Abstract
Background
Apolipoprotein A-IV (ApoA-IV) has been shown to be involved in obesity and diabetes pathogenesis in animal studies, but its role in humans is uncertain.
Objectives
The objective of this study was to determine the relation of ApoA-IV with changes in glucose metabolism and weight after bariatric surgery.
Setting
University Hospital.
Methods
The patients (n = 49) included lean controls (n = 8) and patients before and after a mean of 7 months after laparoscopic adjustable gastric banding (LAGB, n = 12), laparoscopic Roux-en-Y gastric bypass (RYGB, n = 22), or laparoscopic sleeve gastrectomy (SG, n = 11). ApoA-IV and other hormone assays were performed in the fasting and the postprandial state.=Pearson’s correlation analyses controlled for baseline BMI and percent excess weight loss (EWL) were used to determine relationships between ApoA-IV levels and insulin resistance (HOMA-IR).
Results
With all bariatric procedures combined, the change in ApoA-IV [533 versus 518 microg/L, P = .813] or ApoA-IV area under the curve (AUC - 1072 versus 1042, P = .939) was not significant. None of the surgeries individually affected levels of fasting or ApoA-IV=AUC. Bariatric surgery resulted in a decrease in HOMA-IR (5.3 versus 2.0, P < .001). In the RYGB group, higher baseline ApoA-IV levels correlated with decrease in HOMA-IR [r =−.6, P=.008]. This relationship was independent of EWL and was not observed in the LAGB=or SG group. There was no association of ApoA-IV levels with EWL, insulin secretion, Peptide-YY, or leptin levels.
Conclusion
Preoperative ApoA-IV levels, rather than changes in levels, positively correlate with improvements in insulin sensitivity independent of weight loss after RYGB.
Keywords: RYGB, LAGB, LSG, Apolipoprotein A4, insulin resistance, Roux-en-Y gastric bypass, Laparascopic adjustable gastric banding, Sleeve gastrectomy, apolipoprotein A-IV, PYY
Bariatric surgery is currently regarded as the most effective therapy for obesity and more recently an option for the treatment of type 2 diabetes mellitus [1,2]. In addition to causing calorie restriction, some bariatric procedures cause changes in secretion of gastrointestinal hormones [3,4] that regulate appetite and glucose homeostasis through communication with peripheral tissues and the central nervous system [3]. For example, one year after Roux-en-Y gastric bypass surgery (RYGB) there were increases in postprandial levels of the hormones Peptide YY (PYY) and glucagon-like peptide-1 (GLP-1) that did not occur after laparoscopic adjustable gastric banding (LAGB) [5]. These hormones increase insulin sensitivity or secretion in addition to decreasing appetite and food intake. Thus, changes in signaling along the “gut-brain” axis depends upon the mode of weight reduction, and the superior clinical results of RYGB may be due in part to levels of neuroendocrine mediators of glucose and energy balance that are altered by change in gastrointestinal transit time [6], bypassing a segment of intestine and/or delivery of undigested food to distal intestine.
Apolipoprotein A-IV (ApoA-IV) is suspected to be another important component of this gut-brain axis and a mediator of the effects of bariatric surgery on obesity and its co-morbidities. ApoA-IV is a peptide component of lipoproteins, especially high density lipoprotein. It is secreted from the jejunal mucosa in response to fat ingestion. Data from animal studies have shown that ApoA-IV increases insulin secretion [7], reduces hepatic gluconeogenesis [8], and decreases food intake [9]. These favorable metabolic functions and the potential for alteration of levels with changes of the gut anatomy after bariatric surgery led us to hypothesize that ApoA-IV may favorably influence weight loss and insulin sensitivity after bariatric surgery in humans. Thus, the objectives of this study were several-fold: 1) to compare levels of ApoA-IV between lean and obese individuals; 2) to prospectively determine whether ApoA-IV levels change after weight loss induced by LAGB, RYGB, and laparoscopic sleeve gastrctomy (LSG); and 3) to assess if there are relationships between ApoA-IV levels and weight loss or insulin sensitivity and if these relationships are dependent on the type of surgical procedure.
Methods
Study patients
The study was approved by the Columbia University Institutional Review Board and written informed consent was obtained from all patients. Participants were recruited from patients who presented for surgical treatment of obesity. Standard criteria for performing bariatric surgery were used for selection of obese patients – BMI ≥ 40 kg/m2 or BMI ≥ 35 kg/m2 in the presence of obesity related co-morbidities [10]. The choice of surgical procedure was determined by patient and surgeon. The surgical procedures were performed in the standard accepted fashion [11]. A total of 45 patients were studied before and 6 to 12 months after surgery: LAGB (n = 12); RYGB (n = 22); LSG (n = 11). In addition, 8 healthy lean controls with BMI ≥19 and ≤ 25 kg/m2 were included. Five patients in the RYGB group, and 1 subject in the LSG group had type 2 diabetes.
Measurement of meal related responses
In view of the adverse effects related to dumping seen with a standard oral glucose tolerance test, a liquid mixed-meal challenge (Optifast, Novartis, Minneapolis, MN, USA; 474 mL, 320 kcal, 50% carbohydrate, 35% protein, 15% fat) consumed within a 15 minute period was administered. Venous blood was drawn in the fasted state and 30, 60, 90, and 120 min after the meal. Plasma hormone measurements were performed on blood samples collected in EDTA tubes that were centrifuged for 15 min at 4°C and stored at −80°C until assayed in duplicate. ApoA-IV was quantified by ELISA (Millipore, Billerica, MA). Due to discontinuation of the ELISA kit from Millipore, plasma from 4 patients before and after LSG was assayed by another commercial ELISA (Cusabio Biotech, Wuhan, Hubei, China). Blood concentrations of insulin, leptin and total PYY were determined as previously described [12].
Data analysis and statistics
Percent excess weight loss (EWL) was calculated as the weight loss divided by the patient’s actual weight minus the ideal weight (kg) which was calculated from the patient’s height in inches as follows: 50 + (2.3*(Ht-60)) for males and 45.5 + (2.3*(Ht-60)) for females and multiplied by 100. Insulin resistance was measured using Homeostatic Model of Insulin Resistance (HOMA-IR) [13]. Summary statistics are expressed as mean ± standard error of measurement (SEM). Differences between the preoperative groups (surgical groups and lean controls) were assessed using the nonparametric Kruskal Wallis test for continuous variables and Fisher test for dichotomous variables. The differences between preoperative and postoperative parameters were assessed using paired Wilcoxon Rank Sum tests. Kruskal-Wallis test was again applied to detect differences in the effects of various surgical procedures. Pearson’s correlation analysis was used to assess relationships between predefined parameters. Correlations were adjusted for covariates (baseline BMI and EWL) using partial correlations test. The values for ApoA-IV from 4 LSG patients obtained using the new ELISA kit were not included in the correlation analyses due to differences in absolute values between kits, however, they were included in the paired Wilcoxon tests. AUC from 0–120 min was calculated using the trapezoidal rule. All statistical analysis were performed using R software [14]. P < .05 was designated to signify statistical significance.
Results
Baseline characteristics of the subject population are shown in Table 1. The mean preoperative BMI of the obese patients was 46.0 ±1.2 kg/m2 and was lower in LAGB compared to RYGB(P = .005) and LSG patients (P = .037). The mean duration of postoperative follow-up was 7.0±.33 months and was of longer duration in the LAGB (8.8±.9 months) compared to RYGB or LSG groups (P = .002 and .001, respectively). As expected, the lean patients exhibited lower BMI and insulin resistance as measured by HOMA-IR compared with obese patients. HOMA-IR was similar between surgical groups.
Table 1.
Characteristics of lean controls and obese patients before and after surgery
| Groups | P Values | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| LEAN | LAGB | RYGB | SG | ap | bp | |
| n (M/F) | 8 (4/4) | 9 (2/7) | 21 (4/17) | 11 (2/9) | ||
| Age (y) | 31.3 ± 4.7 | 43.4 ± 3.4 | 43.1 ± 2.4 | 39.9 ± 2.9 | .019 | .750 |
| Postop period (mo) | NA | 8.8 ± .9 | 6.6 ± .4 | 6.0 ± .0 | NA | .003 |
| Wt (kg) | ||||||
| Pre | 61.9 ± 3.0 | 123.8 ± 10.5 | 127.5 ± 5.9 | 114.0 ± 7.1 | .000 | .000 |
| Post | 105.2 ± 9.5* | 94.6 ± 5.1* | 94.7 ± 5.6† | |||
| EWL (%) | NA | 29.1 ± 4.5 | 40.4 ± 3.2 | 38.6 ± 4.4 | NA | .085 |
| BMI (kg/m2) | ||||||
| Pre | 21.8 ± .7 | 42.5 ± 2.7 | 47.8 ± 1.7 | 46.4 ± 1.7 | .000 | .000 |
| Post | 36.1 ± 2.5* | 35.3 ± 1.4* | 36.0 ± 1.3* | |||
| Leptin-F (ng/mL) | ||||||
| Pre | 4.5 ± 1.8 | 40.2 ± 4.9 | 42.4 ± 2.9 | 67.9 ± 6.2 | .000 | .002 |
| Post | 25.6 ± 3.6* | 18.6 ± 1.8* | 29.2 ± 7.2* | |||
| Glucose-F (mg/dL) | ||||||
| Pre | 88.5 ± 2.2 | 109.2 ± 5.2 | 117.7 ± 11.6 | 100.4 ± 7.5 | .006 | .875 |
| Post | 94.5 ± 2.6* | 95.5 ± 4.2* | 79.0 ± 2.3‡ | |||
| Glucose-AUC | ||||||
| Pre | 168 ± 5 | 231 ± 22 | 261 ± 28 | 239 ± 24 | .000 | .436 |
| Post | 174 ± 6† | 185 ± 15* | 157 ± 6† | |||
| Insulin-F (μIU/mL) | ||||||
| Pre | 4.5 ± .9 | 19.7 ± 2.9 | 18.3 ± 2.5 | 23.0 ± 3.8 | .023 | .539 |
| Post | 10.6 ± 1.3† | 8.2 ± 1.3* | 9 ± 2.3† | |||
| Insulin-AUC | ||||||
| Pre | 34 ± 5 | 134 ± 17 | 112 ± 15 | 162 ± 29 | .000 | .631 |
| Post | 72 ± 8* | 66 ± 13† | 72 ± 12† | |||
| HOMA-IR | ||||||
| Pre | 1.0 ± .21 | 5.4 ± .8 | 5.5 ± .9 | 5.0 ± 1.2 | .000 | .909 |
| Post | 2.5 ± .3† | 1.9 ± .3* | 1.7 ± .5† | |||
| Apo A-IV F (μg/mL) | ||||||
| Pre | 443 ± 49.6 | 483 ± 68 | 481 ± 57 | 690 ± 131 | .694 | .109 |
| Post | 493 ± 67 | 506 ± 53 | 570 ± 130 | |||
| Apo A-IV AUC (× 103) | ||||||
| Pre | 53.6 ± 5.6 | 61.8 ± 7.6 | 59 ± 5.7 | 76.2 ± 14.7 | .474 | .569 |
| Post | 61.8 ± 7.9 | 61.3 ± 6.6 | 65.6 ± 11.5 | |||
| PYY-F (pg/mL) | ||||||
| Pre | 91 ± 26 | 111 ± 22 | 124 ± 32 | 64 ± 22 | .442 | .302 |
| Post | 101 ± 16 | 127 ± 19 | 71 ± 20 | |||
| PYY-AUC | ||||||
| Pre | 271 ± 46 | 332 ± 69 | 317 ± 56 | 182 ± 36 | .422 | .003 |
| Post | 403 ± 100 | 675 ± 102* | 273 ± 52‡ | |||
F = fasting values; AUC = area under the curve from 0–120 minutes.
Paired analyses before and after surgery are indicated as follows:
P = Comparison between lean and obese patients combined before surgery.
P = Analysis of the pre to postsurgery change between the different surgical groups.
P < .001
P < .01
P < .05.
The changes in various parameters after surgery are depicted in Table 1. LAGB produced less weight loss compared with RYGB (18.6 versus 33.0 kg, P < .001) and LSG (19.3 kg, P = .02). However, the differences in the percent EWL produced by the 3 surgeries did not reach statistical significance (P = .09). HOMA-IR decreased similarly (P = .91) in all 3 surgical groups.
Fasting ApoA-IV or ApoA-IV AUC were not different between lean and obese or between the surgical groups at baseline. There were no significant changes in fasting or postprandial AUC ApoA-IV levels after any of the bariatric procedures (Fig 1). ApoA-IV values postsurgery were not different between groups (P = .99 and P = .99 for fasting and AUC, respectively). In contrast, and as expected, PYY AUC increased after RYGB and SG, but not after LAGB [P = .004]. The increase in PYY AUC was greater with RYGB (P = .008) compared with LSG. Fasting and postprandial PYY levels did not correlate with ApoA-IV levels at any time point (data not shown).
Fig 1.
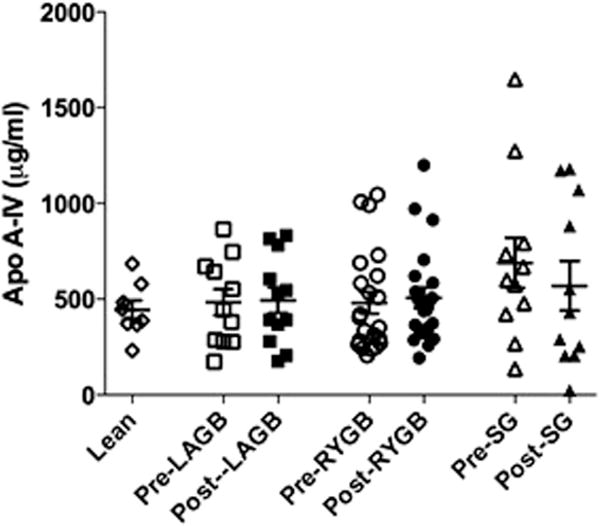
Plasma Levels of fasting ApoA-IV before and after bariatric surgery. P = non-significant for all groups.
Baseline ApoA-IV levels did not correlate with HOMA-IR; however, higher preoperative fasting ApoA-IV correlated with a decrease in HOMA-IR after all surgeries: fasting ApoA-IV (r = −.39, P = .01) and AUC (r =−.45, P = .005) . This relation also held true when RYGB was analyzed separately for fasting ApoA-IV (r = −60, P = .008; Fig. 2) and ApoA-IV AUC (r = −.67=P .002). This relationship was not at all present in the LAGB group (r = .12, P = .76 and r = .2, P = .64 for fasting ApoA-IV and ApoA-IV, respectively). The fewer number of patients in the LSG group for whom data was available (n = 7) may have limited our ability to accurately assess this relationship which was as follows: (r = −.84, P = .16 and r = −.86, P = .14 for fasting and ApoA-IV AUC, respectively). All Pearson’s correlations were controlled for EWL and preoperative BMI. No significant correlations were detected between ApoA-IV levels and EWL%, HOMA-B, plasma leptin, or PYY at baseline or after surgery (data not shown).
Fig 2.
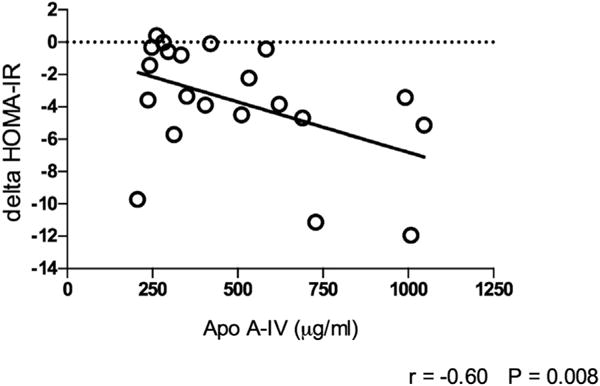
Relation of baseline ApoA-IV to change in HOMA-IR after RYGB. LAGB = laparoscopic adjustable gastric banding; RYGB = laparoscopic Roux-en-Y gastric bypass; SG laparoscopic sleeve gastrectomy; PYY = peptide YY; ApoA-IV = Apoliporotein A-IV; GLP-1 = glucagon-like Peptide −1.
Discussion
The goal of this study was to determine if ApoA-IV may be a candidate mediator of weight loss and some of the positive metabolic effects of bariatric surgery. In this report we measured circulating fasting and postprandial levels of ApoA-IV before and after 3 different surgical procedures to determine if there is a relationship between ApoA-IV levels, the amount of weight loss, the method of weight loss, and insulin sensitivity. Several contrasting factors made it difficult to formulate a hypothesis predicting the effect of bariatric surgery on ApoA-IV levels. For example, RYGB might be expected to decrease postprandial ApoA-IV levels due to decreased nutrient stimulation of enterocytes in the duodenum and proximal jejunum that produce ApoA-IV; on the other hand, PYY has been shown to stimulate ApoA-IV secretion so one might expect postprandial ApoA-IV levels to increase after RYGB due to increased PYY secretion [15]. Similarly, while LSG may be expected to increase postprandial ApoA-IV through stimulation by PYY, the faster intestinal transit time seen with LSG which can decrease proximal intestinal absorption of lipids could conceivably decrease secretion of ApoA-IV [16]. LAGB was included to serve as a control for weight loss without manipulation of gastrointestinal anatomy other than restriction. Another mediator of ApoA-IV in animal studies is leptin which decreases the production of ApoA-IV in the jejunum and ileum [17]. A decrease in leptin levels, which occurred after all 3 surgical procedures, might then be predicted to result in increased secretion of ApoA-IV. We found that mean ApoA-IV fasting and postprandial levels did not change after weight loss with any of the bariatric procedures. Interestingly, however, higher presurgical ApoA-IV levels correlated with a greater decrease in insulin resistance even after weight loss was taken into account, and, therefore, may be a mediator of improved glucose homeostasis after bariatric surgery. This relationship was particularly noteworthy in RYGB and not at all present in LAGB. More patients undergoing LSG will need to be studied to definitively assess whether ApoA-IV levels are predictive of change in insulin resistance. Interestingly, in mice, ApoA-IV was not necessary for metabolic benefits seen after VSG [18]; however, the weight loss may have superseded any detectable effect that ApoA-IV may have on glucose homeostasis.
What might be the mechanism linking ApoA-IV levels and improved insulin sensitivity? ApoA-IV knockout mice have impaired glucose tolerance compared to wild type mice believed to be due to reduced glucose induced β-cell insulin secretion [7]. ApoA-IV is also known to act at the level of hypothalamus to inhibit food intake [14]. In mice, it has an important inhibitory action on the orexigenic Neuropeptide Y and AgRP (agouti-related peptide) neurons and stimulatory action on the anorexigenic pro-opiomelanocortin (POMC) neurons. Food deprivation was found to suppress ApoA-IV levels in the hypothalamus [19]. Chronic fat intake and obesity decrease sensitivity of the hypothalamus to ApoA-IV, thus contributing to obesity [20]. ApoA-IV has been suspected to have a beneficial role through decreasing insulin resistance. An action of ApoA-IV includes transcriptional inhibition of the gluconeogenic genes encoding glucose 6 phosphatase and phosphoenolpyruvate carboxykinase, and thus reduction of hepatic glucose production [8]. Consistent with this action of ApoA-IV, we demonstrated, using HOMA-IR as an indirect measure of hepatic insulin resistance and hepatic glucose production, a relationship between presurgical levels of ApoA-IV and HOMA-IR.
Results of previous studies reporting changes in circulating ApoA-IV levels after RYGB have been inconsistent [21]. Rafaelli et al. demonstrated an increase in ApoA-IV levels after RYGB[22]. An increase was also observed in a study by Culnan et al. who using proteomic analysis detected an increase in plasma levels which correlated with an increase in insulin sensitivity [23]. In contrast to these studies, but consistent with our results, Pardina et al. [24] found decreased levels 1 month after surgery which gradually increased back to baseline levels within 1 year after surgery.
Studying the relationship between baseline ApoA-IV and change in insulin resistance might provide additional information to predict those patients who may have suboptimal control of blood glucose postoperatively and thus help in better choice of the bariatric procedure and better postoperative medical management. Also, this preliminary study paves the way for further studying the mechanisms behind the interindividual variability in improvement of insulin resistance and diabetes after bariatric surgery.
This study has several limitations. HOMA-IR was utilized as a measure of insulin resistance as opposed to more accurate clamp methods. Unfortunately, the number of patients with type 2 diabetes was too few to assess whether ApoA-IV levels would be different in such patients; however, levels did not change in these few individuals after surgery (data not shown). The study did not include obese patients who did not undergo surgery as controls due to which the effects of surgical reconstruction cannot be differentiated from the effects of calorie restriction alone. Blood concentrations may not represent ApoA-IV activity or responsiveness in the brain, and lymphatic or tissue levels not assessed in this study could also be relevant measures of biological effect. Challenges with meals of higher fat content might by expected to reveal different results. Also, our study was limited by sample size, which especially limited the ability to detect differences with each individual surgery. Given the unfortunate discontinuation of the ApoA-IV ELISA toward the latter part of our study, we will need to repeat measurements in our previous patients and continue recruitment of LSG patients using the currently available reagents.
Conclusion
Our study did not find any evidence for change in ApoA-IV levels after bariatric surgery. However, given the association of baseline ApoA-IV with improvements in insulin resistance, this hormone could still have a mechanistic role in improvement in glucose metabolism after RYGB. Future studies should focus on elucidating these mechanisms, performing longer-term follow-up, and studying more patients with type 2 diabetes and additional patients before and after LSG. The goal of further studying the relationship between ApoA-IV and changes in insulin resistance would be not only to provide mechanistic insight into how bariatric surgery results in metabolic improvements but also to aid in the choice of procedure and better predict individual outcomes.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grant DK072011 (J.K.) and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number UL1 TR001873.
Appendix
Supplementary data
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.soard.2016.10.019.
Footnotes
Disclosures
Dr. Korner receives research support from Covidien-Medtronic.
References
- 1.Fisher BL, Schauer P. Medical and surgical options in the treatment of severe obesity. Am J Surg. 2002;184(6B):9S–16S. doi: 10.1016/s0002-9610(02)01173-x. [DOI] [PubMed] [Google Scholar]
- 2.Rubino F, Nathan DM, Eckel RH, et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Diabetes Care. 2016;39(6):861–77. doi: 10.2337/dc16-0236. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen KT, Korner J. The sum of many parts: potential mechanisms for improvement in glucose homeostasis after bariatric surgery. Curr Diab Rep. 2014;14(5):481. doi: 10.1007/s11892-014-0481-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finelli C, Padula MC, Martelli G, Tarantino G. Could the improvement of obesity-related co-morbidities depend on modified gut hormones secretion? World J Gastroenterol. 2014;20(44):16649–64. doi: 10.3748/wjg.v20.i44.16649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korner J, Inabnet W, Febres G, et al. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. Int J Obes (Lond) 2009;33(7):786–95. doi: 10.1038/ijo.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen NQ, Debreceni TL, Bambrick JE, et al. Rapid gastric and intestinal transit is a major determinant of changes in blood glucose, intestinal hormones, glucose absorption and postprandial symptoms after gastric bypass. Obesity (Silver Spring) 2014;22(9):2003–9. doi: 10.1002/oby.20791. [DOI] [PubMed] [Google Scholar]
- 7.Wang F, Kohan AB, Kindel TL, et al. Apolipoprotein A-IV improves glucose homeostasis by enhancing insulin secretion. Proc Natl Acad Sci U S A. 2012;109(24):9641–6. doi: 10.1073/pnas.1201433109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li X, Xu M, Wang F, et al. Apolipoprotein A-IV reduces hepatic gluconeogenesis through nuclear receptor NR1 D1. J Biol Chem. 2014;289(4):2396–404. doi: 10.1074/jbc.M113.511766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu M, Shen L, Tso P. The role of enterostatin and apolipoprotein AIV on the control of food intake. Neuropeptides. 1999;33(5):425–33. doi: 10.1054/npep.1999.0052. [DOI] [PubMed] [Google Scholar]
- 10.American Society for Metabolic and Bariatric Surgery [homepage on the Internet]. Gainesville (FL) Bariatric Surgery Guidelines and Recommendations. 2012:1–2. [cited 2015 Nov 1]. Available from: https://asmbs.org/resources/bariatric-surgery-guidelines-and-recommendations.
- 11.Elder KA, Wolfe BM. Bariatric surgery: a review of procedures and outcomes. Gastroenterology. 2007;132(6):2253–71. doi: 10.1053/j.gastro.2007.03.057. [DOI] [PubMed] [Google Scholar]
- 12.Jackness C, Karmally W, Febres G, et al. Very low-calorie diet mimics the early beneficial effect of Roux-en-Y gastric bypass on insulin sensitivity and β-cell function in type 2 diabetic patients. Diabetes. 2013;62(9):3027–32. doi: 10.2337/db12-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487–95. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 14.R Core Team. A language and environment for statistical computing [homepage on the Internet] R Foundation for Statistical Computing; Vienna, Austria: [updated 2016 Oct 31]. Available from: http://www.r-project.org. [Google Scholar]
- 15.Kalogeris TJ, Qin X, Chey WY, Tso P. PYY stimulates synthesis and secretion of intestinal apolipoprotein AIV without affecting mRNA expression. Am J Physiol. 1998;275(4 Pt 1):G668–74. doi: 10.1152/ajpgi.1998.275.4.G668. [DOI] [PubMed] [Google Scholar]
- 16.Melissas J, Leventi A, Klinaki I, et al. Alterations of global gastrointestinal motility after sleeve gastrectomy: a prospective study. Ann Surg. 2013;258(6):976–82. doi: 10.1097/SLA.0b013e3182774522. [DOI] [PubMed] [Google Scholar]
- 17.Doi T, Liu M, Seeley RJ, Woods SC, Tso P. Effect of leptin on intestinal apolipoprotein AIV in response to lipid feeding. Am J Physiol Regul Integr Comp Physiol. 2001;281(3):R753–9. doi: 10.1152/ajpregu.2001.281.3.R753. [DOI] [PubMed] [Google Scholar]
- 18.Pressler JW, Haller A, Sorrell J, et al. Vertical sleeve gastrectomy restores glucose homeostasis in apolipoprotein A-IV KO mice. Diabetes. 2015;64(2):498–507. doi: 10.2337/db14-0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yan C, He Y, Xu Y, et al. Apolipoprotein A-IV inhibits AgRP/NPY neurons and activates POMC neurons in the arcuate nucleus. Neuroendocrinology. 2016;103(5):476–88. doi: 10.1159/000439436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tso P, Liu M. Apolipoprotein A-IV, food intake, and obesity. Physiol Behav. 2004;83(4):631–43. doi: 10.1016/j.physbeh.2004.07.032. [DOI] [PubMed] [Google Scholar]
- 21.Aminian A, Zelisko A, Kirwan JP, Brethauer SA, Schauer PR. Exploring the impact of bariatric surgery on high density lipoprotein. Surg Obes Relat Dis. 2015;11(1):238–47. doi: 10.1016/j.soard.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 22.Raffaelli M, Guidone C, Callari C, Iaconelli A, Bellantone R, Mingrone G. Effect of gastric bypass versus diet on cardiovascular risk factors. Ann Surg. 2014;259(4):694–9. doi: 10.1097/SLA.0b013e31829d6989. [DOI] [PubMed] [Google Scholar]
- 23.Culnan DM, Cooney RN, Stanley B, Lynch CJ. Apolipoprotein A-IV, a putative satiety/antiatherogenic factor, rises after gastric bypass. Obesity (Silver Spring) 2009;17(1):46–52. doi: 10.1038/oby.2008.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pardina E, López-Tejero MD, Llamas R, et al. Ghrelin and apolipoprotein AIV levels show opposite trends to leptin levels during weight loss in morbidly obese patients. Obes Surg. 2009;19(10):1414–23. doi: 10.1007/s11695-008-9793-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


