Abstract
Catastrophic antiphospholipid syndrome (CAPS) is a rare and fatal condition that is characterized by diffuse venous and/or arterial thromboembolism within a short period of time and histopathological confirmation of small-vessel occlusion in at least one organ or tissue in the presence of positive antiphospholipid antibodies. Here we report the case of a 19-year-old woman with CAPS. During the first week of her hospitalization, she was diagnosed with CAPS on the basis of skin necrosis, pulmonary artery thrombosis, cerebral venous sinus thrombosis, and positive lupus anticoagulant. She was treated with corticosteroids, intravenous immunoglobulins, plasmapheresis, and anticoagulants. Forty days after the onset of CAPS, cutaneous lesions were recurred during skin surgery. She required a high dose of corticosteroids, intravenous immunoglobulins, and rituximab. No further thrombotic events occurred. Rituximab may be an effective treatment option for patients with CAPS.
Keywords: Antiphospholipid syndrome, plasmapheresis, rituximab, skin necrosis
Introduction
Antiphospholipid syndrome (APS) is a state of hypercoagulation that is characterized by recurrent venous or arterial thrombosis and/or morbidity during pregnancy. Antiphospholipid antibodies include three main antibodies: anticardiolipin, lupus anticoagulant, and anti-b2-glycoprotein. Infection, malignancy, and certain drugs may play a crucial role in triggering APS. However, the pathophysiology of this association is unclear (1).
Catastrophic antiphospholipid syndrome (CAPS) is a severe, life-threatening form of APS. CAPS affects only 1% of patients with APS; however, it has a high mortality rate. CAPS predominantly affects the microvasculature and is characterized by small-vessel thrombosis within a short period of time. Early diagnosis and aggressive treatment are important to save the lives of patients with CAPS. Only 20 patients with CAPS have been reported to be treated with rituximab in the CAPS registry (2). Here we report the case of a 19-year-old female patient with CAPS who was successfully treated with rituximab.
Case Presentation
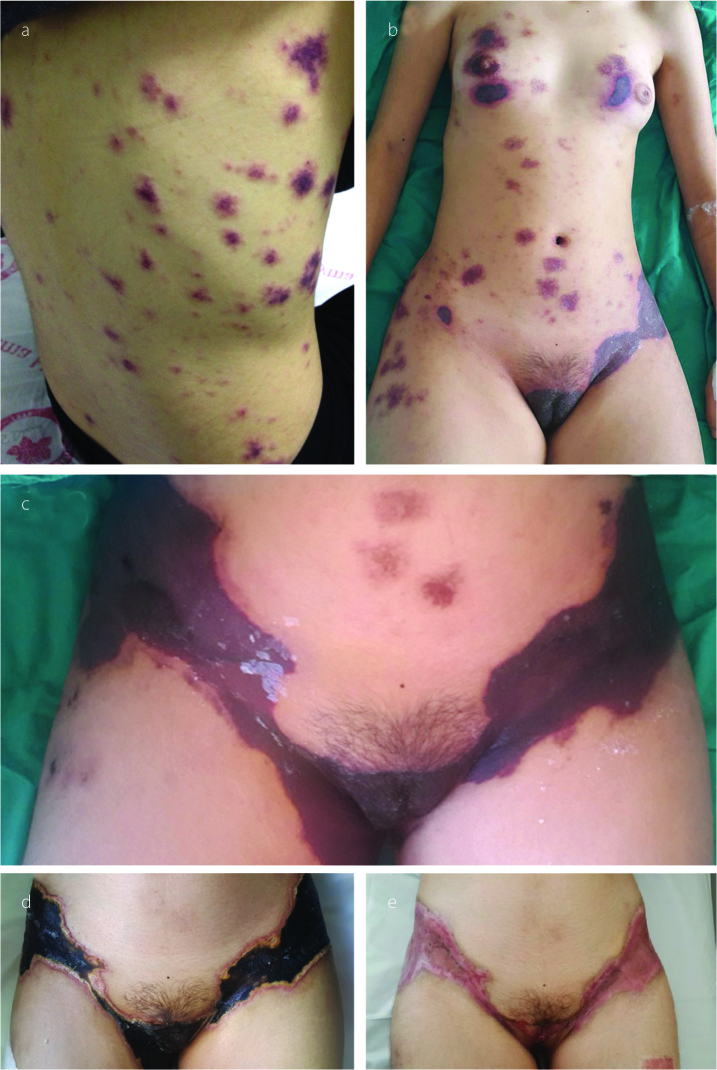
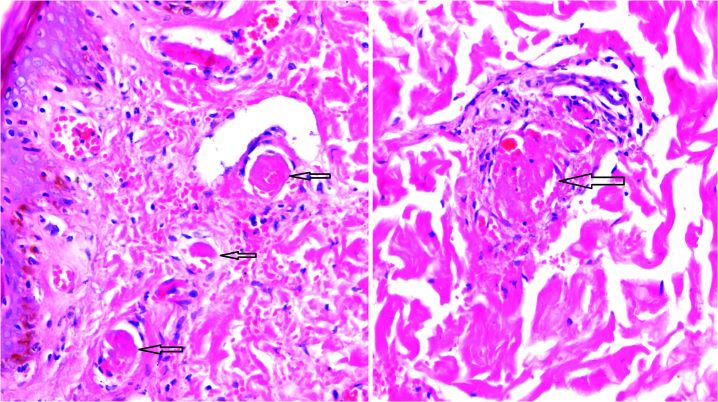
A 19-year-old female patient was admitted with cutaneous lesions that started after taking a non-steroidal anti-inflammatory drug. She took ketoprofen for 2 days to reduce the knee pain. The lesions were irregularly shaped, non-itching red patches that were mainly located on the chest and back (Figure 1a). Constitutional symptoms were not present. Physical examination was unremarkable except for the presence of skin lesions. Laboratory examination revealed microcytic anemia (hemoglobin: 11.2 g/dL and mean corpuscular volume: 77 fL) and increased C-reactive protein level (CRP: 52 mg/dL, N: 0–3). A skin biopsy was performed. However, 1 day after the appearance of the lesions, new painful necrotic skin lesions appeared on the left inguinal and genital areas (Figure 1b). The patient was treated with 1 mg/kg/day enoxaparin. Before treatment, a blood sample was taken to examine the thrombophilia panel, antinuclear antibody (ANA), extractable nuclear antigen antibody (ENA), anti-neutrophil cytoplasmic antibody (ANCA), and antiphospholipid antibody. Further new necrotic lesions in the right inguinal area were observed at the follow-up visit 2 days later (Figure 1c) accompanied by shortness of breath and headache. Thromboses in the superior sagittal and transverse sinus were detected by magnetic resonance venography (Figure 2a, b). Thromboses were also detected in the subsegmental branches of the pulmonary artery (Figure 3a, b). Transthoracic echocardiogram and Doppler scan of the leg and abdominal vessels were normal. The patient’s platelet count decreased to 102×103/μL. Skin biopsies showed vascular thromboses in small arterioles and capillaries of the subcutaneous tissue (Figure 4). Other causes of microangiopathic hemolytic anemia were ruled out. The patient was administered six rounds of plasmapheresis. The patient was also treated with intravenous methyl prednisolone at a dose of 1 g/day for 3 days, followed by intravenous immunoglobulin (IVIg) at a dose of 2 g/kg for 2 days. ANA, ENA, ANCA, cardiolipin antibody, and beta-2-glycoprotein values were later found to be negative. Lupus anticoagulant (LA) was reported as positive, and LA was repeated at 10 weeks; a positive result was detected again. Dilute Russell viper venom test was performed to detect and confirm the presence of LA. On the basis of skin biopsy indicating thrombosis in small arterioles and capillaries, pulmonary artery thrombosis, superior sagittal and transverse sinus thrombosis developing within 1 week, and positive LA, the patient was diagnosed with CAPS. Improvements in clinical and laboratory parameters were observed after the administration of corticosteroids, IVIg, and plasmapheresis. Skin lesions, except in the inguinal area, completely regressed. Corticosteroid therapy was tapered, and hydroxychloroquine was added. Forty days after the CAPS attack, debridement of necrotic areas in the inguinal area and skin graft were performed. Hydroxychloroquine, methylprednisolone at a dose of 16 mg/day, and low-molecular-weight heparin (LMWH) were continued during surgery. Skin lesions on the chest and back, similar to the initial and early lesions, appeared again on the third postoperative day. Five rounds of plasmapheresis were administered again and the patient was also treated with intravenous methyl prednisolone at a dose of 1 g/day for 3 days. After plasmapheresis, rituximab at a dose of 375 mg/m2 IV infusion once weekly for 4 doses was administered. The skin lesions were completely resolved. The dose of methylprednisolone was tapered. The patient was prescribed with hydroxychloroquine and coumadine, and there was no evidence of recurrent skin lesion or thromboembolism at the 10-month follow-up. Informed consent was obtained from the patient.
Figure 1 a–e.
Skin lesions on the chest and back on hospital admission (a). A day after admission, new painful necrotic skin lesions appeared on the left inguinal and genital areas (b). Two days after admission, new necrotic lesions in the right inguinal area were observed (c). Thirty days after admission, before debridement of necrotic areas in the inguinal area and skin graft (d). Four months after admission, after debridement and skin graft (e).
Figure 2 a, b.
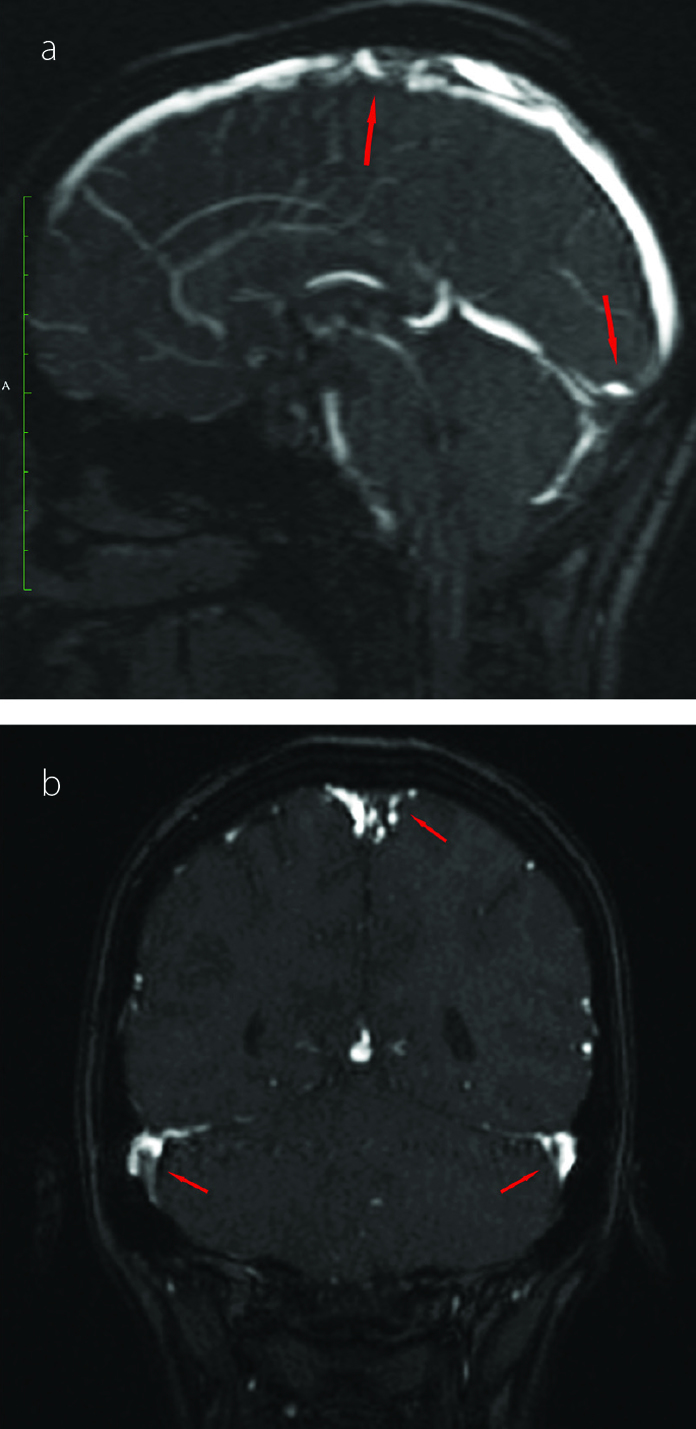
Thromboses in the superior sagittal sinus (a) Thromboses in the transverse sinus (b)
Figure 3 a, b.
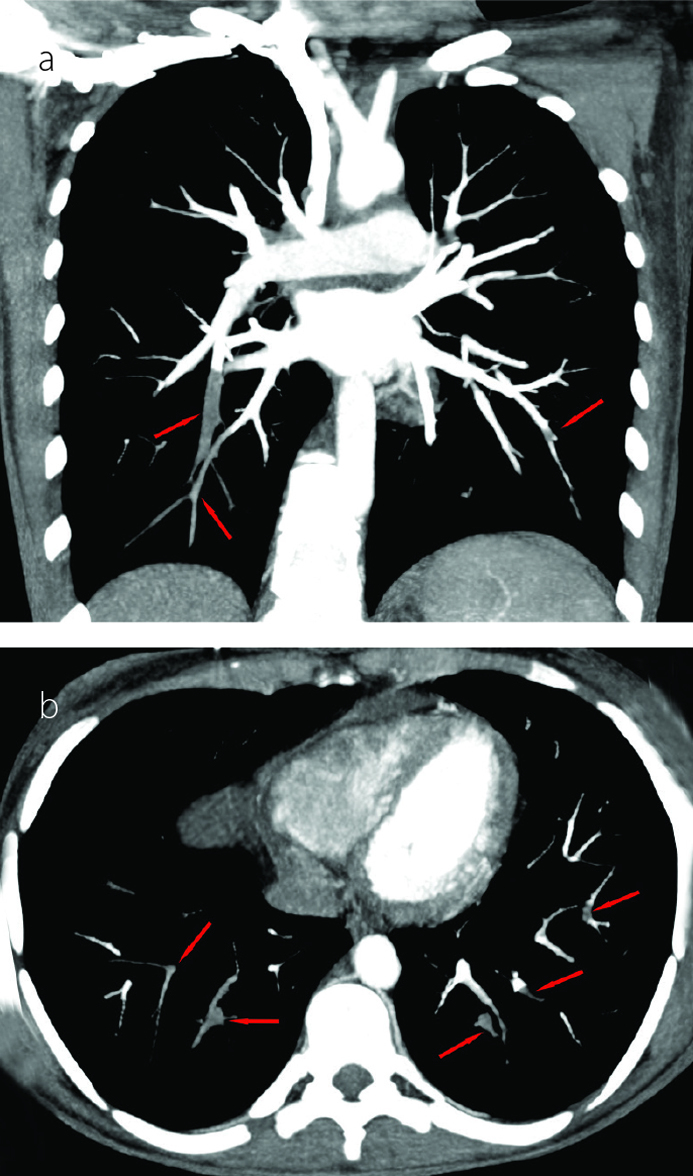
Coronal (a) and axial (b) CT images show thromboses in the subsegmental branches of the pulmonary artery.
Figure 4.
Vascular thromboses in small arterioles and capillaries of the subcutaneous tissue (Hematoxylin and eosin staining; magnification, ×100, ×200)
Discussion
We reported the case of a 19-year-old female patient admitted with cutaneous necrosis. During the first week of her hospitalization, she was diagnosed with CAPS on the basis of skin necrosis, pulmonary artery thrombosis, cerebral venous sinus thrombosis, and positive LA. She was treated with corticosteroids, intravenous immunoglobulins, plasmapheresis, and anticoagulants. Forty days after the CAPS attack, cutaneous lesions were repeated during skin surgery; therefore, rituximab was administered. No further thrombotic events occurred after the rituximab treatment.
Catastrophic antiphospholipid syndrome, a severe manifestation that is observed in a small number of patients with APS, is characterized by widespread thrombosis in many organ systems (3). Being a rare disease, an optimal treatment for CAPS is not yet available. Anticoagulation therapy is the first and most important step of treatment. Unfractionated heparin is an effective anticoagulation agent. Antiplatelet therapy, in particular, can be used in addition to anticoagulant treatment in patients with arterial thrombosis (4). LMWH therapy was initiated in the present study patient in conjunction with the emergence of necrotic skin lesions. Second-line treatments include plasmapheresis and/or immunomodulatory treatments. Plasmapheresis is very frequently used in patients with CAPS. In the present study patient, we have administered seven rounds of plasmapheresis to the patient. In addition to anticoagulant therapy and plasmapheresis, immunomodulatory agents (usually prednisolone and IVIg and less frequently, azathioprine, cyclophosphamide, rituximab, and eculizumab) are used (5). Methylprednisolone at a dose of 1 g/day for 3 days, followed by 1 mg/kg/day was initiated to treat the present study patient. After plasmapheresis, total IVIg at 2g/kg was administered for 2 days. Previous case reports have indicated the use of rituximab in patients with CAPS. The therapeutic effect of rituximab is thought to be caused by a reduction in the number of B cells, which in turn can reduce the production and concentration of cytokines. In a recent case report that reported the case of a patient with CAPS associated with systemic lupus erythematosus (SLE) and treated with rituximab, Sukara et al. (6) reported that the right timing of rituximab administration was uncertain. In this case report, administration of rituximab with steroids, IVIg, and anticoagulant was ineffective, and the patient died. The CAPS registry indicates that rituximab was administered to 20 patients with CAPS. Of these, 15 patients showed a positive response, with no new episodes of thrombosis. Rituximab was the first-line treatment in eight patients (40%). Two of the patients, who used rituximab as a first-line treatment, died. Rituximab was the second-line therapy in 12 patients (60%) who had poor response, relapsing episode, or worsening of clinical finding (2, 7). In the present study patient, the reappearance of skin lesions postoperatively indicated the possibility of refractory CAPS; therefore, rituximab treatment was initiated. A complete remission of the skin lesions was observed.
In conclusion, CAPS is a rare and life-threatening condition. It should be quickly diagnosed, and an aggressive treatment protocol is needed. Rituximab is one of the choices for the effective treatment of CAPS. Because of the rarity of the disease, optimization of the treatment protocol is difficult. In the light of the information that can be received from the CAPS registry in the future, more data may become available on the optimal dose and treatment options of this disease.
Footnotes
Ethics Committee Approval: N/A.
Informed Consent: Written informed consent was obtained from the patient who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - A.D., Y.U.; Design - A.D., Y.U.; Supervision - M.Ş., S.E.T.; Materials - A.D., Y.U., N.K.; Data Collection and/or Processing - A.D., Y.U., N.K.; Analysis and/or Interpretation - A.D., Y.U.; Writing Manuscript - A.D., M.Ş., S.E.T.; Critical Review - M.Ş., S.E.T.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Abdel-Wahab N, Lopez-Olivo MA, Pinto-Patarroyo GP, Suarez-Almozor ME. Systematic review of case reports of antiphospholipid syndrome following infection. Lupus. 2016;25:1520–31. doi: 10.1177/0961203316640912. https://doi.org/10.1177/0961203316640912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berman H, Rodriquez-Pinto I, Cervera R, Morel N, Costedoat-Chalumeau N, Erkan D, et al. Rituximab use in the catastrophic antiphospholipid syndrome: Descriptive analysis of the CAPS registry receiving rituximab. Autoimmun Rev. 2013;12:1085–90. doi: 10.1016/j.autrev.2013.05.004. https://doi.org/10.1016/j.autrev.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Espinosa G, Cervera R, Asherson RA. Catastrophic antiphospholipid syndrome and sepsis. A common link? J Rheumatol. 2007;34:923–6. [PubMed] [Google Scholar]
- 4.Ortel TL, Erkan D, Kitchens CS. How I treat catastrophic thrombotic syndromes. Blood. 2015;126:1285–93. doi: 10.1182/blood-2014-09-551978. https://doi.org/10.1182/blood-2014-09-551978. [DOI] [PubMed] [Google Scholar]
- 5.Cervera R, Rodriguez-Pinto I, Colafrancesco S, Conti F, Valesini G, Rosario C, et al. 14th International Congress on Antiphospholipid Antibodies Task Force Report on Catastrophic Antiphospholipid Syndrome. Autoimmun Rev. 2014;13:699–707. doi: 10.1016/j.autrev.2014.03.002. https://doi.org/10.1016/j.autrev.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Sukara G, Baresic M, Sentic M, Brcic L, Anic B. Catastrophic antiphospholipid syndrome associated with systemic lupus erythematosus treated with rituximab: case report and a review of the literature. Acta Reumatol Port. 2015;40:169–75. [PubMed] [Google Scholar]
- 7.Rodriquez-Pinto I, Cervera R, Espinosa G. Rituximab and its therapeutic potential in catastrophic antiphospholipid syndrome. Ther Adv Musculoskelet Dis. 2015;7:26–30. doi: 10.1177/1759720X14554793. https://doi.org/10.1177/1759720X14554793. [DOI] [PMC free article] [PubMed] [Google Scholar]