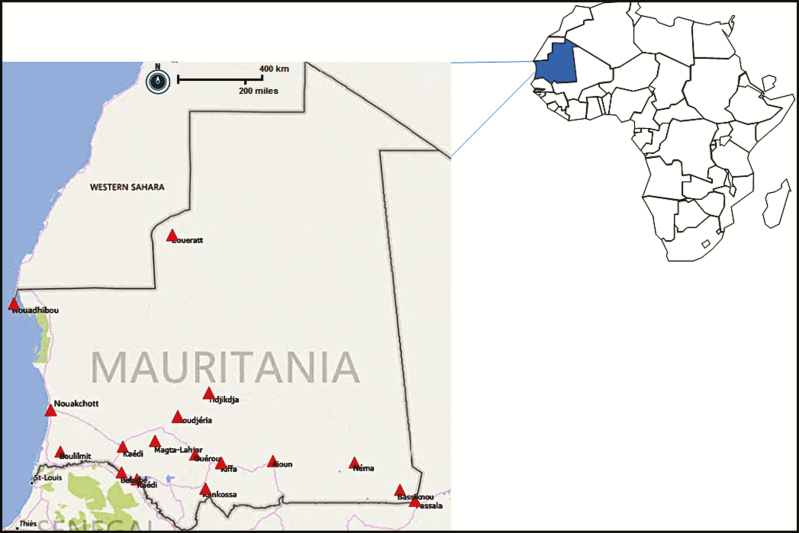
Among the 5 health facilities that reported at least 1 death, lethality varied between 15% for the National Hospital Center (n = 3/20) and 100% for Health Center Moudjeria (n = 1/1). Kiffa’s hospital center recorded the highest number of deaths with a lethality rate of 25% (n = 4/16). Of the 26 health facilities that reported at least 1 suspected case, 30.76% (n = 8/26) were in the southeast, 23.07% (n = 6/26) were in the south, and 30.76% (8/26) were in Nouakchott (Figure 1). The health facilities in the center and north each represented 7.69% (n = 2/26) of cases. Thus, 50.87% (n = 29/57) of the confirmed RVF patients were from the south and southeast parts of the country, and 47.36% (n = 27/57) originated from Nouakchott capital city, where 24% of the national population lives.
Figure 1.
Geographic distribution of suspected cases of Rift Valley fever infection, Mauritania, 2015.
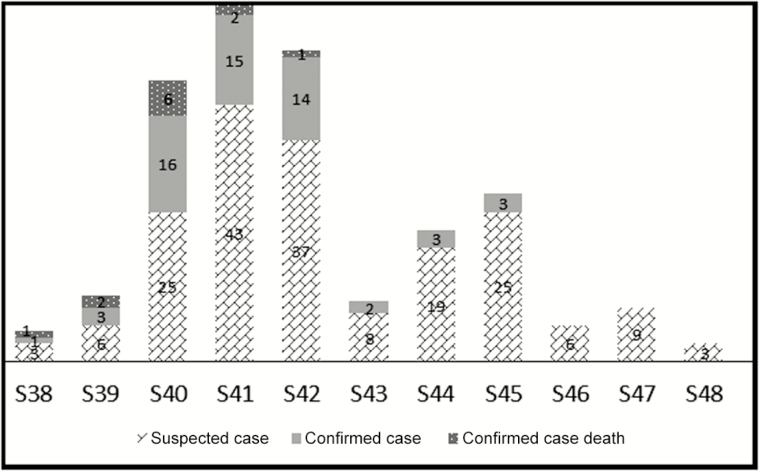
The operating notifications records showed that the first confirmed case was hospitalized on September 14, 2015, at Health Center Maghta Lahjar, and the date of disease onset was September 9, 2015. The last positive case was recorded at Hospital Center Aïoun on November 3 and confirmed by November 13, 2015. The outbreak began at epidemiological week 38 and stopped at week 46, with 2 peaks at weeks 41 and 45. The analysis showed 2 epidemic periods; the first covered weeks 38–43, and all deaths occurred during this period, with the lethality being highest at week 40 (Figure 2).
Figure 2.
Distribution of suspected, confirmed, and fatal Rift Valley fever human cases according to epidemiological weeks in Mauritania in 2015.
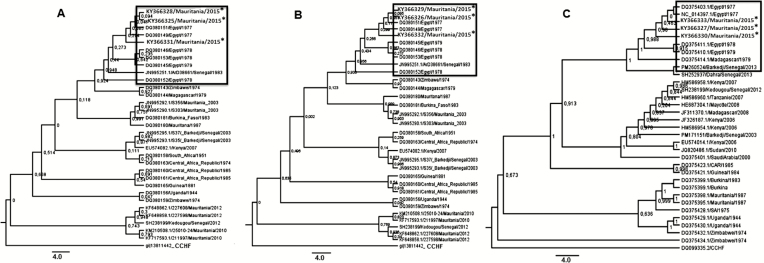
Phylogenetic analysis based on RVFV partial small (709 bp), medium (718 bp), and large RNA (4764 bp) sequences showed that sequences from different areas clustered together with a low level of variation between isolates. The strains were different from the other RVF strains isolated in Mauritania in 2010 and 2012 and more closely related to those from Senegal in 2013 [27] and clustered in the Egyptian lineage (Figure 3).
Figure 3.
Phylogenetic trees for the small (A), medium (B), and long (C) segments of 3 Rift Valley fever virus isolates from Mauritania, 2015 (*) using maximum likelihood with Mega 06, showing relationships among strains isolated from different localities and countries. The strains from 2015 clustered with strains from Egypt and the strain from Senegal in 1983 and 2013.
DISCUSSION
As in 2010 [10] and 2012 [11], Mauritania experienced a new RVF outbreak in 2015, which spread over 8 weeks, with 184 suspected cases, including 57 confirmed cases and 12 deaths. Similar to 2010 and 2012 outbreaks, RVF positive patients were predominantly males (78.95%) and younger individuals (aged <40 years); the fatality rate was greater in male patients. This can be explained by the high exposure risk of men to potentially infected mosquitoes during agricultural work or direct contact with viremic livestock, infected tissues, and aborted animals [15]. The outbreak affected the same areas as the 2012 RVF outbreak (Assaba, Brakna, Hodh El Chargui, Hodh El Gharbi, Tagant, Trarza, and Nouakchott), contrasting with the 2010 outbreak, which was confined to northern regions (Inchiri and Adrar).
Differential diagnosis in IPD revealed co-circulation of RVF and CCHF with 1 coinfection. This was similar to the 2012 outbreak where 1 coinfection with CCHF was also found in Moudjeria [15]. Co-circulation of RVFV with dengue virus was also identified in 8 samples during the 2015 outbreak. In addition to dengue and CCHF, the circulation of West Nile virus was identified for the first time in Mauritania. Taken together, these results indicate circulation of different arboviruses in the region during the RVF outbreak and call for systematic differential diagnosis or a syndromic approach for febrile and hemorrhagic fever surveillance to prevent a large-scale outbreak.
Phylogenetic analysis revealed that the same strain was circulating in the different affected areas. Rift Valley fever virus isolates from the 2015 outbreak clustered in the Northeastern (Egyptian group) lineage with the strains (from mosquito: Aedes ochraceus and human) from Senegal in 2013 [27]. Emergence of RVF strains belonging to the Northeastern African lineage was already notified in West Africa, particularly in Senegal in 1983 (Figure 3), but no outbreak was reported. A reemergence or new introduction of this lineage was identified in Senegal in 2013 [27], and to our knowledge, this is the first evidence of the circulation in Mauritania of strains belonging to the Northeastern African lineage. These phylogenetic analyses suggest that this outbreak may result from an introduction of isolates from Barkedji, Senegal, or Egypt to Mauritania. Because Senegal and Mauritania shared a border and a previous study suggested that Barkedji functions as a hub broadcasting RVFV in the neighboring countries [28], we hypothesized that the outbreak in Mauritania might be due to an introduction of RVFV from Barkedji. This emphasized the need to implement surveillance and control measures after reported cases in Senegal to prevent propagation of RVFV isolates. However, further studies are needed to confirm the precise origin of Mauritania strains. These findings also confirm the existence of RVFV strain mixing between different geographic areas.
In pastoral countries like Mauritania, RVF epizootics are often responsible for serious economic losses and cross-border problems related to livestock transhumance. The reasons for the emergence or reemergence of RVF, the increasing frequency of outbreaks, and the reduced period between consecutive epidemics of RVF in Mauritania remain unknown [29]. However, there is a common observation that the occurrences of outbreaks in Mauritania are often preceded by large inflow of small ruminants when the traditional major Muslim holidays approach and are often associated with heavy rains and hydrographic changes, which result in the proliferation of RVF mosquito vectors.
The mechanism of RVFV spread likely depends on animal population transhumance, and migration routes between West Africa and other areas need to be identified to elucidate the different routes of introduction of the virus.
An RVF outbreak occurred in southern Mauritania in 2015 with 184 suspected cases, of which there were 57 confirmed cases and 12 deaths. Phylogenetic analysis of isolates indicated a virus origin from Northeastern Africa closely related to isolates detected in Senegal in 2013. Surveillance and control measures after reported cases in Senegal are needed to prevent propagation of RVFV isolates in neighboring countries. The identification of CCHV, West Nile, and dengue infections during the RVF outbreak indicates the co-circulation of various arboviruses and calls for systematic differential diagnostics or a syndromic approach for febrile and hemorrhagic fever surveillance to avoid a large-scale outbreak.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Acknowledgments
The authors thank Fatou Dia, Arame Ba, Oumar Ndiaye, Rouguiétou Sylla, Moussa Dia, and Magueye Ndiaye for their excellent technical assistance in laboratory diagnosis.
Financial support. This work was supported by the Institut Pasteur de Dakar.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Gear JHS, De Meillon B, Measarch V et al. Rift virus fever in South Africa. the occurrence of human case in the orange free state, the north-western cap province, the western and the southern transversal B. Field and laboratory investigation. S Afr Med J. 1951;25,908–12. [PubMed] [Google Scholar]
- 2. Linthicum KJ, Britch SC, Anyamba A. Rift Valley fever: an emerging mosquito-borne disease. Annu Rev Entomol. 2016;61:395–415. [DOI] [PubMed] [Google Scholar]
- 3. Daubney R, Hudson JR, Garnham PC. Enzootic hepatitis or Rift Valley fever: an undescribed virus disease of sheep, cattle, and man from east Africa. J Pathol Bacteriol. 1931;34:545–79. [Google Scholar]
- 4. Mansfield KL, Banyard AC, McElhinney L et al. Rift Valley fever virus: a review of diagnosis and vaccination, and implications for emergence in Europe. Vaccine. 2015;33:5520–31. [DOI] [PubMed] [Google Scholar]
- 5. Baudin M, Jumaa AM, Jomma HJ et al. Association of Rift Valley fever virus infection with miscarriage in Sudanese women: a cross-sectional study. Lancet Glob Health. 2016;4:e864–71. [DOI] [PubMed] [Google Scholar]
- 6. World Health Organization. WHO publishes list of top emerging diseases likely to cause major epidemics http://www.who.int/medicines/ebola-treatment/WHO-list-of-top-emerging-diseases/en WHO list of top emerging diseases. http://www.who.int/medicines/ebola-treatment/WHO-list-of-top-emerging-diseases/en. Accessed 10 December 2015.
- 7. El Mamy AB, Baba MO, Barry Y et al. Unexpected Rift Valley fever outbreak, northern Mauritania. Emerg Infect Dis. 2011;17:1894–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Digoutte JP, Peters CJ. General aspects of the 1987 Rift Valley fever epidemic in Mauritania. Res Virol. 1989;140:27–30. [DOI] [PubMed] [Google Scholar]
- 9. Saluzzo JF, Digoutte JP, Chartier C et al. Focus of Rift Valley fever virus transmission in southern Mauritania. Lancet. 1987;1:504. [DOI] [PubMed] [Google Scholar]
- 10. Jouan A, Le Guenno B, Digoutte JP et al. An RVF epidemic in southern Mauritania. Ann Inst Pasteur Virol. 1988;139:307–8. [DOI] [PubMed] [Google Scholar]
- 11. Ndione JA, Lacaux JP, Tourre Y et al. Mares temporaires et risques sanitaires au Ferlo: contribution de la télédétection pour l’étude de la fièvre de la vallée du Rift entre aout 2003 et janvier 2004. Sécheresses. 2009;20:153–60. [Google Scholar]
- 12. Nabeth P, Kane Y, Abdalahi M et al. Rift Valley fever outbreak, Mauritania, 1998: seroepidemiologic, virologic, entomologic, and zoologic investigations. Emerg Infect Dis. 2001;7:1052–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Faye O, Diallo M, Diop D et al. Rift Valley fever outbreak with East-Central African virus lineage in Mauritania, 2003. Emerg Infect Dis. 2007;13:1016–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Faye O, Ba H, Ba Y et al. Reemergence of Rift Valley fever, Mauritania, 2010. Emerg Infect Dis. 2014;20:300–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. .Sow A, Faye O, Ba Y et al. Rift Valley fever outbreak, southern Mauritania, 2012. Emerg Infect Dis. 2014;20:296–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ikegami T. Molecular biology and genetic diversity of Rift Valley fever virus. Antiviral Res. 2012;95:293–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Franceinfo. Ebola: les principales dates de l’épidémie http://www.francetvinfo.fr/sante/maladie/ebola/ebola-les-principales-dates-de-l-epidemie_1243980.html. Accessed 29 December 2015.
- 18. Weidmann M, Mühlberger E, Hufert FT. Rapid detection protocol for filoviruses. J Clin Virol. 2004;30:94–9. [DOI] [PubMed] [Google Scholar]
- 19. Weidmann M, Sanchez-Seco MP, Sall AA et al. Rapid detection of important human pathogenic phleboviruses. J Clin Virol. 2008;41:138–42. [DOI] [PubMed] [Google Scholar]
- 20. Diallo D, Sall AA, Buenemann M et al. Landscape ecology of sylvatic Chikungunya virus and mosquito vectors in southeastern Senegal. PLoS Negl Trop Dis. 2012;6:e1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wagner D, de With K, Huzly D et al. Nosocomial acquisition of dengue. Emerg Infect Dis. 2004;10:1872–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fall G, Faye M, Weidmann M et al. Real-time RT-PCR assays for detection and genotyping of West Nile virus lineages circulating in Africa. Vector Borne Zoonotic Dis. 2016;16:781–9. [DOI] [PubMed] [Google Scholar]
- 23. Weidmann M, Faye O, Faye O et al. Improved LNA probe-based assay for the detection of African and South American yellow fever virus strains. J Clin Virol. 2010;48:187–92. [DOI] [PubMed] [Google Scholar]
- 24. Faye O, Faye O, Dupressoir A et al. One-step RT-PCR for detection of Zika virus. J Clin Virol. 2008;43:96–101. [DOI] [PubMed] [Google Scholar]
- 25. Weidmann M, Sall AA, Manuguerra JC et al. Quantitative analysis of particles, genomes and infectious particles in supernatants of haemorrhagic fever virus cell cultures. Virol J. 2011;8:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tamura K, Stecher G, Peterson D et al. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sow A, Faye O, Ba Y et al. Widespread Rift Valley fever emergence in Senegal in 2013–2014. Open Forum Infect Dis. 2016;3:ofw149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Soumaré POL, Freire CM, Faye O et al. Phylogeography of rift Valley fever virus in Africa reveals multiple introductions in Senegal and Mauritania. PLoS One. 2012; 7:23–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Boushad MB, Savadogo M, Sow MS et al. Forme hémorragique grave de la fièvre de la Vallée du Rift en Mauritanie. Bull Soc Pathol Exot. 2015;108:102–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.