Abstract
Varroa destructor is an ectoparasitic pest of honeybees, and a threat to the survival of the apiculture industry. Several studies have shown that unlike European honeybees, African honeybee populations appear to be minimally affected when attacked by this mite. However, little is known about the underlying drivers contributing to survival of African honeybee populations against the mite. We hypothesized that resistant behavioral defenses are responsible for the survival of African honeybees against the ectoparasite. We tested this hypothesis by comparing grooming and hygienic behaviors in the African savannah honeybee Apis mellifera scutellata in Kenya and A. mellifera hybrids of European origin in Florida, USA against the mite. Grooming behavior was assessed by determining adult mite infestation levels, daily mite fall per colony and percentage mite damage (as an indicator of adult grooming rate), while hygienic behavior was assessed by determining the brood removal rate after freeze killing a section of the brood. Our results identified two additional undescribed damaged mite categories along with the six previously known damage categories associated with the grooming behavior of both honeybee subspecies. Adult mite infestation level was approximately three-fold higher in A. mellifera hybrids of European origin than in A. m. scutellata, however, brood removal rate, adult grooming rate and daily natural mite fall were similar in both honeybee subspecies. Unlike A. mellifera hybrids of European origin, adult grooming rate and brood removal rate did not correlate with mite infestation levels on adult worker honeybee of A. m. scutellata though they were more aggressive towards the mites than their European counterparts. Our results provide valuable insights into the tolerance mechanisms that contribute to the survival of A. m. scutellata against the mite.
Introduction
Varroa destructor Anderson and Trueman (Acari: Varoidae) is an ectoparasitic pest of the Western honeybee, Apis mellifera L. (Hymenoptera: Apidae). It feeds on the fat body of both immature and adult honeybees while transmitting lethal pathogens [1,2] causing severe physical and physiological injuries to individual honeybees [3]. In the absence of appropriate control measures, honeybee colonies heavily infested with the mites succumb within 1–2 years [2]. Interestingly, the mite is a relatively harmless pest on its native host, the Eastern honeybee Apis cerana, found mainly in Asia [4] which has efficient defensive mechanisms including hygienic and grooming behaviors to limit the mite’s reproduction in drone brood cells only which are generally less abundant than worker brood cells in a colony and do not occur throughout the year [5–7]. The mite is an invasive pest of the Western honeybee, A. mellifera which occurs elsewhere in the world (reviewed in [8]). Unlike A. cerana, the mite reproduces successfully in both worker and drone broods of A. mellifera [2]. Additionally, the absence of certain adaptive behavioral and physiological mechanisms that are present in its original host, has made the Western honeybee highly susceptible to the mite [9]. Pathogens associated with the mite are considered responsible for the decline of managed honeybee colonies especially in Europe and North America [10–13]. As a result, beekeepers in most of the affected countries substantially depend on in-hive chemical treatments to keep mite populations below economic thresholds so as to prolong survival of the colonies [2,10,14].
Previous studies carried out in Asia, Europe, South and North America have shown that Apis cerana and some populations of Apis mellifera have developed specific adaptive behaviors that enable them to co-exist with Varroa mite infestations [15]. These adaptive behaviors include hygienic and grooming behaviors, entombing of mites’ infested drone brood, restriction of mite’s reproduction in drone broods, suppression of the mite’s reproductive success, shorter post capping time and less attractive brood for mites.
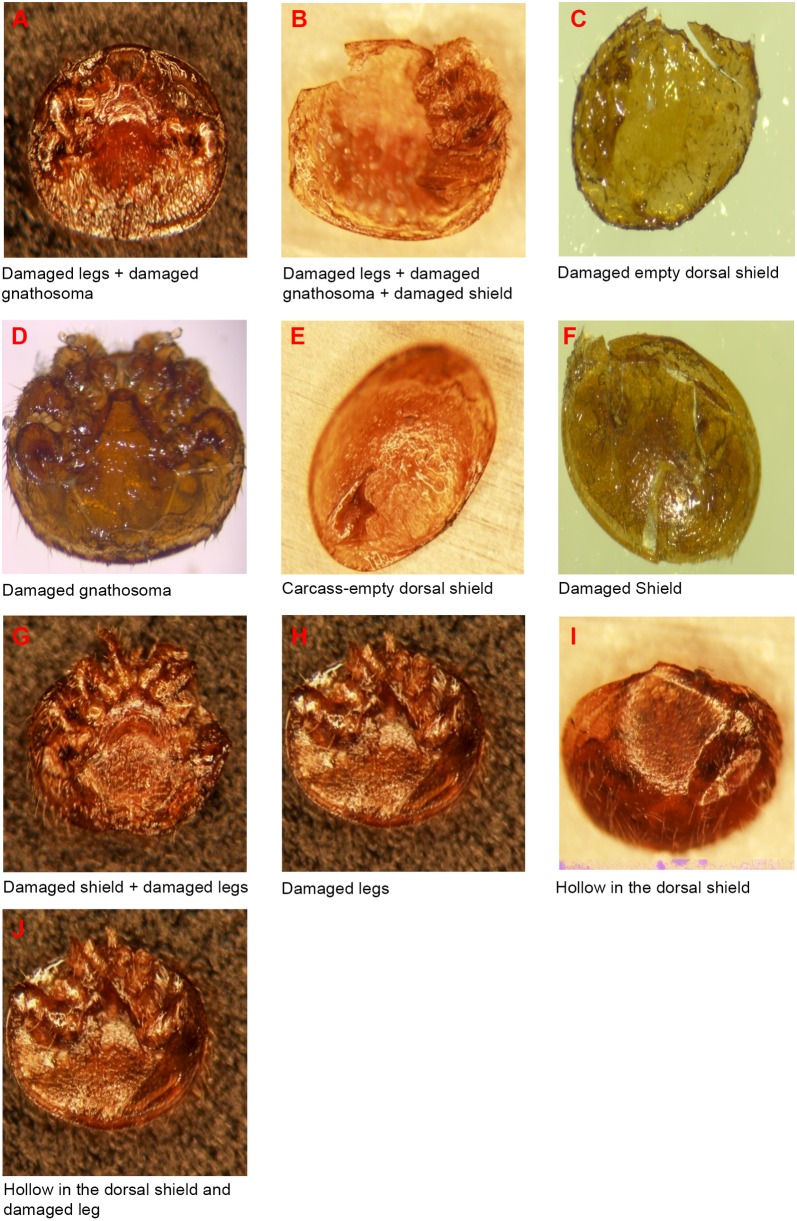
In grooming behavior studies, an estimate of the percentage of damage inflicted on mites by honeybees is used as a measure of the bee’s grooming behavior [16]. This estimation can also be inferred from a damage classification scheme developed by Corrêa-Marques et al.,[17] comprising six different categories: a) damaged legs, b) hollow in the dorsal shield, c) carcass-empty dorsal shield, d) damage shield + damaged legs, e) hollow in the dorsal shield + damaged legs, and f) damaged shield. On the other hand, hygienic behavior is measured as the rate at which nurse bees remove dead or diseased brood [18].
Studies have shown that African honeybee populations survive mite infestation without requiring any managerial inputs by beekeepers. For example, field studies by various researchers demonstrated that survival of the South African Cape honeybee A. m. capensis against Varroa mite was linked to short post-capping stage, hygienic and grooming behaviors of this honeybee subspecies [19–21]. Likewise, survival of the savannah honeybee subspecies A. m. scutellata against the mite was found to be associated with reduced population growth, low viral prevalence, short post-capping stage, low fertility, fecundity and reproductive success of Varroa mite foundresses [19,22–24]. Interestingly, the East African population of A. m. scutellata has also been reported to survive Varroa mite parasitism, requiring no chemical treatment even when coexisting with other pathogens responsible for the losses of colonies in Europe and North America [25,26]. However, it is unknown whether survival of this specific African savannah honeybee population is associated with tolerance (the ability to limit the detrimental effects of the mite) or resistance (the ability to reduce the reproductive fitness of the mite) as part of its behavioral defense mechanisms or both [27]. To test the hypothesis that resistant defense mechanisms confer coping and survival strategies in this specific population of Apis mellifera scutellata, we compared the grooming and hygienic behaviors in this honeybee subspecies with those of A. mellifera hybrids of European origin found in the USA against the mite.
Materials and methods
Study sites
The study was conducted in Nairobi, Kenya from August—September 2015 (the cooler- dry season) and in Gainesville, Florida, United States of America in April 2016 (spring). These periods are characterized by reduced brood rearing in both savannah and European honeybees [28,29]. All the colonies were housed in standard Langstroth hives containing 3 to 4 brood combs and were not treated with acaricides to reduce mite infestations.
In Kenya, seventeen (17) queen right colonies were selected at two sites namely Kithimani (1°8' S, 37°25 E) (N = 10) and Kilimanbogo (1°8' S, 37°21' E) (N = 7) both located within the county of Machakos. These two apiaries were 7.4 Km apart and, contained colonies that originated from locally captured swarms. The colonies in this neighborhood host A. m. scutellata [26,29,30]. In Gainesville, Florida, USA, twenty colonies (20) were selected, with ten (10) each at the University of Florida apiary (29.62°38'N, 82.35°21'W) and USDA- ARS-CMAVE apiary (29.63°38'N, 82.36°21'W). These apiaries were ~ 1.6 Km apart and were bred from honeybee stocks purchased from local commercial queen breeders. The honeybee colonies in the USA were hybrids of different European subspecies (Ellis, personal communication).
Molecular identification of Varroa mite strains
To confirm the strain of Varroa mites present in the savannah and hybrids of European honeybee colonies, two honeybee colonies were randomly selected among the colonies used at the individual apiaries in Kenya and the USA. Five living mites per colony (N = 5) were collected from adult worker honeybees on the brood area of the comb using the standard sugar-roll method [31]and preserved in 95% ethanol for DNA analysis at the USDA-ARS-CMAVE in Gainesville, Florida, USA. In total, eight mites were analyzed, that is, two mites per single colony in each apiary using the methods detailed below. Genomic DNA was extracted from individual mites using the DNeasy Tissue Kit (Qiagen, USA) per the manufacturer’s protocols for the spin-column protocol for Cultured Animal Cells with the following slight modifications: (i) all volumes were reduced to half; (ii) incubation was at 70°C for 1 hour; (iii) final elution was in 50 μL of Buffer AE. Nucleic acid concentrations were measured in each sample and three fragments from the cytochrome oxidase I (cox1), cytochrome oxidase III (cox3) and ATP synthase 6 (atp6) mitochondrial genes were amplified by polymerase chain reaction (PCR). The primers of these selected mitochondrial genes were purchased from Integrated DNA Technologies, Coralville, Iowa, USA [32]. The amplified gene fragment, primer name, primer sequences, product size base pairs (bp) and the annealing temperature for each fragment are presented in S1 Table. Reactions were carried out in 50 μl reactions containing 1X buffer, 0.05 U Taq polymerase (Invitrogen), 0.2 mM dNTPs, 0.4 μM of each oligonucleotide primer, 1.5 mM of MgCl2 and 1 μl of sample DNA. The positive control was gDNA from Varroa destructor samples identified at the study sites in Gainesville, Florida, USA. Cycling conditions involved initial denaturation at 94°C for 4 minutes, followed by 35 cycles of denaturation at 94°C for 30 seconds and annealing for 30 seconds and extension at 72°C for 1 minute. The amplicons were analyzed by gel electrophoreses on a 1.5% agarose gel run for 2 hours at 90 volts. PCR products were cleaned-up using the DNA Clean and Concentrator™-5 kit (Zymo Research) and bi-directionally sequenced by Macrogen (Maryland, USA). Sequences were edited with BioEdit Version 7.2.5.0 software [33]. Sequences obtained from individual mites were compared with those at National Center for Biotechnology Information (NCBI) using the online tool BLASTn to identify the Varroa mite strain. Species-level identification was determined when sequences exhibited ≥ 99% identity.
Assessment of grooming behavior in honeybees of African and hybrids of European origin
Prior to the grooming behavior experiments, the level of infestations with Varroa mites on approximately hundred (100) adult worker honeybees in each colony was determined using the standard sugar-roll method [31]. The percentage of Varroa mite infestation rates in adult honeybees was determined by taking the number of Varroa mite collected divided by 100 adult worker honeybee and then multiplied by 100 [21,22].
Grooming behavior was assessed in the selected colonies in Kenya and USA using the screen bottom board method. Prior to the beginning of the study, the original bottom board of each colony was replaced with a modified bottom equipped with a retractable floor and covered with a screen mesh fine enough to permit only the passage of mites through its openings, thereby restricting the honeybees to further inflict damages on fallen mites. Cardboard white paper coated with sticky non-toxic petroleum jelly (Vaseline®) was smeared on the retractable floor to intercept falling mites and to protect them from being further damaged by predators such as ants, the small hive beetle and wax moth larvae. Natural fallen mites were collected every 24 hours from the debris on the bottom board using a fine Camel hair brush for a duration of 7 days and examined for injuries under a Leica S6E stereo microscope (×40 Magnification). The damaged mites were further grouped into different damage categories using the classification of mite’s damages [17]. The percentage of damaged mite in each colony was determined by dividing the number of damaged mites by the total number of dropped mites collected at the end of the collection period. The average daily natural fallen mite/per colony was determined by dividing the total number of natural fallen mites by the number of days mites were collected [22].
Assessment of the source of physical damage on fallen mites in A. m. scutellata colonies
We investigated whether the recorded mite damages on the screen bottom boards of colonies were due to honeybee’s grooming behavior or other agents such as ants, small hive beetle or wax moth larvae. Varroa mites were collected from the savannah honeybee colonies using the standard sugar-roll method [31] from a subset of colonies at the Kithimani’s apiary in Kenya and freeze-killed at—80°C for 30 minutes. They were subsequently observed under a dissecting microscope to ensure that none was damaged before the beginning of the experiment. The dead, undamaged mites were marked on the dorsal shield with two permanent markers of different colors, blue and black. Three colonies (N = 3) were used for this experiment and grease oil was spread on the wooden platforms to restrict ants present from accessing the hives. In each colony, ten (10) black, marked mites were introduced on a white, glossy cardboard coated with sticky non-toxic petroleum jelly (Vaseline®) (to protect fallen mites from being further damaged by predators such as ants and wax moth larvae); and twenty (20) blue, marked mites were introduced in the brood area of one frame. Fallen mites were collected after 24 hours from the debris on the bottom board using a fine Camel hair brush and examined for injuries under a Leica S6E stereo microscope (×40 Magnification). The experiment was repeated three times.
Assessment of hygienic behavior in honeybees of African and hybrids of European origin
Hygienic behavior was assessed in the selected colonies (N = 17) at each apiary in Kenya and in nine colonies (N = 9) at each apiary in the USA using the standard freeze-killed brood assay method with liquid nitrogen to freeze-kill young pupae (white- to purple-eyed stage with no cuticular tanning) as described by [34]. The number of fully removed freeze-killed brood cells from the test patch was recorded after a period of 24 and 48 hours and expressed as the percentage of the total brood containing cells at the start of the experiment.
Ethical considerations
For field study in Kenya, written informed consents were obtained from the apiary owners. In the United States of America, we used apiaries managed by the USDA/ARS-Centre for Medical, Agricultural and Veterinary Entomology, Gainesville and the University of Florida.
Statistical analyses
Statistical analyses were performed using R-Software version 3.2.5 [35]. In Kenya, a total of six colonies absconded during the experimental period including five from Kithimani and one from Kilimanbogo apiaries respectively. Consequently, these colonies could not be monitored for the entire duration of the experiment. In the USA, none of the colonies absconded during the entire monitoring period. Data from Kithimani and Kilimanbogo apiaries were pooled to obtain average total mite dropped, percentage of damaged mites, Varroa mite-infestation per 100 adult worker honeybees and the proportion of removed freeze-killed brood at 24 and 48 hours in the African savannah honeybee colonies. Likewise, data from the USDA-CMAVE and experimental farm of the University of Florida apiaries were pooled to obtain similar information in the colonies of honeybee hybrids of European origin. The count data were analyzed using generalized linear model (GLM) with log link and binomial distribution error to compare the factors: total number of fallen mites, Varroa mite-infestation level and the daily mites fall between both honeybee subspecies. Meanwhile, the proportion data were analyzed using generalized linear model (GLM) with logit link and binomial distribution error to compare the factors: percentages of damaged mites, different types of damages and freeze-killed brood removed at 24 and 48 hours between both honeybee subspecies. The effect of a factor for a GLM is reflected in the deviance (likelihood ratio test statistic) that has an appropriate chi-square distribution; hence the chi-square values are presented as test statistics. The Mann-Whitney-Wilcoxon test was used to compare the ratio of total natural fallen mite/Varroa mite-infestation level on adult worker honeybee between both honeybee subspecies. Spearman’s rank order correlation analysis was conducted to establish the existence of a relationship between the percentage of mite damage (overall and categorical damage types), total natural fallen mite, daily natural fallen mites/colony and brood removal (after 24 and 48 hours) to Varroa mite-infestation level on adult worker honeybee in each study site.
Results
Molecular identification of Varroa mite strains
Varroa destructor was the only Varroa mite species detected in the colonies of A. m. scutellata and A. mellifera hybrids of European origin and all the haplotypes belonged to the Korean strain (K1 haplotype).
Assessment of grooming behavior in honeybees of African and hybrids of European origin
An infestation rate of 5 ± 1.4 mites/100 adult worker was recorded in the surviving African savannah honeybees which was significantly lower (~three-fold less) than the infestation rate in the susceptible hybrids of European origin honeybees at 14 ± 2.3 mites/ 100 adult worker (df = 32: F = 10.90; P = 0.001 < 0.05, Table 1).
Table 1. Mean ± standard error of mite infestation rates, daily mite fall and percentage of damaged mites on adult honeybee workers in colonies of A. m. scutellata and A. mellifera hybrids of European origin.
| Mean ± SE | |||||
|---|---|---|---|---|---|
| Sites | Honeybee species | Number of colonies | Mite infestation rate/100 adult worker bees (3 replicates/colony) | Daily mite fall/colony | % damaged mites |
| Kenya | Apis mellifera scutellata | 14 | 5.0 ± 1.4 | 18 .1 ± 2.8 | 21.3 ± 1.7 |
| USA | Apis mellifera hybrids of European origin | 20 | 14 ± 2.3 | 15.8 ± 3.9 | 21.3 ± 2.4 |
| P-Valuea | 0.001 | 0.60 | 0.84 | ||
a p values were calculated using the generalized linear model (GLM) with log or logit links
A total of 126.6 ± 3.2 natural fallen mites/colony was collected from the bottom boards of the African savannah honeybee (Kithimani; N = 8 colonies; Kilimanbogo; N = 6 colonies) compared to 110.6 ± 4.2 natural fallen mites/colony collected from the bottom boards of the hybrids European origin honeybees (USDA and University of Florida apiaries-Gainesville: N = 10 colonies in each), which were not significantly different (df = 236: F = 1.97; P = 0.16 > 0.05). Similarly, the daily natural mite fall/colony (df = 32: F = 0.28; P = 0.60 > 0.05) and the percentages of damaged mites (df = 236: F = 0.04; P = 0.84> 0.05) recorded in both honeybee subspecies colonies were not significantly different (Table 1). The ratio of total natural mite fall/mite infestation level was significantly higher in the African savannah honeybee colonies than those recorded in the hybrids of European origin honeybee colonies (W = 52, P = 0.002 < 0.05). There was no significant correlation between the daily natural mite fall/colony (Spearman’s rank correlation: r = -0.17, P = 0.55 > 0.05; Fig 1), total natural mite fall (Spearman’s rank correlation: r = -0.17, P = 0.57 > 0.05; Fig 1) and mite infestation level/colony in the African savannah honeybee colonies. In contrast, a significant and positive correlation was detected between the daily natural mite fall/colony (Spearman’s rank correlation: r = 0.48, P = 0.03 < 0.05; Fig 1), total natural mite fall (Spearman’s rank correlation: r = 0.47, P = 0.04 < 0.05; Fig 1) and mite infestation level/colony in the hybrids of European origin honeybee colonies. Also, there was no significant correlation between the percentage of damaged mites or the percentage of the different types of damages and the Varroa-infestation levels in the African savannah honeybees (Fig 1). A similar result was obtained in the hybrids of European origin honeybees with the exception that there was a significant negative correlation between damage to the mite’s dorsal shield or idiosoma and the adult worker honeybee mite infestation rates (Spearman’s rank correlation: r = -0.46, P = 0.04 < 0.05; Fig 1).
Fig 1. Correlation between daily natural mite fall, total natural mite fall, percentage damaged mites, different categories of damage to the mites and Varroa-mite infestation level per colony in honeybees of African and European origin in Kenya and USA respectively.
Different categories of damages to the mite were recorded in this study (Fig 2) including two additional previously undescribed damage categories to the mite namely; damaged empty dorsal shield and damaged legs + damaged gnathosoma + damaged shield (Fig 2B and 2C). These additional damage categories were present in colonies of both African savannah and hybrids of European origin honeybees (Table 2). Damaged leg (total or partial loss of one or more legs) was the predominant type of physical injury to the mite recorded in the hybrids of European origin honeybee colonies and this was significantly different from those found in the African savannah honeybee colonies (df = 236: F = 9.23; P = 0.003 < 0.05, Table 2). In the African savannah honeybee colonies, damaged legs + damaged gnathosoma was the predominant type of mite injury found and this was significantly different from those found in colonies of European hybrids (df = 236: F = 5.14; P = 0.02 < 0.05, Table 2).
Fig 2. Photographs showing the different damage patterns in mature female Varroa destructor mite (×40 Magnification).
(A and D) Damaged categories from literature [36–38]. (B and C) Additional damage categories reported in this study. (E-J) Previously known classification of damage to the mites reported by Corrêa-Marques et al., [17].
Table 2. Percentages (mean ± SE) for the different categories of damages to Varroa destructor recorded in the colony debris of A. m. scutellata and A. mellifera hybrids of European origin in Kenya and USA respectively.
| Category of damage | Kenya (%) | USA (%) | P- Valuea |
|---|---|---|---|
| Damaged legs (DL) = total or partial loss of one or more legs | 5.7 ± 0.6 | 10 ± 1.4 | 0.003 |
| Hollow in the dorsal shield (HDS) = Depression in the dorsal shield | 0.5 ± 0.2 | 2.3 ± 0.7 | 0.01 |
| Empty dorsal shield (EDS)-carcass = mites that lacked all legs and all or almost all of the ventral shields, generally only the dorsal shield remained | 0.6 ± 0.2 | 0.1 ± 0.7 | 0.01 |
| Damaged shields (DS) = loss of dorsal shields, fissures in and loss pieces of the dorsal shield | 0.6 ± 0.2 | 0.3 ± 0.2 | 0.001 |
| Damaged shield + damaged legs (DS + DL) | 0.2 ± 0.1 | 0.4 ± 0.2 | 0.46 |
| Hollow in the dorsal shield + damaged legs (HDS + DL) | 0.1 ± 0.1 | 0.4 ± 0.2 | 0.08 |
| Damaged gnathosoma (DG) = loss of chelicerae and/or pedipalps | 0.3 ± 0.1 | 0.1 ± 0.1 | 0.001 |
| Damaged empty dorsal shield (DEDS) # = fissures in and loss of pieces of empty dorsal shield | 0.1 ± 0.1 | 0.01 ± 0.01 | 0.44 |
| Damaged legs + damaged gnathosoma (DL + DG) # | 9.5 ± 0.7 | 6.1 ± 1.2 | 0.02 |
| Damaged legs + damaged gnathosoma + damaged shield (DL + DG + DS) # | 3.7 ± 0.4 | 1.6 ± 0.7 | 1.9e-10 |
#New damage categories observed in this study
a p values were calculated using the generalized linear model (GLM) with logit links
Assessment of the source of physical damage on fallen mites in A. m. scutellata colonies
Out of the 90 marked, undamaged, dead mites introduced, four blue (6.7%) and four black (13.3%) marked mites were damaged, representing only 8.9% damaged mites of the overall mites introduced. The damages inflicted on the blue marked mites were likely caused by worker honeybees, while damages inflicted on the black marked mites may have been caused by other agents (e.g. wax moth larvae, small hive beetle adults and ants) found on the white, glossy cardboard fitted on the bottom board of colonies. We recorded only two types of damages to the mites in this experiment namely: damaged legs and damaged legs + damaged gnathosoma.
Assessment of hygienic behavior in honeybees of African and hybrids of European origin
Brood removal rates at 24 and 48 hours were not significantly different between colonies of the African savannah (24 hours = 66.5 ± 8.3% and 48 hours = 81.0 ± 6.2%, mean ± SE) and the hybrids of European origin honeybees (24 hours = 59.1 ± 4.9% and 48 hours = 77.0 ± 3.9%, mean ± SE) (24 hours: F = 0.65, df = 27, P = 0.43 > 0.05; 48 hours: df = 27, F = 0.42, P = 0.52 > 0.05). There was a significant positive correlation between the mite infestation level of adult bees and the brood removal rate at 48 hours (Spearman’s rank correlation: r = 0.48, P = 0.04 < 0.05) though no correlation (Spearman’s rank correlation: r = 0.37, P = 0.13 > 0.05) was detected at 24 hours in the hybrids of European origin. In the African savannah honeybee colonies, there was no correlation between the mite infestation level of adult bees and the brood removal rate at 24 hours (Spearman’s rank correlation: r = -0.43, P = 0.19 > 0.05) and 48 hours (Spearman’s rank correlation: r = -0.30, P = 0.38 > 0.05).
Discussion
Grooming behavior
Our study suggests that European and African honeybees express similar grooming behavior since the percentage of damaged mites recorded on the bottom boards of both subspecies were similar. However, the phoretic mite numbers in both honeybee populations were different, approximately three-fold more in the European than in the African honeybee colonies. With more phoretic mites, one would expect to find more fallen and damaged mites on the bottom board; however, these values were not significantly different between both honeybee subspecies (Table 1). The absence of a significant correlation between the total natural mite fall, the percentage of damaged mites, the different categories of damage to the mite and Varroa mite-infestation levels suggests that grooming behavior may not explain the variability in Varroa mite-infestation levels recorded in the African and European honeybees. Moreover, these measures of grooming behavior (percentage of damaged mites or different categories of damage to mite) might not be sensitive enough to assess grooming behavior at the colony level as previously thought [17,37]. It is important to note that of the total mite population recorded on the bottom boards of honeybee colonies, not all mites which are groomed off by honeybees are damaged [38]. It is likely that damaged mites may also result from hygienic removal of infested capped brood by honeybees, and interactions with other arthropods in the colony such as the small hive beetle, wax moth and/or ants [16,38,39]. The ratio of total natural mite fall/mite infestation level, which represents a fraction of the total mite removed by honeybees off their bodies relative to the total mite population present in their colonies [40], was significantly higher in the African savannah honeybee colonies than those recorded in the colonies of their European counterparts. It appears that, the African savannah honeybee which maintains lower mite colony infestations displays a more efficient grooming behavior than its European counterpart. This finding corroborates results of previous studies which showed that colonies of Varroa-resistant A. mellifera subspecies also maintain lower mite loads and record a higher percentage of injured mites than their susceptible counterparts [41,42].
To further characterize the subspecies differences in grooming behavior, we analyzed the levels and patterns of damage in fallen mites using the previously known classification of damage to mites [17]. We found that the number of mites with only damaged legs was significantly higher in the European honeybee colonies than in colonies of the African counterpart. On the other hand, the numbers of mites with damaged legs and damaged gnathosoma were significantly higher in the African honeybee colonies than the European counterpart. This category of damage was first recorded in mites found in A. m. carnica colonies in Austria [36] but not included as a separate category of damage in the previous classification of damage to mites [17]. In the present study, the category described as legs and gnathosoma damage, was the second most frequent category of damage recorded in mites found in the European honeybee colonies. Moreover, we found two additional undescribed damage categories in mites namely damaged empty dorsal shield and damaged legs + damaged gnathosoma + damaged shield in both honeybee subspecies, and occurring more frequently in the African savannah honeybee than in the European honeybee. Overall, these results suggest that a higher aggressive behavior is displayed by the African savannah honeybee than by their European counterparts towards the mite. Taken together, these results provide additional insights into the grooming behavior of different subspecies of honeybees.
Based on recommendations for the control of Varroa mite in European honeybee colonies in the USA, interestingly, we observed that the Varroa infestation levels recorded in the savannah honeybee colonies were high enough to warrant miticide treatment [43]. Surprisingly, none of the colonies of A. m. scutellata used in the present study showed any signs of collapse. Typically, beekeepers in this region encountering such populations of Varroa mite in honeybee colonies neither administer any mite control measures [26] nor is done by beekeepers elsewhere on the rest of the African continent [44]. The Varroa infestation levels recorded in A. m. scutellata colonies in Kenya was similar to those recorded in colonies of the same honeybee subspecies found in South Africa [45] and no deleterious effects caused by the mites were reported [21,22,44,45]. The suppression of the mite reproductive output which translates into lower mite’s fertility, fecundity and lower reproductive rate (production of at least one viable, mated and mature female offspring) and the lower viral prevalence within honeybees and mites have been demonstrated to explain the slow rate of mite growth in A. m. scutellata colonies and their healthy appearance in colonies in South Africa [23,24]. Hence, other factors such as suppression of the mite’s reproductive success and/or lower viral prevalence within honeybees and mites might better explain the variability in the mite infestation levels observed between both A. mellifera subspecies [46,47]and should be evaluated in future studies.
Hygienic behavior
Our study suggests a similar expression of the hygienic behavior trait in the European and African savannah honeybee since we recorded similar levels of brood removal rates in both honeybee populations. Our findings corroborate results of a previous study [47] which also found a similar expression of hygienic behavior between the Gotland mite-surviving and the local mite-susceptible honeybee populations in Sweden. Hygienic behavior appears not to explain the lower mite infestation rates observed in the savannah honeybee since we recorded no association between the brood removal rate and Varroa mite infestation levels. Our results differ from previously reported results [26] which found that colonies of A. m. scutellata which displayed higher levels of hygienic behavior had lower levels of Varroa mite infestation. These dissimilarities could be due to different climatic zones in which both studies were conducted and this might underline genotypic differences [48]. Nonetheless, hygienic behavior is known to be variable since it can be strongly influenced by environmental and in-hive factors [2]. Thus, the association between this behavior and Varroa mite loads in the savannah honeybee known to have a wide distribution range in Kenya [29] should be investigated in the future.
On the other hand, the significant positive correlation detected between mite infestation rate and brood removal at 48 hours in European honeybee colonies implies that more parasitized/ non-parasitized brood are removed under high Varroa parasitism. Our results suggest that hygienic behavior or brood removal rate is a response to the degree of diseased or parasitized brood found inside the brood cells. We expect that during spring period characterized by the early stages of brood production and lowest mite numbers in colonies [28], few mites will move inside the cells to reproduce, leading to a reduced removal of parasitized brood cells and vice-versa during mid or late summer period [28]. However, previous studies reported that European honeybee colonies bred for hygienic behavior were more efficient at removing Varroa-infested brood only under low mite parasitism and maintain lower mite loads on both adult honeybees and within worker brood cells than unselected colonies [18,49,50]. Under high parasitism (> 15% of both worker brood and adult honeybees), these colonies were found to fail to remove parasitized brood cells efficiently, requiring periodic miticide treatments to reduce their collapse [50]. As has been reported in breeding programs with Russian honeybees, hygienic and Varroa-sensitive hygienic honeybees in the USA, none of these honeybees have provided full protection for susceptible European honeybee colonies against Varroa mite infestation [3]. As such, they periodically require application of in-hive miticide to control the mite [3]. It appears that under high mite parasitism, honeybees invest significant resources into feeding their broods in order to obtain the next generation sub-optional worker honeybees than into other tasks such as grooming or hygienic behavior to remove infesting mites. Another explanation could be that, the build-up of large levels of odor cues released by parasitized broods which signal removal of diseased or parasitized brood cells in the colony might cause habituation and a reduction in receptor sensitivity to further detect odors [50–52]. Nevertheless, a long-term longitudinal study would help shed more light on the hygienic behavior in both the African savannah honeybee and their European counterparts.
Conclusions
In host-parasite interactions, host tolerance is defined as the ability to limit the detrimental effects of the parasite while host resistance is the ability to reduce the reproductive fitness of the parasite [27]. In the present study, we found two additional undescribed damage categories in mites which occur more frequently in the African savannah honeybee than their European counterpart. Grooming behavior was better expressed in A. m. scutellata than in A. mellifera hybrids of European origin and hence, a potential tolerant mechanism displayed by the African savannah honeybee towards V. destructor attack. However, hygienic and grooming behaviors did not significantly differ between subspecies with respect to Varroa mite-infestation levels recorded suggesting that other resistant mechanisms such as suppression of mite reproductive success and/or lower viral prevalence within honeybees and mites might play an important role in honeybee responses to mite infestation.
Supporting information
Amplified gene fragment, product size base pairs (bp) and annealing temperatures (Ta) are indicated [32].
(DOCX)
Acknowledgments
The authors are grateful to C. Nzuki, and S. Mulaeh in Kenya and Dr. James Ellis at the University of Florida, USA for availing their apiaries for this study. Many thanks to Neil Sanscrainte who provided mentorship for the molecular work at the USDA/ARS-CMAVE. We are also grateful to Muema Wilson, Kenya and Bryan Smith, USDA/ARS- CMAVE for their assistance in the field work.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
We gratefully acknowledge the financial support for this research by the following organizations and agencies: United States Department of Agriculture (USDA)/ARS- grant # 58-6615-3-011-f; UK aid from the UK government; Swedish International Development Cooperation Agency (SIDA); the Swiss Agency for Development and Cooperation (SDC); and the Kenyan government. The views expressed herein do not necessarily reflect the official opinion of the donors. Immense gratitude to the German Academic Exchange Service In-Region Scholarship for funding the PhD research work and studies of Beatrice T. Nganso at the International Centre of Insect Physiology and Ecology (ICIPE) and the Office of International Research Programs at USDA-ARS for providing the financial support needed for the research conducted in the USA.
References
- 1.Ramsey SD, vanEngelsdorp D. Varroa destructor feed primarily on honeybee fat body not haemolymph. In Simone-Finstrom M. (Ed). Proceedings of the American Bee Research Conference; 2017 Sep 13–15; Galveston Island Convention Center, Galveston TX. Bee World; 2016.
- 2.Rosenkranz P, Aumeier P, Ziegelmann B. Biology and control of Varroa destructor. J Invertebr Pathol. Elsevier Inc.; 2010;103: S96–S119. doi: 10.1016/j.jip.2009.07.016 [DOI] [PubMed] [Google Scholar]
- 3.Locke B. Host-parasite adaptations and interactions between honeybees, Varroa mites and viruses. Doctoral thesis, Swedish University of Agricultural Sciences, Uppsala. 2012.
- 4.Hepburn HR, Radloff SE. Honeybees of Asia. Springer, Berlin: 2011. [Google Scholar]
- 5.Peng YS, Fang Y, Xu S, Ge L. The resistance mechanism of the Asian honeybee, Apis cerana Fabr., to an ectoparasitic mite, Varroa jacobsoni Oudemans. J Invertebr Pathol. 1987; 49: 54–60. [Google Scholar]
- 6.Rath W. Co-adaptation of Apis cerana Fabr. and Varroa jacobsoni Oud. Apidologie. 1999;30: 97–110. [Google Scholar]
- 7.Boecking O, Spivak M. Behavioral defenses of honeybees against Varroa jacobsoni Oud. Apidologie. 1999;30: 141–158. [Google Scholar]
- 8.Nazzi F, Le Conte Y, Nazzi F, Le Conte Y. Ecology of Varroa destructor, the major ectoparasite of the Western Honeybee, Apis mellifera. Annu Rev Entomol. 2016;61: 417–432 doi: 10.1146/annurev-ento-010715-023731 [DOI] [PubMed] [Google Scholar]
- 9.Ritter W. Varroa disease of the honeybee Apis mellifera. Bee World. 1981; 141–153. [Google Scholar]
- 10.Boecking O, Genersch E. Varroosis—The ongoing crisis in beekeeping. J fur Verbraucherschutz und Leb. 2008;3: 221–228. [Google Scholar]
- 11.Le Conte Y, Ellis M, Ritter W. Varroa mites and honeybee health: can Varroa explain part of the colony losses? Apidologie. 2010;41: 353–363. [Google Scholar]
- 12.Neumann P, Carreck NL. Honeybee colony losses. J Apic Res. 2010;49: 1–6. [Google Scholar]
- 13.Francis RM, Nielsen SL, Kryger P. Varroa-virus interaction in collapsing honeybee colonies. PLoS One. 2013;8: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee KV, Moon RD, Burkness EC, Hutchison WD, Spivak M. Practical sampling plans for Varroa destructor (Acari: Varroidae) in Apis mellifera (Hymenoptera: Apidae) colonies and apiaries. J Econ Entomol. 2010;103: 1039–1050. [DOI] [PubMed] [Google Scholar]
- 15.Locke B. Natural Varroa mite-surviving Apis mellifera honeybee populations. Apidologie. 2015; 1–16. [Google Scholar]
- 16.Bienefeld K, Zautke F, Pronin D, Mazeed A. Recording the proportion of damaged Varroa jacobsoni Oud. in the debris of honey bee colonies. Apidologie. 1999;30: 249–256. [Google Scholar]
- 17.Corrêa-Marques MH, Issa MRC, De Jong D. Classification and quantification of damaged Varroa jacobsoni found in the debris of honeybee colonies as criteria for selection? Am Bee J. American Bee Journal; 2000;140: 820–824. [Google Scholar]
- 18.Spivak M, Reuter GS. Performance of hygienic honeybee colonies in a commercial apiary. Apidologie. 1998;29: 291–302. [Google Scholar]
- 19.Moritz RFA. Heritability of the postcapping stage in Apis mellifera and its relation to varroatosis resistance. J Hered. 1985;76: 267–270. [Google Scholar]
- 20.Moritz RFA, Mautz D. Development of Varroa jacobsoni in colonies of Apis mellifera capensis and Apis mellifera carnica. Apidologie. 1990;21: 53–58. [Google Scholar]
- 21.Allsopp M. Analysis of Varroa destructor infestation of southern African honeybee populations. MSc-thesis, University of Pretoria, South Africa. 2006.
- 22.Strauss U, Pirk CWW, Crewe RM, Human H, Dietemann V. Impact of Varroa destructor on honeybee (Apis mellifera scutellata) colony development in South Africa. Exp Appl Acarol. 2015;65: 89–106. doi: 10.1007/s10493-014-9842-7 [DOI] [PubMed] [Google Scholar]
- 23.Strauss U, Dietemann V, Human H, Crewe RM, Pirk CWW. Resistance rather than tolerance explains survival of savannah honeybees (Apis mellifera scutellata) to infestation by the parasitic mite Varroa destructor. Parasitology. 2016;143: 374–387. doi: 10.1017/S0031182015001754 [DOI] [PubMed] [Google Scholar]
- 24.Strauss U, Human H, Gauthier L, Crewe RM, Dietemann V, Pirk CWW. Seasonal prevalence of pathogens and parasites in the savannah honeybee (Apis mellifera scutellata). J Invertebr Pathol. 2013;114: 45–52. doi: 10.1016/j.jip.2013.05.003 [DOI] [PubMed] [Google Scholar]
- 25.Frazier M, Muli E, Conklin T, Schmehl D, Torto B, Frazier J, et al. A scientific note on Varroa destructor found in East Africa; threat or opportunity?*. Apidologie. 2010;41: 463–465. [Google Scholar]
- 26.Muli E, Patch H, Frazier M, Frazier J, Torto B, Baumgarten T, et al. Evaluation of the distribution and impacts of parasites, pathogens, and pesticides on honey bee (Apis mellifera) populations in East Africa. PLoS One. 2014;9: e94459 doi: 10.1371/journal.pone.0094459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmid-Hempel P. Evolutionary parasitology: The integrated study of infections, immunology, ecology, and genetics Oxford University Press; 2011. [Google Scholar]
- 28.Hood M. Varroa mite control in South Carolina. Entomol Insect Inf Ser. 2000;12: 1–7. [Google Scholar]
- 29.Raina SK, Kimbu DM. Variations in races of the honeybee Apis mellifera (Hymenoptera: Apidae) in Kenya. Int J Trop Insect Sci. 2005;25: 281–291. [Google Scholar]
- 30.Hepburn HR, Radloff SE. Honeybees of Africa. Springer Verlag, Berlin, Heidelberg, New York: 1988. [Google Scholar]
- 31.Dietemann V, Nazzi F, Martin SJ, Anderson DL, Locke B, Delaplane KS, et al. Standard methods for Varroa research. J Apic Res. 2013;52: 1–54. [Google Scholar]
- 32.Navajas M, Migeon A, Alaux C, Martin-Magniette ML, Robinson GE, Evans JD, et al. Differential gene expression of the honeybee Apis mellifera associated with Varroa destructor infection. BMC Genomics. 2008;9: 301 doi: 10.1186/1471-2164-9-301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41: 95–98. [Google Scholar]
- 34.Büchler R, Andonov S, Bienefeld K, Costa C, Hatjina F, Kezic N, et al. Standard methods for rearing and selection of Apis mellifera queens. J Apic Res. 2013;52: 1–30. [Google Scholar]
- 35.R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2015.
- 36.Ruttner F, Hanel H. Active defense against Varroa mites in a Carniolan strain of honeybee (Apis mellifera carnica Pollmann). Apidologie. 1992;23: 173–187. [Google Scholar]
- 37.Lodesani M, Vecchi MA, Tommasini S, Bigliardi M. A study on different kinds of damage to Varroa jacobsoni in Apis mellifera ligustica colonies. J Apic Res. 1996;35: 49–56. [Google Scholar]
- 38.Rosenkranz P, Fries I, Boecking O, Stuermer M. Damaged Varroa mites in the debris of honeybee (Apis mellifera L.) colonies with and without hatching brood. Apidologie. 1997;28: 427–437. [Google Scholar]
- 39.Rinderer TE. Africanized bees: An overview. Am Bee J. 1986; 98–100; 128–129. [Google Scholar]
- 40.Branco M, Kidd N, Pickard R. A comparative evaluation of sampling methods for Varroa destructor (Acari: Varroidae) population estimation*. Apidologie. 2006;37: 452–461. [Google Scholar]
- 41.Invernizzi C, Zefferino I, Santos E, Sanchez L. Multilevel assessment of grooming behavior against Varroa destructor in Italian and Africanized honeybees. J Apic Res. 2016;54: 321–327. [Google Scholar]
- 42.Guzman-novoa E, Emsen B, Unger P, Espinosa-montaño LG, Petukhova T. Genotypic variability and relationships between mite infestation levels, mite damage, grooming intensity, and removal of Varroa destructor mites in selected strains of worker honeybees (Apis mellifera L.). J Invertebr Pathol. Elsevier Inc.; 2012;110: 314–320. doi: 10.1016/j.jip.2012.03.020 [DOI] [PubMed] [Google Scholar]
- 43.Traynor KS, Rennich K, Forsgren E, Rose R, Pettis J, Kunkel G, et al. Multiyear survey targeting disease incidence in US honeybees. Apidologie. 2016; 1–23. [Google Scholar]
- 44.Pirk CWW, Strauss U, Yusuf AA, Demares F, Human H. Honeybee health in Africa-a review. Apidologie. 2016;47: 276–300. [Google Scholar]
- 45.Mortensen AN, Schmehl DR, Allsopp M, Bustamante TA, Kimmel CB, Dykes ME, et al. Differences in Varroa destructor infestation rates of two indigenous subspecies of Apis mellifera in the Republic of South Africa. Exp Appl Acarol. 2016;68: 509–515. doi: 10.1007/s10493-015-9999-8 [DOI] [PubMed] [Google Scholar]
- 46.Mondragon L, Spivak M,Vandame RA. multifactorial study of the resistance of honeybees Apis mellifera to the mite Varroa destructor over one year in Mexico. Apidologie. 2005;36: 345–358. [Google Scholar]
- 47.Locke B, Fries I. Characteristics of honeybee colonies (Apis mellifera) in Sweden surviving Varroa destructor infestation. Apidologie. 2011;42: 533–542. [Google Scholar]
- 48.Meixner MD, Kryger P, Costa C. Effects of genotype, environment, and their interactions on honeybee health in Europe. Curr Opin Insect Sci. 2015;10: 177–184. [DOI] [PubMed] [Google Scholar]
- 49.Ibrahim A, Spivak M. The relationship between hygienic behavior and suppression of mite reproduction as honeybee (Apis mellifera) mechanisms of resistance to Varroa destructor 1. Apidologie. 2006;37: 31–40. [Google Scholar]
- 50.Spivak M, Reuter GS. Varroa destructor infestation in untreated honeybee (Hymenoptera: Apidae) colonies selected for hygienic behavior. J Econ Entomol. 2001;94: 326–331. [DOI] [PubMed] [Google Scholar]
- 51.Masterman R, Smith BH, Spivak M. brood odor discrimination abilities in hygienic honey bees (Apis mellifera L.) using proboscis extension reflex conditioning. J Insect Behav. 2000;13: 87–101. [Google Scholar]
- 52.Spivak M, Gilliam M. Hygienic behaviour of honeybees and its application for control of brood diseases and Varroa. Part II: Studies on hygienic behaviour since the Rothenbuhler era. Bee World. 1998;79: 169–186. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Amplified gene fragment, product size base pairs (bp) and annealing temperatures (Ta) are indicated [32].
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.