Abstract
Background
A post-marketing surveillance (PMS) study was conducted with a 1-year observation period to assess the safety and efficacy of lamotrigine in routine clinical practice in patients with bipolar disorder (BD).
Patients and methods
Central enrollment method was used to recruit patients diagnosed with BD who were being treated for the first time with lamotrigine to prevent the recurrence/relapse of BD mood episodes. Adverse drug reactions (ADRs) and recurrence/relapse were assessed. Improvement of mania and depression was also assessed using the Hamilton’s Rating Scale for Depression (HAM-D) and the Young Mania Rating Scale (YMRS) at treatment initiation, 4–6 months post treatment initiation, and 10–12 months post treatment initiation.
Results
A total of 237/989 patients (24.0%) reported ADRs, most commonly rash (9.1%), and the incidence of serious ADRs was 3.3% (33/989 patients). Skin disorders occurred in 130 patients (13.1%), mostly within 8 weeks post treatment. A total of 237/703 patients (33.7%) experienced recurrence/relapse of mood episodes. The 25th percentile of the time to recurrence/relapse of mood episodes was 105 days. Remission of depression symptoms (HAM-D ≤7) occurred in 147/697 patients (21.1%) at treatment initiation, rising to 361 patients (67.4%) at 10–12 months post treatment. Remission of manic symptoms (YMRS ≤13) occurred in 615/676 patients (91.0%) at treatment initiation, rising to 500 patients (97.3%) at 10–12 months post treatment.
Conclusion
The results of this PMS study suggest that lamotrigine is a well-tolerated and effective drug for preventing recurrence/relapse of BD in clinical practice.
Keywords: lamotrigine, bipolar, mood episode, post-marketing surveillance study, safety, efficacy
Introduction
Lamotrigine is an antiepileptic drug discovered and developed by GlaxoSmithKline Ltd. in the United Kingdom. In the course of the development of the drug for the treatment of epilepsy, it was also found that mood disorders improved in some patients. Development proceeded as a drug for the treatment of BD, and a study confirming the effect in preventing the recurrence/relapse of mood episodes in patients with bipolar I disorder (BDI) suggested that lamotrigine significantly prolonged the time to recurrence/relapse of mood episodes compared with placebo.1–4 The results also suggested that the effect of lamotrigine in preventing recurrence/relapse of mood episodes was more pronounced in the depressive phase than in the manic phase.5 This was followed by more randomized controlled studies in patients with BD, which formed the basis of its marketing authorization approval in BD.
Meanwhile, there have been few reports to date of long-term studies on the safety of lamotrigine or its effect in preventing recurrence/relapse during routine clinical practice. This post-marketing surveillance (PMS) study was conducted to confirm the safety and efficacy of long-term lamotrigine use in routine clinical practice in Japanese patients with BD, and the results of analysis are reviewed.
Patients and methods
Study design and participants
This study was carried out in Japanese patients diagnosed as suffering from BD according to DSM-IV-TR, who were prescribed lamotrigine for the first time to prevent the recurrence/relapse of mood episodes in BD. During the 2-year period from October 2011 to October 2013, investigators registered patients prospectively to a register center within 14 days of starting lamotrigine treatment using a central registration method. The observation period for each patient was 1 year.
The sample size was assumed that 1,000 patients would be needed to detect, with 95% confidence interval; in at least 1 patient, an unexpected adverse drug reaction (ADR) was occurring at the same frequency as the incidence (0.3%) of an ADR that occurred in 1 patient in the Japanese clinical study.3
There was a strict titration schedule of lamotrigine, but no limitation on concomitant drugs. Investigators were requested to prescribe lamotrigine properly based on the Japanese package insert (when lamotrigine was used as a monotherapy, patients began a 6-week escalation of lamotrigine to a target dose of 200 mg/day [weeks 1–2, 25 mg/day; weeks 3–4, 50 mg/day; week 5, 100 mg/day; week 6, 200 mg/day]). When used as an adjunctive therapy with valproate, the starting and target doses of lamotrigine were halved (weeks 1–2, 25 mg/every other day; weeks 3–4, 25 mg/day; week 5, 50 mg/day; week 6, 100 mg/day). When used as an adjunctive therapy with carbamazepine, the starting and target doses of lamotrigine were doubled (weeks 1–2, 50 mg/day; weeks 3–4, 100 mg/day; week 5, 200 mg/day; week 6, 300 mg/day).
Before the implementation of the study, a written contract was finalized with the head of each medical institution (eg, hospital directors), where lamotrigine had been adopted and delivered and the approval for this study had been obtained. Institutional review boards (IRBs) provided approval as needed according to the regulations of the medical institutions that had been requested to conduct the study. This was an observational, interventional PMS study, and the patient consent was obtained according to the regulations at each medical institution (Table S1).
Investigation items
Patient characteristics
Gender, age, diagnosis, duration of BD, mood episodes commonly seen in the past, type of mood episodes at the start of lamotrigine treatment, severity of mood episodes at the start of lamotrigine treatment and complications, etc. were investigated. The diagnoses, as defined by DSM-IV-TR, were “BDI,” “bipolar II disorder (BDII),” “cyclothymic disorder,” “BD not otherwise specified,” and “other.” The type of mood episodes was also diagnosed as “manic,” “hypomanic,” “mixed,” or “major depressive” according to DSM-IV-TR. Manic, hypomanic, or mixed episodes are expressed as “mania-related episodes” in this article.
Lamotrigine treatment status
The dosage and administration, duration of treatment, and concomitant medications were investigated.
Safety assessment
All adverse events (AEs) reported after starting lamotrigine treatment were investigated for the name of the event, date of onset, outcome, seriousness, and the causal relationship with lamotrigine. Serious AEs were defined as follows: an event that 1) results in death; 2) is life-threatening; 3) requires inpatient hospitalization, or prolongation of existing hospitalization, for treatment; 4) causes persistent or significant disability/incapacity; 5) results in a congenital anomaly/birth defect; 6) is another event or reaction that is judged to be a medically significant condition; or 7) is an event in the Important Medical Event Terms list of the European Medicines Agency. AEs causally related to lamotrigine were treated as ADRs and were assessed and tabulated using the verbatim terms by investigators or terms in the MedDRA/J version 18.1 that were closest to the verbatim terms by the investigators.
In addition, the following were defined as priority investigation items: 1) skin disorders; 2) suicide-related events or self-injury; and 3) harmful behavior toward others. The occurrence of events determined by investigators that correspond to the abovementioned information and diseases matching the following MedDRA codes was also investigated: 1) skin disorders: regarded as “skin and subcutaneous tissue disorders” in the MedDRA system organ class (SOC); 2) suicide-related events or self-injury: events related to suicide/self-injury in the MedDRA SMQ; 3) harmful behavior toward others: events included in hostility/aggression in the MedDRA SMQ and events noted in the Food and Drug Administration Talk Paper.6
Efficacy assessment
Patients were asked at every visit about the presence/absence of recurrence/relapse of mood episodes after initiation of lamotrigine treatment. If mood episodes had recurred, the patient was asked about the type of mood episode, time of onset, presence/absence of remission of the episode, and remission date. Patients were also assessed using Hamilton’s Rating Scale for Depression (HAM-D) and the Young Mania Rating Scale (YMRS) at the start of lamotrigine treatment, 4–6 months after treatment initiation, and 10–12 months after treatment initiation.
Statistical analysis
Safety
The number of patients with ADRs was tabulated by SOC and type. The time of the onset of skin disorders was also tabulated by type.
Efficacy
The time to recurrence/relapse of mood episodes was calculated as the time to onset of the first mood episode after lamotrigine treatment. By the type of mood episodes, the time to recurrence/relapse of depressive episodes was defined as the time to onset of the first major depressive episode, and the time to recurrence/relapse of mania-related episodes was defined as the time to onset of manic, hypomanic, or mixed episodes. For patients who had a mood episode at the start of lamotrigine treatment, the duration from the time of remission of the episode to the onset of a subsequent mood episode was estimated as the time to recurrence/relapse, and for those who had not, the duration from the start of lamotrigine treatment to the onset of a subsequent mood episode was used. Remission was defined as no mood episode when a patient visited a medical institution.
The Kaplan–Meier analysis was used to estimate the time to recurrence/relapse of mood episodes. The quartile points for the time to recurrence/relapse of mood episodes were also estimated, and the confidence interval was calculated.
The summary statistics for the total HAM-D and YMRS scores at 4–6 months and 10–12 months after treatment initiation were calculated in patients with scores obtained at 2 points: at the start of lamotrigine treatment and after treatment initiation (either 4–6 months or 10–12 months). The summary statistics for the change from baseline (the day of starting lamotrigine treatment) in total scores were also calculated. The number of patients with an HAM-D score of ≤7 or YMRS score of ≤13, used as an indicator of remission of depressive episodes and manic episodes, respectively, was also tabulated, and the percentage of such patients relative to the number of patients in HAM-D and YMRS analysis was calculated.
Regulatory compliance
This study was conducted according to the “Ordinance on Good Post-marketing Surveillance Practice (GPSP; Ministry of Health, Labour and Welfare Ordinance No 171, December 20, 2004),” which was established to ensure the appropriate conduct of PMS study undertaken by the marketing authorization holder, for example, of a pharmaceutical to confirm the safety or efficacy of the pharmaceutical.
Results
Patient disposition
A total of 1,036 patients were enrolled, and the case report forms (CRFs) were collected from 1,010 patients at 207 medical institutions nationwide. The safety analysis set consisted of 989 patients after the exclusion of a total of 21 patients (no visit after initial prescription: 17, AEs unknown: 3, did not receive lamotrigine: 1). The efficacy analysis set consisted of 966 patients after the exclusion of a total of 23 patients (poor medication adherence: 12, off-label use: 9, efficacy unknown: 2) from the safety analysis set. The time to recurrence/relapse of mood episodes could be analyzed in 703 of these patients. The HAM-D and YMRS analysis sets consisted of 697 and 676 patients, respectively (Figure S1).
Patient characteristics
Females represented 55.9% of the patients. At the start of lamotrigine treatment, the median age was 43 years. In terms of diagnosis, 65.9% were BDII, and 26.1% were BDI. The duration of BD (median) was 9 years. The most common number of mood episodes in the past year was 1 time (36.2%), followed by 2 times (23.0%). The most common type of mood episodes at the start of lamotrigine treatment was major depression (66.7%). The severity of mood episodes was moderate in 52.0%. Concomitant medication was used by 93.5% of the patients, comprising mood stabilizer in 47.3%, antipsychotic in 54.8%, and antidepressant in 47.2% (Table 1). The most commonly used concomitant atypical antipsychotics were aripiprazole (20.2%), olanzapine (16.7%), and quetiapine fumarate (14.6%).
Table 1.
Patient characteristics (safety analysis set)
| Characteristics/category | No of patients (%) |
|---|---|
| Safety analysis set | 989 |
| Gender | |
| Male | 436 (44.1) |
| Female | 553 (55.9) |
| Age (years) | |
| <15 | 2 (0.2) |
| ≥15 to <20 | 13 (1.3) |
| ≥20 to <30 | 146 (14.8) |
| ≥30 to <40 | 238 (24.1) |
| ≥40 to <50 | 255 (25.8) |
| ≥50 to <60 | 174 (17.6) |
| ≥60 to <65 | 64 (6.5) |
| ≥65 | 97 (9.8) |
| Min to max | 13–83 |
| Mean ± SD | 44.2±14.22 |
| Median | 43 |
| Diagnosis | |
| BDI | 258 (26.1) |
| BDII | 652 (65.9) |
| Cyclothymic disorder | 29 (2.9) |
| BD not otherwise specified | 41 (4.1) |
| Othera | 9 (0.9) |
| Duration of BD (years) | |
| <1 | 36 (3.6) |
| ≥1 to <2 | 46 (4.7) |
| ≥2 to <5 | 124 (12.5) |
| ≥5 to <10 | 172 (17.4) |
| ≥10 to <20 | 230 (23.3) |
| ≥20 | 118 (11.9) |
| Unknown | 263 (26.6) |
| Min to max | 0–47 |
| Mean ± SD | 11.0±9.41 |
| Median | 9 |
| No of mood episodes in the past year (times) | |
| 0 | 18 (1.8) |
| 1 | 358 (36.2) |
| 2 | 227 (23.0) |
| 3 | 88 (8.9) |
| ≥4 | 40 (4.0) |
| Unknown | 258 (26.1) |
| Min to max | 0–14 |
| Mean ± SD | 1.8±1.42 |
| Median | 1 |
| Type of mood episodes at the start of lamotrigine treatment | |
| Manic | 37 (3.7) |
| Hypomanic | 75 (7.6) |
| Mixed | 134 (13.5) |
| Major depressive | 660 (66.7) |
| Indeterminate | 17 (1.7) |
| Normal mood | 65 (6.6) |
| Unknown | 1 (0.1) |
| Severity of mood episodes at the start of lamotrigine treatment | |
| Indeterminate | 6 (0.6) |
| Normal (no symptoms) | 46 (4.7) |
| Degree to which the presence of BD cannot be denied | 26 (2.6) |
| Mild | 265 (26.8) |
| Moderate | 514 (52.0) |
| Severe | 116 (11.7) |
| Very severe | 14 (1.4) |
| Extremely severe | 2 (0.2) |
| Concomitant medication | |
| No | 64 (6.5) |
| Yes | 925 (93.5) |
| Concomitant medication (more than 1 in some cases) | |
| Mood stabilizer | 468 (47.3) |
| Lithium | 303 (30.6) |
| Sodium valproate | 188 (19.0) |
| Carbamazepine | 40 (4.0) |
| Concomitant antipsychotic | 542 (54.8) |
| Antipsychotic (atypical) | 460 (46.5) |
| Antipsychotic (typical) | 150 (15.2) |
| Concomitant antidepressant | 467 (47.2) |
| SSRI/SNRI/NaSSA | 432 (43.7) |
| Tricyclic/tetracyclic | 101 (10.2) |
Note:
Depression (n=2), affective disorder (n=1), schizophrenia (n=1), schizoaffective disorder (n=1), Cotard’s syndrome (n=1), adjustment disorder (n=1), acute and transient psychotic disorder (n=1), organic systemic disorders due to late effects of cerebral infarction (n=1).
Abbreviations: SD, standard deviation; BD, bipolar disorder; BDI, bipolar I disorder; BDII, bipolar II disorder; SSRI, selective serotonin reuptake inhibitors; SNRI, serotonin–norepinephrine reuptake inhibitors; NaSSA, noradrenergic and specific serotonergic antidepressant; No, number.
During the lamotrigine maintenance period (maintenance period was defined as 8 weeks after initiation of lamotrigine treatment), the daily dose (median) was 124.42 mg/day, and the total number of treatment days (median) was 360 days.
Safety
ADRs
Of the 989 patients included in the safety analysis set, 354 ADRs were reported in 237 with an ADR incidence of 24.0%. Skin and subcutaneous tissue disorders was 13.1% (n=130/989), nervous system disorders 3.7% (n=37/989), psychiatric disorders 3.6% (n=36/989), and gastrointestinal disorders 2.3% (n=23/989). The most commonly reported ADRs were rash (9.1%), somnolence (1.3%), and pruritus (1.2%; Table S2).
There were 52 serious ADRs in 33 patients, with an incidence of 3.3%. The serious ADRs reported in 2 patients or above were rash (n=8), suicidal ideation and pyrexia (n=4, each), irritability, drug reaction with eosinophilia and systemic symptoms, renal impairment (n=3, each), hepatic function abnormal, stomatitis, erythema, erythema multiforme, and Stevens–Johnson syndrome (SJS; n=2, each). The outcome of the serious ADRs was death in 1 patient (sudden death), unresolved in 1 patient (renal impairment), resolved with sequelae in 1 patient (SJS), unknown in 2 patients (rash and renal impairment in 1 patient each), and resolved or resolving in all other patients.
There were 150 events of skin disorders in 130 patients, with an incidence of 13.1%. Mainly, skin disorders were rash 9.1%, pruritus 1.2%, and drug eruption 1.0%. The outcome was unresolved in 2 patients (rash and rash macular in 1 patient each), resolved with sequelae in 1 patient (SJS), unknown in 7 patients (rash and drug eruption in 2 patients each, and erythema, purpura, and eczema in 1 patient each), and resolved or resolving in all other patients. Serious skin disorders were reported in 16 patients, with an incidence of 1.6% (16/989 patients). Serious skin disorders comprised rash in 8 patients, drug reaction with eosinophilia and systemic symptoms in 3 patients, SJS, erythema, and erythema multiforme in 2 patients each, and toxic skin eruption and drug eruption in 1 patient each. Of the 150 events of skin disorders, 74.0% (111 events) occurred within 8 weeks of starting lamotrigine treatment, and all 20 events of serious skin disorders occurred within 8 weeks of starting lamotrigine treatment (Table S3).
Furthermore, the incidence rate of serious skin disorders in patients using lamotrigine with rapid titration and/or high starting dose was 1.3% (5/376 patients). No increasing trend was seen in the incidence rate of serious skin disorders, compared with that in patients using lamotrigine properly (1.8%; 11/605 patients), and dosage unknown was 0.0% (0/8 patients).
Suicide-related events and self-injury were reported in 5 patients, with an incidence of 0.5%. These comprised suicidal ideation (0.4%) and intentional self-injury (0.1%). All were serious events, and their outcome was resolved.
Harmful behavior toward others was reported, with an incidence of 2.8%. Mainly, harmful behaviors were irritability (1.0%), anger (0.5%), insomnia (0.4%), and anxiety and mania (0.3%) each. Serious events comprised irritability in 3 patients, and aggression, BD, agitation, and anger in 1 patient each. The outcome was unresolved in 2 patients (mania and insomnia), unknown in 1 patient (irritability), and resolved or resolving in all other patients.
Efficacy
Time to recurrence/relapse
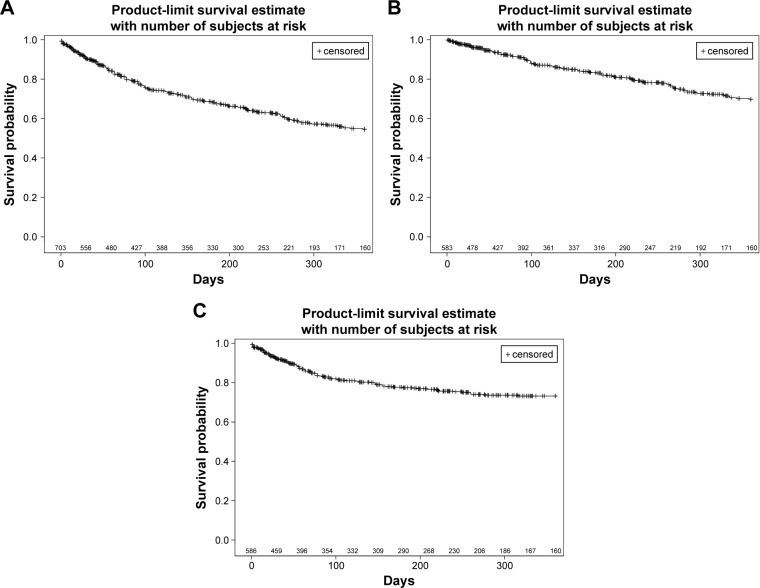
There was no recurrence/relapse of mood episodes in 66.3% (466 patients) of the 703 patients who could be analyzed for the time to recurrence/relapse of mood episodes out of the 966 patients in the efficacy analysis set. The 25th percentile of the time to recurrence/relapse of mood episodes was 105 days in the 237 patients with recurrence/relapse of mood episodes. The 25th percentile of the time to recurrence/relapse of depressive episodes and mania-related episodes was 274 days and 259 days, respectively (Table 2, Figure 1A–C).
Table 2.
Prolongation of time to recurrence/relapse of mood episodes (depressive, mania-related episode)
| Evaluation item | Item | Value |
|---|---|---|
| Recurrence/relapse of mood episodes | N | 703 |
| No of patients with recurrence/relapse of mood episodes (%) | 237 (33.7) | |
| Time to recurrence/relapse of mood episodes, days, 25th percentile | 105 | |
| 95% CI of 25th percentile | 92.0, 147.0 | |
| Recurrence/relapse of depressive episodes | N | 583 |
| No of patients with recurrence/relapse of depressive episodes (%) | 117 (20.1) | |
| Time to recurrence/relapse of depressive episodes, days, 25th percentile | 274 | |
| 95% CI of 25th percentile | 230.0, 343.0 | |
| Recurrence/relapse of mania-related episodesa | N | 586 |
| No of patients with recurrence/relapse of mania-related episodes (%) | 120 (20.5) | |
| Time to recurrence/relapse of mania-related episodes, days, 25th | 259 | |
| percentile | ||
| 95% CI of 25th percentile | 155.0, – |
Note:
Any of the following: manic, hypomanic, or mixed episodes.
Abbreviations: CI, confidence interval; No, number.
Figure 1.
(A) Kaplan–Meier survival curves for time to recurrence/relapse of mood episodes. Kaplan–Meier analysis of days to recurrence/relapse of first mood episode in patients with BD. (B) Kaplan–Meier survival curves for time to recurrence/relapse of depressive episodes. Kaplan–Meier analysis of days to recurrence/relapse of first depressive episode in patients with BD. (C) Kaplan–Meier survival curves for time to recurrence/relapse of mania-related episodes. Kaplan–Meier analysis of days to recurrence/relapse of first mania-related episode in patients with BD.
HAM-D
As shown in Table S4, the score (mean ± SD) was 14.7±8.26 at the start of lamotrigine treatment and was 6.3±7.14 at 10–12 months after treatment initiation, revealing a change of −8.2±8.96. The percentage of patients who achieved remission of depression symptoms increased from 21.1% (147/697 patients) at treatment initiation to 67.4% (361/536 patients) at 10–12 months after treatment initiation.
YMRS
As shown in Table S5, the score (mean ± SD) was 3.8±6.35 at the start of lamotrigine treatment and was 1.7±4.24 at 10–12 months after treatment initiation, revealing a change of −1.8±5.87. The percentage of patients who achieved remission of manic symptoms increased from 91.0% (615/676 patients) at treatment initiation to 97.3% (500/514 patients) at 10–12 months after treatment initiation.
Discussion
The safety and efficacy of lamotrigine in routine clinical practice were studied based on the results of PMS study in patients with BD taking lamotrigine for the first time. The ADR incidence of 24.0% (237/989 patients) in the 989 patients of the safety analysis set was lower than the incidence of 34.4% (74/215 patients) in the Japanese clinical study in Japanese patients with BD. The most commonly reported ADRs were similar to the events that have already been reported in Japanese and overseas clinical studies, such as rash, somnolence, and pruritus.1–4 Although there were differences in assessment criteria, regulatory differences must be taken into account, and there was no increase in the incidence of ADRs or evidence of new ADRs in this study in comparison with the clinical trials. In addition, irritability (1.0%), anger (0.5%), suicidal ideation and insomnia (0.4% each), etc. were reported as ADRs in psychiatric disorders. However, these are the ADRs observed in association with BD and its exacerbation,7 and the difficulty in ruling out the relationship with the primary diseases should be taken into account.
As treatment with lamotrigine may result in serious skin disorders with systemic symptoms, such as toxic epidermal necrolysis, SJS, and drug-induced hypersensitivity syndrome, the occurrence of skin disorders was investigated, revealing that skin disorders were reported in 13.1% of patients (130 patients). The incidence and types of skin disorders were the same as in the results of the Japanese clinical trial (12.1% [26/215 patients]).3 It is known that serious skin disorders caused by lamotrigine typically occur within 8 weeks of treatment initiation.8 All of the serious skin disorders reported in this study occurred within 8 weeks. In this study, SJS was reported in 0.2% of patients (2 patients), but both were hospitalized at another hospital, and no detailed information such as on patient course is available.
Serious skin disorders, result in death, which are possible treatment-related to lamotrigine have been reported in Japan. Therefore, Dear Healthcare Professional Letter of Rapid Safety Communication on serious skin disorders was therefore issued in February 2015 to draw attention to this phenomenon.9 This Dear Healthcare Professional Letter of Rapid Safety Communication recommended following the dosage and administration in the package insert when using lamotrigine, carefully monitoring rashes and other signs of the potential development of a serious skin disorder (such as pyrexia [≥38°C], ocular hyperemia, lip/oral mucosa erosion, pharyngodynia, general malaise, and lymphadenopathy), and ensuring early treatment.
As a US Food and Drug Administration meta-analysis evaluating antiepileptic drugs suggested a relationship between antiepileptic drugs and suicidal ideation or suicidal behavior,10 the occurrence of suicide-related events was investigated, revealing that suicidal ideation was reported in 0.4% of patients (4 patients) and that intentional self-injury was reported in 0.1% (1 patient). The incidence of these events was lower than the incidence of suicidal ideation and suicide attempts in 0.5% each (1/215 patients each) and self-injurious behavior in 1.1% (1/92 patients) in Japanese clinical studies,3,4 and was also lower than in the placebo group in the results of overseas meta-analysis, which showed that lamotrigine did not result in a significant increase in suicide-related events compared with placebo (lamotrigine group: 1.2%; placebo group: 0.9%).11 Harmful behavior toward others was reported in 2.8% of patients (28 patients); the most common events were irritability and anger. There were few reports of events indicative of harmful behavior toward others per se.
Studies tracking the outcome of patients with BD over the course of 10 or more years have shown that patients with BDI experienced depression or depressed state during 31.9% of the observation period and that patients with BDII experienced depression or depressed state during 50.3% of the observation period.12,13 Serious problems associated with depressive episodes are not only that such episodes last longer than manic episodes12,13 but also that the risk of suicide is also greater.14,15 Thus, in addition to acute-phase treatment of mood episodes, controlling recurrence/relapse of mood episodes and maintaining mood stability over the long-term are also important in the treatment of BD.
Recurrence/relapse of mood episodes occurred in 33.7% (237 patients) of the 703 patients, whereas >60% of those patients did not experience any recurrence/relapse of mood disorders after initiation of lamotrigine treatment. As analyzed by type, the rate of recurrence/relapse was approximately 20% for both manic episodes and depressive episodes. Moreover, it is reported that lamotrigine significantly prolonged the time to recurrence/relapse in BDII, compared with BDI, when analyzed by diagnosis of BD.16
The mean HAM-D score in this study was highest at treatment initiation, ranging from 14 to <18 points (indicative of moderate depressive state), but decreased to remission levels (≤7 points) at 10–12 months after treatment initiation. The mean YMRS score remained at remission levels (≤13 points) throughout the observation period, suggesting that mood was stabilized by long-term treatment.
In both HAM-D and YMRS scores, the changes at 1 year after treatment initiation in the study were greater than those in the Japanese clinical study.3,4 It was considered as part of the cause that this PMS study included many patients with severe BD. The percentages of patients whose severity of mood episodes was moderate or more severe at treatment initiation were 65.3% (646/989 patients) in this PMS study and 55.6% (24/45 patients) in the clinical study, and the mean values in both HAM-D and YMRS scores were high at treatment initiation in this PMS study.
Zavodnick and Ali17 indicated that patients with more treatment resistance, comorbid anxiety, and borderline personality disorder may be more able to benefit from lamotrigine, whereas Olesen et al18 suggested that lamotrigine may increase the risk of suicide, and this PMS study did not deal with these effects and side effects of lamotrigine. In addition, there are some limitations to this study. As this is a naturalistic observation and it is not a randomized controlled trial, the lack of control groups and blinding made it difficult to eliminate the possible influence of placebo effect. Moreover, patients were not enrolled consecutively, which could lead to the risk of sample selection bias. To reduce the selection bias, the registration period was set. Therefore, we believe that the sample size of this study (>1,000 patients) provided safety and efficacy data reflecting the actual reality of treatment with lamotrigine in clinical practice, and that these findings are directly applicable to real-life clinical settings in Japan.
Conclusion
The results of this PMS study suggest that lamotrigine is a well-tolerated and effective drug for preventing recurrence/relapse of BD in clinical practice.
Acknowledgments
We would like to express our sincere appreciation for the assistance of all the investigators at 207 medical institutions in Japan who cooperated and provided valuable data for the PMS study of lamotrigine for the treatment of BD.
Footnotes
Disclosure
TT was the medical expert for this study and received honoraria from GlaxoSmithKline K.K. for advice related to this study. The other four authors are employees of GlaxoSmithKline K.K. TH is also a stockholder of GlaxoSmithKline K.K. GlaxoSmithKline K.K. funded this study (study number: 115324). The authors report no other conflicts of interest in this work.
References
- 1.Bowden CL, Calabrese JR, Sachs G, et al. Lamictal 606 Study Group A placebo-controlled 18-month trial of lamotrigine and lithium maintenance treatment in recently manic or hypomanic patients with bipolar I disorder. Arch Gen Psychiatry. 2003;60(4):392–400. doi: 10.1001/archpsyc.60.4.392. [DOI] [PubMed] [Google Scholar]
- 2.Calabrese JR, Bowden CL, Sachs G, et al. Lamictal 605 Study Group A placebo-controlled 18-month trial of lamotrigine and lithium maintenance treatment in recently depressed patients with bipolar I disorder. J Clin Psychiatry. 2003;64(9):1013–1024. doi: 10.4088/jcp.v64n0906. [DOI] [PubMed] [Google Scholar]
- 3.Koyama T, Higuchi T, Yamawaki S, Kanba S, Terao T, Shinohara A. Study SCA104779, an evaluation of BW430C (lamotrigine) versus placebo in the prevention of mood episodes in bipolar I disorder patients. Jpn J Clin Psychiatry. 2011;40(3):369–383. [Google Scholar]
- 4.Koyama T, Higuchi T, Yamawaki S, Kanba S, Terao T, Shinohara A. Study SCA106052, a clinical evaluation of BW430C (lamotrigine) in bipolar I disorder-Long-term extension study. Jpn J Clin Psychiatry. 2011;40(7):981–995. [Google Scholar]
- 5.Popovic D, Reinares M, Goikolea JM, Bonnin CM, Gonzalez-Pinto A, Vieta E. Polarity index of pharmacological agents used for maintenance treatment of bipolar disorder. Eur Neuropsychopharmacol. 2012;22(5):339–346. doi: 10.1016/j.euroneuro.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 6.FDA [webpage on the Internet] FDA Issues Public Health Advisory on Cautions for Use of Antidepressants in Adults and Children. [Accessed October 1, 2011]. Available from: http://www.antidepressantsfacts.com/2004-03-22-FDA-Talk-Paper-use-SSRIs.htm.
- 7.Eberhard J, Weiller E. Suicidality and symptoms of anxiety, irritability, and agitation in patients experiencing manic episodes with depressive symptoms: a naturalistic study. Neuropsychiatr Dis Treat. 2016;12:2265–2271. doi: 10.2147/NDT.S111094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Messenheimer J, Mullens EL, Giorgi L, Young F. Safety review of adult clinical trial experience with lamotrigine. Drug Saf. 1998;18(4):281–296. doi: 10.2165/00002018-199818040-00004. [DOI] [PubMed] [Google Scholar]
- 9.Pharmaceuticals and Medical Devices Agency Dear Healthcare Professional Letter of Rapid Safety Communication PFSB/SD Notification No 0204-1. 2015. [Accessed May 9, 2016]. Available from: http://www.pmda.go.jp/files/000198343.pdf. English Version Available from: https://www.pmda.go.jp/files/000198527.pdf.
- 10.FDA Briefing Document for the July 10, 2008 Advisory Committee Meeting to Discuss Antiepileptic Drugs (AEDs) and Suicidality, Division of Neurology Products. 2008. [Accessed May 9, 2016]. Available from: http://www.fda.gov/ohrms/dockets/ac/08/briefing/2008-4372b1-01-FDA-Katz.pdf.
- 11.GlaxoSmithKline Lamotrigine Suicidality Pooled Analysis. 2007. [Accessed April 13, 2016]. Available from: http://www.gsk-clinicalstudyregister.com/files2/2906.pdf.
- 12.Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530–537. doi: 10.1001/archpsyc.59.6.530. [DOI] [PubMed] [Google Scholar]
- 13.Judd LL, Akiskal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003;60(3):261–269. doi: 10.1001/archpsyc.60.3.261. [DOI] [PubMed] [Google Scholar]
- 14.Baldessarini RJ, Pompili M, Tondo L. Suicide in bipolar disorder: risks and management. CNS Spectr. 2006;11(6):465–471. doi: 10.1017/s1092852900014681. [DOI] [PubMed] [Google Scholar]
- 15.Kupfer DJ, Frank E, Grochocinski VJ, Cluss PA, Houck PR, Stapf DA. Demographic and clinical characteristics of individuals in a bipolar disorder case registry. J Clin Psychiatry. 2002;63(2):120–125. doi: 10.4088/jcp.v63n0206. [DOI] [PubMed] [Google Scholar]
- 16.Terao T, Ishida A, Kimura T, Yarita M, Hara T. Preventive effects of lamotrigine in bipolar disorder II versus I. J Clin Psychiatry. 2017 doi: 10.4088/JCP.16m11404. In press. [DOI] [PubMed] [Google Scholar]
- 17.Zavodnick AD, Ali R. Lamotrigine in the treatment of unipolar depression with and without comorbidities: a literature review. Psychiatr Q. 2012;83(3):371–383. doi: 10.1007/s11126-012-9208-4. [DOI] [PubMed] [Google Scholar]
- 18.Olesen JB, Hansen PR, Erdal J, et al. Antiepileptic drugs and risk of suicide: a nationwide study. Pharmacoepidemiol Drug Saf. 2010;19(5):518–524. doi: 10.1002/pds.1932. [DOI] [PubMed] [Google Scholar]