Abstract
Introduction
The diagnostic utility of in vivo amyloid β (Aβ) imaging to aid in the clinical distinction between frontotemporal dementia (FTD) and Alzheimer's disease remains unclear without data on the prevalence and severity of Aβ in pathologically confirmed FTD syndromes.
Methods
Aβ was assessed in 98 autopsy-confirmed FTD and 36 control cases, and the pathological accuracy of 11C-Pittsburgh compound B (PiB)–positron emission tomography imaging was assessed in a subset of FTD cases (n = 15).
Results
Aβ was identified in a similar proportion of FTD syndromes and age-matched controls and increases with age. Alzheimer's disease pathology was identified in all cases with high PiB retention and in one case with low PiB retention. We further demonstrate a strong regional correlation between volume fraction of histological Aβ with PiB standard uptake value ratio scaled to the white matter.
Discussion
The present study provides a pathologic reference to assist in the interpretation of in vivo assessments in FTD syndromes.
Keywords: Frontotemporal dementia syndromes, Alzheimer's disease, Amyloid β, 11C-Pittsburgh compound B, Diagnostic
1. Introduction
Frontotemporal dementia (FTD) and Alzheimer's disease (AD) are the leading causes of dementia in individuals aged less than 65 years. Despite being characterized by distinct underlying pathologic proteins and spread, the clinical distinction between FTD and AD remains challenging, with ∼25% of patients with a clinical FTD syndrome found to have a pathologic diagnosis of AD at autopsy [1]. Although this is largely driven by patients with the logopenic variant of primary progressive aphasia (lv-PPA), AD is also found in a significant proportion of patients with other FTD syndromes, including the non-fluent variant of primary progressive aphasia (nfv-PPA), corticobasal syndrome (CBS), semantic variant of primary progressive aphasia (sv-PPA) and behavioral variant frontotemporal dementia (bvFTD) [1], [2]. Research diagnostic criteria for clinical AD have recently incorporated in vivo markers of amyloid β (Aβ) pathology such as positron emission tomography (PET) with the 11C-Pittsburgh compound B (PiB) ligand. Amyloid ligands appear to be particularly helpful in identifying patients with AD pathology and may be of particular value in atypically presenting AD cases such as those with lv-PPA or CBS [3]. Importantly, these ligands correlate with overall Aβ burden rather than only neuritic plaques [4], [5], [6], but given that only neuritic plaques were used for diagnostic confirmation of AD before the publication of the updated pathologic criteria for AD in 2012 [7], [8], [9], pathologic data on the prevalence and burden of Aβ in FTD syndromes are lacking. This could have important implications for the use and interpretation of PiB-PET imaging to differentiate pathologic AD in patients presenting with clinical FTD syndromes. To address this, the present study had the following aims: (1) to determine the proportion of Aβ positivity, Thal's topographical distribution [7] and clinical implications of Aβ deposition in a large cohort of pathologically confirmed FTD syndromes that do not meet the threshold for intermediate or high levels of AD neuropathology; (2) to determine if in vivo and postmortem measures of Aβ burden correlate in FTD; and if so, (3) to assess histological Aβ burden in patients with FTD ≤75 years at death (since PiB-PET imaging assessments are performed aged ≤75 years in clinical FTD), with the purpose of determining whether FTD cases demonstrate a Aβ burden similar to pathologic AD.
2. Methods
2.1. Cohort
One hundred thirty-four cases comprising 98 pathologically confirmed FTD and 36 cognitively normal individuals were selected from neuropathologic series collected by the Sydney Brain Bank and the Cambridge Brain Bank through regional brain donor programs. These brain donor programs hold approval from the Human Research Ethics Committees of the University of New South Wales, South Eastern and Illawarra Area Health Service, and the Addenbrooke's Hospital. Patients were diagnosed during life by experienced clinicians using standard clinical diagnostic criteria [10] following a medical interview, cognitive testing, and an informant history. Standardized neuropathologic characterization was performed [7], [11], [12], [13], [14], [15], [16]. Cases were excluded if they had moderate or severe cortical neuritic plaque formation (≥2 modified Consortium to Establish a Registry for Alzheimer's Disease [CERAD] score [7]) and neocortical neurofibrillary tangles (NFTs) (≥2 modified Braak NFT score [7]). This research project was approved by the Human Research Ethics Committee of the University of New South Wales.
2.2. Genetic analyses
Patients were screened for genetic mutations (C9ORF72, GRN, PSEN1, and APP) using previously published methods [17].
2.3. Topographical distribution of amyloid β
Formalin-fixed, paraffin-embedded tissue blocks for the left frontal cortex, temporal cortex, caudate-putamen, and substantia nigra were sectioned at 10 μm and immunostained with an anti–Aβ monoclonal antibody (1:500, M0872; DAKO) according to the updated NIA guidelines [7]. All slides were counterstained with hematoxylin to visualize neurons and other cells. Aβ deposition was graded using the recommended A score, which is the topographical progression according to the modified Thal scoring scheme: A0 = no detectable deposition (Thal phase 0), A1 = any cortical Aβ deposition (Thal phase 1–2), A2 = any Aβ also present in the caudate-putamen (Thal phase 3), and A3 = any Aβ present in the cortex, caudate-putamen, substantia nigra, and cerebellum (Thal phase 4–5) [7].
2.4. PiB-PET assessments
Fifteen autopsy cases that had undergone in vivo PiB-PET and were followed to autopsy were available for this study. All had a clinical diagnosis of a FTD syndrome before imaging, but four (27%, 4/15) were excluded from the previous cohort as they had pathologic AD at autopsy. PET imaging had been performed at the Austin Hospital in Melbourne as previously described [18]. Global and regional standardized uptake value ratios (SUVRs) were obtained from regions of interest (ROIs) for cortical, subcortical, and cerebellar regions defined on a coregistered magnetic resonance imaging, regions corresponding to the Thal stages of Aβ deposition [7]. White matter ROIs were placed at the centrum semiovale, and the cerebellar regions were placed over the cerebellar cortex, taking care to avoid white matter. The orbitofrontal ROI included both the mesial and lateral aspects (including superior, middle, and inferior frontal gyri). Cortical Aβ burden was expressed as the average SUVR of the area-weighted mean of frontal, superior parietal, lateral temporal, lateral occipital, and anterior and posterior cingulate regions. Consistent with previous PiB-PET studies [18], [19], [20], an SUVRCb cutoff of 1.50, using the cerebellar cortex as the reference region, was employed for the categorical classification of patients into a “high” or “low” PiB retention. However, given that Aβ deposition is recognized in the cerebellum of a proportion of patients with an A3 score (Thal phase 5) [7], [21], we assessed which reference region–derived SUVR agreed best with the neuropathology. That is, correlations between the amount of pathologic Aβ deposition and the SUVR scaled to four reference regions (cerebellar cortex, pons, white matter [centrum semiovale], and a composite of white matter and pons) were performed.
2.5. Volume fraction of amyloid β
Volume fraction analysis was performed in all autopsy cases with PiB-PET to assess the association between SUVR and neuropathology (Section 3.3). Volume fraction analysis was also assessed across all pathologically confirmed FTD and control cases that were ≤75 years at death and that had Aβ deposition, and a comparative cohort of AD cases without cerebrovascular disease and ≤75 years at death. The age cutoff of 75 years was applied to reflect in vivo conditions because almost all patients with clinical FTD undergo PiB imaging at the age of ≤75 years. Aβ–immunostained slides were scanned using the Aperio ScanScope XT slide scanner, and the volume fraction of histological Aβ deposits was calculated using a point-counting method as previously described [22] (Supplementary Material) by two raters blind to case details with inter- and intra-rater variances of <5%.
2.6. Statistical analyses
Statistical analyses were performed using SPSS (version 21). Demographic data (age, sex, and postmortem delay), clinical (disease duration, CDR score) variables, and volume fraction of Aβ were assessed across participant groups via one-way analysis of variance (ANOVA) followed by Bonferroni-corrected post hoc tests (P < .05 taken as significant). χ2 analyses were used to compare the proportion of cases with Aβ deposition and the topographical distribution of Aβ deposition between participant groups. Pearson's correlation coefficient was used to assess for associations between demographic features, clinical indices, and the topographical distribution of Aβ within groups. Pearson's correlation coefficient was also used to assess associations between regional Aβ deposition and regional SUVR measures scaled to each of the four reference regions (one analysis per reference region), with corrections for multiple testing using false discovery rate (P < .03 taken as significant). Accuracy was determined as the proportion of cases with matching diagnoses.
3. Results
3.1. Cortical amyloid β in pathologically confirmed FTD and controls
All cases were graded according to the “ABC” score for AD neuropathologic change [7] and cases that met a no or low “ABC” score selected (n = 94 FTD, n = 26 controls). Given that the control cases were significantly older than the FTD cohort (mean ± standard deviation [SD] [years]: FTD 69 ± 9, controls 81 ± 14; P < .001), an age cutoff of 85 years at death was applied to the control cohort to select an age-matched control subset (mean ± SD (years): age-matched controls 71 ± 10; P > .05 compared to FTD cases). Cortical Aβ deposition was identified in 37% (35/94) of FTD and 57% (8/14) of the age-matched control subset (and 77% [20/26] of the entire control cohort). FTD cases with Aβ were older at disease onset and death but demonstrated no significant difference in disease duration, CDR or FTD stage (Supplementary Material). The topographical progression of Aβ increased with age at death (ρ = 0.410, P < .001, n = 94) and disease onset in FTD (ρ = 0.389, P < .001, n = 94). No significant relationship was identified between CDR scores and any measured pathologies in FTD cases. A positive correlation was observed between age at death and the topographical distribution of Aβ across all control cases (ρ = 0.616, P < .001, n = 36) but not within the age-matched control subset (ρ = 0.324, P = .258, n = 14).
3.1.1. Amyloid β in clinical FTD syndromes
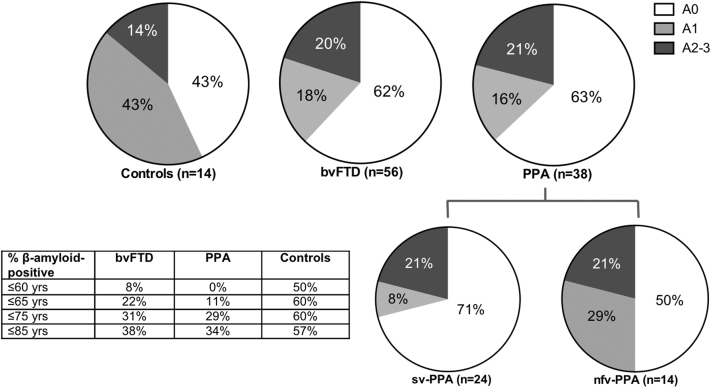
Of the 94 FTD cases, 60% had bvFTD (56/94) and 40% had a PPA (38/94), consisting of 63% with sv-PPA (24/38) and 37% with nfv-PPA (14/38), and age at death was not significantly different across groups [F(3,104) = 2.168; P = .096] (Table 1). Aβ deposition was identified in 38% bvFTD (21/56) and 37% PPA (14/38) (comprised 29% sv-PPA [7/24] and 50% nfv-PPA [7/14]), with no significant difference in these proportions across FTD subtypes [X(2) = 1.646, P = .439, n = 94] nor across FTD subtypes and controls [X(3) = 3.621, P = .305, n = 108] (Fig. 1). In both bvFTD and PPA groups, the topographical progression of Aβ increased with age at death (bvFTD: ρ = 0.448, P = .001, n = 56; PPA: ρ = 0.38, P = .19, n = 38) and disease onset (bvFTD: ρ = 0.375, P = .004, n = 56; PPA: ρ = 0.412, P = .12, n = 38) (Fig. 1).
Table 1.
Demographic, pathologic, and clinical features (mean ± standard deviation) of FTD and age-matched control cohorts
| Age-matched controls | bvFTD | PPA | sv-PPA | nfv-PPA | |
|---|---|---|---|---|---|
| Demographics | |||||
| N (% male) | 14 (57%) | 56 (59%) | 38 (50%) | 24 (50%) | 14 (50%) |
| Age at death (year) | 71 ± 10 | 67 ± 9 | 71 ± 8 | 71 ± 7 | 71 ± 10 |
| Age at disease onset (year) | N/A | 61 ± 8 | 65 ± 8b | 64 ± 7 | 66 ± 11 |
| Disease duration (year) | N/A | 6 ± 4 | 6 ± 4 | 7 ± 4 | 5 ± 3 |
| Postmortem delay (hours) | 21 ± 10 | 108 ± 398 | 20 ± 9 | 17 ± 7 | 22 ± 10 |
| C9ORF72 carrier [% (n)] | 0 (0/14) | 16 (9/56) | 3 (1/38) | 4 (1/24) | 0 (0/14) |
| GRN carrier [% (n)] | 0 (0/14) | 9 (5/56) | 5 (2/38) | 4 (1/24) | 7 (1/14) |
| MAPT carrier [% (n)] | 0 (0/14) | 7 (4/56) | 0 (0/38) | 0 (0/24) | 0 (0/14) |
| Pathologic variables | |||||
| Braak NFT stage (B0–B3) [7] | 0.3 ± 0.6 | 0.1 ± 0.4 | 0.4 ± 0.6 | 0.2 ± 0.4 | 0.5 ± 0.8 |
| CERAD score (C0–C3) [7] | 0.3 ± 0.8 | 0.2 ± 0.6 | 0.2 ± 0.5 | 0.1 ± 0.3 | 0.2 ± 0.6 |
| ABC AD score (0–3) [7] | 0.6 ± 0.5 | 0.4 ± 0.5 | 0.5 ± 0.5 | 0.6 ± 0.5 | 0.5 ± 0.5 |
| Clinical features | |||||
| CDR score | 0.0 ± 0.0 | 2.0 ± 1.0a | 2.0 ± 1.0a | 3.0 ± 0.0a | 1.5 ± 0.7 |
Abbreviations: AD, Alzheimer's disease; bvFTD, behavioral variant frontotemporal dementia; NFT, neurofibrillary tangle; nfv-PPA, non-fluent variant primary progressive aphasia; PPA, primary progressive aphasia; sv-PPA, semantic variant primary progressive aphasia: CERAD, Consortium to Establish a Registry for Alzheimer's Disease; CDR, Clinical Dementia Rating.
NOTE. ABC AD score: low–high represented numerically as 1 to 3.
NOTE. aP < .05 compared to controls; bP < .05 compared to bvFTD.
Fig. 1.
The proportion of Aβ positivity and topographical distribution of Aβ in frontotemporal dementia (FTD) syndromes and age-matched controls. The proportion of patients that demonstrate no Aβ deposition (A0), Aβ in the frontal-temporal cortices (A1), additional Aβ in the basal ganglia (A2), and additional Aβ in the substantia nigra (A3) is shown in patients with behavioral variant frontotemporal dementia (bvFTD), semantic variant primary progressive aphasia (sv-PPA), non-fluent variant primary progressive aphasia (nfv-PPA), and age-matched controls. Table inset demonstrates the percentage of cases with any Aβ deposition by age. As only one nfv-PPA and one control demonstrated an A3 distribution, these have been combined with the A2 group in the present figure.
3.1.2. Amyloid β in FTD cases with and without motor impairment
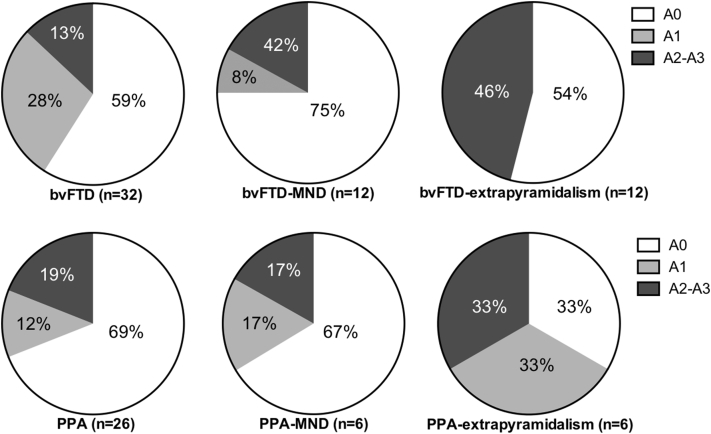
Co-existing motor neuron disease (MND) was present in 21% bvFTD (12/56) and 16% PPA cases (6/38). An extrapyramidal syndrome (CBS/progressive supranuclear palsy [PSP]) was present in a further 21% bvFTD (12/56) and 16% PPA cases (6/38). The age at disease onset, disease duration, and age at death were similar across motor subgroups both within and across the bvFTD and PPA cohorts (Supplementary Material). Collectively, across all FTD cases, the proportion of cases with Aβ deposition was similar between motor subgroups, with Aβ identified in 36% FTD cases (21/58), 28% FTD-MND cases (5/18), and 50% FTD-extrapyramidal cases (9/18) [X(2) = 1.970, P = .373, n = 94] (Fig. 2).
Fig. 2.
The proportion of Aβ positivity and topographical distribution of Aβ in frontotemporal dementia (FTD) syndromes with and without motor impairment. The proportion of patients that demonstrate no Aβ deposition (A0), Aβ in the frontal-temporal cortices (A1), additional Aβ in the basal ganglia (A2), and additional Aβ in the substantia nigra (A3) is shown in patients with behavioral variant FTD cases without motor impairment (bvFTD), primary progressive aphasia cases without motor impairment (PPA), cases with motor neuron disease (MND), and cases with extrapyramidalism. As only one PPA case without motor impairment had an A3 distribution (4%, 1/26), this has been combined with the A2 group.
3.1.3. Amyloid β in FTD cases with and without genetic mutations
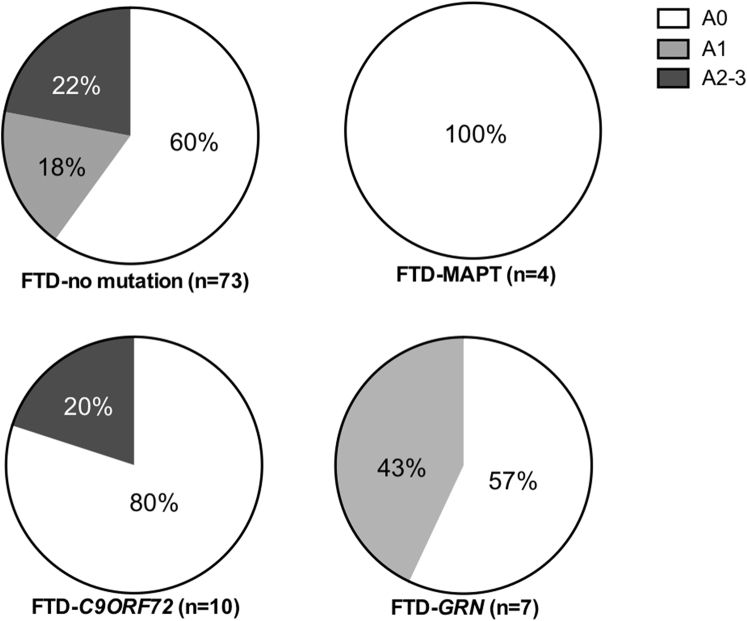
Among FTD cases, a C9ORF72 expansion was identified in 11% (10/94), a GRN mutation in 7% (7/94), and a MAPT mutation in 4% (4/94) (Table 1). FTD cases without a mutation were older at death, but this only reached significance in comparison to cases with a GRN mutation (mean ± SD (years): no mutation 70 ± 8, C9ORF72 expansion 65 ± 9, GRN mutation 60 ± 5, and MAPT mutation 60 ± 4; F(3,88) = 5.254, P < .05 with post hoc tests identifying P = .021 for GRN versus no mutation only). Aβ deposition was identified in 40% of FTD cases without a mutation (bvFTD: 16/38 and PPA: 13/35), 20% of cases with a C9ORF72 expansion (bvFTD: 2/9 and PPA: 0/1), 43% of cases with a GRN mutation (bvFTD: 3/5 and PPA: 0/2), and 0% of cases with a MAPT mutation (Fig. 3). No significant difference was identified in the proportion [X(3) = 4.036, P = .258, n = 94] or topographical distribution [X(9) = 9.410, P = .400, n = 94] of Aβ between mutation groups in FTD cases.
Fig. 3.
The proportion of Aβ positivity and topographical distribution of Aβ in frontotemporal dementia (FTD) cases with and without a genetic mutation. The proportion of patients that demonstrate no Aβ deposition (A0), Aβ in the frontal-temporal cortices (A1), additional Aβ in the basal ganglia (A2), and additional Aβ in the substantia nigra (A3) is shown in FTD cases without a mutation (n = 55), a MAPT mutation (n = 4), a C9ORF72 expansion (n = 10), and a GRN mutation (n = 7) is shown. As only one FTD case without a mutation had an A3 distribution (1%, 1/73), this has been combined with the A2 group.
3.2. PiB-PET SUVR and amyloid β pathology in clinical FTD syndromes
Of the 15 patients with antemortem PiB-PET, postmortem characterization revealed FTD pathology in 73% (11/15) and high levels of AD pathology sufficient to reach a pathological diagnosis of AD in 27% (4/15) (Table 2). We assessed the relationship between (1) the degree of Aβ deposition with the categorical classification of a high/low PiB based on a SUVRCb cutoff of 1.50 and (2) the volume fraction of histological Aβ with cortical SUVR scaled to four different reference regions.
Table 2.
PiB-PET and postmortem pathologic assessment in patients with FTD
| Number | Clin dx | MI | Path dx | M/F | Age at death (years) | Age at onset (years) | Death duration (years) | Mut | SUVRCb | PiB stat | TL (years) | Amyloid β A score [7] | Braak NFT score [7] | CERAD neuritic score [7] | ABC score [7] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | nfv-PPA | No | FTD-tau | M | 53 | 47 | 6 | 0 | 1.03 | Low | 6 | 0 | 0 | 0 | 0 |
| 2 | sv-PPA | MND | FTD-TDP | M | 62 | 62 | 0 | 0 | 1.02 | Low | 1 | 0 | 0 | 0 | 0 |
| 3 | bvFTD | No | FTD-TDP | M | 64 | 59 | 5 | GRN | 0.99 | Low | 3 | 1 | 0 | 0 | 1 |
| 4 | bvFTD | No | FTD-TDP | F | 68 | 64 | 4 | GRN | 0.88 | Low | 4 | 0 | 0 | 0 | 0 |
| 5 | nfv-PPA | EPS | FTD-tau | M | 69 | 65 | 4 | 0 | 1.04 | Low | 3 | 0 | 2 | 0 | 1 |
| 6 | nfv-PPA | No | FTD-tau | F | 72 | 71 | 1 | 0 | 0.97 | Low | 1 | 0 | 1 | 0 | 1 |
| 7 | sv-PPA | No | FTD-tau | F | 73 | 67 | 6 | 0 | 0.99 | Low | 4 | 0 | 0 | 0 | 0 |
| 8 | nfv-PPA | EPS | FTD-tau | M | 73 | 71 | 2 | 0 | 1.18 | Low | 2 | 0 | 0 | 0 | 0 |
| 9 | CBS | No | FTD-tau | F | 74 | 69 | 5 | 0 | 1.06 | Low | 4 | 0 | 0 | 0 | 0 |
| 10 | nfv-PPA | No | FTD-tau | F | 76 | 72 | 4 | 0 | 1.02 | Low | 3 | 2 | 1 | 0 | 1 |
| 11 | nfv-PPA | No | FTD-tau | M | 82 | 78 | 4 | 0 | 1.57 | High | 4 | 3 | 1 | 2 | 1 |
| 12 | lv-PPA | No | AD | F | 73 | 67 | 6 | 0 | 1.25 | Low | 6 | 3 | 3 | 3 | 3 |
| 13 | nfv-PPA | No | AD | M | 74 | 70 | 4 | 0 | 2.30 | High | 3 | 3 | 3 | 3 | 3 |
| 14 | lv-PPA | No | AD | M | 72 | 68 | 4 | 0 | 1.68 | High | 2 | 3 | 3 | 3 | 3 |
| 15 | CBS | No | AD | F | 64 | 59 | 5 | 0 | 1.56 | High | 4 | 3 | 3 | 3 | 3 |
Abbreviations: AD, Alzheimer's disease; CBD, corticobasal degeneration; CBS, corticobasal syndrome; Clin dx, clinical diagnosis; CERAD, Consortium to Establish a Registry for Alzheimer's Disease; EPS, extrapyramidal syndrome; F, female; lv-PPA, logopenic variant primary progressive aphasia; M, male; MI, motor impairment; MND, motor neuron disease; Mut, mutation; N/A, Not available; NFT, neurofibrillary tangle; nfv-PPA, non-fluent primary progressive aphasia; Path dx, pathological diagnosis; PiB stat, 11C-Pittsburgh compound B status; PiD, Pick's disease; PSP, progressive supranuclear palsy; SUVRCb, standardized uptake value ratio; sv-PPA, semantic variant primary progressive aphasia; TDP, transactive response DNA binding protein 43; TL, time lapse (years) between PiB-PET imaging and autopsy.
3.2.1. Categorical classification
At the time of PiB-PET imaging, four patients had a high SUVRCb of >1.5 (#11, #13, #14, and #15) and all four of these patients had severe Aβ deposition (PiB accuracy of 100%), although one (#11) did not have sufficient NFT formation for a neuropathologic diagnosis of AD [7]. A SUVRCb of 1.25 was determined in one patient (#12 who progressed to have high levels of AD pathology at death), and SUVRCb <1.2 were observed in all other patients (10 FTD), including two pathologic FTD cases with moderate cortical Aβ deposition at autopsy (#3 and #10, Table 2). Overall, in cases with a low SUVRCb <1.5, 73% (8/11) demonstrated no Aβ deposition in the frontal and temporal cortices, and 91% (10/11) did not meet pathologic criteria for a diagnosis of AD at autopsy.
3.2.2. Volume fraction of amyloid β and SUVR in patients with a clinical FTD syndrome
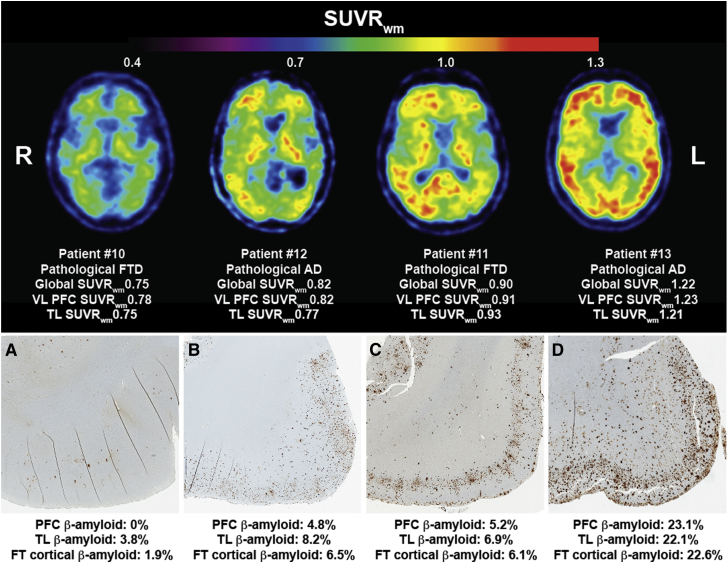
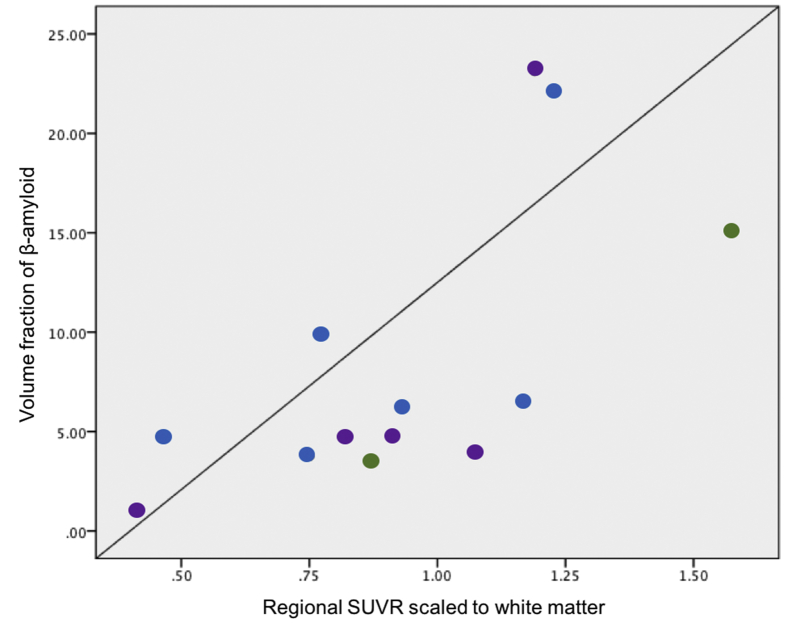
The volume fraction of Aβ in the frontal cortex, temporal cortex, and striatum of patients found to have Aβ pathology in these regions at autopsy (7/15 patients) was assessed against the corresponding regional SUVR scaled to four reference regions. This analysis was performed across cases and regions with Aβ deposition at autopsy, using data from all regions with Aβ pathology. Correlation analyses revealed a significant positive association between regional histological volume fractions of Aβ with SUVR scaled to the white matter (centrum semiovale) (ρ = 0.659; P = .014) (Fig. 4), but not to the corresponding regional SUVR scaled to the cerebellum (ρ = 0.457; P > .5), pons (ρ = 0.513; P > .5) nor to the composite of white matter and pons (ρ = 0.598; P = .031).
Fig. 4.
PiB-PET SUVRwm and cortical Aβ pathology in patients with clinical FTD. A strong regional correlation was identified between the volume fraction of histological Aβ deposited in the frontal and temporal cortices with the corresponding SUVRwm in patients with clinical FTD (P <.05). Abbreviations: FT, frontotemporal; FTD, frontotemporal dementia; PET, positron emission tomography; PiB, 11C-Pittsburgh compound B; SUVRwm, standard uptake value ratio scaled to the white matter; TL, temporal lobe; VL PFC, ventrolateral prefrontal cortex.
3.3. Volume fraction of histological amyloid β in FTD, AD, and controls ≤75 years at death
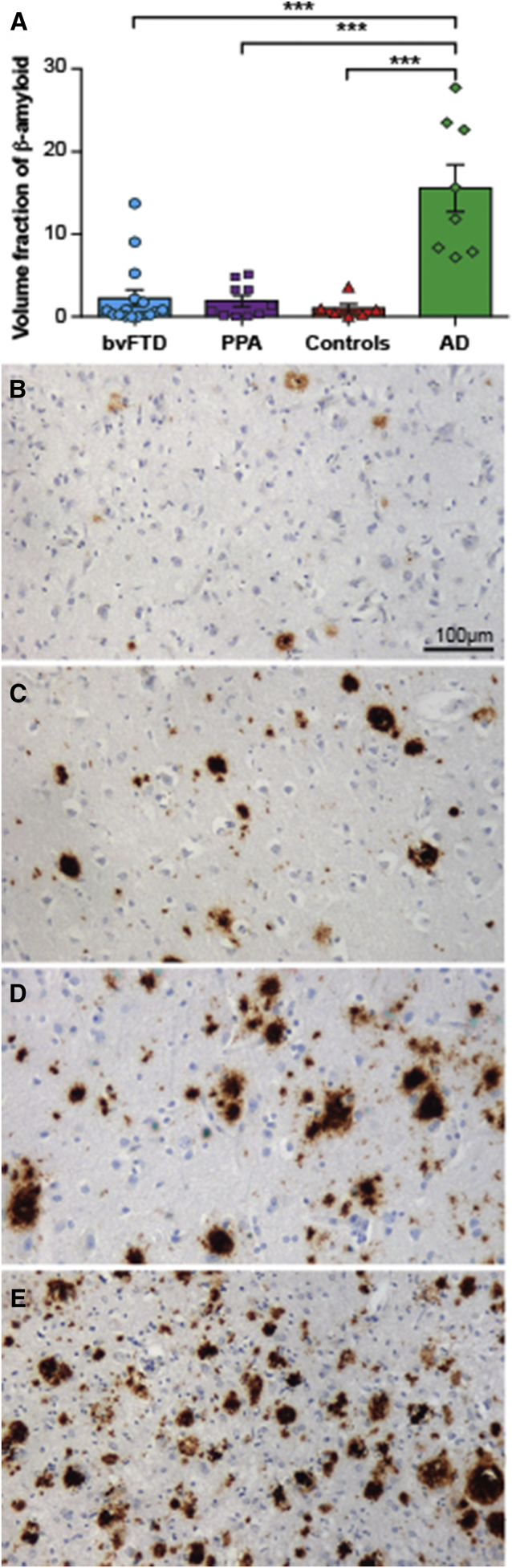
All groups were matched for age at death [F(3,32) = 0.701; P = .558], and patient groups (bvFTD, PPA, and AD) were matched for age at disease onset [F(2,26) = 0.405; P > .6] and disease duration [F(2,26) = 0.388; P > .7] (Table 3). Given that the volume fraction of Aβ deposition in the frontal and temporal cortices was similar within all groups (P > .05 for all), an average of these two regions was taken to calculate the burden of cortical Aβ, which was found to be significantly different across groups [F(3,32) = 17.062; P < .001 due to a significantly greater burden of cortical Aβ in AD cases (P < .001: AD compared to all other groups) (Fig. 5).
Table 3.
Demographic data and volume fraction of cortical amyloid β (mean ± standard deviation) in FTD, age-matched control, and AD cases with amyloid β deposition and ≤75 years at death
| bvFTD | PPA | Age-matched controls | Age-matched AD | |
|---|---|---|---|---|
| Demographics | ||||
| N (% male) | 14 (79%) | 9 (33%) | 6 (66%) | 7 (71%) |
| Age at death (year) | 67 ± 5 | 70 ± 3 | 67 ± 8 | 69 ± 7 |
| Age at disease onset (year) | 62 ± 6 | 64 ± 5 | N/A | 62 ± 6 |
| Disease duration (year) | 6 ± 4 | 7 ± 4 | N/A | 5 ± 2 |
| Postmortem delay (hour) | 17 ± 14 | 21 ± 7 | 18 ± 9 | 22 ± 12 |
| Motor neuron disease [% (n)] | 14% (2/14) | 22% (2/9) | 0% (0/6) | 0% (0/7) |
| Extrapyramidal syndrome [% (n)] | 14% (2/14) | 22% (2/9) | 0% (0/6) | 0% (0/7) |
| C9ORF72 carrier [% (n)] | 14% (2/14) | 0% (0/9) | 0% (0/6) | 14% (1/7) |
| GRN carrier [% (n)] | 21% (3/14) | 0% (0/9) | 0% (0/6) | 14% (1/7) |
| MAPT carrier [% (n)] | 0% (0/14) | 0% (0/9) | 0% (0/6) | 0% (0/7) |
| Topographical distribution of Aβ | ||||
| A1/A2/A3 | 8/6/0 | 4/5/0 | 5/1/0 | 0/0/8 |
| Volume fraction of Aβ | ||||
| Frontal cortex | 2.6 ± 4.0∗ | 1.4 ± 1.2∗ | 1.5 ± 2.0∗ | 16.9 ± 10.0 |
| Temporal cortex | 3.5 ± 5.8∗ | 3.4 ± 3.4∗ | 0.6 ± 0.7∗ | 14.3 ± 6.7 |
| Frontotemporal cortices | 2.8 ± 4.2∗ | 2.2 ± 2.0∗ | 1.0 ± 1.3∗ | 15.6 ± 8.1 |
Abbreviations: bvFTD, behavioral variant frontotemporal dementia; PPA, primary progressive aphasia.
P < .005 compared to AD.
Fig. 5.
Volume fraction of cortical Aβ in frontotemporal dementia (FTD), Alzheimer's disease (AD), and controls ≤75 years at death. Graph demonstrating the mean (±standard error) volume fraction of histological Aβ in the frontal and temporal cortices of behavioral variant frontotemporal dementia (bvFTD), primary progressive aphasia (PPA), controls, and AD (A). The Aβ observed in the frontal cortex of two sporadic FTD cases (B, C); an FTD case with a C9ORF72 expansion (D); and an AD case (E). ***P < .001.
4. Discussion
Although a pathologic diagnosis of AD accounts for ∼25% of patients with an FTD syndrome [1], the pathologic assessment of Aβ in the remaining ∼75% of patients with FTD, particularly, in patients that have also undergone in vivo PiB-PET imaging is scarce. The present study assesses a large pathologic series of FTD syndromes without AD and reports Aβ deposition in 38% (21/56) in patients with bvFTD, 37% (14/38) in patients with PPA, and 57% (8/14) in age-matched controls. The presence and topographical progression of Aβ increased with age in FTD, as observed in controls [23], [24]. In particular, our results are consistent with recent results showing that the transition to amyloidosis in the population without substantive neurodegeneration is greatest between 60 and 75 years of age [25]. In patients with clinical FTD and antemortem PiB-PET imaging, we confirm pathologic Aβ deposition in all patients with a high PiB retention, and the absence of pathologic Aβ deposition in 91% (10/11) of cases with a low PiB retention. Importantly, we demonstrate a strong regional correlation between the volume fraction of histological Aβ with PiB SUVRs scaled to the white matter, but not to the cerebellum and pons. Almost all patients with FTD undergo PiB-PET imaging before the age of 75 years, and we demonstrate here that in these patients that do not have a pathologic diagnosis of AD, a significantly lower volume fraction of histological Aβ is found compared with the patients that have a pathologic diagnosis of AD. Together, this study corroborates the accuracy of PiB-PET imaging in clinical FTD.
Although it is well established that PiB-PET imaging enables the in vivo detection of Aβ burden in individuals, it is less clear as to whether PiB-PET imaging selectively binds to fibrillar Aβ in neuritic plaques only, or if it also binds to fibrillar Aβ in diffuse plaques [5], [6], [26], [27], [28]. Importantly, this contention underscores the lack of pathologic data on Aβ in FTD. By assessing a large pathologic series of 108 autopsied cases without a pathologic diagnosis of AD, the present study demonstrates that Aβ deposition occurs in ∼37% (35/94) of FTD and 57% (8/14) of age-matched controls early within their 7th decade of life, suggesting incidental Aβ plaques in FTD. Despite the younger age at death in patients with a GRN mutation compared to those without, both groups demonstrated a similar proportion of patients with Aβ. This converges with emerging evidence of GRN as a risk factor for AD phenotypes and neuropathology [29] to suggest that a GRN mutation may predispose toward Aβ deposition, particularly with studies in animal models having shown that GRN haploinsufficiency impairs its inhibitory effect on Aβ deposition [30]. In contrast to this, despite the similar age at disease onset and death, Aβ deposition was not observed in patients with a MAPT mutation and was only identified in 20% (2/10) of patients with a C9ORF72 expansion. Future studies in larger cohorts of mutation carriers will shed further light on whether these FTD gene mutations protect against or predispose toward Aβ deposition. As expected, patients with and without MND or extrapyramidal syndromes demonstrated a similar proportion with Aβ deposition.
Amyloidosis represents one of the earliest changes in patients that go on to have intermediate or high levels of AD neuropathology and precedes cognitive impairment by over 15 years [20], [31], [32]. With the use of in vivo markers of Aβ deposition, it has been proposed that a diagnosis of preclinical AD can be made even in the absence of cognitive impairment [33], [34]. Importantly, however, although a PiB retention indicative of AD pathology has been reported in patients with clinical FTD [35], pathologic assessments at autopsy are scarce. In the present series of patients with clinical FTD and high PiB retention (SUVRCb > 1.5) [20], we found significant Aβ deposition in 100% (4/4) cases, where 75% (3/4) also met neuropathologic criteria for a diagnosis of AD. Importantly, the single case (25%,1/4) who did not meet neuropathologic criteria for a diagnosis of AD [7], despite all of them fulfilling current clinical research criteria for atypical AD [3], represents the rare patient with clinical FTD assessed with PiB-PET imaging >75 years of age (Table 2). Significant Aβ deposition and pathologic AD was identified at postmortem in 9% (1/11) of FTD cases with a low PiB retention (SUVRCb < 1.5) (Table 2), either suggesting significant Aβ accumulation in the time that elapsed between PiB-PET imaging and autopsy, which seems unlikely, or, as previously reported, it most likely represents a false negative case [36]. Importantly, we identified a strong regional correlation between the volume fraction of histological Aβ and PiB SUVRs scaled to the white matter. Together with the significantly lower cortical burden of Aβ identified in FTD cases, these findings attest for the first time, the sensitivity of in vivo measures of Aβ in clinical FTD.
The main methodological issue of consideration in the present study is the sample size of the PiB-autopsy cohort. This is one of the larger pathologic evaluations performed in patients with FTD that had undergone in vivo PiB-PET imaging, and our findings support reports in patients with other neurodegenerative conditions [27], [36]. Nevertheless, future studies replicating these findings in larger cohorts of patients with pathologically confirmed FTD will be able to determine the overall sensitivity of PiB-PET imaging in distinguishing between underlying AD and the presence or absence of Aβ deposition in FTD syndromes. In comparison to in vivo measures in which an SUVR is derived from both hemispheres, autopsy assessments of Aβ are often unilateral and could only be performed in the left hemisphere in this series. As such, although we only identified a strong regional correlation with SUVR scaled to white matter, future studies in larger cohorts or sampling greater predilection sites will determine if histological Aβ correlates with SUVR scaled to the cerebellum in FTD, as seen in AD [4]. With the exception of CDR assessments, other more specific neuropsychiatric and cognitive measures were not available for all autopsy cohorts. As such, although Aβ is also found in cognitively normal individuals, its contribution to the deterioration in specific cognitive domains in FTD cannot yet be excluded. Finally, given that the present study assessed patients with pathologically confirmed FTD with no or low levels of AD neuropathology, only the milder end of the amyloidosis spectrum may have been represented here and future clinicopathologic studies including cases with AD neuropathology will be needed to determine the clinical progression and ramifications of increasing Aβ deposition in FTD.
In summary, the present study has assessed for the first time, a large pathologically confirmed series of FTD cases, providing a pathologic reference for the proportion of patients with Aβ and the burden of Aβ in these patients, which may aid the interpretation of future in vivo assessments of Aβ in FTD syndromes. We confirm for the first time, a strong correlation between in vivo and postmortem measures of Aβ in FTD. Future pathologic assessments in larger cohorts of patients with FTD that have undergone PiB-PET imaging, and have been followed longitudinally to autopsy, will enable further refinement of current thresholds for in vivo markers of Aβ in FTD syndromes and represent an important avenue for future research.
Research in Context.
-
1.
Systematic review: The authors reviewed the literature using PubMed. There have been several studies assessing amyloid β (Aβ) using amyloid imaging in patients with frontotemporal dementia (FTD), but pathologic assessments of Aβ in large autopsy-confirmed FTD cohorts are lacking. All relevant citations are appropriately cited.
-
2.
Interpretation: Our findings demonstrate a strong correlation between in vivo and postmortem measures of Aβ in FTD and provide a pathologic reference that will assist in the interpretation of future in vivo assessments of Aβ in FTD syndromes.
-
3.
Future direction: Future clinicopathologic studies in larger cohorts will be needed to determine the clinical progression and ramifications of increasing Aβ deposition in FTD.
Acknowledgments
The authors wish to thank the participating patients and families for their generous donation and support of our research. Tissues were received from the Sydney Brain Bank, the New South Wales Tissue Resource Centre, and the Cambridge Brain Bank. The SBB is supported by the National Health and Medical Research Council of Australia (NHMRC), University of New South Wales, and Neuroscience Research Australia; and the Cambridge Brain Bank is supported by the NIHR Cambridge Biomedical Research Centre. The authors thank Heidi Cartwright for her assistance with preparation of figures, Marianne Hallupp for APOE genotyping, and the Sydney Brain Bank for their assistance with this study.
This work was supported by the Mason Foundation National Medical Program, as well as by funding to ForeFront, a collaborative research group dedicated to the study of FTD and motor neuron disease, from the NHMRC program grant (#1037746) and the Australian Research Council Centre of Excellence in Cognition and its Disorders Memory Node (#CE110001021); R.H.T. is supported by an NHMRC-ARC Dementia Research Development Fellowship (#1110369), C.E.L. is supported by an NHMRC-ARC Dementia Research Development Fellowship (#1102969), V.L.V. is supported by NHMRC Research Fellowship (#1046471), and G.M.H. is a NHMRC Senior Principal Research Fellow (#1079679).
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.dadm.2017.05.005.
Supplementary data
Supplementary Figure 1.
References
- 1.Chare L., Hodges J.R., Leyton C.E., McGinley C., Tan R.H., Kril J.J. New criteria for frontotemporal dementia syndromes: clinical and pathological diagnostic implications. J Neurol Neurosurg Psychiatry. 2014;85:865–870. doi: 10.1136/jnnp-2013-306948. [DOI] [PubMed] [Google Scholar]
- 2.Shelley B.P., Hodges J.R., Kipps C.M., Xuereb J.H., Bak T.H. Is the Pathology of Corticobasal Syndrome Predictable in Life? Movement Disord. 2009;24:1593–1599. doi: 10.1002/mds.22558. [DOI] [PubMed] [Google Scholar]
- 3.Dubois B., Feldman H.H., Jacova C., Hampel H., Molinuevo J.L., Blennow K. Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria. Lancet Neurol. 2014;13:614–629. doi: 10.1016/S1474-4422(14)70090-0. [DOI] [PubMed] [Google Scholar]
- 4.Ikonomovic M.D., Klunk W.E., Abrahamson E.E., Mathis C.A., Price J.C., Tsopelas N.D. Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer's disease. Brain. 2008;131:1630–1645. doi: 10.1093/brain/awn016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lockhart A., Lamb J.R., Osredkar T., Sue L.I., Joyce J.N., Ye L. PIB is a non-specific imaging marker of amyloid-beta (A beta) peptide-related cerebral amyloidosis. Brain. 2007;130:2607–2615. doi: 10.1093/brain/awm191. [DOI] [PubMed] [Google Scholar]
- 6.Niedowicz D.M., Beckett T.L., Matveev S., Weidner A.M., Baig I., Kryscio R.J. Pittsburgh compound B and the postmortem diagnosis of Alzheimer disease. Ann Neurol. 2012;72:564–570. doi: 10.1002/ana.23633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montine T.J., Phelps C.H., Beach T.G., Bigio E.H., Cairns N.J., Dickson D.W. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta neuropathologica. 2012;123:1–11. doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hyman B.T., Phelps C.H., Beach T.G., Bigio E.H., Cairns N.J., Carrillo M.C. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease. Alzheimers Dement. 2012;8:1–13. doi: 10.1016/j.jalz.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mirra S.S., Heyman A., McKeel D., Sumi S.M., Crain B.J., Brownlee L.M. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 10.Rascovsky K., Hodges J.R., Knopman D., Mendez M.F., Kramer J.H., Neuhaus J. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brooks B.R., Miller R.G., Swash M., Munsat T.L. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Mot Neuron Disord. 2000;1:293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- 12.Mackenzie I.R., Neumann M., Baborie A., Sampathu D.M., Du Plessis D., Jaros E. A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol. 2011;122:111–113. doi: 10.1007/s00401-011-0845-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmed Z., Bigio E.H., Budka H., Dickson D.W., Ferrer I., Ghetti B. Globular glial tauopathies (GGT): consensus recommendations. Acta Neuropathol. 2013;126:537–544. doi: 10.1007/s00401-013-1171-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dickson D.W. Neuropathology of Pick's disease. Neurology. 2001;56:S16–S20. doi: 10.1212/wnl.56.suppl_4.s16. [DOI] [PubMed] [Google Scholar]
- 15.Dickson D.W., Bergeron C., Chin S.S., Duyckaerts C., Horoupian D., Ikeda K. Office of Rare Diseases neuropathologic criteria for corticobasal degeneration. J Neuropathol Exp Neurol. 2002;61:935–946. doi: 10.1093/jnen/61.11.935. [DOI] [PubMed] [Google Scholar]
- 16.Hauw J.J., Daniel S.E., Dickson D., Horoupian D.S., Jellinger K., Lantos P.L. Preliminary NINDS neuropathologic criteria for Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy) Neurology. 1994;44:2015–2019. doi: 10.1212/wnl.44.11.2015. [DOI] [PubMed] [Google Scholar]
- 17.Tan R.H., Kril J.J., Fatima M., McGeachie A., McCann H., Shepherd C. TDP-43 proteinopathies: pathological identification of brain regions differentiating clinical phenotypes. Brain. 2015;138:3110–3122. doi: 10.1093/brain/awv220. [DOI] [PubMed] [Google Scholar]
- 18.Leyton C.E., Villemagne V.L., Savage S., Pike K.E., Ballard K.J., Piguet O. Subtypes of progressive aphasia: application of the International Consensus Criteria and validation using beta-amyloid imaging. Brain. 2011;134:3030–3043. doi: 10.1093/brain/awr216. [DOI] [PubMed] [Google Scholar]
- 19.Rowe C.C., Ellis K.A., Rimajova M., Bourgeat P., Pike K.E., Jones G. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging. 2010;31:1275–1283. doi: 10.1016/j.neurobiolaging.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Villemagne V.L., Burnham S., Bourgeat P., Brown B., Ellis K.A., Salvado O. Amyloid beta deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer's disease: a prospective cohort study. Lancet Neurol. 2013;12:357–367. doi: 10.1016/S1474-4422(13)70044-9. [DOI] [PubMed] [Google Scholar]
- 21.Thal D.R., Rub U., Orantes M., Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–1800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- 22.Tan R.H., Wong S., Kril J.J., Piguet O., Hornberger M., Hodges J.R. Beyond the temporal pole: limbic memory circuit in the semantic variant of primary progressive aphasia. Brain. 2014;137:2065–2076. doi: 10.1093/brain/awu118. [DOI] [PubMed] [Google Scholar]
- 23.Glodzik L., Rusinek H., Kamer A., Pirraglia E., Tsui W., Mosconi L. Effects of vascular risk factors, statins, and antihypertensive drugs on PiB deposition in cognitively normal subjects. Alzheimers Dement (Amst) 2016;2:95–104. doi: 10.1016/j.dadm.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jagust W. Is amyloid-beta harmful to the brain? Insights from human imaging studies. Brain. 2016;139:23–30. doi: 10.1093/brain/awv326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jack C.R., Therneau T.M., Wiste H.J., Weigand S.D., Knopman D.S., Lowe V.J. Transition rates between amyloid and neurodegeneration biomarker states and to dementia: a population-based, longitudinal cohort study. Lancet Neurol. 2016;15:56–64. doi: 10.1016/S1474-4422(15)00323-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Driscoll I., Troncoso J.C., Rudow G., Sojkova J., Pletnikova O., Zhou Y. Correspondence between in vivo C-11-PiB-PET amyloid imaging and postmortem, region-matched assessment of plaques. Acta Neuropathol. 2012;124:823–831. doi: 10.1007/s00401-012-1025-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murray M.E., Lowe V.J., Graff-Radford N.R., Liesinger A.M., Cannon A., Przybelski S.A. Clinicopathologic and 11C-Pittsburgh compound B implications of Thal amyloid phase across the Alzheimer's disease spectrum. Brain. 2015;138:1370–1381. doi: 10.1093/brain/awv050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sojkova J., Driscoll I., Iacono D., Zhou Y., Codispoti K.E., Kraut M.A. In vivo fibrillar beta-amyloid detected using [C-11]PiB positron emission tomography and neuropathologic assessment in older adults. Arch Neurol-chicago. 2011;68:232–240. doi: 10.1001/archneurol.2010.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perry D.C., Lehmann M., Yokoyama J.S., Karydas A., Lee J.J., Coppola G. Progranulin mutations as risk factors for Alzheimer disease. JAMA Neurol. 2013;70:774–778. doi: 10.1001/2013.jamaneurol.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Minami S.S., Min S.W., Krabbe G., Wang C., Zhou Y., Asgarov R. Progranulin protects against amyloid beta deposition and toxicity in Alzheimer's disease mouse models. Nat Med. 2014;20:1157–1164. doi: 10.1038/nm.3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bateman R.J., Xiong C., Benzinger T.L., Fagan A.M., Goate A., Fox N.C. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. New Engl J Med. 2012;367:795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jansen W.J., Ossenkoppele R., Knol D.L., Tijms B.M., Scheltens P., Verhey F.R.J. Prevalence of cerebral amyloid pathology in persons without dementia a meta-analysis. JAMA. 2015;313:1924–1938. doi: 10.1001/jama.2015.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jack C.R., Knopman D.S., Jagust W.J., Petersen R.C., Weiner M.W., Aisen P.S. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vos S.J.B., Xiong C.J., Visser P.J., Jasielec M.S., Hassenstab J., Grant E.A. Preclinical Alzheimer's disease and its outcome: a longitudinal cohort study. Lancet Neurol. 2013;12:957–965. doi: 10.1016/S1474-4422(13)70194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Engler H., Santillo A.F., Wang S.X., Lindau M., Savitcheva I., Nordberg A. In vivo amyloid imaging with PET in frontotemporal dementia. Eur J Nucl Med Mol Imaging. 2008;35:100–106. doi: 10.1007/s00259-007-0523-1. [DOI] [PubMed] [Google Scholar]
- 36.Villeneuve S., Rabinovici G.D., Cohn-Sheehy B.I., Madison C., Ayakta N., Ghosh P.M. Existing Pittsburgh Compound-B positron emission tomography thresholds are too high: statistical and pathological evaluation. Brain. 2015;138:2020–2033. doi: 10.1093/brain/awv112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.