Abstract
Background
To assess the clinical outcomes in patients with greater than 4 newly diagnosed brain metastases treated with focal stereotactic radiotherapy alone.
Methods
All patients with five or more brain metastases who received focal radiotherapy without whole brain radiation or resection were included in this retrospective analysis. Distant brain failure (DBF), overall survival (OS) and toxicity were reported.
Results
Thirty-six patients met inclusion with median clinical follow-up of 6.3 months (range: 1.1, 51.4). Twenty-nine patients received stereotactic radiosurgery (SRS) to a median dose of 20 Gy (16-20), and 7 received fractionated stereotactic radiotherapy (FSRT) to a median dose of 30 Gy (25, 30) in five fractions. The median lesion number and total brain metastases volume was 6 (5, 14) and 1.55 cc (0.12, 32.96), respectively. The Kaplan-Meier estimate of DBF at six-month was 58%, and survival probability at 1 year was 49%. Twenty percent of patients experienced systemic death without CNS relapse. Eight percent experienced grade 3 toxicity with no grade 4 or 5 toxicity. Neither tumor volume nor number predicted DBF.
Conclusions
DBF, OS and treatment toxicity were similar to historical controls with fewer than five metastases treated with focal radiation. Focal stereotactic radiotherapy alone without whole brain RT is a reasonable treatment strategy for five or more brain metastases.
Keywords: distant brain failure, radiosurgery, brain metastases
Introduction
Forty percent of all cancer patients will develop brain metastases [1, 2]. Of these patients, a significant proportion present with greater than four lesions [3]. Controversy exists regarding the optimal management of this patient subset. Historically the standard of care has been whole brain radiotherapy (WBRT), yet mounting evidence indicates WBRT results in cognitive dysfunction and worsened quality of life [4-6]. Additionally, WBRT is delivered over weeks in patients who have a limited life expectancy [7] and necessitates systemic therapy breaks in patients who most frequently succumb to their extra-cranial disease [8, 9].
Prospective randomized controlled trials (RCT) have confirmed equivalent local control and overall survival following SRS without adjuvant WBRT in patients with one to four brain metastases [10, 11]. Yet, in patients with five or more lesions, treatment decisions hinge on a lower level of evidence limited to prospective observational studies as prospective RCTs are lacking [9, 12]. Physicians remain reluctant to provide focal therapy alone in those with five or more brain metastases, which may stem from the perception that tumor number dramatically predicts poorer survival, increased distant brain failure (DBF) and treatment toxicity concerns. The present study assesses the clinical outcomes in patients with five or more newly diagnosed brain metastases treated with stereotactic radiosurgery (SRS) or fractionated stereotactic radiotherapy (FSRT) alone without initial whole brain RT.
Materials and Methods
Patient and selection criteria
This institutional review board-approved retrospective analysis included all patients at our institution who received definitive focal stereotactic radiotherapy without prior WBRT, SRS or surgery for five or more newly diagnosed brain metastases beginning in 1995. Small cell lung cancer was excluded. Inclusion required at least one month of radiographic follow-up with brain magnetic resonance imaging (MRI) with contrast. A database of over 5000 radiosurgery courses was queried and reviewed, and 36 patients met study entry criteria. All patients were reviewed at radiosurgery conference, and focal radiotherapy is recommended for patients with good KPS and controlled extra-cranial disease or actively receiving systemic therapy.
Stereotactic radiotherapy technique
SRS was delivered utilizing both a Leksell Model C (Elekta, Stockholm, Sweden) Gamma Knife (GK) and linear accelerator (LINAC)-based volumetric modulated arc therapy (VMAT). LINAC-based VMAT was used to deliver FSRT. For GK therapy a stereotactic frame was placed by a neurosurgeon using local anesthetic, and all patients received thin slice (stealth) MRI with contrast for planning. A neurosurgeon and radiation oncologist delineated the gross tumor volume (GTV) defined as enhancing abnormality on T1 post-contrast sequence. The GTV was equal to the planning target volume (PTV). Treatment plans were devised with a goal of at least 99% of the PTV covered by 100% of prescription dose with dose most commonly prescribed to the 50 to 80% isodose line.
The LINAC-based VMAT radiosurgery utilized a single isocenter [13]. The computed tomography (CT) simulation scan with 0.8 or 1 mm slice thickness was manually fused with the MRI, and the GTV was delineated as aforementioned. All SRS doses ranged from 16-20 Gy depending on PTV diameter with lesions < 2 cm receiving 20 Gy, 2.1-3 cm receiving 18 Gy, 3.1-4 cm receiving 15 Gy and > 4 cm lesions receiving fractionated radiotherapy. Therapy was also fractionated if there was concern for treatment toxicity related to critical structure proximity. FSRT doses ranged from 25-30 Gy delivered in five fractions over one to two weeks, depending on physician preference. Plans were developed in Varian Eclipse treatment planning system utilizing Rapid Arc [13, 14].
Data collection, follow-up evaluation and data analysis
Patient, tumor and treatment characteristics were obtained from electronic medical records. Extra-cranial disease was defined as active systemic disease undergoing therapy. Karnofsky performance status (KPS) was assigned prospectively. Recursive partitioning analysis (RPA) class and disease-specific graded prognostic assessment (dsGPA) score were generated from patient data [15, 16]. Target volume was obtained from GK or Eclipse planning software. Follow-up included clinical examination and MRI at 1 month after treatment then at two to three-month intervals thereafter. Toxicity was scored using the Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0 [17]. Irreversible grade 3, or any grade 4 and 5 neurologic events were recorded.
Time to DBF, defined as new enhancing lesion on MRI or development of leptomeningeal disease, was defined from radiotherapy (RT) start date to DBF date or censored at date of last MRI if no DBF. Salvage modality was recorded. Overall survival (OS) was measured from start of RT to death date or censored at last clinical follow up. The primary end points of DBF and OS were estimated using the Kaplan-Meier method. Cox proportional hazards models were used to evaluate the association between total target volume and number of metastases and the DBF or OS.
Results
Patient and treatment demographics
Patient and treatment characteristics are summarized in Table 1 for the entire cohort, and additional details regarding patients who underwent FSRT given in Table 2. The median clinical follow-up for the living patients was 5.2 months (range: 1.1, 32.9) and 6.3 months (1.1, 51.4) for the entire cohort. Median radiographic follow-up was 3 months (1, 23). Median dsGPA predicted OS was 4.7 months. Of the seven patients treated with FSRT, median cumulative tumor volume was 17.4 cc compared to 1.2 cc in SRS patients (p = 0.001).
Table 1.
Patient and treatment characteristics.
| Characteristic | Study cohort (n = 36) Number of patients, (%) |
| Age at treatment, years | |
| Median [range] | 65.5 [23-82] |
| Gender | |
| Male | 16 (44 %) |
| Female | 20 (56 %) |
| Primary tumor | |
| Non small cell lung cancer | 13 (36 %) |
| Melanoma | 9 (25 %) |
| Breast | 8 (22 %) |
| Renal cell carcinoma | 3 (8 %) |
| Other | 3 (8 %) |
| Extra-cranial disease | |
| Yes | 30 (84 %) |
| No | 6 (16 %) |
| Karnofsky performance status | |
| 70 | 8 (22 %) |
| 80 | 7 (19 %) |
| 90 | 15 (42 %) |
| 100 | 3 (8 %) |
| Not prospectively assigned | 3 (8 %) |
| Recursive partitioning analysis class | |
| II | 33 (92 %) |
| Unclassified1 | 3 (8 %) |
| Diagnosis-specific GPA score | |
| 0-1 | 17 (47 %) |
| 1.5-2.5 | 7 (19 %) |
| 3 | 5 (14 %) |
| 3.5-4 | 1 (0.5 %) |
| Unclassified1 | 3 (8 %) |
| Not applicable | 3 (8 %) |
| SRS | |
| Dose, Gy | |
| Median [range] | 20 [16-20] |
| SRS technique | |
| Gamma knife | 27 (75 %) |
| LINAC-based | 2 (.05 %) |
| Fractionated stereotactic radiotherapy | 7 (19 %) |
| Dose, Gy | |
| Median [range] | 30 [25-30] |
| Number of brain metastases | |
| Median [range] | 6, [5-14] |
| 5 | 15, (42 %) |
| 6 | 8, (22 %) |
| 7 | 4, (11 %) |
| 8 | 5, (14 %) |
| 9 | 3, (8 %) |
| 14 | 1, (.03 %) |
| Cumulative tumor volume, cc | |
| Median [range] | 1.55, [0.12 – 32.96] |
| Salvage treatment | 19, (53 %) |
| SRS2 | 14, (74 %) |
| Whole brain radiotherapy2 | 5, (26 %) |
| Grade 3 toxicity | 3, (8 %) |
GPA, graded prognostic assessment; SRS, stereotactic radiosurgery; LINAC, linear accelerator; Gy, Gray; cc, cubic centimeter
Three patients lacking prospectively assigned KPS.
Denominator adjusted to reflect salvage patients only
Table 2.
Fractionated stereotactic radiotherapy characteristics
| Patient | Metastatic lesion number | Total metastatic volume, cc |
| 1 | 6 | 18.56 |
| 2 | 5 | 5.66 |
| 3 | 5 | 15.12 |
| 4 | 5 | 34.96 |
| 5 | 5 | 17.44 |
| 6 | 6 | 24.83 |
| 7 | 9 | 15.59 |
Treatment outcomes
Sixty-four percent developed DBF during the follow-up period with median time to DBF of 4.2 months. The six-month risk of DBF was 58% for all patients (Fig. 1). On multivariable analysis, neither tumor volume nor tumor number was significantly associated with DBF (Table 3). At the time of analysis, 24 patients (67 %) died with a median OS of 9.1 months (Fig. 2). OS probability at 1 year was 49%. Twenty percent of patients died with controlled central nervous systemic (CNS) disease, never having experienced a local relapse after SRS. Of the 24 patients with DBF, only 1 experienced leptomeningeal disease. Three patients experienced grade 3 toxicity including 2 patients with intolerance to steroid taper and 1 patient requiring inpatient admission for seizures. No patients had grade 4 or 5 toxicity. Of the 19 patients salvaged, 14 received repeat SRS and 5 received WBRT.
Figure 1.
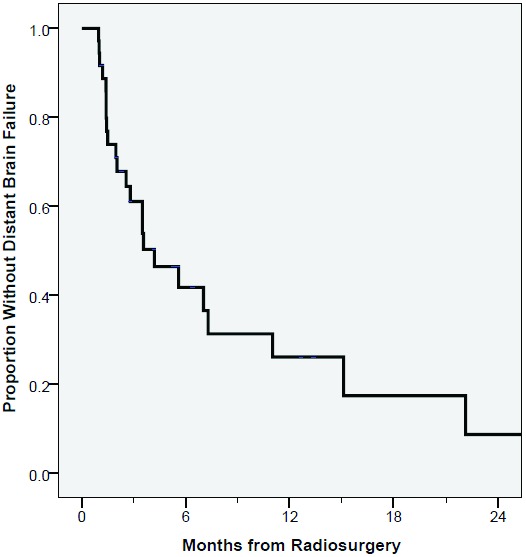
Kaplain-Meier curve of distant brain recurrence from date of radiation.
Table 3.
Multivariable analyses.
| Distant brain failure | Overall survival | |||||
| Covariate | P-value | HR | 95% CI | P-value | HR | 95% CI |
| Cumulative lesion volume, cc | 0.07 | 0.91 | 0.82-1.01 | 0.71 | 0.98 | 0.90-1.08 |
| Lesion number | 0.12 | 1.19 | 0.96-1.46 | 0.84 | 0.84 | 0.65-1.09 |
CC, cubic centimeters
Figure 2.
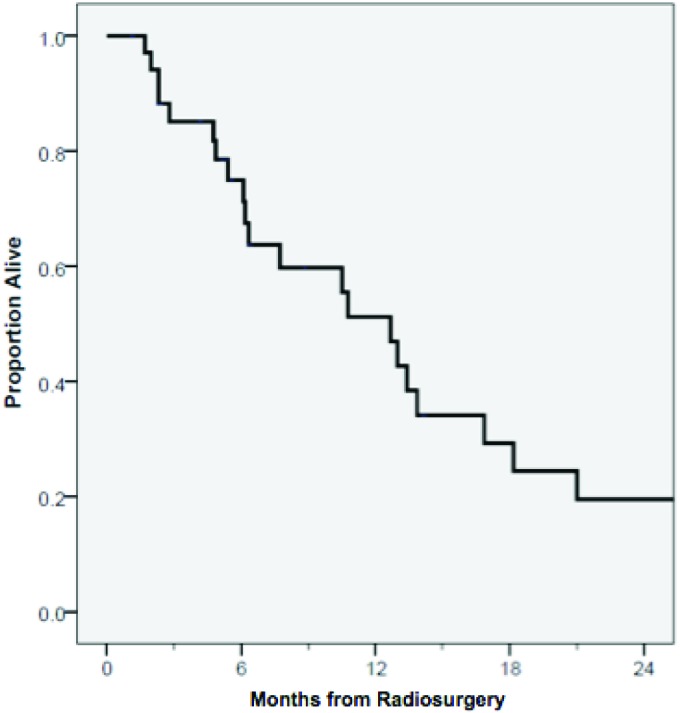
Kaplain-Meier curve of overall survival from date of radiation.
Discussion
In the past decade, radiotherapy for brain metastases has rapidly evolved, with WBRT being less utilized, even in the setting of multiple brain metastases. RCTs including patients with less than five brain metastases failed to show an increase in OS, improvement in KPS or reduction in neurologic deaths with the addition of WBRT to SRS [6, 10, 11, 18]. One randomized controlled trial reported inferior OS of patients treated with SRS plus WBRT vs. SRS alone [4]. Although WBRT reduces DBF, a significant portion of these patients observed after SRS will not fail distantly and can be spared unnecessary adjuvant WBRT without increased morality risk or functional decline. In addition, a majority of brain metastases patients will succumb to their disease due to extra-cranial disease progression. Moreover, prospective data demonstrated WBRT leads to worsened quality of life (QOL) and cognitive deficits such as learning and memory decline as early as 4 months [4, 5]. As a result, the National Comprehensive Cancer Network endorses upfront SRS alone in patients with one to three brain metastases, and the American Society for Radiation Oncology has specifically recommended against the addition of WBRT to SRS [19, 20]. Foregoing adjuvant WBRT in patients with more than five brain metastases remains controversial, as there are no published prospective RCT in this patient subset. A prospective phase III trial comparing SRS versus WBRT in patients with 4 to 10 newly diagnosed non-melanoma brain metastases is currently recruiting participants [21].
Physicians question whether patients with five or more brain metastases have prohibitively high DBF rates in the absence of WBRT. In the present study, 64% of patients experienced DBF, which is comparable to the 42-64% DBF rate observed in patients with one to three brain metastases following SRS alone [4, 10, 11]. The similarity in DBF rates confirms the brain lesion number does not always correlate with DBF risk and should not be the sole factor regarding adjuvant WBRT. Although WBRT would likely reduce DBF by 30%, 20% of patients died of systemic disease without CNS relapse and were presumably spared WBRT’s toxicity. Data suggests WBRT delays DBF for approximately 6 months [10]. Our six-month risk of DBF was 58% indicating almost half of our patients would not have benefited from upfront WBRT. Our results support prior work which claims WBRT can be delayed or excluded in patients with more than four brain metastases [22]. WBRT’s DBF reduction must be weighed against the negative impact on cognition and QOL.
Focal stereotactic radiotherapy for several brain metastases has been criticized as over utilization of resources in a patient population with a limited life expectancy. At the time of analysis, 67% of patients had died with a median OS of 9.1 months which is similar to patients with fewer than five brain metastases [9, 23]. retrospective data suggests number of metastases is not prognostic for OS [23-25]. Despite having five or more brain metastases, our cohort’s OS was better than their median dsGPA predicted survival. OS remains multifactorial, and lesion number should not preclude aggressive focal stereotactic therapy.
The majority of our patients had extra-cranial disease receiving systemic therapy. WBRT requires longer treatment breaks compared to SRS. Given the majority of patients die from extra-cranial disease, it is important to deliver CNS directed therapy expeditiously, allowing for resumption of systemic treatment [8, 9]. For example, BRAF inhibitors given with concurrent WBRT result in severe skin reactions, but concurrent SRS does not result in grade 2 or higher dermatitis [26]. Shortened radiation courses also afford patients fewer clinics visits in a population burdened with frequent medical appointments and limited life expectancy. Despite our cohort’s extra-cranial disease, they had a reasonable life expectancy. As systemic agents improve, CNS control will become even more essential and the toxicities incurred with WBRT may become more apparent.
Toxicity concerns persist regarding SRS for more than four targets. Three patients experienced grade 3 toxicities with no grade 4 toxicity, which is comparable to published toxicity rates in individuals with four or less metastases [4, 8, 9]. These low toxicity rates suggest multiple target SRS was well tolerated, especially in light of the cognitive detriments caused by WBRT.
While the cohort was predominantly lung and breast primaries, representative of the general population, 25% were melanoma histology, which is a risk factor for DBF [27]. Despite the small patient number, CNS directed therapy was homogenous as patients with prior WBRT, SRS or resections were excluded. Seven patients received FSRT rather than SRS due to increased disease volume or critical structure proximity, but published literature suggests equivalent local control and OS in SRS and FSRT [28, 29].
Due to its retrospective nature, our results are subject to selection bias. Despite multiple brain metastases and extra-cranial disease, 92% of the cohort classified as RPA II suggesting poor KPS patients received WBRT as upfront therapy. Neurocognitive and QOL outcomes were not captured. Furthermore cause of death could not be assessed in all patients.
Conclusion
SRS and FSRT were well tolerated and delayed or prevented the need for WBRT in a portion of patients. Our data corroborates prior studies and further supports that focal stereotactic therapy alone in a select group of patients is a safe alternative to upfront WBRT. Given the toxicity concerns with adjuvant WBRT, we assert it is reasonable to provide focal stereotactic therapy and withhold WBRT with close serial imaging.
Acknowledgements
The Department of Radiation Oncology at the University of Alabama at Birmingham supported this study.
Authors’ disclosure of potential conflicts of interest
The authors reported no conflict of interest.
Author contributions
Conception and design: Dr. John Fiveash, Dr. Olivia Claire Barrett, and Dr. Jonathan Thompson
Data collection: Dr. Jonathan Thompson and Dr. Olivia Claire Barrett
Data analysis and interpretation: Dr. Olivia Claire Barrett, Dr. Gerald McGwin, Dr. Andrew McDonald, Dr. Markus Bredel
Manuscript writing: Dr. Olivia Claire Barrett, Dr. Jonathan Thompson, Dr. Andrew McDonald
Final approval of manuscript: Dr. Markus Bredel, Dr. Kristen Riley, Dr. John Fiveash
References
- Posner JB: Management of brain metastases. Rev Neurol (Paris) 1992, 148(6-7):477-487. [PubMed] [Google Scholar]
- Gavrilovic IT, Posner JB: Brain metastases: epidemiology and pathophysiology. J Neurooncol 2005, 75(1):5-14. [DOI] [PubMed] [Google Scholar]
- Delattre JY, Krol G, Thaler HT, Posner JB: Distribution of brain metastases. Arch Neurol 1988, 45(7):741-744. [DOI] [PubMed] [Google Scholar]
- Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, Kornguth DG, Arbuckle RB, Swint JM, Shiu AS, Maor MH, Meyers CA: Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol 2009, 10(11):1037-1044. [DOI] [PubMed] [Google Scholar]
- Soffietti R, Kocher M, Abacioglu UM, Villa S, Fauchon F, Baumert BG, Fariselli L, Tzuk-Shina T, Kortmann RD, Carrie C, Ben Hassel M, Kouri M, Valeinis E, van den, Berge D, Mueller RP, Tridello G, Collette L, Bottomley A: A European Organisation for Research and Treatment of Cancer phase III trial of adjuvant whole-brain radiotherapy versus observation in patients with one to three brain metastases from solid tumors after surgical resection or radiosurgery: quality-of-life results. J Clin Oncol 2013, 31(1):65-72. [DOI] [PubMed] [Google Scholar]
- Brown PD, Asher AL, Ballman KV, Farace E, Cerhan JH, Anderson SK, Carrero XW, Barker FG, Deming RL, Burri S: NCCTG N0574 (Alliance): A phase III randomized trial of whole brain radiation therapy (WBRT) in addition to radiosurgery (SRS) in patients with 1 to 3 brain metastases. In: ASCO Annual Meeting Proceedings: 2015; 2015: LBA4. [Google Scholar]
- Cairncross JG, Kim JH, Posner JB: Radiation therapy for brain metastases. Ann Neurol 1980, 7(6):529-541. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Kawabe T, Sato Y, Higuchi Y, Nariai T, Barfod BE, Kasuya H, Urakawa Y: A case-matched study of stereotactic radiosurgery for patients with multiple brain metastases: comparing treatment results for 1-4 vs >/= 5 tumors: clinical article. J Neurosurg 2013, 118(6):1258-1268. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Serizawa T, Shuto T, Akabane A, Higuchi Y, Kawagishi J, Yamanaka K, Sato Y, Jokura H, Yomo S, Nagano O, Kenai H, Moriki A, Suzuki S, Kida Y, Iwai Y, Hayashi M, Onishi H, Gondo M, Sato M, Akimitsu T, Kubo K, Kikuchi Y, Shibasaki T, Goto T, Takanashi M, Mori Y, Takakura K, Saeki N, Kunieda E, Aoyama H, Momoshima S, Tsuchiya K: Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol 2014, 15(4):387-395. [DOI] [PubMed] [Google Scholar]
- Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, Hatano K, Kenjyo M, Oya N, Hirota S, Shioura H, Kunieda E, Inomata T, Hayakawa K, Katoh N, Kobashi G: Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA 2006, 295(21):2483-2491. [DOI] [PubMed] [Google Scholar]
- Kocher M, Soffietti R, Abacioglu U, Villa S, Fauchon F, Baumert BG, Fariselli L, Tzuk-Shina T, Kortmann RD, Carrie C, Ben Hassel M, Kouri M, Valeinis E, van den Berge D, Collette S, Collette L, Mueller RP: Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol 2011, 29(2):134-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar AK, Flickinger JC, Kondziolka D, Lunsford LD: Stereotactic radiosurgery for four or more intracranial metastases. Int J Radiat Oncol Biol Phys 2006, 64(3):898-903. [DOI] [PubMed] [Google Scholar]
- Clark GM, Popple RA, Young PE, Fiveash JB: Feasibility of single-isocenter volumetric modulated arc radiosurgery for treatment of multiple brain metastases. Int J Radiat Oncol Biol Phys 2010, 76(1):296-302. [DOI] [PubMed] [Google Scholar]
- Clark GM, Popple RA, Prendergast BM, Spencer SA, Thomas EM, Stewart JG, Guthrie BL, Markert JM, Fiveash JB: Plan quality and treatment planning technique for single isocenter cranial radiosurgery with volumetric modulated arc therapy. Pract Radiat Oncol 2012, 2(4):306-313. [DOI] [PubMed] [Google Scholar]
- Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, McKenna WG, Byhardt R: Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys 1997, 37(4):745-751. [DOI] [PubMed] [Google Scholar]
- Sperduto PW, Chao ST, Sneed PK, Luo X, Suh J, Roberge D, Bhatt A, Jensen AW, Brown PD, Shih H, Kirkpatrick J, Schwer A, Gaspar LE, Fiveash JB, Chiang V, Knisely J, Sperduto CM, Mehta M: Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys 2010, 77(3):655-661. [DOI] [PubMed] [Google Scholar]
- Health UDo, Services H: Common terminology criteria for adverse events (CTCAE) version 4.0. National Institutes of Health, National Cancer Institute 2009, 4(03). [Google Scholar]
- Aoyama H, Tago M, Kato N, Toyoda T, Kenjyo M, Hirota S, Shioura H, Inomata T, Kunieda E, Hayakawa K, Nakagawa K, Kobashi G, Shirato H: Neurocognitive function of patients with brain metastasis who received either whole brain radiotherapy plus stereotactic radiosurgery or radiosurgery alone. Int J Radiat Oncol Biol Phys 2007, 68(5):1388-1395. [DOI] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network: Central Nervous System Tumors Version 1.2015. Also available at www nccn org.
- Tsao MN, Rades D, Wirth A, Lo SS, Danielson BL, Gaspar LE, Sperduto PW, Vogelbaum MA, Radawski JD, Wang JZ, Gillin MT, Mohideen N, Hahn CA, Chang EL: Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): An American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol 2012, 2(3):210-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MDACC: A Prospective Phase III Trial to Compare Stereotactic Radiosurgery Versus Whole Brain Radiation Therapy. In: In: ClinicalTrialsgov [Internet] Bethesda (MD): National Library of Medicine (US) 2000- [cited 2016 June 11]. [Google Scholar]
- Ojerholm E, Lee JY, Kolker J, Lustig R, Dorsey JF, Alonso-Basanta M: Gamma Knife radiosurgery to four or more brain metastases in patients without prior intracranial radiation or surgery. Cancer Med 2014, 3(3):565-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang WS, Kim HY, Chang JW, Park YG, Chang JH: Analysis of radiosurgical results in patients with brain metastases according to the number of brain lesions: is stereotactic radiosurgery effective for multiple brain metastases?. J Neurosurg 2010, 113 Suppl:73-78. [DOI] [PubMed] [Google Scholar]
- Salvetti DJ, Nagaraja TG, McNeill IT, Xu Z, Sheehan J: Gamma Knife surgery for the treatment of 5 to 15 metastases to the brain: clinical article. J Neurosurg 2013, 118(6):1250-1257. [DOI] [PubMed] [Google Scholar]
- Serizawa T, Hirai T, Nagano O, Higuchi Y, Matsuda S, Ono J, Saeki N: Gamma knife surgery for 1-10 brain metastases without prophylactic whole-brain radiation therapy: analysis of cases meeting the Japanese prospective multi-institute study (JLGK0901) inclusion criteria. J Neurooncol 2010, 98(2):163-167. [DOI] [PubMed] [Google Scholar]
- Anker CJ, Grossmann KF, Atkins MB, Suneja G, Tarhini AA, Kirkwood JM: Avoiding Severe Toxicity From Combined BRAF Inhibitor and Radiation Treatment: Consensus Guidelines from the Eastern Cooperative Oncology Group (ECOG). Int J Radiat Oncol Biol Phys 2016, 95(2):632-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawrie SM, Guthrie BL, Spencer SA, Nordal RA, Meredith RF, Markert JM, Cloud GA, Fiveash JB: Predictors of distant brain recurrence for patients with newly diagnosed brain metastases treated with stereotactic radiosurgery alone. Int J Radiat Oncol Biol Phys 2008, 70(1):181-186. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Cho KH, Kim JY, Lim YK, Min HS, Lee SH, Kim HJ, Gwak HS, Yoo H, Lee SH: Single-dose versus fractionated stereotactic radiotherapy for brain metastases. Int J Radiat Oncol Biol Phys 2011, 81(2):483-489. [DOI] [PubMed] [Google Scholar]
- Fokas E, Henzel M, Surber G, Kleinert G, Hamm K, Engenhart-Cabillic R: Stereotactic radiosurgery and fractionated stereotactic radiotherapy: comparison of efficacy and toxicity in 260 patients with brain metastases. J Neurooncol 2012, 109(1):91-98. [DOI] [PubMed] [Google Scholar]


