Abstract
Objective
This study aims to assess the efficacy of extracorporeal shockwave therapy (ESWT) and low-intensity pulsed ultrasound (LIPUS) on osteoarthritic rat knees.
Material and Methods
Twenty-four rats were divided into 3 groups: group 1-control (n=8), group 2-LIPUS (n=8) and group 3-ESWT (n=8). Cartilage degeneration was provided using mono-iodo-asetate (MIA). One milligram of MIA was delivered to the right knees in group 1 and both knees in group 2 and 3. A 0.09% saline solution was delivered to the left knees in group 1 for control. Twenty-four hours after the delivery, ESWT was applied once on the right knees in the group 2 rats to the medial tibia plateu with a 1 Hz frequency and 800 impulses. LIPUS was applied to the right knees in the group 2 rats to the medial tibia plateu with a 3 mHz frequency and 40 mW/cm2 intensity for 20 minutes over a period of 15 days. Pain scores were measured with a knee bend test. Bone mineral density measurements and scintigraphic bone scans were performed. Histopathological examination was done using a modified Mankin scale.
Results
There was no difference among the right knee subchondral bone osteoblastic activities (p>0.05). The left knee osteoblastic activities in the LIPUS and extracorporeal shockwave therapy (ESWT) groups were higher than those in the control group (p<0.05), but there was no difference between the LIPUS and ESWT groups. There was no difference among the groups for both knee subchondral bone BMD values (p>0.05). The modified Mankin scores of both the right and left knees of the ESWT and LIPUS groups were lower than those of the control group (p<0.05), but there was no difference between the ESWT and LIPUS groups. The pain scores of both knees of the ESWT and LIPUS groups at day 7 were higher than those of the control group (p<0.05), but there was no difference between the ESWT and LIPUS groups. There was no difference among the pain scores of the right knees at day 14 (p<0.05).
Conclusion
ESWT and LIPUS have systemic proliferative and regenerative effects on cartilage and tissue.
Keywords: Extracorporeal shockwave therapy, low-intensity pulsed ultrasound, osteoarthritis
Introduction
Osteoarthritis (OA) is a heterogenous disease associated with degenerative processes in the joint cartilage and subchondral bone that leads to disintegration of the cartilage along with related clinical signs and symptoms (1). All biochemical and histopathological processes of the joint cartilage may result in cartilage damage with ineffective remodeling during the course of the disease OA may be considered the biochemical and histopathological result of a group of diseases, rather than a single disease (2, 3). Although it is accepted as a noninflammatory disease, moderate inflammation is common, and it results in swelling, effusion, and pain (4). Subchondral bone is a structure that provides mechanical and nutritional support to the overlying joint cartilage. However, the degenerative processes associated with OA originate from the subchondral bone, making it a leading target in the treatment of OA (5).
Traditional treatment modalities of OA are pharmacological (oral and topical non-steroid anti-inflammatory drugs) and non-pharmacological (regulation of daily activities, education and exercises), but none of these can revive the cartilage degeneration. Advanced treatment modalities demonstrating histopathological recovery are required. Low-intensity pulsed ultrasound (LIPUS) and extracorporeal shockwave therapy (ESWT) have been shown to be effective in experimental animal models, but studies have not revealed which one of these modalities is more effective. This study aims to compare the efficacy of these treatment modalities that were shown to be effective in early OA models.
Low-intensity pulsed ultrasound is a representative treatment modality used in the field of orthopedics for the treatment of nonunion (6). Ultrasound has both termogenic and nontermogenic effects. Application of high intensity (1–300 W/cm2) ultrasound continuously generates termogenic effects in living tissues, while low-intensity (<100 mW/cm2) ultrasound generates non-termogenic effects. Recent data suggests that ultrasound treatment encourages cartilage repair as well as bone healing (7). The repair process mechanism is still unclear. Continuous in vitro exposure to 0.14 mW/cm2 leads to increased expression of integrin molecules (especially a5 and b1), chondocyte markers, like Sox molecules (especially Sox 5 and Sox 9), collagen II C telopeptides and aggrecan (8). Concerning the molecular restoration process, Cook et al. (9) reported morphologic recovery in osteoarthritic rabbit knees. Huang et al. (10) obtained similar data about rat osteoarthritic knees. It seems that chondrocyte regeneration and matrix proliferation could be provided by exposure to LIPUS in vivo. However, the current data about in vitro exposure is insufficient.
Extracorporeal shockwaves are energy waves that have both direct and indirect effects on the tissue (11). ESWT is used in the treatment of various tendinopathies, such as calcific tendonitis of the shoulder, epicondylitis, and plantar fasciitis (12, 13). Treatment indications were extended to cover conditions such as femoral head necrosis, delayed unions, and nonunions of fractures (14, 15). Some studies report reactivation and enhancement of bone healing processes (16, 17). Most of them attribute this enhancement to upgrading the revascularization, which leads to the recruitment of growth factors and possibly stem cells that are necessary for the normal healing process (18). ESWT also has beneficial effects on cartilage tissue (19). Furthermore, some studies demonstrate that ESWT has chondroprotective effects in the early stages of knee OA (20). The effects of ESWT are dose dependent, but the optimal chondroprotective dosage is unknown.
Material and Methods
Study design-animals
The study was conducted after approval was granted by the Ethical Board of Experimental Animal Researches of Gulhane Military Medicine Academy, and all the animals were kept in compliance with the ethics rules. Twenty-four Sprague-Dawney, 8-week-old male rats weighing 200–300g were used and were maintained under climate-controlled conditions on a 12-h light-dark cycle at 22°C with a relative humidity of 50–55%.
Experimental design and mono-iodo-asetate (MIA) injection
Twenty-four rats were divided into 3 groups, randomly: group 1-control group (n=8), group 2-LIPUS group (n=8), and group 3-ESWT group (n=8). The cartilage degeneration model was provided chemically by mono-iodo-asetate (MIA). Four rats (2 from the ESWT and 2 from the LIPUS group) died 24 hours after MIA delivery, so the study was completed with 6 rats in each group.
Before MIA application, all rats were anesthetized with 0.2 mg/kg 2% xylazine and 0.5 mg/kg 10% ketamine delivered intraperitoneally. For each rat, 1 mg of MIA was dissolved in 50 μL 0.09% saline and delivered to the right knees in group 1 rats and both knees in group 2 and 3 rats intraarticularly. Fifty microliters of 0.09% saline was delivered to the left knees in group 1 to check to see if osteoarthritis resulted. Applications were performed with a 27G Hamilton injector.
Application of treatments
Twenty-four hours after MIA delivery, LIPUS was applied to the right knees in the group 2 rats. The application was performed 0.5 cm below the medial tibial plateau in the anteroposterior plane with a 3 mHz frequency and 40 mW/cm2 intensity (Chattanooga Group Intelect Advance 2005 Encore Medical Corporation; Austin, Texas, U.S.A) at room temperature (22 °C) for 20 minutes a day for 15 days. Ultrasound gel was applied to the skin contacting the ultrasound probe, and all applications were performed under intraperitoneal anesthesia with 0.2 mg/kg 2% xylazine and 0.5 mg/kg 10% ketamine.
Twenty-four hours after MIA delivery, ESWT was applied only once on the right knees in the group 3 rats. The application was performed 0.5 cm below the medial tibial plateau in the anteroposterior plane with 800 impulses of shockwave at 12 kV (equivalent to 0.18 mj/mm2) (Storz medical duolith SD1; Storz Medical AG, Tagerwillen, Switzerland) at room temperature (22°C). Ultrasound gel was applied to the skin contacting the shockwave tube, and the application was performed under intraperitoneal anesthesia with 0.2 mg/kg 2% xylazine and 0.5 mg/kg 10% ketamine.
Assessments
Bone mineral density (BMD) measurements and scintigraphic scanning’s performed at day 16 before the animals were sacrified under the same anesthesia procedure (0.2 mg/kg 2% xylazine and 0.5 mg/kg 10% ketamine intraperitoneally). The BMD assessment was performed with a DXA device (QDR 4500 Elite Hologic Inc 1999, U.S.A) after calibration with an Anthropomorphic Spine Phantom (Hologic Inc. 35 Crocbydrie) device. Baseline volumetric subchondral BMD measurements of bilateral proximal (subchondral) tibiae were performed, and the mediolateral BMD ratio was calculated. For scintigraphic scanning, 2 mCi technetium-99 m methylenediphosphate (Tc-99 m-MDP) was administered to each rat through the tail vein. Precisely 190 minutes after injection, planar imaging was performed using a gamma camera (Mediso Nucline Spirit; Budapest, Hungary) for bilateral knees. In the scintigraphy results for evaluation purposes, regions of interest (ROI) of the same size were drawn bilaterally on the knees, and the counts were compared using the statistical analysis of differences.
For pain assessment, the bend test was performed on all subjects twice at day 7 and 14 after MIA delivery. During the knee bend test, all subjects were handled to restrict free motion but to permit passive movements in the knee joints. Vocal responses like squeaking were considered over 20 points.
Histomorphological analysis
Rats were sacrified at day 16 after the BMD measurement and scintigraphic scanning. Proximal tibial segments of the knees were taken, including articular cartilage and subchondral bone, and fixed by a 10% formaldehyde solution. The specimens were decalcified in 8% formic acid/hydrochloric acid solution for 12 hours. After the decalcification procedure, the specimens were immersed in paraffine blocks, and 4 μm thickened samples were cut. Samples were stained with heamtoxylin-eosin stain. The microscopic features of the articular cartilage were presented as chondrocyte activation, proliferation, apoptosis, cartilage fissuring, and pannus formation and assessed by a pathologist using a modified Mankin scale over 14 points. The histological evidence of cartilage degeneration was assessed through the structural changes in the articular cartilage (0, normal; 1, surface irregularities; 2, pannus and surface irregularities; 3, clefts to transitional zones; 4, clefts to radial zones; 5, clefts to calcified zones; and 6, complete disorganization), as well as the cell status (0, normal; 1, diffuse hypercellularity; 2, cloning; and 3, hypocellularity). The total cartilage degeneration score ranged from 0 (normal) to 9 (complete disorganization and hypocellularity of the articular cartilage). All cartilage sections were graded by a blinded pathologist.
Statistical analysis
The Statistical Package for the Social Sciences version 15.0 (SPSS Inc.; Chicago, IL, USA) software package was used in the analysis of the data. The variable distribution was analyzed visually and using analytical methods. The median, minimum, and maximum values by a chi-quare test were used for descriptive analysis. Variables among the groups were analyzed with the Kruskal-Wallis test, while dependent variables were compared with the Wilcoxon test. P values <0.05 were accepted as statistically significant.
Results
Radiographs of the knee in the anteroposterior and lateral projections were performed at day 0 and day 16. At day 16, osteoarthritic features like narrowing of the joint space and spur formation were seen.
The osteoblastic activities measured by scintigraphic scanning are given in Table 1. There was no significant difference among the right knee osteoblastic activities (p=0.532). The left knee osteoblastic activity of both the LIPUS and ESWT groups was significantly higher than those in the control group (p=0.003 for each), but there was no difference between the LIPUS and ESWT groups (p=0.631).
Table 1.
Osteoblastic activities of the groups
| Groups | Osteoblastic Activity median (min–max) | p |
|---|---|---|
| Right Knee | ||
| Control | 11290.29 (10380.93–14927.26) | |
| ESWT | 13791.29 (8743.12–16757.09) | 0.532 |
| LIPUS | 12530.29 (10792.66–17139.26) | |
| Left Knee | ||
| Control | 461.515 (358.79–897.29) | |
| ESWT | 13980.85 (9497.53–18653.46) | 0.003 |
| LIPUS | 14129.31 (10805.97–24999.74) |
min-max: minimum-maximum; ESWT: extracorporeal shockwave therapy; LIPUS: low-intensity pulsed ultrasound
The subchondral BMD values are given in Table 2. There was no difference among the groups for the right and left knee BMD values (p=0.557 and p=0.232, respectively).
Table 2.
The subchondral BMD values of the groups
| Groups | Subchondral BMD scores median (min–max) | p |
|---|---|---|
| Right Knee | ||
| Control | 1.460 (1.170–1.830) | |
| ESWT | 1.285 (1.190–2.050) | 0.557 |
| LIPUS | 1.710 (1.350–2.020) | |
| Left Knee | ||
| Control | 1.625 (1.190–1.960) | |
| ESWT | 1.515 (1.140–1.700) | 0.232 |
| LIPUS | 1.455 (1.170–1.860) |
min-max: minimum-maximum; ESWT: extracorporeal shockwave therapy; LIPUS: low-intensity pulsed ultrasound
The pain scores obtained by the knee bend test at day 7 are given in Table 3. The pain scores of both the right and left knees of the ESWT and LIPUS groups are significantly higher than those in the control group (p=0.044 and 0.001, respectively), but there was no significant difference between the ESWT and LIPUS groups (p=0.936 and 0.620, respectively).
Table 3.
The pain scores of the groups at day 7
| Groups | Pain Scores median (min–max) | p |
|---|---|---|
| Right Knee | ||
| Control | 12.0 (8.0–16.0) | |
| ESWT | 15.5 (14.0–18.0) | 0.044 |
| LIPUS | 13.0 (10.0–18.0) | |
| Left Knee | ||
| Control | 5.5 (4.0–8.0) | |
| ESWT | 16.5 (12.0–20.0) | 0.001 |
| LIPUS | 16 (13.0–19.0) |
min–max: minimum-maximum; ESWT: extracorporeal shockwave therapy; LIPUS: low-intensity pulsed ultrasound
The pain scores at day 14 are given in Table 4. There was no difference among the pain scores of the right knees (p=0.774). However, the pain scores of the left knees in both the ESWT and LIPUS groups were significantly higher than those in the control group (p=0.004 for each), but there was no significant difference between the ESWT and LIPUS groups (p=0.216).
Table 4.
Pain scores of the groups at day 14
| Groups | Pain Scores median (min–max) | p |
|---|---|---|
| Right Knee | ||
| Control | 14.5 (12.0–17.0) | |
| ESWT | 13.5 (10.0–17.0) | 0.774 |
| LIPUS | 14.0 (10.0–17.0) | |
| Left Knee | ||
| Control | 3.0 (1.0–4.0) | |
| ESWT | 16.5 (14.0–18.0) | 0.004 |
| LIPUS | 15.0 (13.0–18.0) |
min–max: minimum-maximum; ESWT: extracorporeal shockwave therapy; LIPUS: low-intensity pulsed ultrasound
The modified Mankin scores of the rat knees are given in Table 5. The modified Mankin scores of the right knees in the ESWT and LIPUS groups were significantly lower than those in the control group (p=0.002 for each), but there was no difference between the ESWT and LIPUS groups (p=0.131). The modified Mankin scores of the left knees in the ESWT and LIPUS groups were significantly higher than those in the control group (p=0.002 for each), but there was no difference between the ESWT and LIPUS groups (p=0.072).
Table 5.
Modified Mankin Scale scores of the rats according to groups
| Groups | Modified Mankin Scores median (min–max) | p |
|---|---|---|
| Right Knee | ||
| Control | 9.0 (7.0–10.0) | |
| ESWT | 2.0 (2.0–5.0) | 0.002 |
| LIPUS | 4.0 (2.0–5.0) | |
| Left Knee | ||
| Control | 0.5 (0–2.0) | |
| ESWT | 4.5 (3.0–8.0) | 0.004 |
| LIPUS | 6.5 (5.0–7.0) |
min–max: minimum–maximum; ESWT: extracorporeal shockwave therapy; LIPUS: low-intensity pulsed ultrasound
Figures 1–4 show the microscopic examples of the knee cartilage tissues of the subjects.
Figure 1.
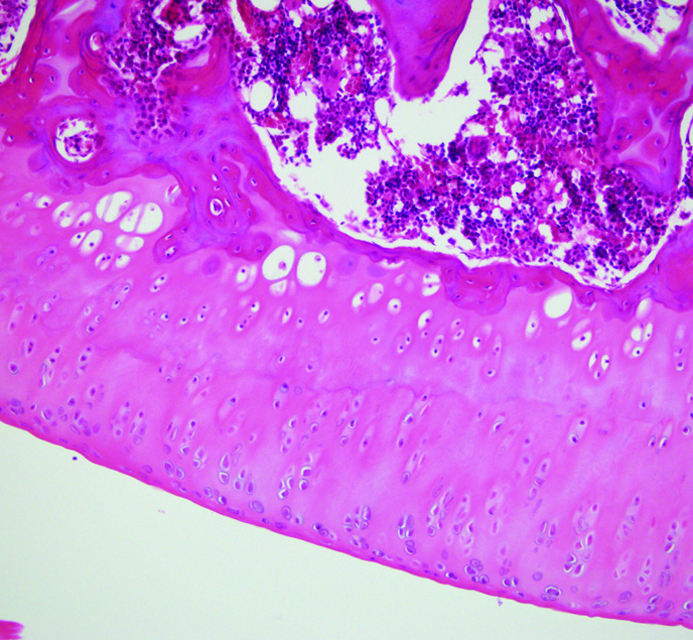
Normal cartilage tissue. Normal carilage surface is smooth. Deep layers of the cartilage tissue consist of proliferating chondroctes, but the density of the cells decreases through the cartilage surface.
Figure 2.
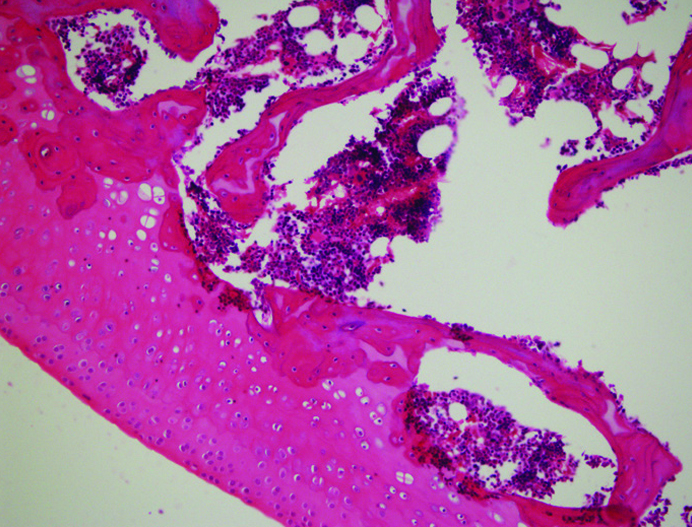
Cellular irregularity and picnosis. The early morphological features of osteoarthritis are cellular irregularity and picnosis. Both picnosis and cellular irregularity are unique findings for chondrocyte apopitosis. Chondrocyte loss results in decreased cartilage regeneration.
Figure 3.
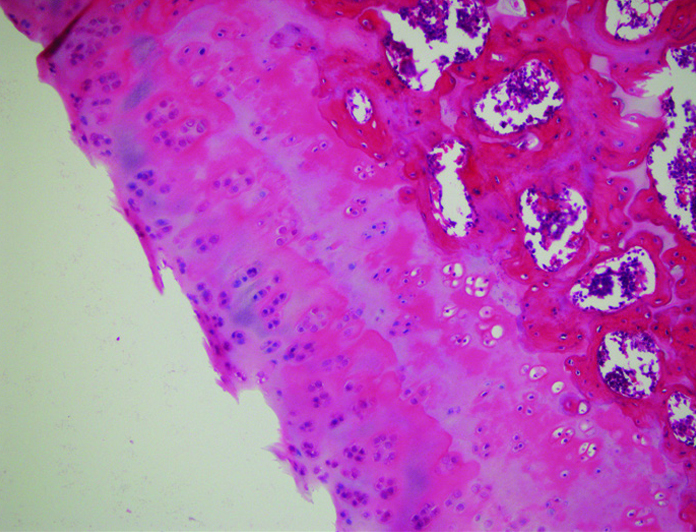
Surface irregularity. Decreased regeneration makes cartilage tissue sensitive to mechanical stress. Mechanical stress results in microfractures in deep layers. Excessive load and mechanical trauma without regeneration of the cartilage tissue results in surface irregularity.
Figure 4.
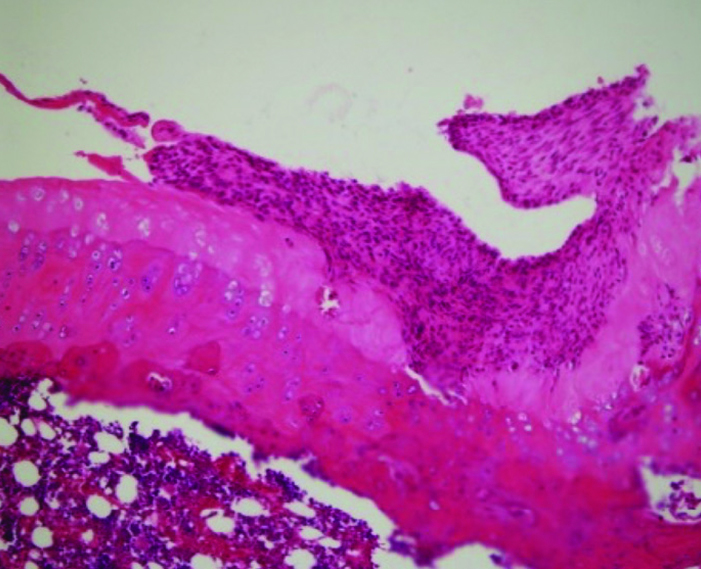
Cleft and pannus formation. Union of the surface irregularity areas results in deep clefts on the surface of the cartilage tissue. Insufficient chondrocytes with poor proliferative response to mechanical trauma evoke pannus formation.
Discussion
Our study results suggest that both ESWT and LIPUS have proliferative, regenerative effects on both bone and cartilage tissue in rats. Both modalities have analgesic effects besides the mitogenic activities. These findings confirmed the results of prior studies. However, we also found that both modalities have systemic effects and, to the best of our knowledge, this is the first study that demonstrates the systemic effect of LIPUS and ESWT in OA in vitro.
Hence, the basic pathological finding of OA is cartilage degeneration, and it has been recently accepted as a joint cartilage disease. There are increasing data indicating that morphological changes in OA arise from subchondral and underlying trabecular bone before cartilage degeneration, which makes subchondral bone an important target for new treatment modalities.
The effects of ESWT on connective tissue are controversial. Although proliferative effects were indicated, some studies have reported no significant effects but some of the recent animal experiments have indicated that ESWT application has chondroprotective effects and relieves symptoms such as pain by improving motor functions (21–24). Zhao et al. (25) suggested that these effects were due to the inhibition of nitric oxide (NO) release and partial apopytosis. Mayer-Wagner et al. (26) reported that ESWT down-regulates Tenascin-C whose expression increases at the superficial layer of the joint cartilage in OA. In the same study, researchers found that the Chi3L1 gene, which mediates the release of proinflammatory cytokines like TNF α and IL-1 regarding the subchondral bone reorganization, upregulates with ESWT application in 10 weeks. In their studies with a dog tendinopathy model, Wang et al. (17, 18) indicated that the chondroprotective effects of ESWT could be attributed to angiogenesis, neovasculation, and osteogenesis mediated by growth factors such as eNOS, VEGF, PCNA, and BMP-2.
The therapeutic use of ultrasound was first introduced in the orthopedic field in nonunion and delayed union cases. The following studies indicated that LIPUS might have beneficial effects on cell metabolism and tissue regeneration (27). Xueping et al. (28) reported that LIPUS inhibited signaling pathways like ERK1 ERK2 and p38, which mediate the matrix metalloproteinase-13 (MMP-13) release.
Our study results suggest that both ESWT and LIPUS have proliferative and regenerative effects on both bone and cartilage tissue in rats. Both modalities have analgesic effects besides the mitogenic activities. These findings confirm prior studies. Despite obvious histopathological recovery in cartilage tissue, there was no significant difference among the control, ESWT and LIPUS groups in subchondral BMD and knee joint osteoblastic activity suggesting that these treatment modalities may not have the same beneficial effects in subchondral bone. This may be due to the fact that LIPUS application was limited to 15 times and ESWT to one time. Longer applications may have more beneficial effects.
We found no difference among the pain scores of the right knees in the control, ESWT and LIPUS groups at day 7 and 14, suggesting that neither of the treatment modalities had an effect on pain in the early OA model. We found that the pain scores at day 14 were significantly lower than those at day 7 in both the ESWT and LIPUS groups. Several mechanisms, such as anti-inflammatory effects and increased angiogenesis, may be involved through the analgesic effects of both modalities (24, 26). In a recent study, Maier et al. (29) reported an increased release of substance P and prosotglandin E2, which might implicate analgesic effects.
The modified Mankin scale scores of the untreated knees in either the ESWT or LIPUS group were significantly lower than those in the right knees of the control group. However, there was no significant difference among the osteoblastic activities. These findings suggest that both treatment modalities have systemic effects.
The LIPUS and ESWT modalities are shown to have proliferative effects and prevent apoptyosis in chondrocyte cell culture models (30). However, these beneficial effects are limited to experimental animal studies in vitro. Moreover, no other studies have indicated their systemic effects before. We found histopathological improvement in both treated and untreated knees. To the best of our knowledge, this is the first study to compare the effects of these two modalities, and we have found that both of them have regenerative, proliferative, and protective effects on osteoarthritic rat knees.
Osteoarthritis is a common cause of morbidity in the world. The disease may be asymptomatic in early stages, and its irreversible features are common when it is diagnosed. Current treatment modalities are palliative and do not improve morphology. Research is still underway for a “disease modifying treatment” supporting morphological recovery. ESWT and LIPUS are effective, safe and cheap treatment modalities for OA. They seem to be effective before irreversible changes take place in the cartilage. Therefore, the early stages of OA must be discovered to determine when the morphologic features become irreversible. Further studies must be designed to assess the efficacy in vitro.
Although a power analysis was performed before the study, the main limitation of this study was the limited number of subjects. Similar studies with a larger number of subjects may have more significant results. Another limitation was the method of ESWT application. Some studies have used several ESWT applications, and some have used only one. We used only one application to present the effect of minimal use. Nevertheless, our results are remarkable, and these modalities seem to be promising for the treatment of OA.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Gülhane Training and Research Hospital.
Informed Consent: N/A.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - V.Y., E.U., Ö.K., T.D., A.Ç., A.K.T.; Design - V.Y., E.U., Ö.K., T.D., A.Ç., A.K.T.; Supervision - V.Y., E.U., Ö.K., T.D., A.Ç., A.K.T.; Resources - V.Y., Ö.K.; Materials - V.Y., E.U.; Data Collection and/or Processing - V.Y., T.D.; Analysis and/or Interpretation - V.Y., A.Ç.; Literature Search - V.Y., A.K.T.; Writing Manuscript - V.Y., E.U.; Critical Review - V.Y., A.K.T.; Other - V.Y, A.K.T.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Altman RD, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee. Arthritis Rheum. 1986;29:1039–49. doi: 10.1002/art.1780290816. https://doi.org/10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 2.Lozada CJ, Altman RD. Chondroprotection in osteoarthritis. Bull Rheum Dis. 1997;46:5–7. [PubMed] [Google Scholar]
- 3.Mow VC, Setton LA, Guilak F, Ratcliffe A. In: Mechanical factors in articular cartilage and their role in osteoarthritis, in osteoarthritic disorders. Kuettner KE, Goldberg VM, editors. American Academy of Orthopaedic Surgeons; Rosemont, IL: 1995. pp. 147–171. [Google Scholar]
- 4.Sukenik S, Henkin J, Zimlichman S, Skibin A, Neuman L, Pras M, et al. Serum and synovial fluid levels of serum amyloid A protein and C-reactive protein in inflammatory and noninflammatory arthritis. J Rheumatol. 1988;15:942–45. [PubMed] [Google Scholar]
- 5.Brandt KD. Defining Osteoarthritis: What it is and what it is not. J Musculoskeletal Med. 2010;27:338–57. [Google Scholar]
- 6.Duarte LR. The stimulation of bone growth by ultrasound. Acta Orthop Traumatol Surg. 1983;101:153–9. doi: 10.1007/BF00436764. https://doi.org/10.1007/BF00436764. [DOI] [PubMed] [Google Scholar]
- 7.Tien YC, Lin SD, Chen CH, Lu CC, Su SJ, Chih TT. Effects of pulsed low intensity ultrasound of human child chondrocytes. Ultrasound Med Biol. 2008;34:1174–81. doi: 10.1016/j.ultrasmedbio.2007.12.019. https://doi.org/10.1016/j.ultrasmedbio.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 8.Hasanova Gl, Noriega SE, Mamedov TG, Thakurta SG, Turner JA, Subramanian A. The effect of ultrasound stimulation on the gene and protein expression of the chondrocytes seeded in chitosan scaffolds. J Tissue Eng Regen Med. 2011;5:815–22. doi: 10.1002/term.384. https://doi.org/10.1002/term.384. [DOI] [PubMed] [Google Scholar]
- 9.Cook SD, Salkeld SD, Popich-Patron LS, Ryaby JP, Jones DG, Barrack RL. Improved cartilage repair after treatment with low intensity pulsed ultrasound. Clin Orthop. 2001;391:S231–43. doi: 10.1097/00003086-200110001-00022. https://doi.org/10.1097/00003086-200110001-00022. [DOI] [PubMed] [Google Scholar]
- 10.Huang MH, Yang RC, Ding HJ, Chai CY. Ultrasound effect on level of stress proteins and arthritic histology in experimental arthritis. Arch Phys Med Rehabil. 1999;80:551–6. doi: 10.1016/s0003-9993(99)90198-3. https://doi.org/10.1016/S0003-9993(99)90198-3. [DOI] [PubMed] [Google Scholar]
- 11.Granz B, Köhler G. What makes shockwaves efficient in lithotripsy. J Stone Dis. 1992;4:123–8. [PubMed] [Google Scholar]
- 12.Ogden JA, Alvarez RG, Levitt R, Marlow M. Shock wave therapy (orthotripsy) in musculoskeletal disorders. Clin Orthop Relat Res. 2001;387:22–40. doi: 10.1097/00003086-200106000-00005. https://doi.org/10.1097/00003086-200106000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Rompe JD, Kirkpatrick CJ, Kullmer K, Schwitalle M, Krischek O. Dose-related effects of shock waves on rabbit tendo Achillis. J Bone Joint Surg Br. 1998;80:546–52. doi: 10.1302/0301-620x.80b3.8434. https://doi.org/10.1302/0301-620X.80B3.8434. [DOI] [PubMed] [Google Scholar]
- 14.Sauer ST, Marymont JV, Mizel MS. What’s new in foot and ankle surgery. J Bone Joint Surg Am. 2004;86:878–86. doi: 10.2106/00004623-200404000-00045. https://doi.org/10.2106/00004623-200404000-00045. [DOI] [PubMed] [Google Scholar]
- 15.Wang CJ, Huang HY, Chen HH, Pai CH, Yang KD. Effect of shock wave therapy on acute fractures of the tibia. Clin Orthop Relat Res. 2001;387:112–8. doi: 10.1097/00003086-200106000-00015. https://doi.org/10.1097/00003086-200106000-00015. [DOI] [PubMed] [Google Scholar]
- 16.Thiel M. Application of shock waves in medicine. Clin Orthop Relat Res. 2001;387:18–21. doi: 10.1097/00003086-200106000-00004. https://doi.org/10.1097/00003086-200106000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Wang CJ, Yang KD, Wang FS, Hsu CC, Chen HH. Shock wave therapy enhances bone mass and bone strength after fracture of femur. Bone. 2004;34:225–30. doi: 10.1016/j.bone.2003.08.005. https://doi.org/10.1016/j.bone.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Wang CJ, Wang FS, Yang KD, Weng LH, Hsu CC, Huang CS, et al. Shock wave therapy induces neovascularization at the tendon-bone junction. J Orthop Res. 2003;21:984–89. doi: 10.1016/S0736-0266(03)00104-9. https://doi.org/10.1016/S0736-0266(03)00104-9. [DOI] [PubMed] [Google Scholar]
- 19.Revenaugh MS. Extracorporeal shock wave therapy for the treatment of osteoarthritis in the horse: clinical application. Vet Clin North Am Equine Pract. 2005;21:609–25. doi: 10.1016/j.cveq.2005.09.001. https://doi.org/10.1016/j.cveq.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Wang CJ, Weng LH, Ko JY, Sun YC, Yang YJ, Wang FS. Extracorporeal shockwave therapy shows chondroprotective effects in osteoarthritic rat knee. Arch Orthop Trauma Surg. 2011;131:1153–8. doi: 10.1007/s00402-011-1289-2. https://doi.org/10.1007/s00402-011-1289-2. [DOI] [PubMed] [Google Scholar]
- 21.Ertürk C, Altay MA, Ozardali I, Altay N, Cece H, Isıkan EU. The effect of extracorporeal shockwaves on cartilage end-plates in rabbits: a preliminary MRI and histopathological study. Acta Orthop Traumatol Turc. 2012;46:449–54. doi: 10.3944/aott.2012.2931. https://doi.org/10.3944/AOTT.2012.2931. [DOI] [PubMed] [Google Scholar]
- 22.Orhan Z, Alper M, Demirkaya M, Ozturan K. The effect of extra-corporeal shock wave therapy (ESWT) on the albino rat tendon tissue. Acta Orthop Traumatol Turc. 2000;34:308–11. [Google Scholar]
- 23.Frisbie DD, Kawcak CE, Mc Ilwraith CW. Evaluation of the effect of extracorporeal shock wave treatment on experimentally induced osteoarthritis in middle carpal joints of horses. Am J Vet Res. 2009;70:449–54. doi: 10.2460/ajvr.70.4.449. https://doi.org/10.2460/ajvr.70.4.449. [DOI] [PubMed] [Google Scholar]
- 24.Ochiai N, Ohtori S, Sasho T, Nakagawa K, Takahashi K, Takahashi N, et al. Extracorporeal shock wave therapy improves motor dysfunction and pain originating from knee osteoarthritis in rats. Osteoarthritis Cartilage. 2007;15:1093–96. doi: 10.1016/j.joca.2007.03.011. https://doi.org/10.1016/j.joca.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 25.Zhao Z, Huiri J, Rufang J, Liu C, Wang M, Zhai L, et al. Extracorporeal shock wave therapy reduces progression of knee osteoarthritis in rabbits by reducing nitric oxide level and chondrocyte apopitosis. Arch Orthop Trauma Surg. 2012;132:1547–53. doi: 10.1007/s00402-012-1586-4. https://doi.org/10.1007/s00402-012-1586-4. [DOI] [PubMed] [Google Scholar]
- 26.Mayer-Wagner S, Ernst J, Maier M, Chiquet M, Joos H, Müller PE, et al. The effect of high energy extracorporeal shockwaves on hyaline cartilage of adult rats in vivo. J Orthop Res. 2010;28:1050–6. doi: 10.1002/jor.21074. [DOI] [PubMed] [Google Scholar]
- 27.Kristiansen TK, Ryaby JP, McCabe J, Frey JJ, Roe LR. Accelerated healing of distal radial fractures with the use of specific low intensity ultrasound. A multicenter, prospective, randomized, double blinded placebo controlled study. J Bone Joint Surg Am. 1997;79:961–73. doi: 10.2106/00004623-199707000-00002. https://doi.org/10.2106/00004623-199707000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Li X, Li J, Cheng K, Lin Q, Wang D, Zhang H, et al. Effect of low intensity pulsed ultrasound on MMP-13 and MAPKs signaling pathway in rabbit knee osteoarthritis. Cell Biochem Bio Phys. 2011;61:427–34. doi: 10.1007/s12013-011-9206-4. https://doi.org/10.1007/s12013-011-9206-4. [DOI] [PubMed] [Google Scholar]
- 29.Maier M, Averbeck B, Milz S, Reifor HJ, Schimitz C. Substance P and Prostaglandin E2 release after shockwave application to the rabbit femur. Clin Orthop Relat Res. 2003;406:237–45. doi: 10.1097/01.blo.0000030173.56585.8f. https://doi.org/10.1097/00003086-200301000-00034. [DOI] [PubMed] [Google Scholar]
- 30.Yuan LJ, Niu CC, Lin SS, Yang CY, Chan YS, Chen WJ, et al. Effects of low intensity pulsed ultrasound and hyperbaric oxygen on human osteoarthritic chondrocytes. J Orthop Surg Res. 2014;9:5. doi: 10.1186/1749-799X-9-5. https://doi.org/10.1186/1749-799X-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


