Abstract
Fatigue is one of the most common and debilitating side effects of cancer and cancer treatment, and yet its etiology remains elusive. The goal of this study is to understand the role of chronic inflammation in fatigue following repeated stress from radiotherapy. Fatigue and non-fatigue categories were assessed using ≥ 3-point change in Functional Assessment of Cancer Therapy-Fatigue questionnaire (FACT-F) administered to participants at baseline/before radiotherapy and one year post-radiotherapy. Whole genome microarray and cytokine multiplex panel were used to examine fatigue-related transcriptome and serum cytokine changes, respectively. The study included 86 subjects (discovery phase n = 40, validation phase n = 46). The sample in the discovery phase included men with prostate cancer scheduled to receive external-beam radiotherapy. A panel of 48 cytokines were measured and the significantly changed cytokine found in the discovery phase was validated using sera from a separate cohort of men two years after completing radiotherapy for prostate cancer at a different institution. Effects of the significantly changed cytokine on cell viability was quantified using the MTT assay. During the discovery phase, tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and TRAIL decoy receptor, TNFRSF10C (TRAIL-R3), were significantly upregulated in fatigued (≥ 3-point decrease from baseline to 1yr-post radiotherapy) subjects (n=15). In the validation phase, TRAIL correlated with fatigue scores 2yrs post-radiotherapy. TRAIL caused selective cytotoxicity in neuronal cells, but not in microglial and muscle cells, in vitro. Late-onset inflammation directed by TRAIL may play a role in fatigue pathogenesis post-repeated stress from irradiation.
Fatigue is one of the most common and debilitating symptoms that affect up to 80% of cancer patients (Berger et al., 2015; Hall et al., 2015). Despite its prevalence, fatigue is underdiagnosed and poorly managed, and the underlying mechanism remains elusive (Ahlberg et al., 2005; Feng et al., 2016a; Feng et al., 2016b). Several factors that contribute to fatigue development have been identified, such as anemia, hypothalamic–pituitary–adrenal (HPA) axis dysregulation, and inflammation (Bower and Lamkin, 2013; Dantzer et al., 2014; Feng et al., 2015).
Radiotherapy is considered standard care for patients with localized prostate cancer (Zelefsky et al., 2008). Although radiotherapy is an effective treatment for localized cancer, side effects arise when ionizing radiation produces reactive oxygen species (ROS) leading to oxidative damage in late responding normal tissues (Zhao and Robbins, 2009). For example, inflammatory damage as a result of genomic instability caused by a single dose of radiation has been observed in both irradiated and bystander tissues (Limoli et al., 2003; Nugent et al., 2007; Zhao and Robbins, 2009). The initial burst of ROS can subsequently lead to point mutations in mitochondrial DNA, which further propagates ROS production by dysfunctional mitochondria causing an accumulation of oxidative stress leading to a prolonged inflammatory state (Azzam et al., 2012; Prithivirajsingh et al., 2004; Zhao and Robbins, 2009). This process can manifest in late onset inflammatory symptoms such as urinary flare which have been observed one to two years post-radiotherapy (Keyes et al., 2009; Yu et al., 2014).
Effects of repeated stress on symptoms collectively known as “sickness behavior” are well-documented in the literature (Dantzer, 2001; Dantzer et al., 2014; Miller and Raison, 2016). Prolonged inflammation triggered by repeated stress has been shown to result in psychiatric symptoms such as depression and fatigue (Dantzer et al., 2014; Miller and Raison, 2016). Radiotherapy-related fatigue especially in men treated for prostate cancer often co-occurs with other symptoms including urinary problems, depressive symptoms, and cognitive deficits, which often provide clues to shared and distinct pathways that can explain underlying mechanisms of fatigue (Laird et al., 2011; Piper and Cella, 2010).
Previous studies observed chronic inflammation as evidenced by nuclear and cytoplasmic changes in transurethrally resected prostate and reports of urinary inflammatory symptoms one to two years after repeated radiation exposure delivered by both intensity-modulated and hypofractionated treatment modalities (Suy et al., 2010; Yu et al., 2014). We hypothesize that chronic inflammation initially triggered by repeated stress from radiotherapy can persist long after treatment completion, which results in a multitude of symptoms including fatigue. The goal of this hypothesis generating study is to understand the role of chronic inflammation in fatigue following repeated stress from radiotherapy. To generate the hypothesis, the study was driven primarily by a discovery phase, followed by a validation phase to confirm the initial findings. In the discovery phase, we first identified an inflammation-related biomarker associated with fatigue in cancer survivors using whole transcriptome microarray and multiplex immunoassay. We then validated our finding in a separate cohort of subjects. Finally, we used in vitro models to describe the toxic effects of the identified fatigue-related cytokine on different cell lines. The focus of this study was to demonstrate a novel mechanism that explains the role of chronic inflammation on fatigue induced by repeated stress from radiotherapy, and to identify a biological substrate for fatigue that can serve as a promising therapeutic target for managing this debilitating condition.
Methods
Participants
A total of 86 participants were included in the study. In the discovery phase, 40 participants were recruited at the National Institutes of Health (NIH), Bethesda, Maryland. The biomarker found in the discovery phase was confirmed in the validation phase using sera from 46 participants recruited at MedStar Georgetown University Hospital.
Discovery phase
The study (NCT00852111) was approved by the Institutional Review Board (IRB) of the NIH. All participants enrolled in this study were men, 18 years of age or older, diagnosed with non-metastatic prostate cancer with or without prior prostatectomy, and scheduled to receive external beam radiation therapy (EBRT). The entire EBRT treatment lasted 38–42 days, depending on the clinical stage of the prostate disease. Potential participants were excluded if they had progressive diseases causing significant fatigue, psychiatric disease within the past five years, uncorrected hypothyroidism or anemia, or a second malignancy. Individuals who used sedatives, steroids, or non-steroidal anti-inflammatory agents were also excluded. Participants were recruited from September 2009 to November 2015 at the Magnuson Clinical Research Center at the NIH. Signed written informed consents were obtained prior to study participation. Several questionnaires were administered to participants in the discovery phase to assess various symptoms and a panel of 48 cytokines (described below) was used to select a biomarker that is correlated with fatigue.
Validation phase
The selected inflammatory biomarker in the discovery phase was validated in a community-based population. A collaborative activity between NIH and MedStar Georgetown University Hospital was established and approved by the NIH Office of Human Subjects Research Protections (Approval # 11443). This collaborative activity allowed MedStar to share pre-collected data and samples to NIH to validate the findings in the discovery phase. The data and samples shared by MedStar were obtained from subjects enrolled in a study that was approved by the IRB of MedStar Georgetown University Hospital, Washington, DC. Both the NIH and Georgetown studies had the same eligibility criteria. However, subjects enrolled in the Georgetown study were treated with CyberKnife (Accuray Inc., Sunnyvale CA) to deliver fiducial-based image-guided stereotactic beam radiation therapy. Treatment planning and delivery were conducted as previously described (Chen et al., 2013; Suy et al., 2010).
Instruments
Fatigue, as the primary outcome measure, was assessed in all participants using the 13-item Functional Assessment of Cancer Therapy–Fatigue (FACT-F), which is a frequently used, validated, reliable, stand-alone measure of fatigue in cancer therapy (coefficient alpha = 0.95–0.96) (Yellen et al., 1997). Each item response is rated on a 0–4 scale, where a 0 represents “not at all” and a 4 indicates that the respondent relates to the corresponding statement “very much.” Total scores range from 16–53 with lower scores reflecting high fatigue intensity. Subjects were considered to be fatigued when there is a clinically significant decrease (worsening of fatigue symptom) in FACT-F score of ≥ 3 points from baseline (prior to radiotherapy initiation) to one year following radiotherapy (1yr post-radiotherapy) (Cella et al., 2002).
Depressive symptoms were measured using the validated, 24-item Hamilton Depression Rating Scale (HAM-D) (Lydiatt et al., 2008; Milam et al., 2015). Scores ranged from 0–54 with high scores reflecting severe depression; a score of 0–7 indicated no depression, 8–16 indicated mild depression, and a score of ≥ 17 indicated moderate to severe depression (Zimmerman et al., 2013). HAM-D has good internal consistency (standardized Cronbach alpha = 0.67 – 0.80) and test-retest reliability (Pearson correlation coefficient = 0.88, p < 0.001) (González-Pinto et al., 2009).
Lower urinary tract symptoms (LUTS) were measured using the American Urological Association (AUA) symptom score. The AUA symptom index is a 7-item global measurement of subjects’ urinary problems that is internally consistent (Cronbach’s alpha = 0.86) and has good test-retest reliability (r = 0.92) (Barry et al., 1992). An AUA score ≥ 8 indicates moderate/severe LUTS, and an AUA score < 8 means mild to no LUTS (Barry et al., 1992; Lepor and Kaci, 2004).
The Revised Piper Fatigue Scale (rPFS) is a 22-item multidimensional questionnaire that measures four domains of fatigue: (1) behavioral (6 items relating to the severity, distress, and degree of disruption in activity of daily living), (2) affective (5 items relating to the emotional meaning attributed to fatigue), (3) sensory (5 items relating to the physical symptoms of fatigue), and (4) cognitive/mood (6 items relating to mental and mood states). The rPFS has a standardized α of 0.97 and an inter-item correlation between 0.30–0.70 (Piper et al., 1998). Each item is coded on a 0–10 numeric scale and each subscale is scored individually (Piper and Cella, 2010; Piper et al., 1998; Reeve et al., 2012).
Sleep disturbance was measured using the Patient-Reported Outcomes Measurement Information System (PROMIS) Sleep Disturbance (PROMIS-SD) short form. The PROMIS-SD item bank consists of 27 items and was developed as part of the NIH Roadmap Initiative from more than 1000 datasets from multiple disease populations including cancer, heart disease, rheumatoid arthritis and osteoarthritis, psychiatric conditions, spinal cord injury, and chronic obstructive pulmonary disease (for detailed information, see www.nihpromis.org). The PROMIS-SD short form consists of 8 items and has demonstrated good validity (0.83) and internal consistency (Cronbach’s alpha > 0.90) (Mahieu et al., 2016; Yu et al., 2011). The PROMIS measures are reported on a T-score metric that is anchored to the mean score of a healthy American general population (Rothrock et al., 2010).
Cytokine Measurement
During the discovery phase, a panel of 48 cytokines (interleukin-2 receptor alpha chain/IL2Ra, interleukin-3/IL3, Interleukin-12 (p40)/IL12(p40), interleukin-16/IL16, Interleukin-18/IL18, cutaneous T cell-attracting chemokine/CTACK, growth-regulated alpha protein/GROa, hepatocyte growth factor/HGF, Interferon alpha-2/IFNa2, leukemia Inhibitory Factor/LIF, monocyte chemotactic protein-3/MCP3, macrophage colony-stimulating factor/MCSF, macrophage migration inhibitory factor/MIF, monokine induced by IFN-gamma/MIG, beta nerve growth factor/bNGF, stem cell factor/SCF, stem cell growth factor beta/SCGFb, stromal cell-derived factor 1a/SDF1a, TNF-related apoptosis-inducing ligand/TRAIL, interleukin-1 beta/IL1b, interleukin-1 receptor antagonist/IL1ra, interleukin-2/IL2, interleukin-4/IL4, interleukin-5/IL5, interleukin-6/IL6, interleukin-7/IL7, interleukin-8/IL8, interleukin-9/IL9, interleukin-10/IL10, interleukin-12 (p70)/IL12(p70), interleukin-13/IL13, interleukin-17/IL17, Eotaxin, fibroblast growth factor basic/FGFbasic, granulocyte colony-stimulating factor/GCSF, granulocyte-macrophage colony-stimulating factor GMCSF, interferon gamma/IFNg, Interferon gamma-induced protein 10/IP10, monocyte chemotactic protein 1/MCP1, macrophage inflammatory protein 1 alpha/MIP1a, platelet-derived growth factor bb/PDGFbb, macrophage inflammatory protein 1beta/MIP1b, regulated on activation normal T cell expressed and secreted/RANTES, tumor necrosis factor alpha/TNFa, vascular endothelial growth factor/VEGF) was measured in 50 μl of non-diluted serum samples collected from each participant 1yr post-radiotherapy using the Bio-Plex Pro Human Cytokine Kits (Bio-Rad Laboratories, Hercules, CA) according to manufacturer’s instructions.
During the validation phase, serum samples collected two years after treatment (2yrs post-radiotherapy) from a separate cohort were used to confirm the identified biomarker in the discovery phase. Serum TRAIL levels were measured using the TRAIL Quantikine ELISA Kit according to manufacturer’s instructions (R&D Systems, Minneapolis, MN).
Gene Expression Profile by Microarray and RT-qPCR Validation
A total of 2.5 ml of blood was collected from each subject in a RNA PAXGene tube (Qiagen, Frederick, MD). RNA extraction and Affymetrix microarray chips (HG U133 Plus 2.0, Santa Clara, CA) were processed as previously described (Saligan et al., 2013). Affymetric GeneChip Command Console (AGCC, 3.0V) was used to scan images during data acquisition. Affymetrix CEL files containing raw intensity data were imported into Partek Genomics Suite 6.6 (Partek Inc., St. Louis, MO), log transformed, and normalized using the robust multiarray average (RMA) algorithm. Because the chips were processed on different days, Partek batch removal analysis of variance (ANOVA) was used to eliminate differences due to batch variation. ANOVA with false discovery rate (FDR) correction was used to identify differentially expressed genes between groups (FDR < 5%). The gene of interest was further confirmed with a TaqMan-based real-time quantitative PCR (RT-qPCR) using gene-specific primers ((Thermo Fisher Scientific, Waltham, MA). Following genomic DNA elimination, first-strand RNA-cDNA PCR template was generated from 150 ng of RNA using the High-capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific). RT-qPCR was performed on a QuantStudio 6 Flex instrument (Thermo Fisher Scientific) and gene of interest was normalized to GAPDH endogenous control.
Cell culture
Neuro-2A (N2a, neuroblastoma cell line, ATCC CCL-131) and Sol8 (myoblast cell line, ATCC CRL-2174) cells were directly purchased from American Type Culture Collection (ATCC, Manassas, VA); BV2 cells (microglial cell line) were kindly provided by Dr. Wai Wong (National Eye Institute, NIH). All cells were cultured based on standard ATCC protocols. N2a, Sol8, and BV2 cells were plated in a 96-well plate 24 hours prior to treatment with recombinant TNF-Related Apoptosis-Inducing Ligand (TRAIL/Apo2L, PeproTech, Rocky Hill, NJ) diluted in phosphate-buffered saline (PBS) at various concentrations. Cell viability was quantified 24 hours after treatment using the MTT (3-[4,5-dimethylthiazol]-2,5-diphenyltetrazolium) assay (Thermo Fisher Scientific, Waltham, MA) based on manufacturer’s instructions. Each experiment was performed with n = 8, and was repeated at least three times. Data were expressed as percentage of cell death with respect to control untreated cells.
Statistical analysis
Descriptive analyses were used to describe demographic characteristics of the sample. All data were expressed as mean ± SEM. One-way analysis of variance (ANOVA) was used to determine significant differences in comparisons involving more than 2 groups. Post hoc non-directional Student’s t-test with Bonferroni correction was used for between group comparisons. False Discovery Rate (FDR) adjustment using the Benjamini-Hochberg procedure was applied for analyses of whole transcriptome microarray and multiplex immunoassay data. Pearson’s correlation with Bonferroni correction for multiple comparisons was used to analyze correlations between variables. P values < 0.05 were considered significant. Statistical analyses were performed with SPSS statistics software version 23 (IBM SPSS, Purchase, NY).
Results
Clinical characteristics of participants in the discovery phase
FACT-F scores of fatigued subjects (n = 15, 37.5% of total subjects) were significantly lower than those of non-fatigued subjects (n = 25) one year following radiotherapy (Figure 1A; p = 3.5×10−7). Both fatigued and non-fatigued participants were predominately Caucasian with an average age of 65.6 ± 1.9 and 66.0 ± 1.9, respectively. There were no significant differences in clinical characteristics between the two groups including body mass index (BMI), Gleason scores, T stage, anemia status, and PSA levels (Table 1). The percentages of subjects who received ADT were similar between the two groups (77% of fatigued subjects and 76 of non-fatigued subjects; Table 2). In addition, ADT usage did not significantly affect fatigue scores between the two groups (FACT-Ffatigued = 48.6 ± 1.4, FACT-Fnon-fatigued = 42.15 ± 1.9, p = 0.1). It is worth pointing out that even though none of the study participants experienced clinically significant depression at any of the study time points, as the HAM-D scores were well below 3 (Table 1), FACT-F scores significantly correlated with HAM-D scores (r = −0.64, p = 2.59 × 10−6). Of the symptoms assessed using HAM-D, depressed mood (r = −0.51, p = 0.0004), insomnia (r = −0.45, p = 0.002), difficulties with work and activities (r = −0.53, p = 0.0002), as well as gastrointestinal symptoms (r = −0.51, p = 0.0004) significantly correlated with FACT-F scores. Subjective cognitive/mood impairment as measured by rPFS-cognitive/mood subscale was significantly worse in fatigued subjects 1yr post-radiotherapy (Figure 1B).
Figure 1.
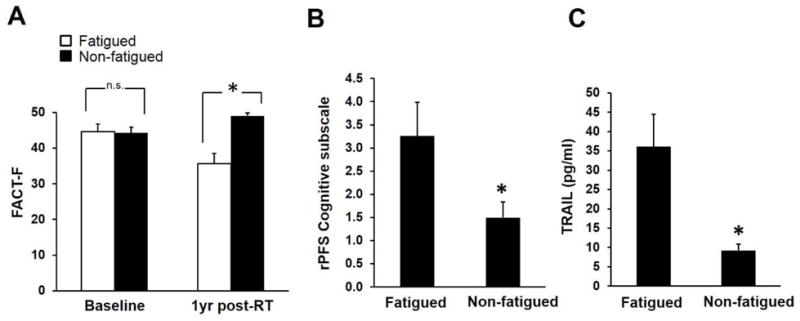
Discovery phase: TRAIL was upregulated in fatigued subjects. (A) One year after EBRT, 37.5% of the 40 subjects recruited at the NIH continued to experience fatigue as indicated by lower FACT-F scores compared to non-fatigued subjects (p = 3.5×10−7); whereas baseline fatigue symptoms were similar between the two groups (p = 0.41). (B) Cognitive impairment in CF subjects was more severe compared to NF subjects (p = 0.0001). Mean ± SEM. * p < 0.05. (C) TRAIL concentrations were significantly higher in fatigued subjects compared to non-fatigued controls (p = 0.02).
Table 1.
Characteristics of participants in the discovery phase.
| Fatigued (n = 15) | Non-fatigued (n = 25) | P Value | |
|---|---|---|---|
| Age (years) | 65.6 ± 1.9 | 66.0 ± 1.9 | 0.79 |
| Race/ethnicity | |||
| White | 60.0% | 72.0% | |
| Black/African American | 26.7% | 20.0% | NA |
| Asian | 6.7% | 4.0% | |
| Hispanic/Latino | 6.7% | 4.0% | |
| BMI | 31.5 ± 1.6 | 29.0 ± 1.2 | 0.13 |
| Gleason Score | 7.7 ± 0.3 | 7.5 ± 0.2 | 0.28 |
| T stage | |||
| T1c | 28.6% | 37.5% | NA |
| T2a-c | 50.0% | 58.3% | |
| T3a-c | 21.4% | 4.2% | |
| HAM-D | |||
| Baseline | 0.9 ± 0.5 | 1.0 ± 0.4 | 0.92 |
| One year post-RT | 2.2 ± 0.7 | 1.1 ± 0.4 | 0.05 |
| PSA (ng/mL) | |||
| Baseline | 9.41 ± 6.86 | 7.38 ± 1.82 | 0.53 |
| One year post-RT | 0.25 ± 0.17 | 0.19 ± 0.08 | 0.95 |
BMI: Body Mass Index; cm: centimeter; kg: kilogram; HAM-D: Hamilton Depression Rating Scale; PSA: prostate specific antigen; RT: radiotherapy. Values are mean ± standard error.
Table 2.
Characteristics of participants in the validation phase.
| Fatigued (n = 13) | Non-fatigued (n = 46) | P Value | |
|---|---|---|---|
| Age (years) | 74.0 ± 2.8 | 76.3 ± 1.1 | 0.37 |
| Race/ethnicity | |||
| White | 46.2% | 56.5% | |
| Black/African American | 53.8% | 32.6% | NA |
| Asian | 0% | 8.7% | |
| Hispanic/Latino | 0% | 2.2% | |
| BMI | 30.7 ± 1.7 | 27.7 ± 0.6 | 0.051 |
| ADT | 77% | 76% | NA |
| Gleason Score | 7.3 ± 0.4 | 7.0 ± 0.1 | 0.49 |
| T stage | |||
| T1c | 46.2% | 50% | NA |
| T2a-c | 53.8% | 50% | |
| PSA (ng/mL) | |||
| Baseline | 9.18 ± 1.69 | 11.98 ± 2.75 | 0.60 |
| Two year post- RT | 0.20 ± 0.04 | 0.86 ± 0.28 | 0.28 |
BMI: Body Mass Index; cm: centimeter; kg: kilogram; HAM-D: Hamilton Depression Rating Scale; ADT: androgen deprivation therapy; PSA: prostate specific antigen; RT: radiotherapy Values are mean ± standard error.
Discovery phase: cytokines and fatigue
Of the 48 cytokines measured to identify a late onset inflammatory response marker that is associated with fatigue, only TNF-related apoptosis-inducing ligand (TRAIL) concentrations were found to be significantly higher in fatigued subjects compared to non-fatigued subjects (Figure 1C; FDR corrected p value = 0.0195). Further supporting the role of TRAIL in the persistence of fatigue post-radiotherapy, we found an upregulation of the mRNA transcript level of a TRAIL receptor, TNFRSF10C (fold change = 1.39, p = 2.49 × 10−5) in fatigued subjects using microarray-based expression profiling. The expression levels of TNFRSF10C were confirmed with a TaqMan-based RT-qPCR (fold change = 1.41, p = 0.04). The upregulation of the TRAIL receptor was possibly due to compensatory mechanisms in response to increased concentrations of circulating TRAIL.
One year post-radiotherapy, fatigue (FACT-F) was significantly clustered with urinary symptoms (AUA) (Figure 2A; r = −0.549, p = 0.00009) and sleep disturbance (PROMIS-SD) (Figure 2C; r = −0.585, p = 0.00002). In addition, TRAIL serum concentration was also correlated with urinary symptoms (Figure 2D; r = 0.430, p = 0.008) but not with sleep disturbance (r = 0.07, p = 0.68) 1 year post-radiotherapy, suggesting that inflammation is a shared pathway influencing fatigue and urinary symptoms.
Figure 2.
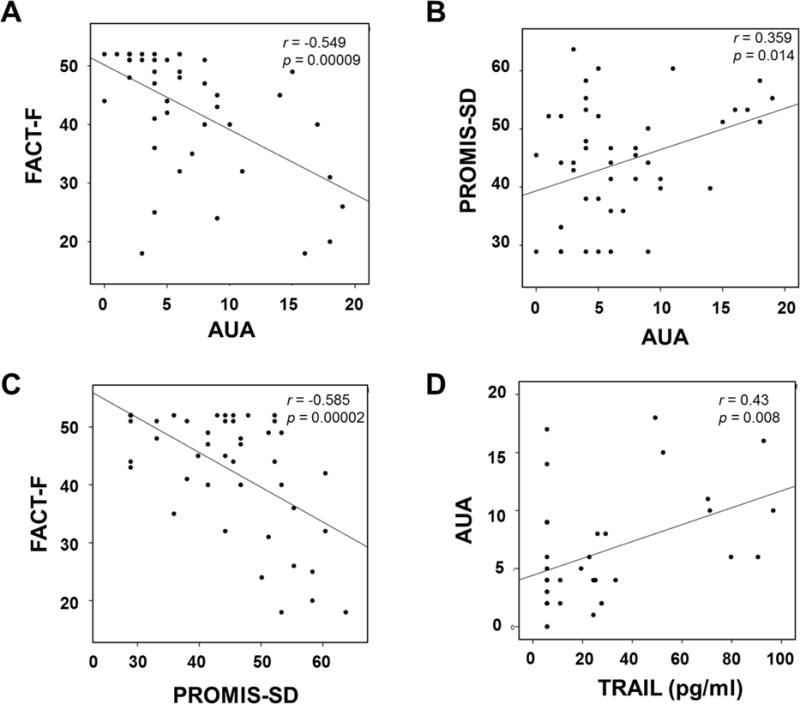
Lower urinary tract symptoms (LUTS) contribute to fatigue in prostate cancer survivors 1 year after EBRT (N = 40; subjects recruited at the NIH). (A) General urinary bother as measured by AUA symptom scores significantly correlated with FACT-F scores (r = −0.549, p = 0.00009) suggesting urinary symptoms contributed to fatigue related to sleep disturbance. (B) PROMIS-SD scores negatively correlated with FACT-F scores (r = −0.585, p = 0.00002) suggesting fatigue was in part attributable to sleep loss. AUA scores correlated significantly with (C) PROMIS-SD scores (r = 0.359, p = 0.01), as well as (D) TRAIL concentrations (r = 0.43, p = 0.008).
Clinical characteristics of participants in the validation phase
Similar to subjects in the discovery phase, fatigued subjects in the validation phase experienced significantly worse fatigue than non-fatigued subjects 1yr (p = 0.000002) and 2yrs post-radiotherapy (p = 0.02); whereas no difference in FACT-F scores was observed at baseline (Figure 3A). Of the validation subjects who experienced fatigue 1yr post-radiotherapy, 54.4% continued to experience fatigue 2 years post-treatment (Figure 3B). Other clinical characteristics of the validation subjects are described in Table 2.
Figure 3.
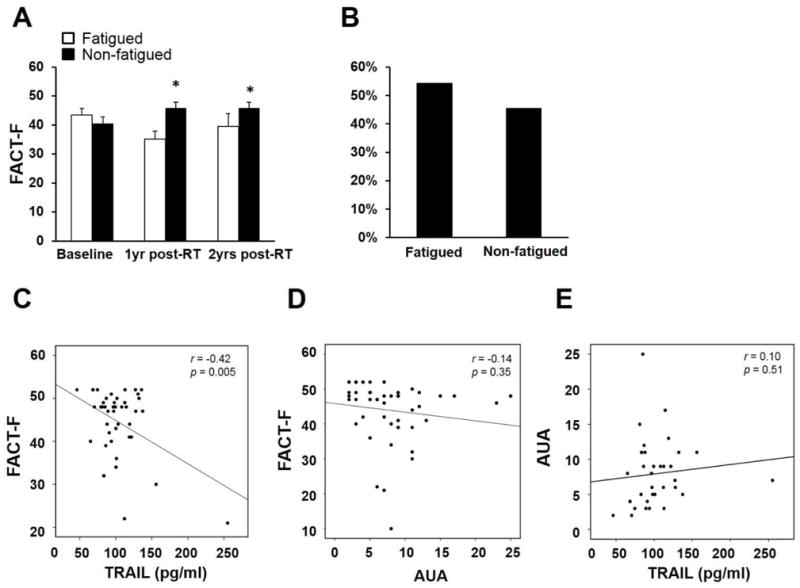
Validation phase: fatigue at 2 years post-radiotherapy was associated with TRAIL but not urinary symptoms (n = 46). (A) FACT-F scores differed significantly between fatigued and non-fatigued groups at 1 year (p = 0.000002) and 2 years post-radiotherapy (p = 0.02), but no difference was observed at baseline (T1). (B) Of the fatigued subjects 1 year post-radiotherapy, 54.4% were still fatigued 2 years post-radiotherapy. (C) TRAIL significantly correlated with FACT-F scores 2 years post-radiotherapy (r = −0.42, p = 0.005). AUA scores did not correlate with FACT-F scores (D) or TRAIL concentrations (E).
Validation phase: TRAIL and fatigue
TRAIL correlated significantly with FACT-F scores 2 years post-radiotherapy (Figure 3C; r = −0.42, p = 0.005). Interestingly, urinary symptoms did not correlate with either FACT-F (Figure 3D; r = −0.14, p = 0.35) or TRAIL (Figure 3E; r = 0.10, p = 0.51), suggesting that TRAIL-induced inflammation may be a distinct pathway that can influence fatigue.
Neuronal cells’ selective vulnerability to TRAIL
Previous studies have established the selective toxicity that TRAIL exerts on neurons leading to neurotoxicity and neuroinflammation (Cantarella et al., 2003; Kichev et al., 2014). Since fatigue correlated with depressive symptoms and neurotoxicity is involved in the pathogenesis of depressive symptoms (Banasr et al., 2011; Hurley and Tizabi, 2013), we hypothesized that TRAIL may contribute to fatigue by inducing neurotoxicity. To test this hypothesis, we compared the effects of TRAIL on cell viability in N2a (neuronal cell line), BV2 (microglial cell line), and Sol8 (myoblast cell line) cells. Within 24 hours, TRAIL induced neuronal cell death at 5 μg/ml (p = 0.006) and 10 μg/ml (p = 0.0001) (Figure 4A). In contrast, TRAIL did not affect the cell viability of BV2 (Figure 4B) or Sol8 cells (Figure 4C). The in vitro finding that neurons are vulnerable to TRAIL may explain the significantly worse cognitive scores of fatigued subjects 1yr post-radiotherapy in the discovery cohort.
Figure 4.
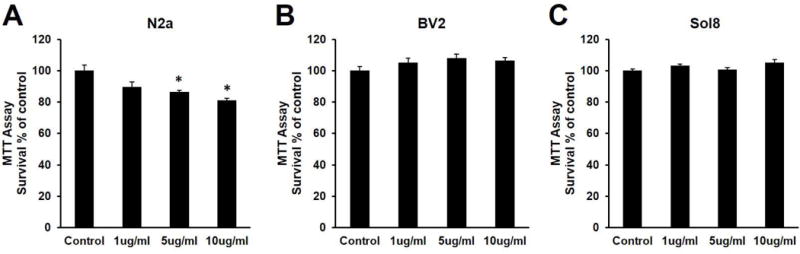
Neuronal cells are selectively vulnerable to TRAIL. (A) TRAIL induces cell death in N2a cells at 5 μg/ml and 10 μg/ml. F3,28 = 9.197, p = 0.0002. Bonferroni post hoc p = 0.057 at 1 μg/ml, p = 0.0065 μg/ml, p = 0.0001 at 10 μg/ml. TRAIL did not affect cell viability in (B) BV2 (F3,28 = 1.902, p = 0.152) or (C) Sol8 cells (F3,28 = 2.718, p = 0.063).
Discussion
Fatigue is one of the most common and distressing complications of cancer and cancer treatment. To investigate the role of chronic inflammation on fatigue induced by repeated stress from radiotherapy and its co-occurring symptoms, we measured a panel of cytokines in serum samples collected from fatigued participants in the discovery phase. Despite having received similar radiation dosage as non-fatigued participants, fatigued participants showed an upregulation of TRAIL and its receptor, TRAIL-R3. The correlation of TRAIL with fatigue and urinary symptoms suggest that a shared mechanism for both of these symptoms may be related to repeated stress from daily localized radiotherapy to the pelvic area. Long-term complications of radiotherapy often stem from biological responses in non-irradiated tissues/cells, referred to as the “bystander effects” (Kadhim and Hill, 2015). Previous work has shown that even localized radiotherapy targeting the prostate can result in oxidative damage to bystander tissues surrounding the prostate, especially the urinary tract, lasting up to one to two years (Keyes et al., 2009; Morgan and Sowa, 2015; Suy et al., 2010; Yu et al., 2014). Our own work expands the “bystander damage” hypothesis of radiotherapy-induced inflammation by showing a link between TRAIL and a centrally-driven behavior, like fatigue. In the validation phase of the study, we showed that fatigue significantly correlated with TRAIL, but no longer correlated with urinary symptoms 2 years post-radiotherapy, suggesting a distinct mechanism for fatigue that is not directly attributable to urinary difficulties. Interestingly, cognitive difficulties were also observed only in fatigued subjects, which can be supported by the in vitro finding that neuronal cells are selectively vulnerable to TRAIL.
TRAIL, also named Apo2 ligand, induces apoptosis by activating the caspase-3-dependent pathway (Kimberley and Screaton, 2004; LeBlanc and Ashkenazi, 2003). TNFRSF10C, or TRAIL-R3 (decoy receptor), lacks the intracellular death domain that is necessary for inducing TRAIL-induced apoptosis (Degli-Esposti et al., 1997). TRAIL-R3 is highly expressed in a variety of peripheral blood cells (Deligezer and Dalay, 2007; Hasegawa et al., 2004; Hebb et al., 2011) and high expression of TRAIL-R3 protects cells from TRAIL-induced apoptosis (Kimberley and Screaton, 2004). Therefore, the upregulated TRAIL-R3 mRNA seen in the fatigued subjects could be attributed to this compensatory mechanism in response to serum TRAIL upregulation. TRAIL and TRAIL receptors have been found to be expressed in bladder tissues and are thought to mediate inflammatory conditions of the bladder (Kutlu et al., 2010). It is possible that radiotherapy-induced oxidative tissue damage resulted in secondary tissue injury and chronic inflammation as demonstrated by TRAIL upregulation, which in turn induced more tissue damage and bladder irritation. Consistent with this hypothesis, a previous study demonstrated that radiation-induced tissue damage can elicit an autoimmune attack (Schaue and McBride, 2012). Circulating TRAIL may enter the CNS through openings in the blood-brain-barrier; TRAIL can be expressed by microglia and astroglia as a secondary response to chronic inflammation (Yarlagadda et al., 2009) (Kichev et al., 2014). When bound to their respective ligands, the intracellular death domain of these transmembrane proteins recruit DISC (death-inducing signaling complex) leading to caspase activation and eventual apoptosis. Although neurons are not typically exposed to TRAIL, activated microglia as well as CD4+ T cells invading the CNS have been shown to produce TRAIL (Kichev et al., 2014). Unlike other cell types, neurons only express the apoptosis-inducing TRAIL-R2 (Aktas et al., 2005). Further supporting the neurotoxic role of TRAIL, in vivo studies showed that lymphocytes from TRAIL-deficient mice exhibited decreased toxicity to neurons and less propensity for microglial activation (Vogt et al., 2009). Therefore, while TRAIL can play an important immunoregulatory role in the periphery and induce apoptosis in a variety of transformed tumor cells, its main effect in the CNS is neurotoxicity (Lemke et al., 2014; Liblau et al., 2013; Oh et al., 2012). In fact, TRAIL has been shown to play a pathogenic role in multiple neurological disorders including multiple sclerosis, Alzheimer’s disease, stroke, and HIV-associated dementia (Aktas et al., 2007).
It is possible that fatigue and urinary symptoms are both downstream events of TRAIL-mediated chronic inflammation, and the two independent symptoms resolved at different times. The strength of this study is its longitudinal, hypothesis-generating design for identifying shared and distinct pathways that can explain a complex symptom like fatigue. Fatigue is a multidimensional concept, hence its etiology may be related to multiple factors (Bower et al., 2000). Accordingly, fatigue should not be considered as one symptom with a single underlying mechanism. Instead, fatigue symptoms in patients with various types of cancer receiving different therapies may originate from different pathobiologic mechanisms. Our work suggests that chronic inflammation mediated by TRAIL may contribute to urinary symptoms leading to sleep disturbance and, subsequently, fatigue in our euthymic participants. At the same time, TRAIL upregulation as a result of chronic inflammation may result in neuronal stress further contributing to fatigue and cognitive impairment. The main clinical implication of our study is the identification of a novel molecular substrate associated with fatigue. Not only does TRAIL provide clues to pathways involved in post-radiotherapy fatigue pathogenesis, it also provides a target for development of future therapeutic interventions, where therapies employing TRAIL receptor antagonists or neutralizing TRAIL antibodies may potentially help alleviate fatigue symptoms. Another advantage of our study is the relative homogeneity of the study population – all subjects were men treated with radiotherapy for non-metastatic prostate cancer. Compared to chemotherapy and whole-body radiotherapy, which can have global effects including cognitive alterations, local radiotherapy serves as an optimal model for assessing effects of peripherally triggered inflammatory events on a central phenomenon such as fatigue. The lack of differences in main clinical factors between the fatigued and non-fatigued groups allowed us to rule out variables such as age, BMI, thyroid profile, treatment type, and stage of cancer as contributors of fatigue. We previously found that ADT played a role in fatigue during radiotherapy, possibly related to the effect of ADT on hemoglobin levels (Feng et al., 2015). This effect of ADT on fatigue pathogenesis appears to be limited to fatigue during radiation treatment, but not the more persistent fatigue in our cohort. Considering the study’s small sample size, further investigation of the effects of long-term ADT usage when combined with radiotherapy is warranted.
Previous studies on cancer-related fatigue have typically focused on markers such as IL-1 and IL-6 (Bower, 2014; Illi et al., 2012; Miaskowski and Aouizerat, 2012). A strength of the current study is the unbiased approach of measuring a panel of 48 cytokines at the same time. Both IL-1 and IL-6 as well as other commonly studied markers were included in the cytokine multiplex panel. After multiple comparison correction, only TRAIL was significantly upregulated in fatigued subjects. It is possible that the upregulation of TRAIL is specific for fatigue in subjects with localized prostate cancer after receiving radiation therapy. Future studies will investigate the role of TRAIL in other types of cancer and other cancer treatment modalities. In addition, it is possible that due to the large number of cytokines measured in the discovery phase and the stringent multiple comparison correction, cytokines commonly found in other studies were undetected as false negative. Nonetheless, the novel finding that TRAIL correlated with fatigue demonstrates the strength of a broad and unbiased approach in biomarker discovery.
One caveate is that subjects in our discovery phase were followed only up to one year post-RT. Future studies will continue to follow the same subjects for a longer period of time in order to examine the longitudinal progression and evolvement of fatigue. Second, urinary symptoms, sleep disturbance, and fatigue symptoms were all measured by self-report, the inevitable subjectivity may explain the relatively modest correlation. Although the primary outcome was measured by the same instrument (FACT-F) in both phases of the study, the other secondary measures (e.g. HAM-D, rPFS) were not administered in the MedStar study, so were not included in the analysis. Further, the mechanism of fatigue found in this study may be unique to prostate cancer survivors post-radiotherapy, because urinary symptoms experienced by fatigued subjects in our study stemmed from tissue damage due to radiation localized to the prostate region. Future studies will aim to examine whether TRAIL plays a role in other types of cancer-related fatigue. Another limitation of the study is that EBRT was used in the discovery phase at NIH, whereas the more hypofractionated CyberKnife was the treatment modality used at MedStar Georgetown University Hospital in the validation phase. While the two radiation treatment modalities have different short-term and long-term toxicity profiles, our evidence suggests that regardless of which method of treatment is used, TRAIL may be a major factor in the development of persistent fatigue.
We observed a small but significant difference in depressive symptoms as measured by HAM-D (Table 1; fatigued: 2.2 ± 0.4, non-fatigued: 1.1 ± 0.4, p = 0.05). In addition, fatigue and depressive symptoms correlated significantly even though the HAM-D scores were far below the clinical threshold for depression (HAM-D scores of 0–7 indicate no depression). The significant correlation between fatigue and depressive symptoms indicates a high degree of commonality between the two symptoms. Fatigue and depressive symptoms are difficult to dissociate, both subjectively and objectively, and psychiatrists are increasingly consulted regarding cancer-related fatigue (Levy, 2008). Therefore, it may be beneficial for current and future studies to characterize fatigue by its association with other depressive symptoms. In fact, the National Institute of Mental Health has defined fatigue as a Research Domain Criteria (RDoC) element (https://www.nimh.nih.gov/research-priorities/rdoc/index.shtml) as part of a framework of studying psychiatric conditions in recent years.
The revised Piper Fatigue Scale is a widely used instrument for measuring fatigue in cancer populations with a cognitive subscale (Piper and Cella, 2010; Piper et al., 1998). This instrument allows for the assessment of perceived cognitive difficulties associated with fatigue without having to administer a separate cognitive test in the small window of time during each study visit. Accordingly, even though the rPFS-cognitive/mood subscale does not differentiate between cognitive impairment and mood disorder, the difference observed between fatigued and non-fatigued subjects was likely due to cognitive impairment, because individuals with psychiatric conditions (e.g., mood disorders) were ineligible to participate in this study.
In conclusion, we identified a novel connection between fatigue and post-radiotherapy inflammation in prostate cancer survivors. Repeated stress related to chronic inflammation mediated by TRAIL may contribute to neuronal damage resulting in cognitive impairment and chronic fatigue. The selective vulnerability of neurons to TRAIL may help explain fatigue-related symptoms post-radiotherapy that persists long after treatment.
Acknowledgments
The authors thank Dr. Steve Soldin and Mr. Brian Stolze, and Ms. Alexandra Espina for assistance with data acquisition. The authors also thank Douglas Joubert, NIH Library Writing Center, for manuscript editing assistance. This study is fully supported by the Division of Intramural Research of the National Institute of Nursing Research of the NIH, Bethesda, Maryland.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Li Rebekah Feng, National Institute of Nursing Research, National Institutes of Health, Bethesda, Maryland.
Simeng Suy, Department of Radiation Medicine, Georgetown University Hospital, Washington, DC.
Sean P. Collins, Department of Radiation Medicine, Georgetown University Hospital, Washington, DC.
Leorey N. Saligan, National Institute of Nursing Research, National Institutes of Health, Bethesda, Maryland.
References
- Ahlberg K, Ekman T, Gaston-Johansson F. The experience of fatigue, other symptoms and global quality of life during radiotherapy for uterine cancer. International Journal of Nursing Studies. 2005;42(4):377–386. doi: 10.1016/j.ijnurstu.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Aktas O, Smorodchenko A, Brocke S, Infante-Duarte C, Topphoff US, Vogt J, Prozorovski T, Meier S, Osmanova V, Pohl E, Bechmann I, Nitsch R, Zipp F. Neuronal Damage in Autoimmune Neuroinflammation Mediated by the Death Ligand TRAIL. Neuron. 2005;46(3):421–432. doi: 10.1016/j.neuron.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Aktas O, Ullrich O, Infante-Duarte C, Nitsch R, Zipp F. Neuronal damage in brain inflammation. Arch Neurol. 2007;64(2):185–189. doi: 10.1001/archneur.64.2.185. [DOI] [PubMed] [Google Scholar]
- Azzam EI, Jay-Gerin JP, Pain D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer letters. 2012;327(0):48–60. doi: 10.1016/j.canlet.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banasr M, Dwyer JM, Duman RS. Cell atrophy and loss in depression: reversal by antidepressant treatment. Current Opinion in Cell Biology. 2011;23(6):730–737. doi: 10.1016/j.ceb.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry MJ, Fowler FJ, Jr, O’Leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK, Cockett AT. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148(5):1549–1557. doi: 10.1016/s0022-5347(17)36966-5. discussion 1564. [DOI] [PubMed] [Google Scholar]
- Berger AM, Mooney K, Alvarez-Perez A, Breitbart WS, Carpenter KM, Cella D, Cleeland C, Dotan E, Eisenberger MA, Escalante CP, Jacobsen PB, Jankowski C, LeBlanc T, Ligibel JA, Loggers ET, Mandrell B, Murphy BA, Palesh O, Pirl WF, Plaxe SC, Riba MB, Rugo HS, Salvador C, Wagner LI, Wagner-Johnston ND, Zachariah FJ, Bergman MA, Smith C. Cancer-Related Fatigue, Version 2.2015. Journal of the National Comprehensive Cancer Network : JNCCN. 2015;13(8):1012–1039. doi: 10.6004/jnccn.2015.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE. Cancer-related fatigue-mechanisms, risk factors, and treatments. Nat Rev Clin Oncol. 2014;11(10):597–609. doi: 10.1038/nrclinonc.2014.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, Lamkin DM. Inflammation and cancer-related fatigue: Mechanisms, contributing factors, and treatment implications. Brain, Behavior, and Immunity. 2013;30(Supplement(0)):S48–S57. doi: 10.1016/j.bbi.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantarella G, Uberti D, Carsana T, Lombardo G, Bernardini R, Memo M. Neutralization of TRAIL death pathway protects human neuronal cell line from [beta]-amyloid toxicity. Cell Death Differ. 2003;10(1):134–141. doi: 10.1038/sj.cdd.4401143. [DOI] [PubMed] [Google Scholar]
- Cella D, Eton DT, Lai JS, Peterman AH, Merkel DE. Combining Anchor and Distribution-Based Methods to Derive Minimal Clinically Important Differences on the Functional Assessment of Cancer Therapy (FACT) Anemia and Fatigue Scales. Journal of Pain and Symptom Management. 2002;24(6):547–561. doi: 10.1016/s0885-3924(02)00529-8. [DOI] [PubMed] [Google Scholar]
- Chen LN, Suy S, Uhm S, Oermann EK, Ju AW, Chen V, Hanscom HN, Laing S, Kim JS, Lei S, Batipps GP, Kowalczyk K, Bandi G, Pahira J, McGeagh KG, Collins BT, Krishnan P, Dawson NA, Taylor KL, Dritschilo A, Lynch JH, Collins SP. Stereotactic Body Radiation Therapy (SBRT) for clinically localized prostate cancer: the Georgetown University experience. Radiation Oncology. 2013;8(1):1–10. doi: 10.1186/1748-717X-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-Induced Sickness Behavior: Mechanisms and Implications. Annals of the New York Academy of Sciences. 2001;933(1):222–234. doi: 10.1111/j.1749-6632.2001.tb05827.x. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Heijnen CJ, Kavelaars A, Laye S, Capuron L. The neuroimmune basis of fatigue. Trends in Neurosciences. 2014;37(1):39–46. doi: 10.1016/j.tins.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degli-Esposti MA, Smolak PJ, Walczak H, Waugh J, Huang CP, DuBose RF, Goodwin RG, Smith CA. Cloning and Characterization of TRAIL-R3, a Novel Member of the Emerging TRAIL Receptor Family. The Journal of Experimental Medicine. 1997;186(7):1165–1170. doi: 10.1084/jem.186.7.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deligezer U, Dalay N. Expression of the TRAIL receptors in blood mononuclear cells in leukemia. Pathol Oncol Res. 2007;13(4):290–294. doi: 10.1007/BF02940307. [DOI] [PubMed] [Google Scholar]
- Feng LR, Chen M-k, Lukkahatai N, Hsiao CP, Kaushal A, Sechrest L, Saligan LN. Clinical Predictors of Fatigue in Men With Non-Metastatic Prostate Cancer Receiving External Beam Radiation Therapy. Clinical Journal of Oncology Nursing. 2015;19(6):744–750. doi: 10.1188/15.CJON.744-750. [DOI] [PubMed] [Google Scholar]
- Feng LR, Dickinson K, Kline N, Saligan LN. Different phenotyping approaches lead to dissimilar biologic profiles in men with chronic fatigue following radiation therapy. Journal of Pain and Symptom Management. 2016a doi: 10.1016/j.jpainsymman.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng LR, Wolff BS, Lukkahatai N, Espina A, Saligan LN. Exploratory Investigation of Early Biomarkers for Chronic Fatigue in Prostate Cancer Patients Following Radiation Therapy. Cancer Nursing Publish Ahead of Print. 2016b doi: 10.1097/NCC.0000000000000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Pinto A, Mosquera F, Reed C, Novick D, Barbeito S, Vega P, Bertsch J, Alberich S, Haro JM. Validity and Reliability of the Hamilton Depression Rating Scale (5 Items) for Manic and Mixed Bipolar Disorders. The Journal of Nervous and Mental Disease. 2009;197(9):682–686. doi: 10.1097/NMD.0b013e3181b3b3a0. [DOI] [PubMed] [Google Scholar]
- Hall DL, Antoni MH, Lattie EG, Jutagir DR, Czaja SJ, Perdomo D, Lechner SC, Stagl JM, Bouchard LC, Gudenkauf LM, Traeger L, Fletcher M, Klimas NG. Perceived Fatigue Interference and Depressed Mood: Comparison of Chronic Fatigue Syndrome/Myalgic Encephalomyelitis Patients with Fatigued Breast Cancer Survivors. Fatigue : biomedicine, health & behavior. 2015;3(3):142–155. doi: 10.1080/21641846.2015.1039289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa H, Yamada Y, Harasawa H, Tsuji T, Murata K, Sugahara K, Tsuruda K, Masuda M, Takasu N, Kamihira S. Restricted expression of tumor necrosis factor-related apoptosis-inducing ligand receptor 4 in human peripheral blood lymphocytes. Cellular Immunology. 2004;231(1–2):1–7. doi: 10.1016/j.cellimm.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Hebb ALO, Moore CS, Bhan V, Robertson GS. Effects of IFN-B on TRAIL and Decoy Receptor Expression in Different Immune Cell Populations from MS Patients with Distinct Disease Subtypes. Autoimmune Diseases. 2011;2011:485752. doi: 10.4061/2011/485752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LL, Tizabi Y. Neuroinflammation, Neurodegeneration, and Depression. Neurotoxicity Research. 2013;23(2):131–144. doi: 10.1007/s12640-012-9348-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illi J, Miaskowski C, Cooper B, Levine JD, Dunn L, West C, Dodd M, Dhruva A, Paul SM, Baggott C, Cataldo J, Langford D, Schmidt B, Aouizerat BE. Association between pro- and anti-inflammatory cytokine genes and a symptom cluster of pain, fatigue, sleep disturbance, and depression. Cytokine. 2012;58(3):437–447. doi: 10.1016/j.cyto.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadhim MA, Hill MA. Non-targeted effects of radiation exposure: recent advances and implications. Radiation protection dosimetry. 2015;166(1–4):118–124. doi: 10.1093/rpd/ncv167. [DOI] [PubMed] [Google Scholar]
- Keyes M, Miller S, Moravan V. Urinary symptom flare in 712 125I prostate brachytherapy patients: long-term follow-up. International journal of radiation oncology, biology, physics. 2009;75 doi: 10.1016/j.ijrobp.2008.11.043. [DOI] [PubMed] [Google Scholar]
- Kichev A, Rousset CI, Baburamani AA, Levison SW, Wood TL, Gressens P, Thornton C, Hagberg H. Tumor Necrosis Factor-related Apoptosis-inducing Ligand (TRAIL) Signaling and Cell Death in the Immature Central Nervous System after Hypoxia-Ischemia and Inflammation. Journal of Biological Chemistry. 2014;289(13):9430–9439. doi: 10.1074/jbc.M113.512350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberley FC, Screaton GR. Following a TRAIL: Update on a ligand and its five receptors. Cell Res. 2004;14(5):359–372. doi: 10.1038/sj.cr.7290236. [DOI] [PubMed] [Google Scholar]
- Kutlu O, Akkaya E, Koksal IT, Bassorgun IC, Ciftcioglu MA, Sanlioglu S, Kukul E. Importance of TNF-related apoptosis-inducing ligand in pathogenesis of interstitial cystitis. International urology and nephrology. 2010;42(2):393–399. doi: 10.1007/s11255-009-9632-z. [DOI] [PubMed] [Google Scholar]
- Laird BJA, Scott AC, Colvin LA, McKeon AL, Murray GD, Fearon KCH, Fallon MT. Pain, Depression, and Fatigue as a Symptom Cluster in Advanced Cancer. Journal of Pain and Symptom Management. 2011;42(1):1–11. doi: 10.1016/j.jpainsymman.2010.10.261. [DOI] [PubMed] [Google Scholar]
- LeBlanc HN, Ashkenazi A. Apo2L//TRAIL and its death and decoy receptors. Cell Death Differ. 2003;10(1):66–75. doi: 10.1038/sj.cdd.4401187. [DOI] [PubMed] [Google Scholar]
- Lemke J, von Karstedt S, Zinngrebe J, Walczak H. Getting TRAIL back on track for cancer therapy. Cell Death Differ. 2014;21(9):1350–1364. doi: 10.1038/cdd.2014.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepor H, Kaci L. The Impact of Open Radical Retropubic Prostatectomy on Continence and Lower Urinary Tract Symptoms: A Prospective Assessment Using Validated Self-Administered Outcome Instruments. The Journal of Urology. 2004;171(3):1216–1219. doi: 10.1097/01.ju.0000113964.68020.a7. [DOI] [PubMed] [Google Scholar]
- Levy M. Cancer fatigue: a review for psychiatrists. General Hospital Psychiatry. 2008;30(3):233–244. doi: 10.1016/j.genhosppsych.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Liblau RS, Gonzalez-Dunia D, Wiendl H, Zipp F. Neurons as targets for T cells in the nervous system. Trends in Neurosciences. 2013;36(6):315–324. doi: 10.1016/j.tins.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Limoli CL, Giedzinski E, Morgan WF, Swarts SG, Jones GDD, Hyun W. Persistent Oxidative Stress in Chromosomally Unstable Cells. Cancer Research. 2003;63(12):3107–3111. [PubMed] [Google Scholar]
- Lydiatt WM, Denman D, McNeilly DP, Puumula SE, Burke WJ. A randomized, placebo-controlled trial of citalopram for the prevention of major depression during treatment for head and neck cancer. Archives of otolaryngology–head & neck surgery. 2008;134(5):528–535. doi: 10.1001/archotol.134.5.528. [DOI] [PubMed] [Google Scholar]
- Mahieu MA, Ahn GE, Chmiel JS, Dunlop DD, Helenowski IB, Semanik P, Song J, Yount S, Chang RW, Ramsey-Goldman R. Fatigue, patient reported outcomes, and objective measurement of physical activity in systemic lupus erythematosus. Lupus. 2016 doi: 10.1177/0961203316631632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miaskowski C, Aouizerat BE. Biomarkers: Symptoms, Survivorship, and Quality of Life. Seminars in oncology nursing. 2012;28(2):129–138. doi: 10.1016/j.soncn.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milam JE, Meeske K, Slaughter RI, Sherman-Bien S, Ritt-Olson A, Kuperberg A, Freyer DR, Hamilton AS. Cancer-related follow-up care among Hispanic and non-Hispanic childhood cancer survivors: The Project Forward study. Cancer. 2015;121(4):605–613. doi: 10.1002/cncr.29105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16(1):22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan WF, Sowa MB. Non-targeted effects induced by ionizing radiation: mechanisms and potential impact on radiation induced health effects. Cancer letters. 2015;356(1):17–21. doi: 10.1016/j.canlet.2013.09.009. [DOI] [PubMed] [Google Scholar]
- Nugent SME, Mothersill CE, Seymour C, McClean B, Lyng FM, Murphy JEJ. Increased Mitochondrial Mass in Cells with Functionally Compromised Mitochondria after Exposure to both Direct γ Radiation and Bystander Factors. Radiation Research. 2007;168(1):134–142. doi: 10.1667/RR0769.1. [DOI] [PubMed] [Google Scholar]
- Oh Y, Jeon YJ, Hong GS, Kim I, Woo HN, Jung YK. Regulation in the targeting of TRAIL receptor 1 to cell surface via GODZ for TRAIL sensitivity in tumor cells. Cell Death Differ. 2012;19(7):1196–1207. doi: 10.1038/cdd.2011.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper BF, Cella D. Cancer-Related Fatigue: Definitions and Clinical Subtypes. Journal of the National Comprehensive Cancer Network. 2010;8(8):958–966. doi: 10.6004/jnccn.2010.0070. [DOI] [PubMed] [Google Scholar]
- Piper BF, Dibble SL, Dodd MJ, Weiss MC, Slaughter RE, Paul SM. The revised Piper Fatigue Scale: psychometric evaluation in women with breast cancer. Oncol Nurs Forum. 1998;25(4):677–684. [PubMed] [Google Scholar]
- Prithivirajsingh S, Story MD, Bergh SA, Geara FB, Kian Ang K, Ismail SM, Stevens CW, Buchholz TA, Brock WA. Accumulation of the common mitochondrial DNA deletion induced by ionizing radiation. FEBS Letters. 2004;571(1–3):227–232. doi: 10.1016/j.febslet.2004.06.078. [DOI] [PubMed] [Google Scholar]
- Reeve BB, Stover AM, Alfano CM, Smith AW, Ballard-Barbash R, Bernstein L, McTiernan A, Baumgartner KB, Piper BF. The Piper Fatigue Scale-12 (PFS-12): Psychometric Findings and Item Reduction in a Cohort of Breast Cancer Survivors. Breast Cancer Res Treat. 2012;136(1):9–20. doi: 10.1007/s10549-012-2212-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothrock NE, Hays RD, Spritzer K, Yount SE, Riley W, Cella D. Relative to the general US population, chronic diseases are associated with poorer health-related quality of life as measured by the Patient-Reported Outcomes Measurement Information System (PROMIS) Journal of Clinical Epidemiology. 2010;63(11):1195–1204. doi: 10.1016/j.jclinepi.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saligan LN, Hsiao CP, Wang D, Wang XM, John LS, Kaushal A, Citrin D, Barb JJ, Munson PJ, Dionne RA. Upregulation of α-synuclein during localized radiation therapy signals the association of cancer-related fatigue with the activation of inflammatory and neuroprotective pathways. Brain, Behavior, and Immunity. 2013;27(0):63–70. doi: 10.1016/j.bbi.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaue D, McBride WH. T lymphocytes and normal tissue responses to radiation. Frontiers in Oncology. 2012;2 doi: 10.3389/fonc.2012.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suy S, Oermann E, Hanscom H, Lei S, Vahdat S, Yu X, Park HU, Chen V, Collins BT, McGeagh K, Dawson N, Jha R, Azumi N, Dritschilo A, Lynch J, Collins SP. Histopathologic Effects of Hypofractionated Robotic Radiation Therapy on Malignant and Benign Prostate Tissue. Technology in Cancer Research & Treatment. 2010;9(6):583–587. doi: 10.1177/153303461000900606. [DOI] [PubMed] [Google Scholar]
- Vogt J, Paul F, Aktas O, Müller-Wielsch K, Dörr J, Dörr S, Bharathi BS, Glumm R, Schmitz C, Steinbusch H, Raine CS, Tsokos M, Nitsch R, Zipp F. Lower motor neuron loss in multiple sclerosis and experimental autoimmune encephalomyelitis. Annals of Neurology. 2009;66(3):310–322. doi: 10.1002/ana.21719. [DOI] [PubMed] [Google Scholar]
- Yarlagadda A, Alfson E, Clayton AH. The Blood Brain Barrier and the Role of Cytokines in Neuropsychiatry. Psychiatry (Edgmont) 2009;6(11):18–22. [PMC free article] [PubMed] [Google Scholar]
- Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. Journal of Pain and Symptom Management. 1997;13(2):63–74. doi: 10.1016/s0885-3924(96)00274-6. [DOI] [PubMed] [Google Scholar]
- Yu JB, Cramer LD, Herrin J, Soulos PR, Potosky AL, Gross CP. Stereotactic Body Radiation Therapy Versus Intensity-Modulated Radiation Therapy for Prostate Cancer: Comparison of Toxicity. Journal of Clinical Oncology. 2014;32(12):1195–1201. doi: 10.1200/JCO.2013.53.8652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Buysse DJ, Germain A, Moul DE, Stover A, Dodds NE, Johnston KL, Pilkonis PA. Development of short forms from the PROMIS sleep disturbance and Sleep-Related Impairment item banks. Behavioral sleep medicine. 2011;10(1):6–24. doi: 10.1080/15402002.2012.636266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelefsky MJ, Levin EJ, Hunt M, Yamada Y, Shippy AM, Jackson A, Amols HI. Incidence of Late Rectal and Urinary Toxicities After Three-Dimensional Conformal Radiotherapy and Intensity-Modulated Radiotherapy for Localized Prostate Cancer. International Journal of Radiation Oncology*Biology*Physics. 2008;70(4):1124–1129. doi: 10.1016/j.ijrobp.2007.11.044. [DOI] [PubMed] [Google Scholar]
- Zhao W, Robbins ME. Inflammation and chronic oxidative stress in radiation-induced late normal tissue injury: therapeutic implications. Current medicinal chemistry. 2009;16(2):130–143. doi: 10.2174/092986709787002790. [DOI] [PubMed] [Google Scholar]
- Zimmerman M, Martinez JH, Young D, Chelminski I, Dalrymple K. Severity classification on the Hamilton depression rating scale. Journal of Affective Disorders. 2013;150(2):384–388. doi: 10.1016/j.jad.2013.04.028. [DOI] [PubMed] [Google Scholar]


