Abstract
While recent studies demonstrate that cancer can arise from mutant stem cells, this hypothesis does not explain why tissues without defined stem cell populations are susceptible to inflammation-driven tumorigenesis. We propose that chronic inflammatory diseases, such as colitis and pancreatitis, predispose to gastrointestinal (GI) adenocarcinoma by reprogramming differentiated cells. Focusing on colon and pancreas, we discuss recently discovered connections between inflammation and loss of cell differentiation, and propose that dysregulation of cell fate may be a novel rate-limiting step of tumorigenesis. We review studies identifying differentiation mechanisms that limit tumor initiation and that, upon reactivation, can prevent or revert the cancer cell transformed phenotype. Together, these findings suggest that differentiation-targeted treatments hold promise as a therapeutic strategy in GI cancer.
Keywords: inflammation, colorectal cancer, pancreatitis, pancreatic ductal adenocarcinoma, differentiation
Inflammation, Stem Cells, and Metaplasia in GI Tumor Initiation
Chronic inflammatory conditions such as ulcerative colitis (UC), chronic pancreatitis (CP, see Glossary), and chronic viral hepatitis increase the lifetime risk of cancer development. Why exactly this might be has puzzled researchers and physicians for some time. Given the regenerative capacity of many GI organs, it has been hypothesized that chronic inflammation causes the gradual accumulation of mutations and epigenetic changes in resident stem cells, leading to their emergence as cancer-initiating cells [1]. However, this argument does not fully explain why organs that apparently lack a major stem cell population, such as the pancreas, are so susceptible to inflammation-driven tumorigenesis [2]. Epithelial metaplasia, the process by which one cell type appears to turn into another, is a hallmark of many chronic inflammatory diseases that predispose to cancer [3]. The ability of cells to change their differentiation state in response to injury and stress may explain recent discoveries that the phenotype of a tumor cell – from histology to gene expression profile – may not provide an accurate account of its site of origin.
In various organ systems, numerous elegant studies now support the longstanding hypothesis that resident tissue stem cells can directly give rise to cancer [4]. Colorectal cancer (CRC), in particular, is generally considered to be GI disease of mutated stem cells [5], which is underscored by the high proliferation capacity of the colon. An interesting observation supporting this hypothesis is that the number of stem cell divisions within a given organ appears to correlate with the lifetime cancer risk [6], suggesting that cancer initiation is mostly due to the ‘bad luck’ of DNA replication errors in stem cells. Chronic inflammation, by promoting additional rounds of stem cell proliferation to facilitate tissue repair, could increase the chance of replication errors and mutation.
This conclusion is not without controversy, however, because an independent analysis of the same data argues that extrinsic factors play a much larger role in cancer initiation than predicted by the ‘bad luck’ hypothesis, favoring alternative models of carcinogenesis in the GI tract and elsewhere that are potentially independent of stem cell division [7]. Stem cell-independent tumor development has been directly observed in animal models: for example, in a zebrafish model of melanoma development driven by mutant BRAF and Trp53, tumors initiate from differentiated melanocytes that reacquire a progenitor-like identity [8]. Indeed, it is debatable whether stem cells even exist in the pancreas and liver: both organs are generally quiescent in adults, with no need for continuous replenishment, and lineage-tracing studies have not yet identified convincing markers of a stem cell population in either organ [9]. Nonetheless, each of these accounts for more new cancer cases in the USA than the indisputably stem cell-driven stomach (http://seer.cancer.gov/).
Consequently, the question emerges: because stem cells engineered with cancer-causing mutations are capable of giving rise to cancer in mouse [5,10], does that necessarily mean that this is the only mechanism by which human cancer can arise? Could changes in differentiation state, cued by intrinsic mutation or local inflammatory signals, represent a physiologically plausible mechanism to initiate cancer in organs both with and without defined stem cell populations? Based on recent work in colon, pancreatic, and liver cancers, we hypothesize that inflammatory signals alter the transcriptional networks controlling differentiation in these organs, reprogramming mature cells toward a progenitor-like state that is uniquely sensitive to tumor initiation. Box 1 provides an overview of reprogramming and tumorigenesis in the liver.
Box 1. Reprogramming and Tumorigenesis in the Liver.
The past several years have seen remarkable progress in understanding the cellular basis of regeneration in the liver, next-door-neighbor to the pancreas and intestine [9]. As in the exocrine pancreas, hepatocytes and biliary cells of the liver are predominantly maintained by slow self-renewal, although hepatocyte proliferation occurs preferentially in a pericentral domain exposed to endothelial Wnt ligands [84]. This placid picture changes dramatically after injury, however. First, although new hepatocytes continue to self-renew, the locale of proliferation changes to a periportal domain [85]. Second, new biliary cells are generated by reprogramming of hepatocytes, also in this periportal domain [86]. Biliary cells themselves, by contrast, can self-renew after injury but do not contribute to hepatocytes [9].
The two deadly cancers of the liver, hepatocellular carcinoma (HCC) and cholangiocarcinoma (CC), are thought to represent transformed counterparts of normal hepatocytes and biliary cells. Indeed, lineage-tracing studies indicate that mouse HCC arises from hepatocytes [87,88]. The origins of CC are more complex and present remarkable parallels to those of PDAC. In one study, carcinogen-induced CC was found to develop strictly from hepatocytes, but not from biliary cells [89]. A separate study found that deletion of the hepatocyte differentiation factor Hnf4a enhances CC development [90], paralleling the role of acinar differentiation factors in suppressing PDAC initiation. Another study, however, found that biliary cells could contribute to CC, but only after homozygous deletion of Trp53 [91], analogous to the Trp53-suppressed contribution of pancreatic duct cells to PDAC [64]. Taken together, these data suggest that cellular reprogramming contributes to at least one form of liver cancer, possibly accounting for the increased risk of human HCC and CC associated with chronic inflammation.
CRC Can Arise from Both Stem and Non-Stem Cells
Undergoing continuous cellular turnover, the small and large intestine represent a paradigm of stem cell-driven organ homeostasis [11]. These tissues comprise multiple mature cell types, including enterocytes, goblet cells, enteroendocrine cells, and, in the small intestine, Paneth cells. All of these emerge from multipotent stem cells located in the basal crypts (Figure 1). There appear to be two classes of stem cells, separated by several cells diameters and expressing several unique markers, including the Wnt target gene leucine-rich repeat-containing G protein-coupled receptor 5, Lgr5 (‘crypt basal cells’, the most basally located stem cells and the best-studied to date) and the transcription factor Bmi1 (‘+4 cells’, so-called for their position relative to the crypt base). Cells exiting the crypt, moving toward the luminal surface of the intestine, first pass through a transit-amplifying (TA) phase in which they undergo rapid expansion, followed by differentiation. The restriction of stem cell activity to the basal crypt suggests that this microenvironment represents a niche for multipotency and self-renewal, maintained in part by paracrine Wnt signaling. Departing from this niche, both the TA and mature cells are restricted in their lineage and in their lifespan, and are destined to be shed into the lumen and replenished by new progeny of the stem cells [11,12].
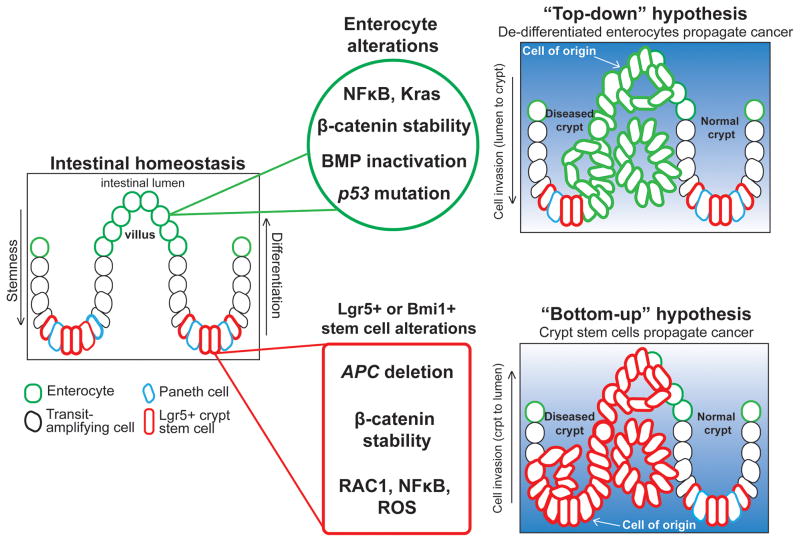
Figure 1.
Top-Down Versus Bottom-Up Hypotheses of Colorectal Cancer (CRC) Initiation. During intestinal homeostasis, Lgr5+ or Bmi1+ basal crypt stem cells proliferate and give rise to transit-amplifying (TA) cells (black outline) and, subsequently, differentiated enterocytes and other mature cell types (green). Alterations in non-stem cells, such as simultaneous NF-κB activation and β-catenin stabilization, can produce adenomas, suggesting that CRC can initiate from the top of the villus and grow down into the crypt (top right). This ‘top-down’ hypothesis proposes that differentiated or committed cells acquire stem-like characteristics to drive tumor growth from the luminal side of the colon. According to the more-traditional ‘bottom up’ model, mutations occurring directly in Lgr5+ or Bmi1+ crypt stem cells, such as loss of Apc, initiate adenoma and CRC formation. The ‘bottom-up’ hypothesis suggests that mutations accumulate in crypt stem cells as they continually proliferate, and then can serve as the effective cell of origin in CRC; they propagate disease from the base of the crypt, growing toward the lumen (bottom right).
Conditional deletion and lineage-tracing experiments in mice indicate that intestinal neoplasia can propagate directly from genetically mutated Lgr5+ or Bmi1+ crypt stem cells [5,10]. For example, deletion of the adenamatous polyposis coli (Apc) tumor-suppressor gene specifically from adult crypt stem cells using an inducible Cre recombinase (Lgr5–CreERT2) results in the rapid formation of β-cateninhigh, cMyc+ microadenomas throughout the mouse intestine [5], resembling the genetic cancer syndrome familial adenomatous polyposis. These results support the bottom-up hypothesis of CRC initiation and progression in which polyp growth initiates from a crypt stem cell and then propagates toward the lumen, eventually predisposing to CRC (Figure 1, bottom panel). The long life of the crypt stem cell makes it particularly susceptible to a high burden of cancer-causing mutations because the same mutations occurring in TA or differentiated cells would be predicted to be lost with the cells themselves.
As early as the 1960s, however, physicians observed that early adenomatous polyps are often found at the top of the colonic crypts, suggesting that mature cells in the villus or mucosal layer may dedifferentiate, proliferate, and replace the normal mucosa from the luminal side [13–15] (Figure 1, top panel). Supporting this top-down hypothesis of CRC initiation, a recent study demonstrated that CRC initiates from epithelial cells outside the stem cell niche in a mouse model of hereditary mixed polyposis syndrome (HMPS), a familial CRC susceptibility syndrome. HMPS is caused by a 40 kb duplication upstream of the BMP antagonist gene Grem1 that leads to its ectopic expression in the intestinal epithelium [16]. Misexpression of epithelial Grem1 in mice results in ‘top-down’ intestinal polyp formation, originating from an Lgr5-negative progenitor cell population that forms outside the crypts [17]. Notably, these Grem1-induced, Lgr5-negative progenitor-like cells appear to be dramatically sensitized to CRC initiation, as indicated by a synergistic increase in polyp numbers on an Apc mutant background [17]. These results suggest that dysregulation of differentiation by altered microenvironmental signaling, in this case loss of BMP activity, can allow the persistence or reacquisition of stem-like properties such that cells outside of the stem cell niche can serve as tumor initiating cells. Notably, these tumor-initiating cells need not have all the properties of normal stem cells (e.g., Lgr5 expression); instead, their phenotype may reflect stabilization of a normally transient intermediate cell fate or else the adoption of a new, non-physiological gene expression program that wild-type cells cannot access.
Whether fully differentiated cells ultimately serve as cells of origin for the top-down polyps seen in Grem1-misexpressing mice is as yet unknown, in part owing to the scarcity of Cre deletor lines that specifically mark non-stem cells in the intestine. Recent studies have begun to address this issue, and reveal that ‘stemness’ in the intestine is a product of both intrinsic and extrinsic factors, including inflammatory signals that can reprogram differentiation and induce tumor-initiating properties [15]. For example, the Greten laboratory has demonstrated that expression of oncogenic versions of the Kras proto-oncogene or activation of nuclear factor κB (NF-κB), in combination with Wnt/β-catenin activation, confers tumor-initiating properties on otherwise quiescent and differentiated intestinal villi [18]. In this system NF-κB acts downstream of oncogenic Kras as a target of Kras-induced inflammatory signaling. Using an Xbp1s-CreERT2 deletor mouse, which allows Cre-mediated recombination specifically outside the Lgr5+ stem cell domain, this group found that coactivation of Wnt/β-catenin and NF-κB could induce dedifferentiation of villus cells and rapid polyp development. Interestingly, mutation of the tumor-suppressor Trp53 promotes NF-κB-dependent inflammation in the mouse intestine [19,20], suggesting that this key inhibitor of CRC and other cancers may act in part by limiting inflammation and subsequent dedifferentiation.
Notwithstanding these results, is it plausible that non-stem cells, with their inherently limited lifespan, could serve as cells of origin for CRC under physiological conditions? Of note, clone-marking studies in the intestine indicate that a subset of TA cells, particularly those restricted to the goblet cell lineage, can persist for months after leaving the crypt [12]. The location of such cells would make them a logical source for top-down polyp generation, particularly if mechanisms exist to further extend their lifespan. Importantly, recent studies indicate that inflammation and tissue damage can override the normal commitment process in the intestine, and rekindle stem cell potential in otherwise lineage-restricted TA populations [21]. For example, enterocyte-restricted TA cells, expressing the alkaline phosphate intestinal (Alpi) gene, are capable of reacquiring stem-like properties when Lgr5+ cells are specifically ablated [22]. An earlier study by this group found that secretory lineage-restricted TA cells, marked by Delta-like-1 (Dll1) expression, could reconstitute Lgr5+ stem cells after radiation-induced crypt damage [23], although dedifferentiation in this model appears to be less efficient than is observed with Alpi+ cells. These studies suggest that TA and recently differentiated cells can act as a stem cell reserve in response to injury, as has been observed in several Drosophila organs [24]. Whether this process is driven by NF-κB is unknown; however, these injury models do not appear to be associated with widespread inflammation. In contrast to the observations made with Xbp1s–CreERT2, above, Alpi+ cells appear to be resistant to transformation in vivo, even by the combination of Apc loss and KrasG12D activation [22]. However, the oncogenic potential of these cells was not tested following Lgr5+ stem cell ablation, or in the context of inflammatory injury. It will be important to determine if NF-κB and other inflammatory pathways can induce tumors from Alpi+ or Dll1+ cells by reprogramming their differentiation state.
These findings support a model in which tumors arising within inflammatory microenvironments, such as that of colitis, arise from dedifferentiation of TA or mature cells rather than from stem cells. While more work is needed to determine whether dedifferentiation occurs during intestinal regeneration, suggestive findings indicate significant cell fate rearrangement during this process. In human ulcerative colitis, for example, expression of the intestinal stem cell marker OLFM4 expands into the superficial epithelium overlying lesions [18]. Meanwhile, mice subjected to experimental colitis exhibit almost complete loss of Lgr5+ cells in the distal colon, followed by reappearance of stem cells as the injury is resolved [25]. Whatever their origin, these newly formed stem cells could promote regeneration by supporting tissue remodeling and epithelial barrier restoration [21], while at the same time representing an expanded pool of cells susceptible to tumor initiation.
Going forward, it will be interesting to interrogate the mechanisms of reprogramming in the intestine, including how pro-tumorigenic signaling pathways such as NF-κB, Kras, and Wnt/β-catenin interact with pro-differentiation transcription factors, such as Hnf1a, Hnf1b, and Klf4 [26,27]. It will also be important to determine whether there is any relationship between mutational ‘drivers’ and the cell of origin. A recent study indicates that CRC arising in patients with preexisting inflammatory bowel disease has a unique mutational spectrum compared to sporadic CRC, including a lower prevalence of APC mutations and a higher prevalence of mutations in SOX9, a transcription factor that, in the intestine, is expressed in crypts and required for normal differentiation [28,29]. It should be possible to determine whether Sox9-knockout intestines exhibit a pro-tumorigenic response to colitis, and whether this reflects cell fate plasticity in TA or mature cells.
Pancreatic Cancer Is Initiated from Differentiated Epithelial Cells
Unlike the intestine, the pancreas is a quiescent organ with no obvious need for a dedicated stem cell population. Numerous lineage-tracing studies of both exocrine and endocrine cells support a model in which each cell type is maintained by self-renewal rather than by stem cell replenishment [9]. The pace of self-renewal is accelerated by injury, but influx of newly differentiated cells appears to be minimal or non-existent even during regeneration. For example, animal models of acute pancreatitis exhibit widespread destruction of digestive enzyme-producing acinar cells, which are replaced upon injury resolution by proliferation of surviving differentiated cells [30]. In considering the potential origins of pancreatic ductal adenocarcinoma (PDAC), therefore, attention has focused on differentiated cells.
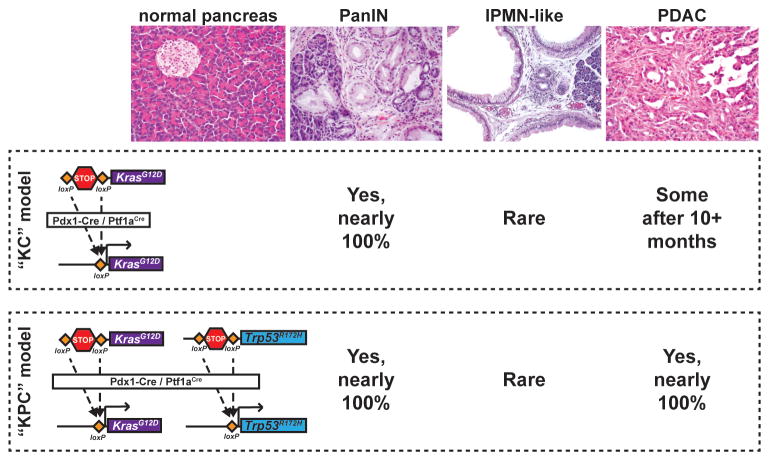
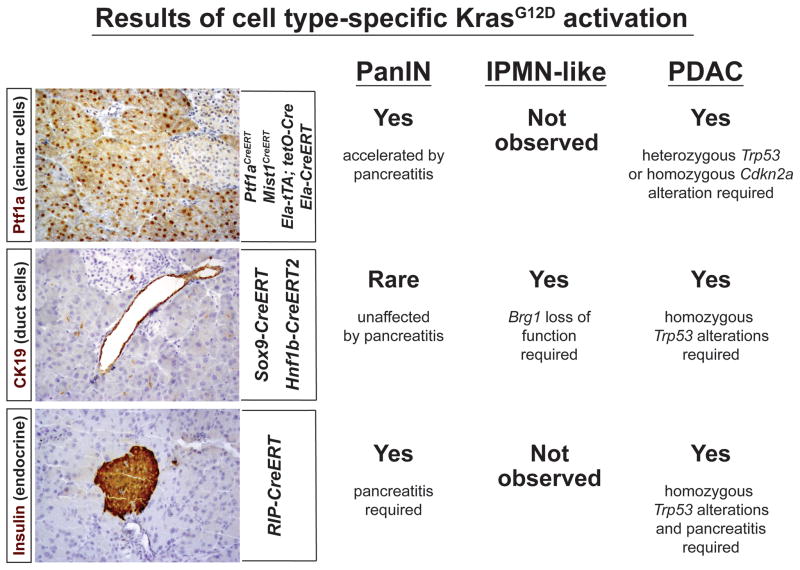
Activation of the KRAS proto-oncogene occurs in ~90% of all human PDAC cases [31,32] and mouse models reveal that KrasG12D drives the formation and maintenance of both PDAC and pre-cancerous pancreatic intraepithelial neoplasia (PanIN) [33]. In the ‘KC’ (KrasG12D, Cre) mouse model, activation of a KrasG12D allele in the embryonic pancreas by Pdx1–Cre or Ptf1a/p48–Cre causes focal PanIN formation [34] (Figure 2). When subjected to additional genetic ‘hits’ in tumor-suppressor genes, such as Trp53 or Cdkn2a (p16-INK4A/p19-ARF), mice develop invasive PDAC [35–37] (Figure 2). Because the Pdx1 and p48/Ptf1a promoters drive Cre expression in nearly every pancreatic epithelial cell beginning in utero (Figure 2), it is not possible to determine the PDAC cell of origin using these models. While duct cells might seem to be an obvious source for duct-like PanINs and PDAC, driving KrasG12D expression specifically within ducts generates very few, if any, PanINs or alternative PDAC precursors, such as intraductal papillary mucinous neoplasm (IPMN) [38,39] (Figure 3). Conversely, when KrasG12D is expressed exclusively in adult acinar cells, PanINs, and [with mutation of Trp53 or Cdkn2a (p16-INK4A/p19-ARF)] PDACs are generated with nearly 100% penetrance [38,40–42] (Figure 3).
Figure 2.
Mouse Models of Pancreatic Ductal Adenocarcinoma (PDAC) Replicate Human Pathology. To activate cancer-causing alleles specifically within the mouse pancreatic epithelium, Cre recombinase is expressed under the control of the Pdx1 or Ptf1a/p48 promoter, both of which are active in embryonic pancreatic progenitor cells. In the ‘KC’ (Kras/Cre) model, one copy of the endogenous Kras allele is mutated to change amino acid 12 from glycine to aspartic acid, locking the Kras protein in its GTP-bound (active) form. A loxP–STOP–loxP cassette (LSL) is placed upstream of this mutation and, before recombination, inhibits transcription of the mutant allele. When Cre is expressed it excises the STOP cassette, activating KrasG12D in all epithelial cells of the early pancreas. This produces fully-penetrant pancreatic intraepithelial neoplasia (PanIN) formation, rare intraductal papillary mucinous neoplasia (IPMN) formation, and occasional PDAC (in ~50% of aged animals). In the ‘KPC’ (Kras/p53/Cre) model of invasive PDAC, the same oncogenic Kras allele is activated along with a Trp53 ‘gain-of-function’ (neomorphic) allele. The R172H point mutation in this Trp53 allele models a mutation frequently found in human PDAC. Expression of both KrasG12D and p53R172H using Pdx1–Cre results in fully-penetrant PanIN and PDAC development in young animals.
Figure 3.
Outcome of KrasG12D Activation in Different Adult Pancreatic Cell Types. Expression of KrasG12D specifically in acinar cells (Ptf1a+ nuclei, top panel) using Ptf1aCreERT, Mist1CreERT, elastase (Ela)–tTA tetO–Cre, or Ela–CreERT, results in focal PanIN formation, which can be greatly accelerated and enhanced by pancreatitis. When compounded with mutations in either p53 (heterozygous) or p16-INK4a/p19-ARF (homozygous), PDAC develops within 2–6 months. Activating KrasG12D using a duct-specific Sox9–CreERT or Hnf1b–CreERT2 transgene (CK19+ duct cells, middle panel) only leads to rare PanIN formation even in the presence of pancreatitis. Combining duct-specific KrasG12D expression and Brg1 deletion, however, induces lesions that are reminiscent of IPMNs, while combining ductal KrasG12D with homozygous p53R172H/R172H induces rapid PDAC development without intermediate PanIN precursors. Finally, one study has found that combining KrasG12D expression in β-cells (insulin+ cells, bottom panel) with pancreatitis is sufficient to induce PanIN formation, while the combination of β cell-specific KrasG12D, pancreatitis, and homozygous Trp53 deletion can produce PDAC.
While these findings suggest that acinar cells are susceptible to transformation, it is noteworthy that most acinar cells are not detectably altered by KrasG12D expression, with only a small minority giving rise to PanINs. The fact that acinar cells preserve their normal identity in the face of oncogenic Kras leads to the hypothesis that pro-differentiation factors restrain transformation by preserving cell identity [33,43]. Interestingly, caerulein-induced pancreatitis, in which acinar cells transiently upregulate progenitor-like gene expression [44,45], greatly accelerates PanIN development from Kras-mutant acinar cells [46–48]. The effects of caerulein pancreatitis are normally transient in wild-type acinar cells, but acinar cells expressing mutant Kras are incapable of regenerating normally after this acute injury, and the disease evolves into a chronic form similar to that associated with increased PDAC risk in humans [2,49]. Interestingly, a high-fat diet can also promote PanIN formation from Kras-mutant acini in a COX-2-dependent manner, perhaps modeling the PDAC-promoting inflammatory effects of obesity in humans [50]. By contrast, PanIN formation is not accelerated from KrasG12D mutant duct cells following caerulein-induced pancreatitis [38]. These findings suggest that acinar but not duct cells initiate PDAC in response to the combination of mutant Kras and pancreatitis, and further suggest that human pancreatitis-associated PDAC may arise from acinar cells.
As in the intestine, pancreatic injury is associated with changes in differentiation that facilitate recovery. In particular, the expression and secretion of acinar digestive enzymes that drive tissue damage are downregulated during experimental pancreatitis, preventing further damage and allowing regeneration [44,45]. This is accompanied by activation of inflammatory pathways such as NF-κB and JAK/STAT; in the context of oncogenic Kras expression, these pathways can stimulate tumor initiation via dedifferentiation. For example, KrasG12D cooperates with inflammatory stimuli to induce NF-κB activation in acinar cells; in turn, the upstream NF-κB activators IKK2 and NEMO are necessary to maintain high levels of signaling downstream of KrasG12D, creating a proinflammatory positive feedback loop that is required for acinar reprogramming and for PanIN and PDAC development [49,51,52]. It should be noted that a very recent study identified an unexpected PanIN-suppressive role of the NF-κB family member RelA, in other words the opposite knockout phenotype to that of Ikk2 and Nemo [53]. RelA appears to be essential for the activation of the senescence-associated secretory phenotype (SASP), in which high oncogene activity stimulates release of proinflammatory cytokines to both enforce local senescence and promote clearance of potential tumor-initiating cells. These results suggest that IKK2 and Nemo have important targets apart from RelA, whether other NF-κB family members or entirely novel factors that are essential for reprogramming of acinar cells.
What are the pro-differentiation factors that might act to preserve acinar cell identity in the face of oncogenic Kras? In adult acini, a network of transcription factors cooperate to promote the expression of acinar-specific digestive enzymes and maintain cell differentiation [30]. Among these are MIST1 (also known as BHLHA15), NR5A2 (also known as LRH-1), and the multiprotein PTF1 complex. The core of this complex is the bHLH transcription factor PTF1A, which is essential to maintain acinar cell differentiation through adulthood [54–57]. Indeed, conditional deletion of Ptf1a, Mist1, or Nr5a2 greatly accelerates KrasG12D-mediated PanIN formation, causing rapid acinar reprogramming as well as widespread inflammation similar to that seen in caerulein-treated KC mice [54,58–60]. In addition, genetic deletion of Nr5a2 or Ptf1a in the context of acute pancreatitis is sufficient to reprogram the acinar epithelium to a ductal fate, with increased (wild-type) RAS signaling and persistent inflammation [54,59]. These findings indicate that the acinar transcriptional network is necessary for regeneration and may restrain the positive feedback between activated Kras and inflammation that drives PDAC initiation [61].
Therefore, we have proposed that two distinct levels of tumor suppression exist during the evolution of PDAC. Under homeostatic conditions, a mutually reinforcing transcriptional network maintains acinar differentiation and suppresses both intrinsic and extrinsic programs that promote PDAC initiation [54,58–60]. Kras mutations are tolerated at the cellular level, but may furnish the raw material for later inflammation-induced carcinogenesis. Local or widespread injury to the pancreas acts in Kras-mutant acinar cells to silence pro-differentiation factors and facilitate a dramatic switch to a duct-like, precancerous phenotype. The initial formation of PanINs thus represents evasion of this first, epigenetic, tumor-suppression mechanism. The subsequent progression of PanINs to invasive cancer is opposed by a second, canonical mechanism of tumor suppression (e.g., TRP53 and INK4A mutation). The fact that epidemiological risk factors for PDAC such as pancreatitis and obesity appear to impact on the acinar reprogramming process highlights the importance of this novel tumor-suppressor mechanism.
Are Acinar Cells the Only Cell of Origin for PDAC?
While acinar cells are the most plausible cell of origin for PanINs in mice, one study reported that insulin-producing β-cells could give rise to PanINs in the context of combined KrasG12D activation and pancreatitis [62] (Figure 3). Because islets remain generally intact and functional in most PDAC and pancreatitis models, this finding may reflect the properties of a unique subset of β cells. Interestingly, the RIP–CreERT mouse line used to express KrasG12D in the endocrine compartment has previously been shown to recombine 3–6% of acinar cells in addition to islet cells [63], and this could account for the PanIN formation observed in these studies. Whether non-exocrine cells may contribute to pancreatitis-associated PDAC in human patients remains unknown, and these results require follow-up studies to exclude technical artifacts.
Interestingly, other recent studies suggest that exocrine duct cells can also give rise to PDAC independently of the conventional PanIN progression model. To test whether duct cells could give rise to invasive PDAC, Bailey and colleagues generated a novel inducible Cre line to express KrasG12D and Trp53R172H specifically in the pancreatic ductal epithelium. Whereas expressing KrasG12D and a single copy of Trp53R172H in ducts had little effect, compounding KrasG12D mutation with homozygous expression of Trp53R172H led to lethality from PDAC only 8 weeks after induction (Figure 3). Intriguingly, duct-derived PDAC in KrasG12D Trp53R172H/R172H mice developed without associated PanIN precursors, suggesting that invasive carcinoma arises directly from these mutated cells [64]. While these results confirm that ducts are relatively resistant to transformation, they also demonstrate that these cells can initiate PDAC in the context of homozygous Trp53 mutations. However, because even heterozygous TP53 mutations are uncommon in human PanINs [65], these results also imply that the conventional PanIN–PDAC sequence represents a uniquely acinar-derived entity.
Recent studies from the Hebrok laboratory additionally suggest that the PDAC precursor lesion IPMN arises from mutated duct cells. For example, deletion of Brg1, a component of the SWI/SNF chromatin remodeling complex (also called SMARCA2) in a ‘KC’ (KrasG12D activated throughout the pancreas) background produces lesions similar to IPMNs [66] (Figure 3). Deleting Brg1 in adult acinar cells inhibits Kras-induced PanIN development, while its deletion in KrasG12D-expressing adult duct cells drives IPMN-like dysplasia, which is otherwise never seen with duct-specific Kras activation. Further studies demonstrated that Brg1 is necessary to maintain duct cell differentiation and thus attenuate the formation of IPMN-like lesions [67]. Intriguingly, the antagonism between differentiation and tumor initiation observed in acinar cells also appears to apply in ducts. While duct differentiation is less well studied than that of acinar cells, the transcription factor Sox9 is important in the pancreas for developmental specification of the duct lineage, and is thereafter restricted in expression to these cells [68,69]. Sox9 is downregulated in the IPMN-like lesions that develop from Brg1-deleted, KrasG12D-expressing ducts, and ectopic expression of Sox9 prevents lesion development and preserves duct differentiation [67].
In contrast to ducts, ectopic Sox9 expression in acinar cells promotes metaplasia, and Sox9 is required for acinar-derived PanIN formation [38]. In addition, as noted above, deletion of Brg1 within KrasG12D-expressing acini restrains PanIN formation [66]. These observations suggest divergent roles for Brg1 and Sox9 in the initiation of PanINs and IPMNs, and highlight the importance of understanding the cell of origin of PDAC precursors. They also raise the possibility that the mutational profile of human PDAC may indicate its origin: approximately one-third of PDAC samples harbor somatic mutations affecting the SWI/SNF complex [70,71]. Do these mutations identify tumors of ductal origin, and would those tumors fail to respond to hypothetical drugs targeting Sox9 or Brg1? Moving forward, we propose that understanding the differential role of these and other genes in tumor initiation will provide new and selective treatment approaches.
It should be noted that the reprogramming that occurs in pancreatic tumor initiation is not as simple as a switch between mature cell fates. PanIN lesions, in particular, are known to express markers not only of pancreatic ducts but also of stomach and bile duct epithelium [72,73]. Thus, pancreatic cells that lose their capacity for normal differentiation may adopt non-physiological gene expression programs, possibly analogous to the aberrant, Lgr5-negative progenitor cells that populate HMPS colon polyps [17]. In addition to regulating mature cell functions, an important function of differentiation regulators such as Ptf1a, Brg1, and Sox9 may be to close-off aberrant cell fates that, while accommodating to the growth of the cell, are harmful to the life of the organism.
Can Dedifferentiation and Redifferentiation Be Exploited as Therapeutic Targets?
We propose that acinar and ductal differentiation determinants form an early, epigenetic barrier to PDAC initiation, and it will be important to determine whether reactivating their expression could halt PDAC progression even after genetic mutation of canonical tumor suppressors. Most of the work to date has focused on the initiation of tumorigenesis, leaving open the question of whether fully malignant cells are susceptible to redifferentiation. On this front, Itkin-Ansari and colleagues recently demonstrated that expressing the bHLH transcription factor E47 (a component of the PTF1 complex) in human PDAC cell lines actively triggers G0/G1 arrest, induces the expression of acinar differentiation markers, and inhibits tumorigenesis in mouse xenografts. Thus, PDAC cells retain an important degree of plasticity and, given the proper conditions, can be reprogrammed back to a benign phenotype [74]. This concept is also supported by in vivo mouse genetic experiments in which sustained Mist1 expression was found to prevent KrasG12D-induced acinar cell reprogramming and PanIN formation [75]. Taken together, these findings suggest that enhancing acinar transcription factor activity could serve as differentiation-based therapy to treat or prevent human PDAC. Going forward, it will be necessary to test whether activation of PTF1A and other acinar network components can reverse PDAC progression and metastatic lesions in vivo.
Although less is known about the transcriptional basis for dedifferentiation in the intestine, the signaling pathways driving regeneration and tumorigenesis in this organ are remarkably overlapping with those implicated in the pancreas. These include EGFR, Hippo/YAP, JAK/STAT, Notch, and NF-κB signaling [21,30]. The common role of NF-κB in intestinal and pancreatic tumorigenesis is particularly striking: in both organs, this proinflammatory transcription factor is activated by the small GTPase Rac1, acting through elevated reactive oxygen species (ROS) in an evolutionarily ancient regenerative pathway [76–78]. In other cell types, including endothelium and adipocytes, NF-κB inhibits differentiation-specific gene expression by titrating away essential transcriptional coactivators [79,80]. It will be interesting to determine whether such a model accounts for the importance of this pathway in CRC and PDAC, and whether the requirement for Rac1 and NF-κB in preclinical models of these cancers reflects a role in cellular reprogramming.
Concluding Remarks
While the intestine maintains its epithelium via stem cell replenishment, other organs with regenerative capacity, such as the pancreas and liver, do not have well-defined stem cell populations that contribute to epithelial cell turnover. These differences in tissue maintenance have led to diverging hypotheses about how cancer might initiate in these different organs. Indeed, intestinal stem cells engineered with cancer-causing mutations are able to give rise to tumors; however, inflammatory signaling can induce a cancer-susceptible state via reprogramming of non-stem and differentiated cells in the colon and pancreas alike.
This model does not exclude an effect for stem cells in the tumor-promoting effects of inflammation. In the intestine, for example, one can imagine a scenario in which one allele of Apc is inactivated in a stem cell, while loss of the second allele in a more-differentiated daughter cell results in adenoma initiation only when inflammatory cues are present. While true stem cells have yet to be identified in the pancreas, it is possible that a subset of differentiated cells are specialized for a longer proliferative lifetime, and might present a target for mutation accumulation analogous to stem cells in the crypt. Whether the initial mutational events occur in stem or non-stem cells, we propose that the emergence of cancer-initiating activity in differentiated cells is due to positive feedback between intrinsic changes, such as Apc and Kras mutations, and extrinsic signals from inflammatory cells. These alterations destabilize or limit the complete maturation of differentiated cells and render them susceptible to oncogenic transformation. Even in the absence of chronic disease such as colitis and pancreatitis, shared risk factors between CRC and PDAC, such as obesity and smoking, are also known to promote systemic inflammation, which could stimulate cellular reprogramming in cooperation with genetic changes.
The studies discussed here leave numerous important issues unresolved and awaiting future work (see Outstanding Questions). Among the most central is the question of how to translate these findings to improved clinical outcomes. Defining the epigenetic changes that divert quiescent or short-lived cells to immortal tumor precursors may identify new targets for therapy based on restoring the memory of the cell of origin and thus neutralizing the effect of otherwise irreversible genetic lesions.
Outstanding Questions.
Will determining cell of origin inform clinical treatment decisions? Do tumors that arise via different cellular mechanisms have different expression profiles? Different ‘Achilles heels’? Different potential targets?
Do alternative cells of origin have different routes to tumorigenesis?
Are intestinal stem cells generated de novo during recovery from colitis or do cells that reactivate Lgr5 expression post-injury derive from pre-existing Lgr5+ stem cells?
Why are Alpi+ TA cells able to form tumor organoids in culture, but not tumors when directly mutated in vivo? Could forced dedifferentiation or inflammatory stimuli drive these cells to produce tumors?
What is the role of p53 in determining tumor cell of origin and tumor progression pathway (e.g., PanIN-dependent vs -independent PDAC)?
Do precancerous adenomas (colon) and PanINs/IPMNs (pancreas) limit tumor progression by restraining potentially oncogenic cells? Is the inflammatory and stromal response associated with dedifferentiation and reprogramming favorable to the host in this regard (Box 2)?
Can differentiation-based therapy revert cancerous cells to a benign state in vivo? What is the most efficient way to screen for drugs that would have this benefit? Could gene therapy be beneficial?
Are there stem cell populations within the pancreas and liver? What cues during injury activate these cells? Can these cell populations also give rise to cancer?
Box 2. Does Inflammation Help To Restrain Precancerous Lesions?
The observation that PDAC arises rapidly in the KrasG12D Trp53R172H/R172H ductal model, without precursor lesions and in the absence of extrinsic injury [64], suggests the intriguing hypothesis that the formation of pre-cancerous lesions, such as PanINs (in PDAC) and polyps (in CRC), can actually be part of a protective host response that physically isolates potential carcinoma cells. Mathematical models of human pancreatic cancer suggest that the progression from initial tumor-driving mutation (most likely KRAS) to invasive carcinoma takes over a decade, an interval generally attributed to the accumulation of additional mutations [81]. However, a recent study demonstrates that mouse and human PDAC cells, following organoid culture and orthotopic transplantation, initially form PanIN-like structures, lacking the dysplastic features of carcinoma, and only progress to invasive PDAC after a delay of several months [82]. Thus, even fully endowed with cancer-causing mutations sufficient to kill human patients, PDAC cells can be constrained to a preinvasive phenotype by the local microenvironment. Interestingly, the invasiveness of orthotopic transplanted mouse organoids may depend on the p53 status of the tumor cells, mirroring what is seen in the KrasG12D Trp53R172H/R172H ductal model. Specifically, cells with homozygous p53 mutations become invasive within weeks, bypassing the hypothetical constraint of PanINs, while cancerous cells that are heterozygous for p53 initially form PanINs and are contained for months. Previous work suggests that the fibroinflammatory reaction associated with PanINs and PDAC may act to inhibit, rather than to promote, PDAC progression [83]. This may be mediated in part by the dual role of inflammatory cytokines in promoting pre-tumor senescence and tumor growth, as revealed in the pancreas by deletion of RelA [53]. A hypothetical tumor-suppressive role for inflammation may explain why PanINs take decades to progress from initial genetic ‘hit’ to invasive and metastatic carcinoma [81]. Together, these findings raise the possibility that inflammation may have both positive and negative effects on tumorigenesis, and that PanINs and other preneoplastic lesions may serve as host-protective ‘dead ends’ in which mutation-bearing cells are encapsulated and denied the opportunity to invade and metastasize.
Trends.
Genetic deletion of differentiation determinants allows rapid oncogenic transformation of cells and may be a rate-limiting step in tumor initiation. This transformation is associated with a robust inflammatory response; it remains unclear if the inflammation associated with dedifferentiation is unique in composition.
Colorectal cancer can arise from direct mutation of crypt stem cells, but can also arise via NF-κB mediated dedifferentiation of mature enterocytes
Pancreatic ductal adenocarcinoma can arise via reprogramming of acinar cells into pancreatic intraepithelial neoplasia (PanIN) or directly from duct cells without a ‘pre-cancerous’ PanIN-like intermediate
NF-κB signaling, as well as pathways crucial for endodermal development, play crucial roles in dedifferentiation and subsequent oncogenesis in the colon, pancreas, and liver.
Reintroduction of acinar differentiation determinants to pancreatic cancer cells prevents proliferation and could hold promise as a therapeutic strategy.
Acknowledgments
We would like to thank Drs Nilotpal Roy and Matthias Hebrok for providing images of IPMN lesions (Figure 2). This work was supported by a National Institutes of Health (NIH) predoctoral award to N.M.K. (F30-CA192819) and NIH grants (R01-DK061220, R01-CA194941) to L.C.M.
Glossary
- Adenomatous polyposis coli (Apc)
an intestinal tumor-suppressor gene that encodes a negative regulator of Wnt/β-catenin signaling. Inherited mutations in human APC cause familial CRC predisposition, while sporadic mutations often represent the first genetic ‘hits’ in sporadic CRC.
- Bottom-up hypothesis
the idea that CRC is initiated from a mutated crypt stem cell and propagated from the bottom of the crypt towards the lumen.
- Caerulein
oligopeptide similar to cholecystokinin (CCK); stimulates pancreatic secretion and, at supraphysiological levels, induces intrapancreatic activation of digestive enzymes and subsequent pancreatitis.
- Chronic pancreatitis (CP)
longstanding inflammation of the exocrine pancreas, causing malabsorption and abdominal persistent pain, and significantly increasing the risk of PDAC.
- Colorectal cancer (CRC)
cancer originating from the epithelium of the colon or rectum. It is the most commonly diagnosed cancer of the gastrointestinal (GI) tract, with over 140 000 cases presenting each year in the USA.
- Cre recombinase
bacteriophage enzyme that catalyzes site-specific DNA recombination between nearby loxP sites. Commonly utilized for tissue-specific recombination in mouse genetics.
- Intraductal papillary mucinous neoplasia (IPMN)
putative PDAC precursor lesion that grows within pancreatic ducts and is characterized by thick fluid production. Recent studies suggest that IPMNs arise from cells of the ductal epithelium.
- Kras proto-oncogene
an oncogene precursor that is mutated to an ‘active’ form in 90–95% of PDAC and 30–50% of CRC cases. Once in an active (GTP-bound) conformation, KRAS propagates growth factor signals mediated by RAF/MEK/ERK and PI3K/AKT, among other pathways.
- Leucine-rich repeat-containing G protein-coupled receptor 5 (lgr5)
a Wnt target gene, encoding a cell-surface receptor, that is expressed exclusively in adult stem cells of the intestine, stomach, and hair follicle.
- Metaplasia
from Greek, ‘change in form’, refers to the reversible change of one tissue type into another. This can occur through multiple mechanisms including transdifferentiation of mature cells, alterations to stem cells such that they produce inappropriately differentiated offspring, or the death of one tissue type and replacement by adjacent cells of another type.
- Nuclear factor κB (NF-κB)
family of transcription factors rapidly activated by diverse mediators of injury and infection, often regulating downstream inflammatory responses.
- Pancreatic ductal adenocarcinoma (PDAC)
the most common cancer of the pancreas, often comprising cells with duct-like morphology. About 46 000 cases are diagnosed in the USA each year and the 5 year survival rate is ~6%.
- Pancreatic intraepithelial neoplasia (PanIN)
putative precursor lesion of PDAC. Recent studies suggest PanINs arise from reprogramming of mature acinar cells.
- Top-down hypothesis
the idea that CRC initiates from a differentiated cell on the luminal surface of the colon and subsequently invades the crypt.
- Transit-amplifying (TA) cell
daughter cell of a stem cell, destined to undergo a burst of proliferation followed by differentiation. Often committed to a limited range of cell fates.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Quante M, Wang TC. Inflammation and stem cells in gastrointestinal carcinogenesis. Physiology. 2008;23:350–359. doi: 10.1152/physiol.00031.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lowenfels AB, et al. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N Engl J Med. 1993;328:1433–1437. doi: 10.1056/NEJM199305203282001. [DOI] [PubMed] [Google Scholar]
- 3.Merrell AJ, Stanger BZ. Adult cell plasticity in vivo: de-differentiation and transdifferentiation are back in style. Nat Rev Mol Cell Biol. 2016;17:413–425. doi: 10.1038/nrm.2016.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blanpain C. Tracing the cellular origin of cancer. Nat Cell Biol. 2013;15:126–134. doi: 10.1038/ncb2657. [DOI] [PubMed] [Google Scholar]
- 5.Barker N, et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- 6.Tomasetti C, Vogelstein B. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science. 2015;347:78–81. doi: 10.1126/science.1260825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu S, et al. Substantial contribution of extrinsic risk factors to cancer development. Nature. 2016;529:43–47. doi: 10.1038/nature16166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaufman CK, et al. A zebrafish melanoma model reveals emergence of neural crest identity during melanoma initiation. Science. 2016;351:aad2197. doi: 10.1126/science.aad2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kopp JL, et al. Stem cells versus plasticity in liver and pancreas regeneration. Nat Cell Biol. 2016;18:238–245. doi: 10.1038/ncb3309. [DOI] [PubMed] [Google Scholar]
- 10.Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet. 2008;40:915–920. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clevers H. The intestinal crypt, a prototype stem cell compartment. Cell. 2013;154:274–284. doi: 10.1016/j.cell.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Bjerknes M, Cheng H. Clonal analysis of mouse intestinal epithelial progenitors. Gastroenterology. 1999;116:7–14. doi: 10.1016/s0016-5085(99)70222-2. [DOI] [PubMed] [Google Scholar]
- 13.Cole JW, McKalen A. Studies on the morphogenesis of adenomatous polyps in the human colon. Cancer. 1963;16:998–1002. doi: 10.1002/1097-0142(196308)16:8<998::aid-cncr2820160806>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 14.Shih IM, et al. Top-down morphogenesis of colorectal tumors. Proc Natl Acad Sci U S A. 2001;98:2640–2645. doi: 10.1073/pnas.051629398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huels DJ, Sansom OJ. Stem vs non-stem cell origin of colorectal cancer. Br J Cancer. 2015;113:1–5. doi: 10.1038/bjc.2015.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaeger E, et al. Hereditary mixed polyposis syndrome is caused by a 40-kb upstream duplication that leads to increased and ectopic expression of the BMP antagonist GREM1. Nat Genet. 2012;44:699–703. doi: 10.1038/ng.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis H, et al. Aberrant epithelial GREM1 expression initiates colonic tumorigenesis from cells outside the stem cell niche. Nat Med. 2015;21:62–70. doi: 10.1038/nm.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwitalla S, et al. Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-cell-like properties. Cell. 2013;152:25–38. doi: 10.1016/j.cell.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 19.Cooks T, et al. Mutant p53 prolongs NF-kappaB activation and promotes chronic inflammation and inflammation-associated colorectal cancer. Cancer Cell. 2013;23:634–646. doi: 10.1016/j.ccr.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwitalla S, et al. Loss of p53 in enterocytes generates an inflammatory microenvironment enabling invasion and lymph node metastasis of carcinogen-induced colorectal tumors. Cancer Cell. 2013;23:93–106. doi: 10.1016/j.ccr.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 21.Karin M, Clevers H. Reparative inflammation takes charge of tissue regeneration. Nature. 2016;529:307–315. doi: 10.1038/nature17039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tetteh PW, et al. Replacement of lost Lgr5-positive stem cells through plasticity of their enterocyte-lineage daughters. Cell Stem Cell. 2016;18:203–213. doi: 10.1016/j.stem.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 23.van Es JH, et al. Dll1+ secretory progenitor cells revert to stem cells upon crypt damage. Nat Cell Biol. 2012;14:1099–1104. doi: 10.1038/ncb2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Losick VP, et al. Drosophila stem cell niches: a decade of discovery suggests a unified view of stem cell regulation. Dev Cell. 2011;21:159–171. doi: 10.1016/j.devcel.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davidson LA, et al. Alteration of colonic stem cell gene signatures during the regenerative response to injury. Biochim Biophys Acta. 2012;1822:1600–1607. doi: 10.1016/j.bbadis.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D’Angelo A, et al. Hepatocyte nuclear factor 1alpha and beta control terminal differentiation and cell fate commitment in the gut epithelium. Development. 2010;137:1573–1582. doi: 10.1242/dev.044420. [DOI] [PubMed] [Google Scholar]
- 27.Yu T, et al. Kruppel-like factor 4 regulates intestinal epithelial cell morphology and polarity. PLoS One. 2012;7:e32492. doi: 10.1371/journal.pone.0032492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bastide P, et al. Sox9 regulates cell proliferation and is required for Paneth cell differentiation in the intestinal epithelium. J Cell Biol. 2007;178:635–648. doi: 10.1083/jcb.200704152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robles AI, et al. Whole-exome sequencing analyses of inflammatory bowel disease-associated colorectal cancers. Gastroenterology. 2016;150:931–943. doi: 10.1053/j.gastro.2015.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murtaugh LC, Keefe MD. Regeneration and repair of the exocrine pancreas. Annu Rev Physiol. 2015;77:229–249. doi: 10.1146/annurev-physiol-021014-071727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bailey P, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531:47–52. doi: 10.1038/nature16965. [DOI] [PubMed] [Google Scholar]
- 32.Waddell N, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518:495–501. doi: 10.1038/nature14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murtaugh LC. Pathogenesis of pancreatic cancer: lessons from animal models. Toxicol Pathol. 2014;42:217–228. doi: 10.1177/0192623313508250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hingorani SR, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 35.Hingorani SR, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 36.Aguirre AJ, et al. Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev. 2003;17:3112–3126. doi: 10.1101/gad.1158703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bardeesy N, et al. Both p16(Ink4a) and the p19(Arf)–p53 pathway constrain progression of pancreatic adenocarcinoma in the mouse. Proc Natl Acad Sci U S A. 2006;103:5947–5952. doi: 10.1073/pnas.0601273103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kopp JL, et al. Identification of Sox9-dependent acinar-to-ductal reprogramming as the principal mechanism for initiation of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;22:737–750. doi: 10.1016/j.ccr.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ray KC, et al. Epithelial tissues have varying degrees of susceptibility to Kras(G12D)-initiated tumorigenesis in a mouse model. PLoS One. 2011;6:e16786. doi: 10.1371/journal.pone.0016786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De La O JP, et al. Notch and Kras reprogram pancreatic acinar cells to ductal intraepithelial neoplasia. Proc Natl Acad Sci U S A. 2008;105:18907–18912. doi: 10.1073/pnas.0810111105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Habbe N, et al. Spontaneous induction of murine pancreatic intraepithelial neoplasia (mPanIN) by acinar cell targeting of oncogenic Kras in adult mice. Proc Natl Acad Sci U S A. 2008;105:18913–18918. doi: 10.1073/pnas.0810097105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ji B, et al. Ras activity levels control the development of pancreatic diseases. Gastroenterology. 2009;137:1072–1082. doi: 10.1053/j.gastro.2009.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rooman I, Real FX. Pancreatic ductal adenocarcinoma and acinar cells: a matter of differentiation and development? Gut. 2012;61:449–458. doi: 10.1136/gut.2010.235804. [DOI] [PubMed] [Google Scholar]
- 44.Jensen JN, et al. Recapitulation of elements of embryonic development in adult mouse pancreatic regeneration. Gastroenterology. 2005;128:728–741. doi: 10.1053/j.gastro.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 45.Karki A, et al. Silencing Mist1 gene expression is essential for recovery from acute pancreatitis. PLoS One. 2015;10:e0145724. doi: 10.1371/journal.pone.0145724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De La O JP, Murtaugh LC. Notch and Kras in pancreatic cancer: at the crossroads of mutation, differentiation and signaling. Cell Cycle. 2009;8:1860–1864. doi: 10.4161/cc.8.12.8744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guerra C, et al. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell. 2007;11:291–302. doi: 10.1016/j.ccr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 48.Morris JPt, et al. Beta-catenin blocks Kras-dependent reprogramming of acini into pancreatic cancer precursor lesions in mice. J Clin Invest. 2010;120:508–520. doi: 10.1172/JCI40045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Daniluk J, et al. An NF-kappaB pathway-mediated positive feedback loop amplifies Ras activity to pathological levels in mice. J Clin Invest. 2012;122:1519–1528. doi: 10.1172/JCI59743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Philip B, et al. A high-fat diet activates oncogenic Kras and COX2 to induce development of pancreatic ductal adenocarcinoma in mice. Gastroenterology. 2013;145:1449–1458. doi: 10.1053/j.gastro.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maier HJ, et al. Requirement of NEMO/IKKgamma for effective expansion of KRAS-induced precancerous lesions in the pancreas. Oncogene. 2013;32:2690–2695. doi: 10.1038/onc.2012.272. [DOI] [PubMed] [Google Scholar]
- 52.Maniati E, et al. Crosstalk between the canonical NF-kappaB and Notch signaling pathways inhibits PPARgamma expression and promotes pancreatic cancer progression in mice. J Clin Invest. 2011;121:4685–4699. doi: 10.1172/JCI45797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lesina M, et al. RelA regulates CXCL1/CXCR2-dependent oncogene-induced senescence in murine Kras-driven pancreatic carcinogenesis. J Clin Invest. 2016;126:2919–2932. doi: 10.1172/JCI86477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krah NM, et al. The acinar differentiation determinant PTF1A inhibits initiation of pancreatic ductal adenocarcinoma. Elife. 2015;4:e07125. doi: 10.7554/eLife.07125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Holmstrom SR, et al. LRH-1 and PTF1-L coregulate an exocrine pancreas-specific transcriptional network for digestive function. Genes Dev. 2011;25:1674–1679. doi: 10.1101/gad.16860911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Masui T, et al. Replacement of Rbpj with Rbpjl in the PTF1 complex controls the final maturation of pancreatic acinar cells. Gastroenterology. 2010;139:270–280. doi: 10.1053/j.gastro.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hoang CQ, et al. Transcriptional maintenance of pancreatic acinar identity, differentiation and homeostasis by PTF1A. Mol Cell Biol. 2016 doi: 10.1128/MCB.00358-16. Published online October 3, 2016 http://dx.doi.org/10.1128/MCB.00358-16. [DOI] [PMC free article] [PubMed]
- 58.Flandez M, et al. Nr5a2 heterozygosity sensitises to, and cooperates with, inflammation in KRas(G12V)-driven pancreatic tumourigenesis. Gut. 2014;63:647–655. doi: 10.1136/gutjnl-2012-304381. [DOI] [PubMed] [Google Scholar]
- 59.von Figura G, et al. Nr5a2 maintains acinar cell differentiation and constrains oncogenic Kras-mediated pancreatic neoplastic initiation. Gut. 2014;63:656–664. doi: 10.1136/gutjnl-2012-304287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shi G, et al. Loss of the acinar-restricted transcription factor Mist1 accelerates Kras-induced pancreatic intraepithelial neoplasia. Gastroenterology. 2009;136:1368–1378. doi: 10.1053/j.gastro.2008.12.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.di Magliano MP, Logsdon CD. Roles for KRAS in pancreatic tumor development and progression. Gastroenterology. 2013;144:1220–1229. doi: 10.1053/j.gastro.2013.01.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gidekel Friedlander SY, et al. Context-dependent transformation of adult pancreatic cells by oncogenic K-Ras. Cancer Cell. 2009;16:379–389. doi: 10.1016/j.ccr.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blaine SA, et al. Adult pancreatic acinar cells give rise to ducts but not endocrine cells in response to growth factor signaling. Development. 2010;137:2289–2296. doi: 10.1242/dev.048421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bailey JM, et al. p53 mutations cooperate with oncogenic Kras to promote adenocarcinoma from pancreatic ductal cells. Oncogene. 2016;35:4282–4288. doi: 10.1038/onc.2015.441. [DOI] [PubMed] [Google Scholar]
- 65.Murphy SJ, et al. Genetic alterations associated with progression from pancreatic intraepithelial neoplasia to invasive pancreatic tumor. Gastroenterology. 2013;145:1098–1109. doi: 10.1053/j.gastro.2013.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.von Figura G, et al. The chromatin regulator Brg1 suppresses formation of intraductal papillary mucinous neoplasm and pancreatic ductal adenocarcinoma. Nat Cell Biol. 2014;16:255–267. doi: 10.1038/ncb2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roy N, et al. Brg1 promotes both tumor-suppressive and oncogenic activities at distinct stages of pancreatic cancer formation. Genes Dev. 2015;29:658–671. doi: 10.1101/gad.256628.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shih HP, et al. A Notch-dependent molecular circuitry initiates pancreatic endocrine and ductal cell differentiation. Development. 2012;139:2488–2499. doi: 10.1242/dev.078634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kopp JL, et al. Sox9+ ductal cells are multipotent progenitors throughout development but do not produce new endocrine cells in the normal or injured adult pancreas. Development. 2011;138:653–665. doi: 10.1242/dev.056499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shain AH, et al. Convergent structural alterations define switch/sucrose nonfermentable (SWI/SNF) chromatin remodeler as a central tumor suppressive complex in pancreatic cancer. Proc Natl Acad Sci U S A. 2012;109:E252–259. doi: 10.1073/pnas.1114817109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Witkiewicz AK, et al. Whole-exome sequencing of pancreatic cancer defines genetic diversity and therapeutic targets. Nat Commun. 2015;6:6744. doi: 10.1038/ncomms7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Delgiorno KE, et al. Identification and manipulation of biliary metaplasia in pancreatic tumors. Gastroenterology. 2014;146:233–244. doi: 10.1053/j.gastro.2013.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Prasad NB, et al. Gene expression profiles in pancreatic intraepithelial neoplasia reflect the effects of Hedgehog signaling on pancreatic ductal epithelial cells. Cancer Res. 2005;65:1619–1626. doi: 10.1158/0008-5472.CAN-04-1413. [DOI] [PubMed] [Google Scholar]
- 74.Kim S, et al. The basic helix-loop-helix transcription factor E47 reprograms human pancreatic cancer cells to a quiescent acinar state with reduced tumorigenic potential. Pancreas. 2015;44:718–727. doi: 10.1097/MPA.0000000000000328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shi G, et al. Maintenance of acinar cell organization is critical to preventing Kras-induced acinar-ductal metaplasia. Oncogene. 2013;32:1950–1958. doi: 10.1038/onc.2012.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Myant KB, et al. ROS production and NF-kappaB activation triggered by RAC1 facilitate WNT-driven intestinal stem cell proliferation and colorectal cancer initiation. Cell Stem Cell. 2013;12:761–773. doi: 10.1016/j.stem.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liou GY, et al. Mutant Kras-induced mitochondrial oxidative stress in acinar cells upregulates EGFR signaling to drive formation of pancreatic precancerous lesions. Cell Rep. 2016;14:2325–2336. doi: 10.1016/j.celrep.2016.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu CY, et al. PI3K regulation of RAC1 is required for KRAS-induced pancreatic tumorigenesis in mice. Gastroenterology. 2014;147:1405–1416. doi: 10.1053/j.gastro.2014.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brown JD, et al. NF-kappaB directs dynamic super enhancer formation in inflammation and atherogenesis. Mol Cell. 2014;56:219–231. doi: 10.1016/j.molcel.2014.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schmidt SF, et al. Acute TNF-induced repression of cell identity genes is mediated by NFkappaB-directed redistribution of cofactors from super-enhancers. Genome Res. 2015;25:1281–1394. doi: 10.1101/gr.188300.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yachida S, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467:1114–1117. doi: 10.1038/nature09515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Boj SF, et al. Organoid models of human and mouse ductal pancreatic cancer. Cell. 2015;160:324–338. doi: 10.1016/j.cell.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rhim AD, et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014;25:735–747. doi: 10.1016/j.ccr.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang B, et al. Self-renewing diploid Axin2+ cells fuel homeostatic renewal of the liver. Nature. 2015;524:180–5. doi: 10.1038/nature14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Font-Burgada J, et al. Hybrid periportal hepatocytes regenerate the injured liver without giving rise to cancer. Cell. 2015;162:766–779. doi: 10.1016/j.cell.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yanger K, et al. Robust cellular reprogramming occurs spontaneously during liver regeneration. Genes Dev. 2013;27:719–724. doi: 10.1101/gad.207803.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mu X, et al. Hepatocellular carcinoma originates from hepatocytes and not from the progenitor/biliary compartment. J Clin Invest. 2015;125:3891–3903. doi: 10.1172/JCI77995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shin S, et al. Genetic lineage tracing analysis of the cell of origin of hepatotoxin-induced liver tumors in mice. Hepatology. 2016;64:1163–1177. doi: 10.1002/hep.28602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sekiya S, Suzuki A. Intrahepatic cholangiocarcinoma can arise from Notch-mediated conversion of hepatocytes. J Clin Invest. 2012;122:3914–3918. doi: 10.1172/JCI63065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Saha SK, et al. Mutant IDH inhibits HNF-4alpha to block hepatocyte differentiation and promote biliary cancer. Nature. 2014;513:110–114. doi: 10.1038/nature13441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Guest RV, et al. Cell lineage tracing reveals a biliary origin of intrahepatic cholangiocarcinoma. Cancer Res. 2014;74:1005–1010. doi: 10.1158/0008-5472.CAN-13-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]