Abstract
Pantothenic acid, a precursor of the crucial enzyme cofactor coenzyme A, is one of a relatively few nutrients for which the intraerythrocytic parasite has an absolute and acute requirement from the external medium. In some organisms the provitamin pantothenol can serve as a source of pantothenic acid; however, this was not the case for the human malaria parasite Plasmodium falciparum. Instead, pantothenol inhibited the in vitro growth of P. falciparum via a mechanism that involves competition with pantothenate and which can be attributed to inhibition of the parasite's pantothenate kinase. Oral administration of pantothenol to mice infected with the murine parasite Plasmodium vinckei vinckei resulted in a significant inhibition of parasite proliferation. This study highlights the potential of the coenzyme A biosynthesis pathway in general, and pantothenate kinase in particular, as an antimalarial drug target.
Vitamin B5, pantothenic acid, is a precursor of the important enzyme cofactor coenzyme A (CoA). Analogs of pantothenic acid have been known for over half a century to inhibit the growth of a range of microorganisms (6, 11, 17). In early work on the pantothenic acid requirement of malaria parasites (Plasmodium spp.) it was shown that in chickens infected with Plasmodium gallinaceum and either fed on a pantothenic acid-deficient diet or dosed orally with pantothenic acid analogs, there was significant suppression of the proliferation of blood-stage parasites (7). Similarly, in early in vitro culture experiments Trager showed that a pantothenate analog caused a marked reduction in the proliferation of a number of different malaria parasite strains, including the most virulent of the human malaria parasites, P. falciparum (18, 19).
Biochemical analyses on both uninfected and Plasmodium lophurae-infected duck erythrocytes, as well as on P. lophurae parasites isolated from their host erythrocytes, revealed that duck erythrocytes have all of the enzymes for CoA biosynthesis, whereas the intraerythrocytic parasites themselves have none (5, 8). This led to the proposal that the parasite takes up preformed CoA from the host cell. Consistent with this, the survival of isolated P. lophurae parasites in axenic culture was shown to be enhanced by the inclusion of CoA (but not a number of its precursors) in the extracellular medium (20).
Following the establishment of methodology for the long-term culture of P. falciparum in human erythrocytes, Divo et al. (10) showed that sustained culture required the presence of pantothenate in the external medium. Pantothenate is not taken up by uninfected human erythrocytes but enters parasitized erythrocytes via the new permeability pathways induced by the parasite in the host erythrocyte membrane (15). The human erythrocyte has limited ability to metabolize pantothenate, and the vitamin is taken up by the intracellular parasite (via a low-affinity H+-coupled transporter [16]) and phosphorylated (by a high-affinity kinase [16]). Genes encoding candidates for most (if not all) of the enzymes involved in the conversion of pantothenate to CoA are present in the P. falciparum genome (12). The available biochemical and bioinformatic evidence therefore both point to the human parasite P. falciparum, unlike the avian parasite P. lophurae, synthesizing CoA from pantothenate taken up from the host cell cytosol.
Pantothenol, or provitamin B5, is widely used in the health-care and cosmetics industries; it is a common ingredient of multivitamin supplements, shampoos, and skin-care products. Pantothenol has been administered at high doses, orally, to humans (10 to 15 g per day for a prolonged period for the treatment of lupus) without significant side effects (22, 23) and has been shown to inhibit the growth of a range of different bacteria in vitro (17). In this study we have investigated the effect of pantothenol on the proliferation of malaria parasites. We show that the provitamin is an effective inhibitor of the growth of P. falciparum parasites in vitro, exerting its influence via an effect on pantothenate metabolism, as well as suppressing the proliferation of parasites in an in vivo model.
MATERIALS AND METHODS
Reagents.
Pantothenol was obtained from Daiichi Pharmaceutical Co. Ltd., Tokyo, Japan. Stock solutions of pantothenol were prepared in water. [14C]pantothenate (51.5 mCi/mmol) was purchased from New England Nuclear.
Cell culture and in vitro growth assays.
In vitro experiments involving malaria parasites were carried out using the P. falciparum 3D7 or FAF6 strains maintained in continuous culture as described previously (2). Experiments designed to test the ability of the parasite to grow in vitamin-free RPMI 1640 in the presence or absence of pantothenate (1 μM) or pantothenol (1 μM) were carried out under conditions similar to those described by Divo and colleagues (10) except that premixed amino acid and vitamin mixtures (Sigma-Aldrich) were used to make up the RPMI 1640 culture medium. The media used for the in vitro growth assays were supplemented with 25 mM HEPES, 11 mM glucose, 2.4 μM hypoxanthine, 24 μg of gentamicin/ml, and 0.6% Albumax II.
Parasite proliferation was monitored using a standard [3H]hypoxanthine incorporation assay (9). Experiments were carried out in 96-well plates, starting with parasites in the ring stage and with the hematocrit and parasitemia both set at 1%. Twofold serial dilutions of pantothenol were made in duplicate or triplicate wells. The final volume in each well was 200 μl. Uninfected cells (1% hematocrit) served as blank controls, and parasites cultured in the absence of drug served to estimate 100% parasite growth. The plates were placed in a desiccator cabinet, flushed with a gas mixture comprised of 1% oxygen, 3% carbon dioxide, and 96% nitrogen, and incubated for 72 h at 37°C before adding 0.4 μCi of [3H]hypoxanthine in a volume of 25 μl of vitamin-free RPMI 1640 and incubating for a further 24 h under the same conditions. The cells were then harvested onto glass fiber filters, and the amount of [3H]hypoxanthine incorporated into nucleic acids was measured by scintillation counting.
Jurkat cells (a human T-cell leukemia cell line) were grown in RPMI 1640 supplemented with 25 mM HEPES, 11 mM glucose, 24 μg of gentamicin/ml, and 10% fetal calf serum. Viability assays were carried out using [3H]hypoxanthine as described above, except that the cells were seeded at a density of 5,000 cells/ml (in a volume of 200 μl per well) and were incubated in a 5% CO2 incubator. Doxycycline, an antimalarial shown previously to inhibit Jurkat cell growth (13), was included as a positive control (50% inhibitory concentration [IC50] = 13.6 ± 0.9 μM [mean ± standard error]; n = 3).
[14C]pantothenate accumulation, transport, and phosphorylation.
The effect of pantothenol on the accumulation of [14C]pantothenate by P. falciparum parasites isolated from the host erythrocytes by saponin permeabilization of the erythrocyte and parasitophorous vacuole membranes (3, 4) was measured using a protocol described previously (15). Briefly, isolated parasites suspended in saline (125 mM NaCl, 5 mM KCl, 20 mM glucose, 25 mM HEPES, 1 mM MgCl2; pH 7.1) were combined with [14C]pantothenate (0.1 μCi/ml) in the presence or absence of 1 mM pantothenol and incubated at 37°C. At predetermined time intervals, 300 μl of the suspension was layered onto 300 μl of oil (a 5:4 mixture of dibutyl phthalate-dioctyl phthalate). The accumulation of [14C]pantothenate was terminated by centrifugation (2 min, 15,800 × g), and the pellets were processed as described previously (15, 16). The amount of radioactivity trapped in the extracellular portion of the cell pellet was estimated in a separate batch of cells treated with 800 μM phloretin in order to inhibit the pantothenate transporter (16). The phloretin-treated cells were mixed with [14C]pantothenate (in the same proportions as in the uptake experiment), and then aliquots were sampled immediately for centrifugation through the oil mix and subsequent processing. The counts per minute in the pellets derived from phloretin-treated cells were subtracted from those obtained in untreated samples.
The H+-coupled transport of [14C]pantothenate across the parasite plasma membrane was measured in ATP-depleted parasites (in order to prevent the phosphorylation and hence the “metabolic trapping” of the radiolabeled compound) as described previously (15, 16). Briefly, the parasites were depleted of ATP by preincubation (for 15 min at 37°C and pH 7.3) in a glucose-free medium. At time zero the parasites were added to a medium of pH 5.5 (thereby imposing a large inward [H+] gradient) containing [14C]pantothenate, and the uptake of [14C]pantothenate within the parasites was measured over 20 s. Under these conditions the radiolabel accumulates within the parasite as a direct result of the H+-coupled transport mechanism. Compounds that inhibit the transporter reduce the accumulation (16).
The rate of phosphorylation of [14C]pantothenate (0.1 μM) by pantothenate kinase in parasite lysates was measured at 37°C as described previously (15, 16). [14C]pantothenate phosphorylation time courses were carried out in the presence or absence of different concentrations of pantothenol, and the rate of phosphorylation was estimated from the linear portion of the time course. The duration of the linear portion varied between different batches of lysates but was usually at least 30 min.
P. vinckei vinckei experiment.
The standard 4-day suppressive test (14) was used to assess the efficacy of pantothenol against malaria parasites in vivo. Female BALB/c mice, between 8 and 9 weeks old, were infected intraperitoneally with 107 P. vinckei vinckei parasites in a volume of 200 μl of saline. On the same day (about 2 h later), mice were selected at random, weighed, and then administered orally 1.4 g of pantothenol/kg of body weight (pantothenol was dissolved in H2O at a concentration of 70 mg/ml) or an equivalent volume of water (typically 300 to 400 μl) by gavage. The mice were treated with pantothenol or water for an additional 3 days (once daily). The day after the administration of the fourth dose, blood smears were made from all the mice to monitor the parasitemia. The parasitemia was estimated by counting on average of >500 cells/slide. Subsequently, the parasitemia was monitored daily and mice showing a parasitemia higher than 25% were euthanized immediately. The experiment was carried out twice independently and involved a total of 19 control mice and 14 pantothenol-treated mice. No toxic side effects or weight loss was detected in mice treated with pantothenol (average weight = 17.6 ± 0.4 g at the start of the experiment and 17.8 ± 0.4 g 4 days later; P = 0.7).
RESULTS
Pantothenol inhibits the growth of P. falciparum in vitro.
The chemical structure of pantothenic acid and its provitamin, pantothenol, are shown in Fig. 1A. Pantothenic acid is one of a number of water-soluble vitamins present in culture medium (and in blood plasma). As shown in Fig. 1B, removal of all of the water-soluble vitamins from the medium resulted in a complete cessation of parasite growth. Addition of pantothenic acid (by itself) to the medium, at a physiological concentration (1 μM), restored growth to control levels. Pantothenic acid is therefore the only water-soluble vitamin for which the parasite has an absolute requirement in the medium. Pantothenol (1 μM) was unable to substitute for pantothenate in supporting parasite growth (Fig. 1B).
FIG. 1.
(A) Chemical structures of pantothenic acid and its alcohol, pantothenol. (B) Pantothenate dependence of the growth of P. falciparum in in vitro culture. Removal of the water-soluble vitamins from the culture medium resulted in a cessation of parasite growth. Restoration of just one of these, pantothenate, restored parasite growth to control levels. Pantothenol was unable to substitute for pantothenate in supporting parasite growth. The data are averaged from three or more independent experiments. Error bars represent the SEM.
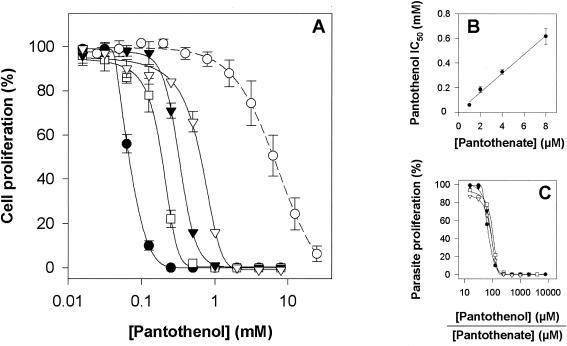
Following on from the finding that the parasite was unable to utilize pantothenol as a vitamin source, we tested the effect of pantothenol on the growth of parasites in normal (vitamin-containing) culture medium. As shown in Fig. 2A, pantothenol inhibited parasite growth in a concentration-dependent manner. Under physiological conditions, the IC50 for parasite growth inhibition (i.e., the concentration of pantothenol required to reduce [3H]hypoxanthine incorporation by 50%) was 60 ± 4 μM (mean ± the standard error of the mean [SEM]; n = 3). Much higher concentrations of pantothenol were required to inhibit the proliferation of a human cell line (Jurkat cells; IC50 = 7.1 ± 1.5 mM; n = 4) (Fig. 2A). Increasing the concentration of pantothenate in the parasite culture medium caused a rightward shift of the dose-response curves (Fig. 2A), with the IC50 values increasing linearly with pantothenate concentration (Fig. 2B). The data are consistent with the antiplasmodial effect of pantothenol being due to an effect on pantothenate utilization, rather than to a nonspecific effect, and with the vitamin and provitamin competing for one or more common sites in the parasite. This is borne out by the observation that when the growth inhibition data are plotted as a function of the ratio of the concentrations of pantothenol and pantothenate in the medium, the curves overlie one another (Fig. 2C), with 50% growth inhibition observed at a pantothenol/pantothenate ratio of approximately 100 and full inhibition of growth at ratios of >200.
FIG. 2.
(A) Inhibition of the in vitro growth of P. falciparum and human Jurkat cells by pantothenol. The dose-response curves show the effect of increasing concentrations of pantothenol on [3H]hypoxanthine incorporation by parasites cultured (for 96 h) in medium containing 1 (filled circles), 2 (open squares), 4 (filled triangles), or 8 μM pantothenate (open triangles) or by Jurkat cells cultured (for 96 h) in medium containing 1 μM pantothenate (open circles). (B) Linear relationship between the IC50 for inhibition of [3H]hypoxanthine incorporation by pantothenol and the concentration of pantothenate in the culture medium. (C) Dose-response curves for the inhibition of [3H]hypoxanthine incorporation as a function of the ratio of the concentration of pantothenol to the concentration of pantothenate in the medium (symbols represent the same pantothenate concentrations as in panel A). The dose-response curves are superimposable, demonstrating the competitive and specific nature of the effect of pantothenol on parasite proliferation. The data are averaged from three independent experiments. Error bars in panels A and B represent SEM and, where not shown, fall within the symbols. Error bars are omitted from panel C for clarity.
Pantothenol inhibits the phosphorylation of pantothenate by the parasite.
The effect of pantothenol on pantothenate utilization by the parasite was investigated directly by measuring the uptake of [14C]pantothenate into mature trophozoite-stage parasites isolated from their host erythrocytes by saponin permeabilization of the erythrocyte and parasitophorous vacuole membranes. The pantothenate concentration was 2 μM. As shown in Fig. 3A, pantothenol had a profound effect on the accumulation of [14C]pantothenate by the parasite. In the absence of pantothenol, [14C]pantothenate accumulation increased throughout the incubation period, reaching a distribution ratio (i.e., the ratio of the concentration of radiolabel in the parasite to that in the extracellular solution) of >20 by 30 min. By contrast, in the presence of pantothenol (1 mM) the [14C]pantothenate simply equilibrated across the parasite plasma membrane, with the estimated distribution ratio leveling off, after 10 min, at a value of approximately 1 (i.e., the estimated concentration inside the parasite was approximately equal to that in the external medium).
FIG. 3.
(A) Accumulation of [14C]pantothenate into isolated P. falciparum trophozoites in the presence (open circles) or absence (closed circles) of 1 mM pantothenol. [[14C]Pantothenate]i/[[14C]Pantothenate]o is the ratio of the concentration of [14C]pantothenate inside the parasite to that in the extracellular medium. (B) H+-coupled transport of [14C]pantothenate across the parasite plasma membrane in the presence or absence of 1 mM pantothenol or 0.2 mM phloretin. (C) Effect of pantothenol on the rate of phosphorylation of [14C]pantothenate in a parasite lysate. The dashed line in panel C represents the dose-response curve for the effect of pantothenol on [3H]hypoxanthine incorporation by the parasite (averaged from Fig. 2C). Complete inhibition of pantothenate phosphorylation in the lysate coincided with full inhibition of [3H]hypoxanthine incorporation. The data in panels A and B are averaged from two independent experiments (error bars represent the range divided by 2), and those in panel C are averaged from three experiments (error bars represent SEM). Where not shown, error bars fall within the symbols.
The data of Fig. 3A are consistent with pantothenol exerting its effect on [14C]pantothenate accumulation via an effect on the phosphorylation process by which pantothenate is trapped within the parasite, but they do not preclude the possibility that the compound also inhibits the H+-coupled transport of pantothenate. The effect of pantothenol on the H+-coupled transport of pantothenate across the parasite plasma membrane was investigated in parasites depleted of ATP (by preincubation in a glucose-free medium) and suspended under conditions of a large inward H+ concentration gradient. In the absence of ATP the parasite is unable to phosphorylate pantothenate and accumulation is attributable wholly to the transporter (16). As shown in Fig. 3B, pantothenol (1 mM) had a very small inhibitory effect (∼10%) on the transport of pantothenate (2 μM). The small effect of pantothenol contrasts with the profound effect of phloretin, an effective inhibitor of the transporter (included here as a positive control) which, under the conditions of the experiment, reduced [14C]pantothenate accumulation by >90% (Fig. 3B).
The effect of pantothenol on the phosphorylation of pantothenate in the parasite cytosol was investigated directly by testing the effect of pantothenol on the rate of phosphorylation of [14C]pantothenate in a parasite lysate. As can be seen in Fig. 3C, pantothenol was an effective inhibitor of [14C]pantothenate phosphorylation, with a 50% decrease in the phosphorylation rate observed at a [pantothenol]/[pantothenate] ratio of approximately 10. For comparison, Fig. 3C also shows the (average) dose-response curve for the inhibition of the in vitro growth of the parasites (from Fig. 2C). Growth inhibition was observed at [pantothenol]/[pantothenate] ratios sufficient to give ≥50% inhibition of pantothenate phosphorylation, and parasite growth was inhibited fully at [pantothenol]/[pantothenate] ratios at which phosphorylation was inhibited by ≥90%.
Pantothenol suppresses the proliferation of P. vinckei vinckei in vivo.
The effect of pantothenol on the proliferation of the murine parasite P. vinckei vinckei, in vivo, was investigated using a standard 4-day suppression test. As shown in Fig. 4, daily oral administration of pantothenol for the 4 days immediately following infection resulted in a significant (∼85%) decrease in parasitemia relative to controls (as measured on the fourth day postinfection) (Fig. 4A) and prolonged the average survival time of the mice by ∼5 days (Fig. 4B).
FIG. 4.
Effect of oral administration of pantothenol on the proliferation of P. vinckei vinckei in an in vivo 4-day suppression test. (A) Parasitemia in mice measured the day after 4 days of treatment with pantothenol or solvent (water) control. Error bars represent SEM. (B) Survival of mice in the days following the end of the 4-day treatment regimen with pantothenol (open circles) or solvent (water; filled circles).
DISCUSSION
With the emergence and spread of malaria parasites that are resistant to most of the antimalarial drugs presently available, there is an urgent need for the identification of new drug targets and the development of new antimalarial strategies. The intraerythrocytic parasite's rapid growth and proliferation are underpinned by a high rate of metabolism and are dependent on the uptake of a number of key nutrients from the external medium. One of these is pantothenic acid, the precursor of CoA (10). In early studies carried out primarily, though not exclusively, on avian malaria a number of pantothenate analogs were shown to inhibit parasite proliferation; however, there has been relatively little work in this area in mammalian systems.
In Plasmodium-infected avian erythrocytes, pantothenate is converted to CoA in the erythrocyte compartment and the CoA is then taken up across the parasite plasma membrane (8). By contrast, in P. falciparum-infected human erythrocytes pantothenic acid is taken up by and metabolized within the parasite (15, 16). There are therefore fundamental differences between the pathways of pantothenate utilization in the avian and mammalian parasites.
In some organisms and cell types, pantothenol is converted (by alcohol dehydrogenase [1]) to pantothenate and therefore serves as a provitamin. However, this is evidently not the case in P. falciparum-infected human erythrocytes. Pantothenol failed to support parasite growth when substituted for pantothenate in the medium (Fig. 1), consistent with neither the parasite nor its host cell being able to convert pantothenol to pantothenate. Furthermore, pantothenol actually inhibited parasite growth (at concentrations at which it had no effect on the growth of a human cell line) through a mechanism that involved competition with pantothenate. Pantothenol did not prevent the H+-coupled transport of pantothenate across the parasite plasma membrane (Fig. 3A and B) but inhibited its subsequent phosphorylation (by pantothenate kinase, the first of five enzymes involved in the synthesis of CoA [21]) both in intact parasites and parasite lysates (Fig. 3C). The nature of the interaction of pantothenol with the kinase, whether pantothenol actually undergoes phosphorylation, and whether this is the sole site of action within the parasite are as yet unclear.
In the in vivo model, pantothenol caused a significant inhibition of parasite proliferation (Fig. 4A), but it did not elicit a cure in any of the mice in the group (Fig. 4B). A crucial consideration in the in vivo situation is the rate at which pantothenol in the serum is converted to pantothenate. Given the competitive nature of the interaction between pantothenol and pantothenate, the conversion of pantothenol to pantothenate (and the consequent decrease in the [pantothenol]/[pantothenate] ratio) will act to mitigate the effectiveness of the provitamin. In this context, nonmetabolizable analogs of pantothenate offer obvious advantages, and such compounds may have significant promise as new and much-needed antimalarials.
Acknowledgments
This work was supported by a grant from the Australian National Health and Medical Research Council (224245).
We are grateful to the Canberra Branch of the Australian Red Cross Blood Service for the provision of blood and to Daiichi Pharmaceutical Co. Ltd. for the provision of pantothenol.
REFERENCES
- 1.Abiko, Y., M. Tomikawa, and M. Shimizu. 1969. Enzymatic conversion of pantothenylalcohol to pantothenic acid. J. Vitaminol. 15:59-69. [DOI] [PubMed] [Google Scholar]
- 2.Allen, R. J. W., and K. Kirk. 2004. The membrane potential of the intraerythrocytic malaria parasite Plasmodium falciparum. J. Biol. Chem. 279:11264-11272. [DOI] [PubMed] [Google Scholar]
- 3.Ansorge, I., J. Benting, S. Bhakdi, and K. Lingelbach. 1996. Protein sorting in Plasmodium falciparum-infected red blood cells permeabilized with the pore-forming protein streptolysin O. Biochem. J. 315:307-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ansorge, I., K. Paprotka, S. Bhakdi, and K. Lingelbach. 1997. Permeabilization of the erythrocyte membrane with streptolysin O allows access to the vacuolar membrane of Plasmodium falciparum and a molecular analysis of membrane topology. Mol. Biochem. Parasitol. 84:259-261. [DOI] [PubMed] [Google Scholar]
- 5.Bennett, T. P., and W. Trager. 1967. Pantothenic acid metabolism during avian malaria infection: pantothenate kinase activity in duck erythrocytes and in Plasmodium lophurae. J. Protozool. 14:214-216. [DOI] [PubMed] [Google Scholar]
- 6.Bird, O. D., E. L. Wittle, R. Q. Thompson, and V. M. McGlohon. 1955. Pantothenic acid antagonists. Am. J. Clin. Nutr. 3:298-304. [DOI] [PubMed] [Google Scholar]
- 7.Brackett, S., E. Waletzky, and M. Baker. 1946. The relation between pantothenic acid and Plasmodium gallinaceum infections in the chicken and the antimalarial activity of analogues of pantothenic acid. J. Parasitol. 32:453-462. [PubMed] [Google Scholar]
- 8.Brohn, F. H., and W. Trager. 1975. Coenzyme A requirement of malaria parasites: enzymes of coenzyme A biosynthesis in normal duck erythrocytes and erythrocytes infected with Plasmodium lophurae. Proc. Natl. Acad. Sci. USA 72:2456-2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desjardins, R. E., C. J. Canfield, J. D. Haynes, and J. D. Chulay. 1979. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob. Agents Chemother 16:710-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Divo, A. A., T. G. Geary, N. L. Davis, and J. B. Jensen. 1985. Nutritional requirements of Plasmodium falciparum in culture. I. Exogenously supplied dialyzable components necessary for continuous growth. J. Protozool. 32:59-64. [DOI] [PubMed] [Google Scholar]
- 11.Drell, W., and M. S. Dunn. 1948. Inhibition of lactic acid bacteria by analogs of pantothenic acid. J. Am. Chem. Soc. 70:2057-2063. [DOI] [PubMed] [Google Scholar]
- 12.Genschel, U. 2004. Coenzyme A biosynthesis: reconstruction of the pathway in archaea and an evolutionary scenario based on comparative genomics. Mol. Biol. Evol. 21:1242-1251. [DOI] [PubMed] [Google Scholar]
- 13.Liu, J., C. A. Kuszynski, and B. T. Baxter. 1999. Doxycycline induces Fas/Fas ligand-mediated apoptosis in Jurkat T lymphocytes. Biochem. Biophys. Res. Commun. 260:562-567. [DOI] [PubMed] [Google Scholar]
- 14.Peters, W. 1975. The chemotherapy of rodent malaria. XXII. The value of drug-resistant strains of P. berghei in screening for blood schizontocidal activity. Ann. Trop. Med. Parasitol. 69:155-171. [PubMed] [Google Scholar]
- 15.Saliba, K. J., H. A. Horner, and K. Kirk. 1998. Transport and metabolism of the essential vitamin pantothenic acid in human erythrocytes infected with the malaria parasite Plasmodium falciparum. J. Biol. Chem. 273:10190-10195. [DOI] [PubMed] [Google Scholar]
- 16.Saliba, K. J., and K. Kirk. 2001. H+-coupled pantothenate transport in the intracellular malaria parasite. J. Biol. Chem. 276:18115-18121. [DOI] [PubMed] [Google Scholar]
- 17.Snell, E. E., and W. Shive. 1945. Growth inhibition by analogues of pantothenic acid: pantothenyl alcohol and related compounds. J. Biol. Chem. 158:551-559. [DOI] [PubMed] [Google Scholar]
- 18.Trager, W. 1966. Coenzyme A and the antimalarial action in vitro of antipantothenate against Plasmodium lophurae, P. coatneyi and P. falciparum. Trans. N. Y. Acad. Sci. 28:1094-1108. [DOI] [PubMed] [Google Scholar]
- 19.Trager, W. 1971. Further studies on the effects of antipantothenates on malaria parasites (Plasmodium coatneyi and P. falciparum) in vitro. J. Protozool. 18:232-239. [DOI] [PubMed] [Google Scholar]
- 20.Trager, W., and F. H. Brohn. 1975. Coenzyme A requirement of malaria parasites: effects of coenzyme A precursors on extracellular development in vitro of Plasmodium lophurae. Proc. Natl. Acad. Sci. USA 72:1834-1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vallari, D. S., and C. O. Rock. 1987. Isolation and characterization of temperature-sensitive pantothenate kinase (coaA) mutants of Escherichia coli. J. Bacteriol. 169:5795-5800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Welsh, A. L. 1952. Lupus erythematosus. Treatment by combined use of massive amounts of calcium pantothenate or panthenol with synthetic vitamin E. AMA Arch. Derm. Syphilol. 65:137-148. [DOI] [PubMed] [Google Scholar]
- 23.Welsh, A. L. 1954. Lupus erythematosus. Treatment by combined use of massive amounts of pantothenic acid and vitamin E. AMA Arch. Derm. Syphilol. 70:181-198. [DOI] [PubMed] [Google Scholar]