Abstract
Individuals with schizophrenia exhibit problems with understanding the figurative meaning of language. This study evaluates neural correlates of diminished humor comprehension observed in schizophrenia. The study included chronic schizophrenia (SCH) outpatients (n = 20), and sex, age and education level matched healthy controls (n = 20). The fMRI punchline based humor comprehension task consisted of 60 stories of which 20 had funny, 20 nonsensical and 20 neutral (not funny) punchlines. After the punchlines were presented, the participants were asked to indicate whether the story was comprehensible and how funny it was. Three contrasts were analyzed in both groups reflecting stages of humor processing: abstract vs neutral stories - incongruity detection; funny vs abstract - incongruity resolution and elaboration; and funny vs neutral – complete humor processing. Additionally, parametric modulation analysis was performed using both subjective ratings separately. Between-group comparisons revealed that the SCH subjects had attenuated activation in the right posterior superior temporal gyrus (BA 41) in case of irresolvable incongruity processing of nonsensical puns; in the left dorsomedial middle and superior frontal gyri (BA 8/9) in case of incongruity resolution and elaboration processing of funny puns; and in the interhemispheric dorsal anterior cingulate cortex (BA 24) in case of complete processing of funny puns. Additionally, during comprehensibility ratings the SCH group showed a suppressed activity in the left dorsomedial middle and superior frontal gyri (BA 8/9) and revealed weaker activation during funniness ratings in the left dorsal anterior cingulate cortex (BA 24). Interestingly, these differences in the SCH group were accompanied behaviorally by a protraction of time in both types of rating responses and by indicating funny punchlines less comprehensible. Summarizing, our results indicate neural substrates of humor comprehension processing impairments in schizophrenia, which is accompanied by fronto-temporal hypoactivation.
Keywords: Communication skills, Figurative meaning, Functional magnetic resonance imaging, Humor, Schizophrenia
Abbreviations: ABS, absurd/nonsensical punchline; ACC, anterior cingulate cortex; BA, Brodmann's area; CON, healthy controls/control group; dACC, dorsal anterior cingulate cortex; dlPFC, dorsolateral prefrontal cortex; dmMFG, dorsomedial Middle Frontal Gyrus; EEG, electroencephalography; ERPs, EEG event-related potentials; FDR, False Discovery Rate; fMRI, functional magnetic resonance imaging; fNIRS, functional near-infrared spectroscopy; FUN, funny punchline; FWHM, full-width-at-half-maximum; GLM, general linear model; IFG, inferior frontal gyrus; IPL, Inferior Parietal Lobule; ISI, interstimulus-interval; k, number of voxels in analyzed cluster size; L, left hemisphere; MFG, medial frontal gyrus; MNI, Montreal Neurological Institute coordinates; MoCA, Montreal Cognitive Assessment; MOG, middle occipital gyrus; MRI, magnetic resonance imaging; MTG, middle temporal gyrus; NEU, neutral/unfunny punchline; SOA, stimulus onset asynchrony; ns, non-significant group difference; PANSS, Positive and Negative Syndrome Scale; PFC, prefrontal cortex; pSTG, posterior Superior Temporal Gyrus; R, right hemisphere; RHLB, Right Hemisphere Language Battery; RT, reaction time; SCH, schizophrenia outpatients/clinical group; SD, standard deviations; SEM, standard error of the mean; sLORETA, standardized low resolution brain electromagnetic tomography analysis; SFG, Superior Frontal Gyrus; STG, superior temporal gyrus; TP, temporal pole; TPJ, temporoparietal junction; ToM, theory of mind.
Highlights
-
•
Schizophrenia outpatients revealed impairments in humor comprehension processing.
-
•
Neural processing was impaired at three stages of the humor comprehension process.
-
•
Incongruity detection - hypofunction in the right temporal lobe in schizophrenia.
-
•
Resolution & Elaboration - hypofunction in the left frontal lobe in schizophrenia.
-
•
Complete process - hypofunction in the anterior cingulate cortex in schizophrenia.
1. Introduction
Schizophrenia is a mental illness characterized by various psychopathological symptoms with a number of cognitive, emotional and communication impairments which together influence social functioning of the patients (Cechnicki, 2011, Howes and Murray, 2014, Nutt and Need, 2014). Recent reports indicate that diminished communication skills may be considered one of the most important features of schizophrenia outcome and recovery (Adamczyk et al., 2016, Falkenberg et al., 2007, Niznikiewicz et al., 2013, Titone, 2010, Wible, 2012).
Figurative meaning of speech (e.g. humor, metaphor, irony) is a key part of human language abilities. For example, the ability to use and experience humor is an important quality of human social functioning, which enriches social relations and improves cooperation between people (Goel and Dolan, 2001, Mobbs et al., 2003, Polimeni and Reiss, 2006a, Vrticka et al., 2013). Studies on humor appreciation in schizophrenia published to date point unequivocally toward disturbed processes related to comprehension and/or appreciation of humor, along with occasional contradictory conclusions concerning the relationship between the occurrence of this deficit and the severity of psychopathological symptoms (Adamczyk et al., 2016, Bozikas et al., 2007, Corcoran et al., 1997, Davenport, 2008, Falkenberg et al., 2007, Marjoram et al., 2006, Polimeni and Reiss, 2006b, Polimeni et al., 2010, Tsoi et al., 2008). It is notable that certain tests indicate that people with schizophrenia may benefit from humor training intervention during the recovery process, which improves their ability to understand and use humor (Cai et al., 2014) or reduces psychopathology and improves self-esteem and coping (Falkenberg et al., 2007, Gelkopf et al., 1993, Gelkopf et al., 1994, Gelkopf et al., 2006, Witztum et al., 1999). However, there is still insufficient data to fully explain this deficit and its causes. Aside from behavioral evidence, we do not know much about neural mechanisms of humor processing impairments in people with schizophrenia. Importantly, only one exceptional study to date has examined schizophrenia-related functional magnetic resonance imaging (fMRI) responses during humor processing. Namely, the study of Marjoram et al. (2006) on high-risk relatives of individuals with schizophrenia indicates reduced prefrontal cortex (PFC) activations during theory of mind (ToM) humor processing related to history of psychotic-like positive symptoms.
A literature review suggests that the nature of humor impairments observed in schizophrenia is related to deficiencies in set shifting and semantic cognition in a given linguistic context, with a general tendency to use literal language and difficulties in understanding figurative meanings (Kircher et al., 2007, Kuperberg and Caplan, 2003, Kuperberg et al., 1998, Polimeni et al., 2010, Rapp et al., 2013). Our previous results are in line with the above, since in a group of people with schizophrenia who were assessed with the Right Hemisphere Language Battery (RHLB, Bryan, 1995; RHLB-PL, Łojek, 2007) we observed a poorer performance of the humor subtest among others diminished specific communication skills, i.e. metaphors (Adamczyk et al., 2016). In the RHLB humor test, consisting of matching one of three available endings to stories in order to create jokes, people with schizophrenia made more mistakes than healthy controls by choosing mainly neutral or sometimes absurd endings. This may suggest that people with schizophrenia exhibit problems primarily with understanding figurative meanings and/or semantic reorganization of funny endings.
Humor seems to involve certain cognitive mechanisms involving set shifting, unexpected stimuli and social assessments. In humor research various forms of stimuli such as cartoons, short films or funny stories, i.e. jokes, were used (for an extended review see: Vrticka et al., 2013). In theory, a joke is complex linguistic material and its humorous nature is manifested by surprising endings. Comprehension of a joke elicits an emotional response of amusement. Theoretically, this response results from a correct reinterpretation of the story in agreement with the surprising ending. In other words, to comprehend a joke one needs to successfully resolve the surprising incongruity of the punchline and the remaining content of the joke (Suls, 1972, Wyer and Collins, 1992). The process of ‘getting a joke’ can be therefore divided into two major phases: comprehension and elaboration. In the comprehension phase a person detects the surprising incongruity of the punchline with the previous content of the story (setup) and then restores the coherence by a reinterpretation of the story. In the elaboration phase the implications resulting from this reinterpretation cause the emotional response of amusement (Wyer and Collins, 1992). In a revised model of neuronal networks involved in verbal joke processing, developed by Chan et al. (2013) based on the theoretical approach by Suls (1972), Wyer and Collins (1992) and previous neuroimaging studies (i.e. Bartolo et al., 2006, Bekinschtein et al., 2011, Chan et al., 2012, Chou et al., 2009, Goel and Dolan, 2001, Goel and Dolan, 2007, Mobbs et al., 2003, Moran et al., 2004, Samson et al., 2008, Samson et al., 2009, Wild et al., 2003), emphasis was placed on a clear division of the two separate steps of the comprehension phase between incongruity detection and incongruity resolution. Finally, this hypothetical three-step model of the ‘getting a joke’ process consists of incongruity detection, incongruity resolution and elaboration, which evoke amusement. In a novel procedure implemented by the authors of this paper, subjects were presented with one of three possible endings to garden-path designed stories they have read: unfunny, nonsensical or funny. This made it possible to investigate separate processes in humor comprehension: the incongruity detection and resolution stages. Thus, this three-step model of humor comprehension may be considered a hypothetical model for studying verbal humor processing with fMRI. However, considering complex nature of humor as a specific psycho-biological phenomenon (Veatch, 1998) and considering pioneering nature of neuroimaging studies on humor, it should be recognized, that this theoretical approach is still highly speculative and there is a lack of a fully acceptable model of humor processing. On the other hand, the theoretical approach of Suls (1972) and Wyer and Collins (1992) is commonly used across humor fMRI study design (see Vrticka et al., 2013).
Data from healthy controls indicates that humor processing involves neural network connections including frontal, temporal and parietal region activation as a response to the cognitive component of humor processing. In particular, neuroimaging studies by Chan et al. (2013) showed activation in the middle temporal gyrus (MTG) and medial frontal gyrus (MFG) of the right hemisphere during incongruity detection. Other research indicated engagement of the right temporal cortices in the processing of surprising, unexpected or less probable word meanings and its integration within semantic context (Federmeier and Kutas, 1999, Goel and Dolan, 2001, St George et al., 1999). Incongruity resolution activates the left hemisphere and includes the dorsomedial MFG and Superior Frontal Gyrus (SFG), temporoparietal junction (TPJ) and precuneus (Bartolo et al., 2006, Chan et al., 2013, Marjoram et al., 2006, Samson et al., 2008, Samson et al., 2009). Successful comprehension of jokes is related to TPJ activation, which is greater if the humor content is related to ToM processing (Bartolo et al., 2006, Campbell et al., 2015, Goel and Dolan, 2001, Kohn et al., 2011, Mobbs et al., 2003, Samson et al., 2008, Wild et al., 2003). This region is also activated during the detection and processing of unexpected stimuli, such as the process of incongruity detection and resolution (Neely et al., 2012, Vrticka et al., 2013). Other studies revealed activation of the SFG in relation to humor appreciation (Campbell et al., 2015). Neural correlates of the emotional component (e.g. feeling of amusement/mirth) were found in the orbitofrontal/ventromedial prefrontal cortex (PFC), ventral anterior cingulate cortex (ACC), insula, amygdala and parahippocampal gyri (Bartolo et al., 2006, Chan et al., 2012, Franklin and Adams, 2011, Goel and Dolan, 2001, Mobbs et al., 2003, Samson et al., 2008, Samson et al., 2009, Wild et al., 2003, Wild et al., 2006). Activation of the caudate nuclei correlates with enjoyable successful processing of jokes (Franklin and Adams, 2011, Mobbs et al., 2005). Lastly, cerebellum is also engaged in humor perception and appreciation and in laughter (Bartolo et al., 2006, Frank et al., 2012, Frank et al., 2013, Franklin and Adams, 2011, Goel and Dolan, 2007, Wild et al., 2003). It should be noted that activated regions differ across different studies, which may be due to differences in procedures implemented (for an extended review see Vrticka et al., 2013).
Although analysis of current literature data unequivocally indicates the presence of communication deficits in schizophrenia, the neural basis of the observed impairments of humor as an important figurative aspect of language is not sufficiently studied. To the best of our knowledge, there has been no fMRI research conducted to date into diminished humor comprehension in schizophrenia. A single fMRI study on relatives of individuals with schizophrenia (with an enhanced risk of schizophrenia) reported hypoactivation in the PFC (BA 6/8/9) during ToM humor processing (Marjoram et al., 2006).
Interestingly, scarce data exists on metaphor processing, indicating functional and structural disturbances in the neural network during metaphor processing in schizophrenia, as assessed by neuroimaging methods, e.g. fMRI (Kircher et al., 2007, Mashal et al., 2013, Mashal et al., 2014, Straube et al., 2013, Straube et al., 2014) and electroencephalography (EEG, Schneider et al., 2015). Pioneering fMRI research in this topic showed that people with schizophrenia exhibited greater activation in the left inferior frontal gyrus (IFG, BA 45/47) alongside suppressed activation in the right posterior superior temporal gyrus (STG, BA 39) and the right precuneus (BA 7) during metaphor processing (Kircher et al., 2007). Concluding their findings, authors indicate that hypoactivation in these brain regions underlies the clinical symptom of concretism in schizophrenia. Next, this impaired neural activity may be responsible for disturbances in understanding of figurative meaning of speech (e.g. non-literal, semantically complex language structures; Kircher et al., 2007).
Another experimental investigation found that people with schizophrenia are characterized by diminished activation and abnormal connectivity in the left fronto-temporal regions (IFG – MTG) during metaphorical gesture processing (Straube et al., 2013, Straube et al., 2014) and suppressed activation in the right IFG, together with compensatory hyperactivation in the left fronto-parietal regions (e.g. IFG/MFG – Precuneus) observed during metaphorical content analysis as compared to healthy controls (Mashal et al., 2013, Mashal et al., 2014). The latest research (Schneider et al., 2015) employing simultaneous functional near-infrared spectroscopy (fNIRS) and EEG event-related potentials (ERPs) reveals that schizophrenia patients show different cortical electrophysiological and hemodynamic activations which relate to impairments in the processing of figurative meaning of language. Non-specific alterations in the N400 amplitude and differences in language comprehension activity in the left hemisphere (Schneider et al., 2015) were observed.
Based on our previous findings regarding schizophrenia-related impairments in humor processing (Adamczyk et al., 2016), this study evaluated neural substrates of diminished humor comprehension observed in schizophrenia. To investigate the nature of this deficit and to localize cortical areas involved in humor processing we used fMRI. In order to reveal activation differences in humor-related brain neural network processing during verbal humor comprehension and elaboration, the study included comparisons of patterns of brain activations between healthy controls and schizophrenia subjects.
2. Materials and methods
2.1. Subjects
All subjects gave their informed consent to participate and were tested individually by psychologists (interview and MoCA assessment), psychiatrists (clinical interview and PANSS assessment) and technicians (fMRI scanning). Additionally, each participant signed a further form about risks and exclusion criteria of fMRI scanning which were provided by the Małopolska Centre of Biotechnology at the Jagiellonian University, Kraków. The procedures were designed in accordance with the ethical standards of the World Medical Association Declaration of Helsinki (2013) and approved by the Bioethical Committee of Collegium Medicum at the Jagiellonian University in Kraków. All participants (n = 45) were remunerated for their participation in the experiment immediately after MR data acquisition (€16) and received a DVD with their own anatomical brain scans.
The study included two groups: 25 clinical subjects (SCH) and 20 healthy controls (CON). The clinical group consisted of people diagnosed with ICD-10 schizophrenia (World Health Organization, 2011), who were participants in a complex psychotherapeutically-oriented community psychiatry treatment and rehabilitation program in Kraków, Poland (Adamczyk et al., 2016, Cechnicki, 2011) and who were recruited through advertising in the local network of outpatients clinics and rehabilitation centers. Diagnoses were made by experienced psychiatrists based on clinical interviews and medical documentation. The mean dose of antipsychotics for each subject from the clinical group was calculated as chlorpromazine equivalents (according to: Atkins et al., 1997, Gardner et al., 2010, Woods, 2003). The severity of psychopathological symptoms was assessed using the Positive and Negative Syndrome Scale (PANSS; Kay et al., 1987). It assesses five factors: positive symptoms, negative symptoms, disorganization symptoms, excitement and emotional distress. Outcomes were calculated based on results presented in the meta-analysis by van der Gaag et al. (2006). Only the items which proved significant in all factor analyses were included in the calculation for each syndrome.
All clinical subjects were in a stable psychopathological condition for several weeks before undergoing the MRI scan and no complaints concerning the worsening of the subjects' mental condition were received following the study. Throughout the MRI procedure, each clinical subject was additionally accompanied by a psychologist for on-demand psychological assistance, if necessary.
None of the participants had a history of head injuries, seizures, substance dependence or any serious, current somatic illnesses. All were right-handed native Polish speakers. The Polish adaptation of the Montreal Cognitive Assessment (MoCA, available at www.mocatest.org; Nasreddine et al., 2005) was used as a general measure of basic cognitive skills.
The groups did not differ in terms of age, sex distribution or level of education, although we found significant differences in total MoCA scores. The SCH group obtained a lower total score than the CON group, revealing the cognitive deficits which are prominent and characteristic of the schizophrenia population (Adamczyk et al., 2016, Fisekovic et al., 2012, Levy et al., 2014, Rodríguez-Bores Ramírez et al., 2014). The clinical data on the SCH group indicates a long-term course of illness and a relatively stable psychopathological profile. The group is treated in a therapeutic program utilizing atypical neuroleptics in 96% of the cases. Due to motor artifacts during MRI scanning, five SCH subjects were excluded from further fMRI data analysis. Demographic and clinical data is presented in Table 1.
Table 1.
Demographic and clinical data.
| Demographic and clinical data | Healthy controls (n = 20) | Schizophrenia outpatients (n = 20) | Test for between-group differences |
|---|---|---|---|
| Age | Mean (± SD) | Mean (± SD) |
t = 0.13; ns Z adj. = 0.28; ns |
| 39.55 (9.50) | 39.95 (9.49) | ||
| Sex | n (%) | n (%) | Chi^2 = 0.10; ns |
| Male | 11 (55%) | 10 (50%) | |
| Female | 9 (45%) | 10 (50%) | |
| Education (in years) | 13.80 (2.21) | 14.80 (2.38) |
t = 1.38; ns Z adj. = 1.42; ns |
| MoCA | 26.55 (2.93) | 25.00 (2.36) |
t = − 1.84; p = 0.073 Z adj. = − 2.08; p = 0.037 |
| Schizophrenia diagnosis: | n (%) | n (%) | |
| Paranoid | n/a | 16 (80%) | |
| Undifferentiated | n/a | 2 (10%) | |
| Schizoaffective disorder | n/a | 2 (10%) | |
| Type of pharmacotherapy: | n (%) | n (%) | |
| Atypical antipsychotics | n/a | 14 (70%) | |
| Typical and atypical antypsychotics mixed | n/a | 5 (25%) | |
| Anxiolytics | n/a | 2 (10%) | |
| Antidepressants | n/a | 2 (10%) | |
| Mood stabilizers | n/a | 4 (20%) | |
| Characteristic of the illness: | Mean (± SD) | Mean (± SD) | |
| Duration of psychosis (in years) | n/a | 16.65 (8.17) | |
| Number of relapses | n/a | 9.35 (8.02) | |
| Number of hospitalizations | n/a | 7.90 (5.54) | |
| Chlorpromazine equivalent (mg/day) | n/a | 495.00 (339.08) | |
| PANSS | Mean (± SD) | Mean (± SD) | |
| Total | n/a | 55.35 (16.14) | |
| Positive | n/a | 10.60 (5.04) | |
| Negative | n/a | 14.95 (5.04) | |
| Disorganization | n/a | 8.40 (4.24) | |
| Excitement | n/a | 5.90 (2.43) | |
| Emotional distress | n/a | 8.20 (2.84) |
Subjects demographics and clinical data were presented as n (%) for nominal variable and as mean ± standard deviation (SD) for quantitative data. Schizophrenia diagnosis was indicated by ICD-10 and contains: paranoid schizophrenia (F.20.0), undifferentiated schizophrenia (F20.3) and schizoaffective disorder (F25.0). All but one patient were taking antipsychotic medication, including conventional (1st generation: flupentixol, haloperidol, levomepromazine, promazine) and/or atypical (2nd generation: amisulpride, clozapine, olanzapine, risperidone, sulpiride, quetiapine; 3rd generation: aripiprazole) neuroleptics. Additionally, some patients received antidepressants (escitalopram, paroxetine), anxiolytics (hydroxyzine) and/or mood stabilizers (carbamazepine, lithium, valproic acid). The significance level in all statistical analyses equaled alpha = 0.05. n/a – non available; ns - non-significant group difference.
2.2. Experimental procedure
2.2.1. Stimuli
120 funny stories in Polish were selected from vast collections of jokes found online, taking care to avoid excessively vulgar and/or sexist, racist, religious and political content. After pre-selection, all stories in their original form were adapted to create three types of endings, following the procedure described by Chan et al. (2013). The three conditions were: funny (FUN) - original endings; neutral (NEU) - unfunny endings; and abstract (ABS) - nonsensical endings. When the original (e.g. semantically congruent and funny) punchlines were replaced with neutral or nonsensical ones, NEU was semantically congruent and unfunny, while ABS was nonsensical sentences with irresolvable incongruities and which were unfunny. This partially reflects three stages of humor processing (Chan et al., 2012, Chan et al., 2013, Vrticka et al., 2013). Significantly, differences in examined stimuli construction which were used in the present fMRI assessments (e.g. complex punchline based stories vs garden-path stories (Chan et al., 2012, Chan et al., 2013) make both contrasts designs only partially parallel. Specifically, contrasts designed in this study formed the basis of the incongruity detection (ABS vs NEU), incongruity resolution and elaboration (FUN vs ABS) and complete humor processing (FUN vs NEU) containing all three phases of humor processing (e.g. incongruity detection, incongruity resolution and elaboration). Next, the stories were presented for pre-selective judgment to 60 healthy people who rated all three conditions for their comprehensibility and funniness on the 1–9 Likert scale (from 1 = totally incomprehensible or unfunny to 9 = totally comprehensible or funny). Three separate sets were prepared prior to the judgment to ensure that each person rated only one version of the same story (setup). As a result, three sets containing 120 stories with various endings were created (40 funny, 40 unfunny, 40 abstract). The pre-selected stories were presented on a computer screen and ratings were provided using a keyboard.
Next, the funny stories were chosen only when they were rated more highly than 6 on the funniness scale and > 6 on the comprehensibility scale. Neutral stories were chosen when they scored no more highly than 3 on the funniness scale, and more highly than 6 on the comprehensibility scale. Abstract endings were chosen when the rating was no > 3 on the funniness and comprehensibility scales. Ratings for chosen stories are presented in Table 2.
Table 2.
Means and standard deviations of funniness and comprehensibility ratings for every type of punchline in pre-selective judgements.
| Type of punchline | Funniness rating (Welch's F, p < 0.001)⁎ |
Comprehensibility rating (Welch's F, p < 0.001)⁎⁎ |
||
|---|---|---|---|---|
| Mean (n = 20) | ± SD | Mean (n = 20) | ± SD | |
| Funny (FUN) | 6.75 | 0.50 | 8.41 | 0.32 |
| Neutral (NEU) | 2.22 | 0.36 | 6.70 | 0.53 |
| Abstract (ABS) | 1.82 | 0.49 | 2.59 | 1.08 |
Scores of pre-selective ratings assessed by a panel of judges were presented as mean ± standard deviation (SD) for all types of punchlines. All differences between punchlines proved significant when assessed with Welch's ANOVA and post-hoc Tukey test, both for funniness and comprehensibility ratings.
Abstract vs Neutral difference at p = 0.018, other Tukey's post-hoc tests p < 0.001.
All Tukey's post-hoc tests p < 0.001.
Finally, each stimulus contained two components: a setup and a punchline. The setups were between six and 41 words long (mean = 20.63; SD = 8.08) and the punchlines were between one and 18 words long (mean = 6.03; SD = 3.19). Each type of punchline was presented 20 times so that the functional run contained the same 60 stimuli for every subject (20 FUN, 20 NEU, 20 ABS). The presentation order was randomized. All procedures were designed and presented using the PsychoPy v1.82.01 software (Peirce, 2009).
2.2.2. Experimental task
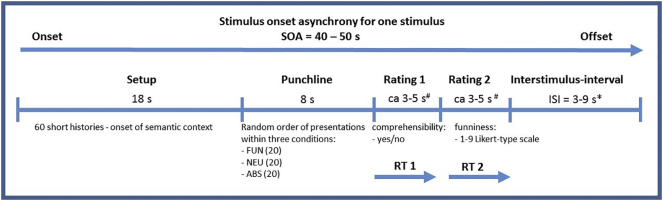
Before entering the MRI scanner all participants were advised to keep their heads very still during the procedure. The experimental task was presented on an MRI compatible screen and responses were collected using fiber-optic response button grips (Nordic Neuro Lab, Bergen, Norway). At the start of the functional run, participants read short instructions on the screen about how to provide ratings for the comprehension and funniness scales. Comprehensibility was rated on a dichotomous scale (0 = no/“not understandable” vs 1 = yes/“understandable”), whereas funniness was rated on a 9-point Likert-type scale (1 = “not funny at all” to 9 = “very funny”). They were then shown three (or more, if necessary) examples to train their responses before the actual test. Ratings were provided by the right hand index and middle fingers (as left and right arrows, respectively) and thumb to accept the chosen answer. Reaction times (RT) of each rating were collected (i.e. the time from the appearance of the rating scale until the subject's rating confirmation by pressing the response-key under their thumb); however, no time restrictions were given. On completion of the training, the test was started by displaying the word ‘start’ and fixation-cross. Each participant was presented 60 stories in a randomized order, with 20 items for each of the three endings (FUN, NEU, ABS). Each setup was shown for 18 s. Each punchline was shown for 8 s. After the punchline was presented, the participants were asked to provide a subjective judgment on the comprehensibility and funniness of the stories. The trials were separated by interstimulus intervals (ISI) randomly varying from 3 to 9 s. One functional run was provided for max 45 min (see Fig. 1). The total time for the structural and functional run in the MRI scanner was approx. 1 h per person.
Fig. 1.
Scheme of experimental stimuli presentation. Abbreviations: # - no time limit for rating responses; * - random order of presentations; ISI – interstimulus-interval; RT - reaction time measurement; SOA – stimulus onset asynchrony; experimental conditions description: ABS – absurd punchline (incomprehensible and unfunny content); FUN – funny punchline (comprehensible and funny content); NEU – neutral punchline (comprehensible and unfunny content).
2.3. MRI data acquisition
Magnetic resonance imaging (MRI) was performed using a 3T scanner (Magnetom Skyra, Siemens). High-resolution, anatomical images were acquired using the T1 MPRAGE sequence (sagittal slices; 1 × 1 × 1.1 mm3 voxel size; TR = 2300 ms, TE = 2.98 ms).
Functional images were acquired using a 32-channel head coil and EPI sequence. The scan parameters were as follows: 3 mm isotropic voxel, TR = 2000 ms, TE = 27 ms, flip angle = 90°, FOV 192 × 192 mm2, GRAPPA acceleration factor 2, and phase encoding A/P. Whole brain images (cerebellum included) were covered with 37 axial slices with a 20% gap between slices (distant factor = 0.6 mm), taken in an interleaved, ascending fashion. Due to magnetic saturation effects, the first four volumes (dummy scans) of each run were discarded.
Additionally, a B0 inhomogeneity field map was acquired with a dual echo gradient echo sequence matched spatially with fMRI scans (TE1 = 4.92 ms, TE2 = 7.38 ms, TR = 466 ms).
2.3.1. fMRI data preprocessing
Data processing was performed using the SPM12 software (Statistical Parametric Mapping, Wellcome Department of Cognitive Neurology, London, UK). The processing pipeline included calculation of voxel displacement using FieldMap, unwarping through a field map correction, motion correction (realignment) of functional images using a six-parameter rigid body transformation, co-registration to the anatomical reference image, segmentation into separate tissues (white matter, grey matter and cerebrospinal fluid), normalization to a standard MNI stereotaxic space with 3 mm isotropic voxels using a 12-parameter affine transformation and spatial smoothing using an 8 mm Gaussian kernel. The ART toolbox was used to detect and reject scans with excessive movements with the following threshold parameters, relative to the previous scan: global intensity 3 z, linear movements (x, y, z) 1 mm, and rotations (p, r, y) 0.02°. Subjects with scan rejection rates > 20% were eliminated from further analysis. Five subjects were eliminated, all of them from the SCH group. Low frequency signal components were removed using a high-pass filter with a cutoff of 128 s.
2.4. Statistical analysis
2.4.1. Behavioral data
Individual ratings of comprehensibility and funniness and reaction times (RTs) were collected for every stimulus. Individual means for each condition (FUN, ABS, NEU) were computed for RTs for both funniness and comprehensibility answers. We followed the same procedure when analyzing funniness ratings. A slightly different procedure was used for comprehensibility ratings. We expected the subjects to rate NEU and FUN punchlined stories as comprehensible, whereas ABS punchlined stories as incomprehensible. Therefore, in case of NEU and, separately, FUN punchlines, for each subject we calculated a sum of responses rating the story as comprehensible, whereas for ABS punchlines we summed up ratings indicating that the story was incomprehensible. Given that each punchline appeared 20 times, each subject could have a maximum score of 20 and a minimum of 0 for each type of punchline. To analyze the differences we used the U Mann-Whitney test, as the data violated assumptions for ANOVA's planned comparisons (i.e. non-normal distributions and unequal variances).
2.4.2. fMRI within-group and between-group contrasts and parametric modulation of the rating responses and a 2-stage masking procedure for incongruity resolution and elaboration
The general linear model (GLM) was applied in a canonical pattern of the hemodynamic function. Three separate models were applied. The first included the setup, the punchline (with three levels: FUN, ABS, NEU), and the response period. The other two models included the same stages, but the punchline was not distinguished between the conditions. Instead, the individual ratings on either comprehensibility or funniness for each punchline were included as parametric modulators. At the 2nd level of analysis, the groups were analyzed separately except for the comparisons between the CON and SCH groups. A non-parametric whole-brain voxel-wise Pseudo-t-test with variance smoothed with full-width-at-half-maximum (FWHM) 6 × 6 × 6 mm and 10,000 permutations using the Statistical NonParametric Mapping (SnPM13) toolbox (http://warwick.ac.uk/snpm) was used. Localizations were reported as a local maximum threshold with k ≥ 10 voxels threshold and with False Discovery Rate (FDR) correction at alpha = 0.050, or uncorrected at alpha = 0.001. Three main within-group and between-group contrasts were provided separately: 1) incongruity detection (ABS vs NEU); 2) incongruity resolution and elaboration (FUN vs ABS); 3) complete humor processing (FUN vs NEU). Additionally, in order to further differentiate the areas involved in processing the incongruity resolution and elaboration, parametric modulation analysis of the within-group and between-group contrasts was performed using both subjective ratings separately (i.e. comprehensibility and funniness). Finally, a 2-stage masking procedure was performed in order to provide more precise and specific information about incongruity resolution and elaboration phases. Masks of two contrast maps (FUN vs ABS and FUN vs NEU) thresholded at significant level (p = 0.001, uncorrected) were created. Then, conjunction of those binary masks was done resulting in a map of brain regions activated in both contrast.
Each contrast included bidirectional comparisons, e.g. hyperactivated regions were reported under a positive contrast (a > b) and hypofunction under a negative one (a < b).
3. Results
3.1. Behavioral data: ratings of comprehensibility and funniness and RT
The between-group comparisons of the means of comprehensibility and funniness ratings for the three types of punchlines revealed just one significant difference in the comprehensibility of funny punchlines (Z-adj. = − 2.29, p = 0.022), with SCH subjects revealing a lower level of understanding of the jokes. Noteworthy, although non-significant, trends toward differences are visible in the funniness ratings for absurd and funny punchlines with SCH subjects finding them more and less funny than controls, respectively. Data for the ratings of all types of punchlines are presented in Table 3.
Table 3.
Ratings of comprehensibility and funniness for the punchline types.
| Ratings/type of punchline | Healthy controls (n = 20) |
Schizophrenia outpatients (n = 20) |
Between-group difference (Mann-Whitney test) |
|||
|---|---|---|---|---|---|---|
| Mean | ± SD | Mean | ± SD | Z adjusted | p-value | |
| Comprehensibilitya | ||||||
| FUN | 19.35 | 1.60 | 17.80 | 2.69 | − 2.29 | 0.022 |
| NEU | 18.55 | 3.97 | 19.05 | 1.57 | 0.03 | 0.976 |
| ABS | 19.40 | 0.99 | 17.95 | 3.95 | − 1.32 | 0.188 |
| Funninessb | ||||||
| FUN | 6.86 | 1.15 | 6.16 | 1.28 | − 1.89 | 0.058 |
| NEU | 2.49 | 1.62 | 3.13 | 2.11 | 1.20 | 0.228 |
| ABS | 1.66 | 0.73 | 2.73 | 1.98 | 1.92 | 0.055 |
Scores of ratings were presented as mean ± standard deviation (SD) for all types of punchlines. The significance level in all statistical analyses equaled alpha = 0.05.
Sum of responses indicating the story was rated as comprehensible in case of NEU and FUN punchlines, or non-comprehensible in case of ABS punchlines, with max score = 20 (min = 0).
Mean of responses on a 1–9 Likert type scale (max score: 9 - very funny).
RT data showed a significant increase of RT across the SCH group, with the subjects reacting more slowly than the control group, with significant differences present in all except one comparison. RT data is presented in Table 4.
Table 4.
Reaction times (RT) for all types of ratings.
| RT for ratings | Healthy controls (n = 20) |
Schizophrenia outpatients (n = 20) |
Between-group difference (Mann-Whitney test) |
|||
|---|---|---|---|---|---|---|
| Mean | ± SD | Mean | ± SD | Z adjusted | p-value | |
| Comprehensibility | ||||||
| FUN | 2.53 | 1.05 | 3.96 | 2.73 | 2.61 | 0.009 |
| NEU | 2.73 | 0.99 | 3.89 | 2.50 | 1.77 | 0.076 |
| ABS | 2.45 | 0.80 | 3.33 | 1.44 | 2.23 | 0.026 |
| Funniness | ||||||
| FUN | 2.41 | 0.61 | 3.25 | 1.32 | 2.20 | 0.027 |
| NEU | 2.62 | 0.89 | 3.34 | 1.11 | 2.20 | 0.027 |
| ABS | 2.61 | 0.63 | 3.37 | 1.40 | 2.50 | 0.012 |
Reaction times (RT) for ratings were presented as mean ± standard deviation (SD) for all types of punchlines. The significance level in all statistical analyses equaled alpha = 0.05. Mean and SD are presented in seconds.
3.2. fMRI results
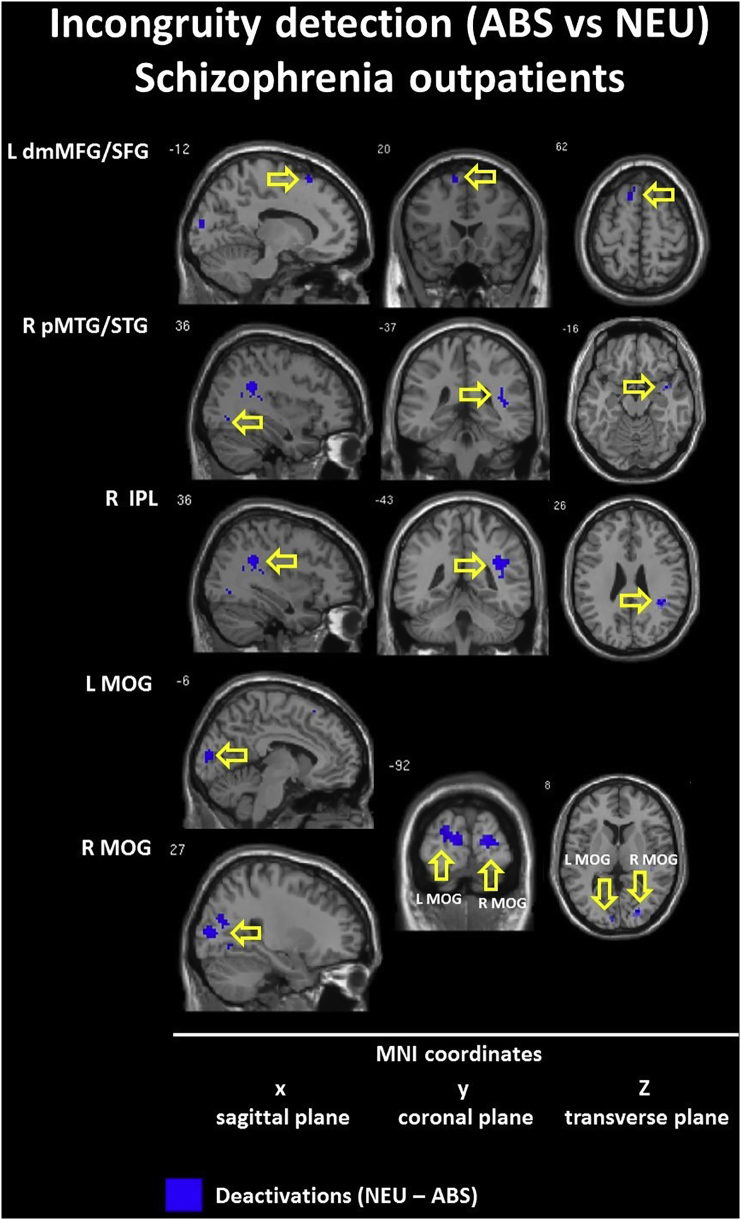
3.2.1. Incongruity detection: the nonsensical (ABS) vs unfunny (NEU) conditions
The within-group contrasts for the ABS vs NEU conditions revealed hyperactivation (ABS > NEU) in the bilateral ventromedial IFG (BA 11/47) and in the right dorsal ACC (BA 24) in the CON group but not within the SCH group.
Next, we found hypoactivation (ABS < NEU) in the CON group including bilateral ventrolateral IFG (BA 10/47) and bilateral dorsolateral MFG (BA 8/9) which were not presented in the SCH group. However, both groups showed somewhat similar hypoactivation in the left dorsomedial SFG (BA 8/9 - CON; BA 6 - SCH); the right MTG/Temporal Pole (anterior MTG/TP, BA 21/38 - CON; posterior MTG/STG, BA 21/22 – SCH); and the bilateral Middle Occipital Gyrus (MOC, BA 18 - CON; BA - 19 SCH). Interestingly, while within the CON group we observed deactivation in the left dorsolateral Inferior Parietal Lobule (IPL, BA 40), activation in the SCH group appeared contralaterally in the right subgyral part of the IPL (BA 40). Detailed information is presented in Table 5 and Inline Supplementary Figures (Fig. 1, Fig. 2).
Table 5.
Results of the fMRI data on the three-step model of humor comprehension contrasts.
| Contrast/side/brain region | Healthy controls (n = 20) |
Schizophrenia outpatients (n = 20) |
||||||
|---|---|---|---|---|---|---|---|---|
| BA | k | MNI (x; y; z) | Pseudo-t | BA | k | MNI (x; y; z) | Pseudo-t | |
| 1. INCONGRUITY DETECTION (ABS vs NEU) | ||||||||
| Activations (ABS > NEU) | ||||||||
| L ventromedial Inferior Frontal Gyrus | 11/47 | 15 | − 24; 41; − 10 | 5.38 | ||||
| R ventromedial Inferior Frontal Gyrus | 11/47 | 13 | 27; 38; − 10 | 4.56 | ||||
| R dorsal Anterior Cingulate Cortex | 24 | 12 | 9; − 4; 29 | 3.88 | ||||
| Deactivations (ABS < NEU) | ||||||||
| L ventrolateral Inferior Frontal Gyrus | 47/10 | 62 | − 42; 47; − 7 | 4.99 | ||||
| R ventrolateral Inferior Frontal Gyrus | 47/10 | 17 | 48; 47; − 7 | 4.62 | ||||
| L > R dorsomedial Superior Frontal Gyrus/Middle Frontal Gyrus | 8/9 | 828 | − 12; 32; 59 | 6.46 | 6 | 10 | − 12; 20; 62 | 3.44 |
| L dorsolateral Middle Frontal Gyrus | 8/9 | 18 | − 42; 8; 50 | 4.12 | ||||
| R dorsolateral Middle Frontal Gyrus | 8/9 | 30 | 42; 23; 38 | 3.89 | ||||
| R anterior Middle Temporal Gyrus/Temporal Pole | 21/38 | 52 | 51; 11; − 28 | 6.25 | ||||
| R posterior Middle Temporal Gyrus/Superior Temporal Gyrus | 21/22 | 16 | 36; − 1; − 16 | 3.37 | ||||
| 15 | 42; − 37; 11 | 3.90 | ||||||
| L dorsolateral Inferior Parietal Lobe/Angular/Supramarginal Gyrus | 40 | 41 | − 51; − 58; 44 | 4.13 | ||||
| R Inferior Parietal Lobe | 40 | 50 | 36; − 43; 26 | 4.51 | ||||
| L Middle Occipital Gyrus | 18 | 22 | − 15; − 100; 5 | 4.44 | 19 | 14 | − 6; − 94; 8 | 3.81 |
| R Middle Occipital Gyrus | 18 | 15 | 15; − 97; 11 | 3.79 | 19 | 38 | 27; − 85; 8 | 4.35 |
| 12 | 27; − 73; 20 | 3.76 | ||||||
| 16 | 36; − 70; − 7 | 3.30 | ||||||
| 2. INCONGRUITY RESOLUTION AND ELABORATION (FUN vs ABS) | ||||||||
| Activations (FUN > ABS) | ||||||||
| L > R dorsomedial Superior Frontal Gyrus | 8/9 | 1608 | − 6; 38; 41 | 10.40 | 8/9 | 46 | − 12; 44; 47 | 4.60 |
| L posterior Middle Temporal Gyrus | 21 | 23 | − 63; − 37; − 1 | 3.70 | ||||
| L posterior Superior Temporal Gyrus | 22 | 40 | − 48; − 28; − 1 | 4.58 | ||||
| R anterior Middle Temporal Gyrus/Temporal Pole | 21/38 | 701 | 51; 11; − 28 | 7.38 | 38 | 37 | 48; 8; − 22 | 4.59 |
| R posterior Middle Temporal Gyrus/Superior Temporal Gyrus | 21/22 | 110 | 51; − 19; − 7 | 5.22 | ||||
| 14 | 72; − 19; − 4 | 3.77 | ||||||
| L Temporo-Parietal Junction | 39 40 | 2060 | − 51; − 64; 32 | 8.24 | 39/40 | 236 | − 57; − 58; 17 | 5.47 |
| R Temporo-Parietal Junction | 39/40 | 355 | 54; − 52; 26 | 7.36 | 39/40 | 107 | 54; − 61; 23 | 4.62 |
| L > R Precuneus | 31 | 189 | − 9; − 49; 38 | 5.27 | 31 | 31 | 0; − 55; 32 | 4.55 |
| L posterior Cerebellum | – | 64 | − 24; − 79; − 31 | 5.07 | ||||
| L > R Caudate nucleus | – | 131 | − 12; 14; 14 | 4.83 | ||||
| Deactivations (FUN < ABS) | ||||||||
| R dorsolateral Inferior Frontal Gyrus | 46/10 | 20 | 42; 41; 11 | 4.71 | ||||
| R ventromedial Inferior Frontal Gyrus | 11/47 | 34 | 21; 41; − 7 | 4.70 | ||||
| L ventromedial Inferior Frontal Gyrus | 47/11 | 25 | − 21; 44; − 10 | 4.55 | ||||
| 3. COMPLETE HUMOR PROCESSING (FUN vs NEU) | ||||||||
| Activations (FUN > NEU) | ||||||||
| L ventrolateral Inferior Frontal Gyrus | 45 | 12 | − 45; 23; − 1 | 3.73 | ||||
| L anterior Middle Temporal Gyrus/Superior Temporal Gyrus | 21 | 16 | − 57; − 4; − 13 | 4.06 | ||||
| R anterior Middle Temporal Gyrus/Temporal Pole | 21/38 | 119 | 51; − 1; − 31 | 4.38 | ||||
| R posterior Middle Temporal Gyrus/Superior Temporal Gyrus | 22 | 17 | 54; − 16; − 7 | 4.34 | ||||
| L Temporo-Parietal Junction | 39/40 | 381 | − 54; − 61; 26 | 5.49 | 39/40 | 191 | − 57; − 55; 14 | 4.33 |
| R Temporo-Parietal Junction | 39/40 | 59 | 60; − 52; 26 | 4.68 | 39/40 | 11 | 57; − 55; 20 | 4.58 |
| Deactivations (FUN < NEU) | ||||||||
| R dorsolateral Inferior Frontal Gyrus | 46/10 | 21 | 42; 44; 17 | 4.51 | ||||
| R orbital Superior Frontal Gyrus | 10 | 11 | 24; 56; − 1 | 3.62 | ||||
List of brain regions revealed by within-group's contrasts during humor comprehension process. Statistical analysis utilized a non-parametric whole-brain voxel-wise Pseudo-t-test. Localizations are reported as local maximum threshold with k ≥ 10 voxels extent threshold and with False Discovery Rate (FDR) correction at alpha = 0.0500, or uncorrected at alpha = 0.0010. L – left hemisphere; R – right hemisphere; BA – Brodmann's area; k – number of voxels in analyzed cluster size; MNI - Montreal Neurological Institute coordinates.
3.2.2. Incongruity resolution and elaboration: Funny (FUN) vs nonsensical (ABS) conditions
The within-group contrast for the FUN vs ABS conditions revealed hyperactivation (FUN > ABS) in similar brain regions for both groups: the left dorsomedial SFG (BA 8/9); the right anterior TP (TP/MTG, BA 21/38 - CON; TP/STG, BA 38 - SCH); bilateral TPJ (BA 39/40); and the left precuneus (BA 31). Within the CON group we found additional activations in the left posterior cerebellum and the caudate nucleus. Within the SCH group we found additional activations in the bilateral posterior temporal lobe (MTG/STG, BA 21/22). Hypoactivaction (FUN < ABS) was found in the CON group only and included the bilateral ventromedial (BA 11/47) and the right dorsolateral (BA 10/46) IFG. Detailed information is presented in Table 5 and Inline Supplementary Figures (Fig. 3, Fig. 4).
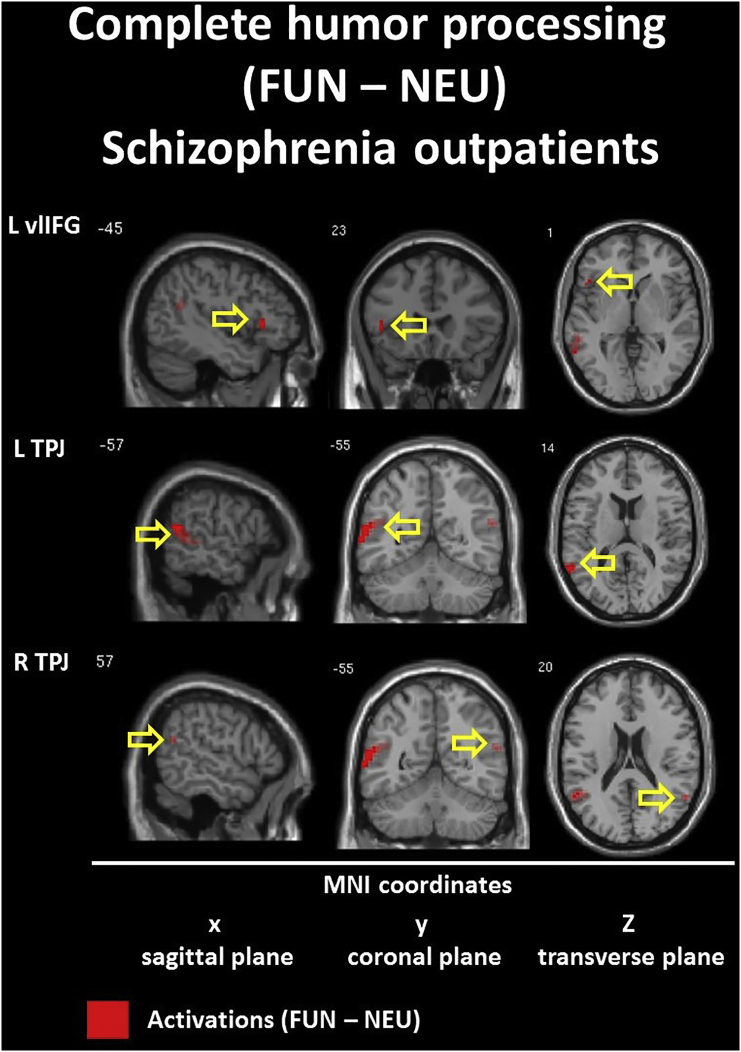
3.2.3. Complete humor processing: the funny (FUN) vs unfunny (NEU) conditions
The within-group contrast for the FUN vs NEU conditions revealed hyperactivation (FUN > NEU) in the bilateral TPJ (BA 39/40) for both groups. Within the CON group we found additional activations in the left anterior MTG/STG (BA 21) and the right anterior TP (BA 21/38) and posterior MTG/STG (BA 22). Additionally, we found hypoactivation (FUN < NEU) in the right dorsolateral IFG (BA 10/46) and orbital SFG (BA 10). Within the SCH group, the only additional activation appeared in the left ventrolateral IFG (BA 45). Detailed information is presented in Table 5 and Inline Supplementary Figures (Fig. 5, Fig. 6).
3.2.4. Parametric modulations of comprehensibility and funniness ratings and 2-stage masking procedure for incongruity resolution and elaboration
3.2.4.1. Comprehensibility rating
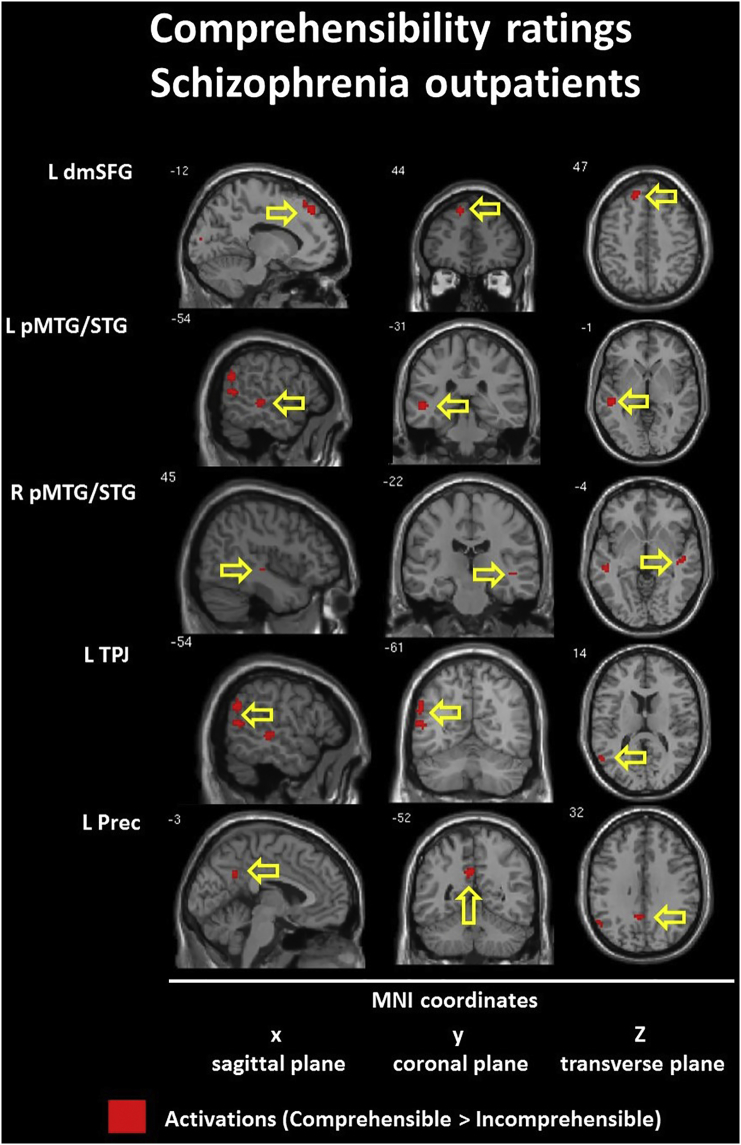
The model with comprehensibility ratings as a parametric modulator revealed different patterns of brain hyperactivation (comprehensible > incomprehensible) in both groups. In the CON group we found interhemispheric (L > R) activation of the dorsomedial MFG/SFG (BA 8/9/10) with activation of the right ventrolateral IFG (BA 47) and bilateral deactivation in the ventromedial IFG (10/11/47). Within the SCH group frontal activation was found in the left dorsomedial SFG (BA 8/9) only. Next, the CON group revealed bilateral activations in the anterior temporal cortices (MTG/TP, BA 21/38) with deactivation in the left posterior STG (BA 22). Within the SCH group we found bilateral activation in the posterior MTG/STG (BA 21/22). The TPJ (BA 39/40) was activated bilaterally (L > R) within the CON group. In the SCH group only left TPJ (BA 39/40) was activated. Finally, within the CON group we found additional activation in the left caudate nucleus with activation of the left and deactivation of the right precuneus (BA 31). Within the SCH group only the left precuneus (BA 31) was activated. Detailed information is presented in Table 6 and Inline Supplementary Figures (Fig. 7, Fig. 8).
Table 6.
Results of the fMRI data on the parametric modulation of the rating responses for comprehensibility and funniness and 2-stage masking for incongruity resolution and elaboration.
| Contrast/side/brain region | Healthy controls (n = 20) |
Schizophrenia outpatients (n = 20) |
||||||
|---|---|---|---|---|---|---|---|---|
| BA | k | MNI (x;y;z) | Pseudo-t | BA | k | MNI (x; y; z) | Pseudo-t | |
| 1. Comprehensibility ratings | ||||||||
| Activations (comprehensible > incomprehensible) | ||||||||
| Interhemispheric (L > R) dorsomedial Superior Frontal Gyrus/Middle Frontal Gyrus | 8/9/10 | 1830 | 0; 50; 35 | 7.48 | ||||
| L dorsomedial Superior Frontal Gyrus | 8/9 | 35 | − 12; 44; 47 | 3.81 | ||||
| R ventrolateral Inferior Frontal Gyrus | 47 | 124 | 51; 44; − 7 | 4.34 | ||||
| L anterior Middle Temporal Gyrus/Temporal Pole/Inferior Frontal Gyrus | 21/38 | 1272 | − 54; 2; − 25 | 7.62 | ||||
| L posterior Middle Temporal Gyrus/Superior Temporal Gyrus | 21/22 | 30 | − 54; − 31; − 1 | 4.00 | ||||
| R anterior Middle Temporal Gyrus/Temporal Pole | 21/38 | 609 | 51; 11; − 28 | 8.44 | ||||
| R posterior Middle Temporal Gyrus/Superior Temporal Gyrus | 22 | 12 | 45; − 22; − 4 | 3.58 | ||||
| L Temporo-Parietal Junction | 39/40 | 576 | − 57; − 58; 29 | 7.35 | 39/40 | 20 | − 54; − 61; 29 | 4.43 |
| 23 | − 54; − 61; 14 | 4.11 | ||||||
| R Temporo-Parietal Junction | 39/40 | 216 | 57; − 55; 29 | 6.07 | ||||
| L Precuneus | 31 | 87 | − 9; − 49; 38 | 4.46 | 31 | 12 | − 3; − 52; 32 | 3.47 |
| L Caudate | – | 42 | − 12; 11; 14 | 4.73 | ||||
| Deactivations (comprehensible < incomprehensible) | ||||||||
| L ventromedial Inferior Frontal Gyrus | 11/47 | 37 | − 24; 41; − 10 | 5.13 | ||||
| R ventromedial Inferior Frontal Gyrus | 10/47 | 32 | 24; 38; − 10 | 4.73 | ||||
| L posterior Superior Temporal Gyrus | 22 | 13 | − 30; − 52; 11 | 4.08 | ||||
| R Precuneus | 7/31 | 20 | 18; − 61; 38 | 4.18 | ||||
| 2. Funniness ratings | ||||||||
| Activations (funny > unfunny) | ||||||||
| Interhemispheric dorsomedial Superior Frontal Gyrus | 9/10 | 1824 | 0; 53; 32 | 7.93 | ||||
| R ventrolateral Inferior Frontal Gyrus | 47 | 91 | 54; 29; − 7 | 4.03 | ||||
| L posterior Superior Temporal Gyrus / Middle Temporal Gyrus | 21/22 | 98 | − 54; − 31; − 1 | 4.81 | ||||
| 13 | − 60; − 52; − 2 | 3.73 | ||||||
| R anterior Middle Temporal Gyrus/Temporal Pole | 21/38 | 551 | 48; 8; − 28 | 6.48 | ||||
| R posterior Middle Temporal Gyrus | 21 | 17 | 48; − 19; − 10 | 3.29 | ||||
| L Temporo-Parietal Junction/Middle Temporal Gyrus/Temporal Pole | 39/40 | 2729 | − 48; − 67; 32 | 8.65 | 39/40 | 11 | − 48; − 46; 26 | 3.01 |
| R Temporo-Parietal Junction | 39/40 | 1396 | 57; − 55; 26 | 6.75 | 39/40 | 54 | 51; − 58; 23 | 4.81 |
| L Precuneus | 31 | 566 | 0; − 55; 38 | 5.84 | 31 | 12 | − 6; − 58; 26 | 3.63 |
| L posterior Cerebellum | – | 600 | − 21; − 79; − 34 | 4.99 | ||||
| R Caudate nucleus | – | 37 | 15; 2; 20 | 3.88 | ||||
| Deactivations (funny < unfunny) | ||||||||
| R dorsolateral Inferior Frontal Gyrus | 10 | 44 | 42; 44; 14 | 4.87 | ||||
| R ventromedial Inferior Frontal Gyrus | 11 | 11 | 21; 44; − 10 | 4.42 | ||||
| 3. 2-Stage masking for incongruity resolution and elaboration (FUN – ABS masked by FUN – NEU) | ||||||||
| Activations (funny > unfunny and absurd) | ||||||||
| L anterior Middle Temporal Gyrus | 21 | 10 | − 54, 2, − 25 | 6.43 | ||||
| R anterior Temporal Pole/Middle Temporal Gyrus/Superior Temporal Gyrus | 21/38 | 107 | 51, 11, − 28 | 7.38 | ||||
| R posterior Middle Temporal Gyrus/Superior Temporal Gyrus | 21/22 | 22 | 51, − 13, − 7 | 4.82 | ||||
| 15 | 57, − 31, − 1 | 5.69 | ||||||
| L Superior Temporal Gyrus/Angular | 22/39 | 108 | − 57, − 58, 17 | 5.47 | ||||
| L Temporo-Parietal Junction | 39/40 | 277 | − 51, − 64, 32 | 8.24 | ||||
| R Temporo-Parietal Junction | 39/40 | 47 | 54, − 52, 26 | 7.36 | ||||
List of brain regions revealed by within-group's contrasts with comprehensibility and funniness ratings as parametric modulators and the 2-stage masking for incongruity resolution and elaboration. Statistical analysis utilized a non-parametric whole-brain voxel-wise Pseudo-t-test. Localizations are reported as local maximum threshold with k ≥ 10 voxels extent threshold and with False Discovery Rate (FDR) correction at alpha = 0.0500, or uncorrected at alpha = 0.0010. L – left hemisphere; R – right hemisphere; BA – Brodmann's area; k – number of voxels in analyzed cluster size; MNI - Montreal Neurological Institute coordinates.
3.2.4.2. Funniness rating
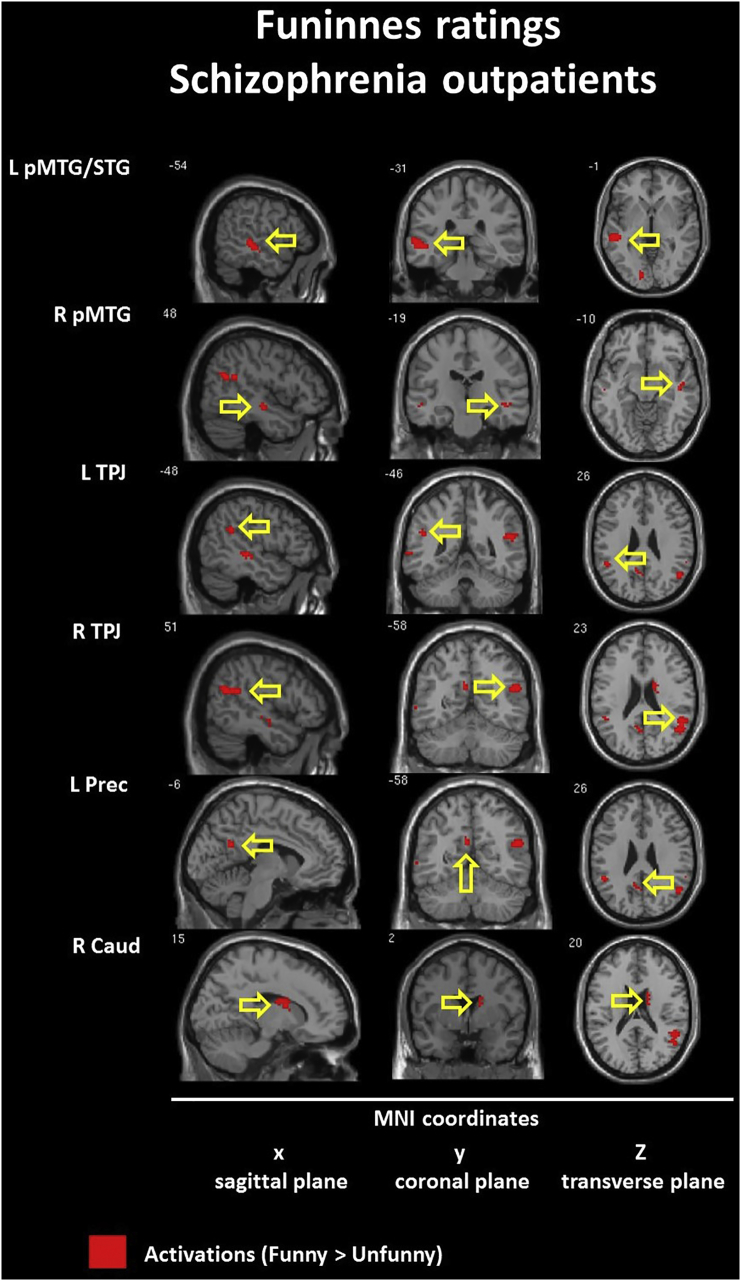
The within-group model with funniness ratings as the parametric modulator revealed different patterns of brain hyperactivation (funny > unfunny) in both groups. Within the CON group we found interhemispheric activation in the dorsomedial STG (BA 9/10) and the right ventrolateral IFG (BA 47) with deactivation in the right dorsolateral (BA 10) and ventromedial (BA 11) IFG. Within the SCH group there were no frontal responses. In the temporal lobe, the CON group revealed activations in the right anterior MTG/TP (BA 21/38). In the SCH group activation appeared in the right posterior MTG (BA 21). Next, we observed activation in the left posterior cerebellum within the CON group only and in the right caudate nucleus within the SCH group only. The similar activations in both groups included only the bilateral TPJ (BA 39/40) and the left precuneus (BA 31). Detailed information is presented in Table 6 and Inline Supplementary Figures (Fig. 9, Fig. 10).
3.2.4.3. 2-stage masking procedure for incongruity resolution and elaboration
The within-group model with a 2-stage masking procedure revealed different pattern of hyperactivation (FUN – ABS masked by FUN - NEU) in both groups. Within the CON group we found activation in the left anterior MTG (BA 21), the right anterior TP/MGT/STG (BA 21/38) and posterior MTG/STG (BA 21/22), and the TPJ (BA 39/40) in both hemispheres. Within the SCH group we found only the one preserved activation in the left posterior STG (including Angular) (BA 22). Detailed information is presented in Table 6 and Inline Supplementary Figure (Fig. 11).
3.2.5. Between-group differences in brain activations during humor processing
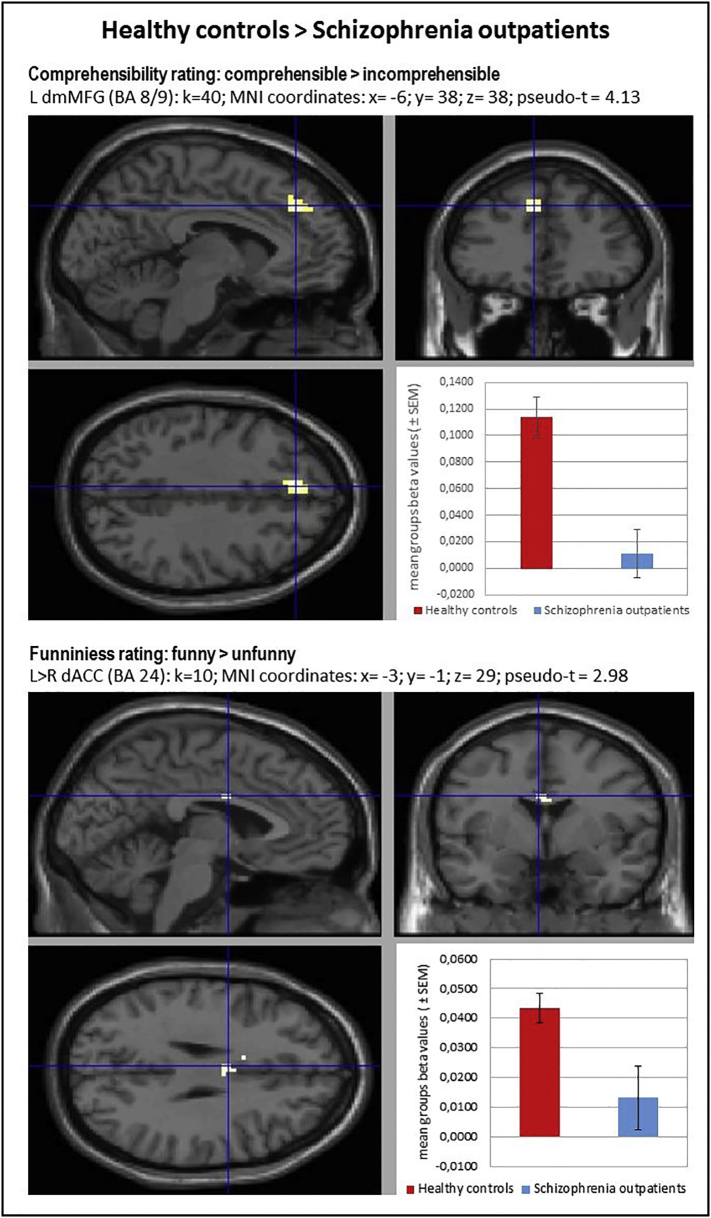
Between-group comparison contrast (CON > SCH) revealed impaired activation in the SCH group during all used contrasts. The BOLD responses of subjects in the SCH group were characterized by hypofunction of the right posterior STG (BA 41) during processing of irresolvable incongruities in absurd punchlines (ABS vs NEU), the left dorsomedial MFG/SFG (BA 8/9) during resolution and elaboration of incongruities for funny punchlines (FUN vs ABS) and the interhemispheric dorsal ACC (BA 24) in complete humor processing contrast (FUN vs NEU). Hypoactivation in the last two brain regions was reaffirmed by parametric modulation analysis for comprehension and funniness ratings. This indicates engagement of the left dorsomedial frontal lobe in the successful incongruity resolution and elaboration process together with a subjective comprehensibility rating and the important role of the ACC in complete humor processing related with subjective funniness rates of the jokes. Detailed information is presented in Table 7 and Fig. 2, Fig. 3.
Table 7.
Between-group differences in brain activations during humor processing.
| Contrast | Healthy controls > Schizophrenia outpatients |
||||
|---|---|---|---|---|---|
| side/brain region | BA | k | MNI (x; y; z) | Pseudo-t | |
| 1. Incongruity detection (ABS vs NEU) | R posterior Superior Temporal Gyrus | 41 | 40 | 42; − 40; 11 | 3.78 |
| 2. Incongruity resolution and elaboration (FUN vs ABS) & Comprehension rating* | L dorsomedial Middle Frontal Gyrus/Superior Frontal Gyrus | 8/9 | 21 | − 9; 38; 38 | 4.34 |
| 40* | − 6; 38; 38* | 4.13* | |||
| 3. Complete humor processing (FUN vs NEU) & Funniness rating* | L > R interhemispheric dorsal Anterior Cingulate Cortex | 24 | 35 | − 6; 2; 26 | 3.75 |
| 10* | − 3; − 1; 29* | 2.98* | |||
List of brain regions revealed by between-group contrasts during humor comprehension process and with comprehensibility and funniness ratings analyzed as parametric modulators (*). Statistical analysis utilized a non-parametric whole-brain voxel-wise Pseudo-t-test. Localizations are reported as local maximum threshold with k ≥ 10 voxels extent threshold and with False Discovery Rate (FDR) correction at alpha = 0.0500, or uncorrected at alpha = 0.0010. L – left hemisphere; R – right hemisphere; BA – Brodmann's area; k – number of voxels in analyzed cluster size; MNI - Montreal Neurological Institute coordinates.
Fig. 2.
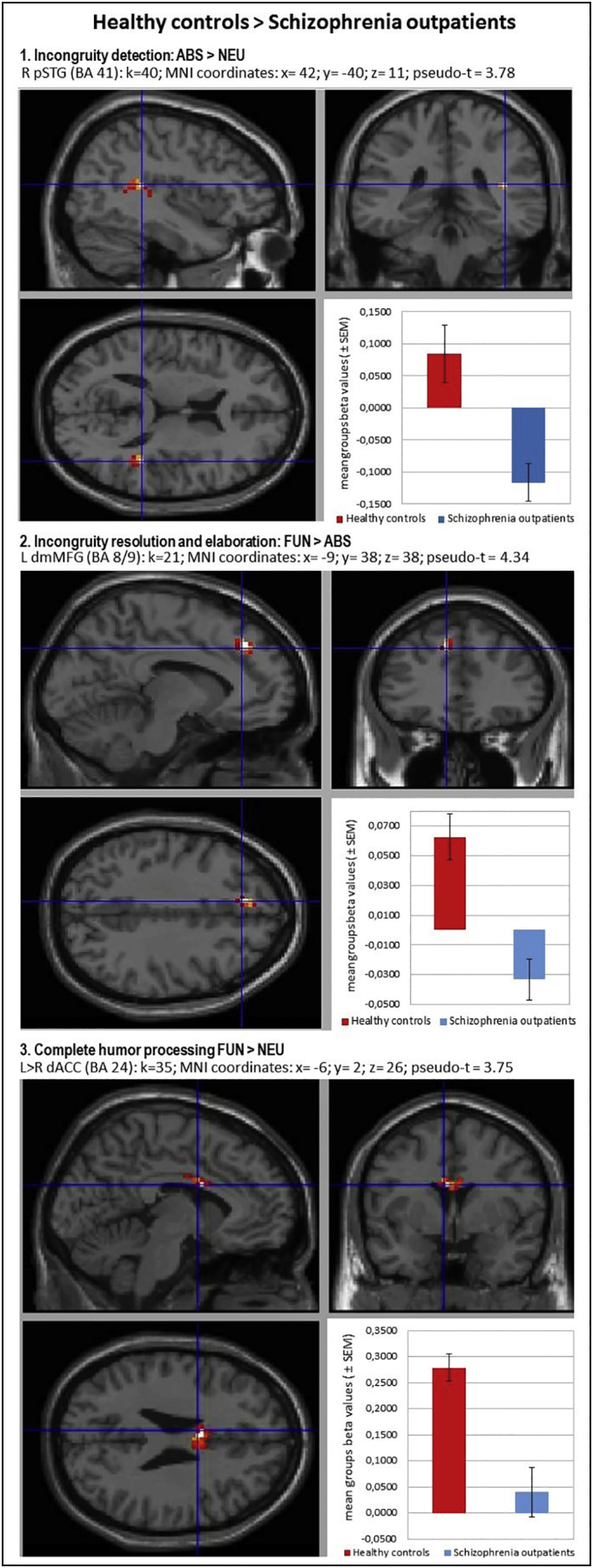
Localizations of brain regions revealed by between-group contrasts during humor comprehension process. Images of the localizations of brain regions activations (upper panels: sagittal and coronal planes; left bottom: transverse plane) were obtained by XjView toolbox (http://www.alivelearn.net/xjview). Each right bottom panel: mean group beta values (± SEM) averaged from the whole analyzed cluster volume presented as arbitrary units. Statistical analysis utilized a non-parametric whole-brain voxel-wise Pseudo-t-test. Localizations are reported as local maximum threshold with k ≥ 10 voxels extent threshold and with False Discovery Rate (FDR) correction at alpha = 0.0500, or uncorrected at alpha = 0.0010. L – left hemisphere; R – right hemisphere; BA – Brodmann's area; k – number of voxels in analyzed cluster size; MNI - Montreal Neurological Institute coordinates; pSTG – posterior Superior Temporal Gyrus; dmMFG – dorsomedial Middle Frontal Gyrus; dACC – dorsal Anterior Cingulate Cortex.
Fig. 3.
Localizations of brain regions revealed by between-group contrasts during comprehensibility and funniness ratings analyzed as parametric modulators. Images of the localizations of brain regions activations (upper panels: sagittal and coronal planes; left bottom: transverse plane) were obtained by XjView toolbox (http://www.alivelearn.net/xjview). Each right bottom panel: mean group beta values (± SEM) averaged from the whole analyzed cluster volume presented as arbitrary units. Statistical analysis utilized a non-parametric whole-brain voxel-wise Pseudo-t-test. Localizations are reported as local maximum threshold with k ≥ 10 voxels extent threshold and with False Discovery Rate (FDR) correction at alpha = 0.0500, or uncorrected at alpha = 0.0010. L – left hemisphere; R – right hemisphere; BA – Brodmann's area; k – number of voxels in analyzed cluster size; MNI - Montreal Neurological Institute coordinates; dmMFG – dorsomedial Middle Frontal Gyrus; dACC – dorsal Anterior Cingulate Cortex.
4. Discussion
To the best of our knowledge, this study is the first to indicate the neural correlates of impaired verbal humor comprehension in schizophrenia outpatients. Our results reveal that in comparison to healthy controls, people with schizophrenia manifest different patterns of activity throughout the neural circuits engaged in verbal humor processing. This is reflected especially by the decreased activations in the frontal, temporal and limbic cortices during processing of written jokes. In particular, between-group contrasts reveal that the most important alterations in schizophrenia are associated with fronto-temporal hypofunction in the right posterior temporal lobe during incongruity detection, in the left dorsomedial frontal cortex during incongruity resolution and elaboration as well as during comprehensibility ratings, and in the dorsal ACC during the complete humor processing and funniness ratings. Thus, we show that the humor comprehension process in schizophrenia seems to be deviant at all phases of the humor comprehension process.
4.1. Impaired verbal humor comprehension in schizophrenia: a behavioral level of expression
When analyzing behavioral data, we can consider our fMRI results as neural substrates of a lower level of humor comprehension in schizophrenia outpatients. The behavioral data related to humor comprehension shows subtle yet significant impairments in the comprehension of funny punchlines, indicating the same diminished ability to understand jokes in schizophrenia. This is consistent with previous studies on humor in schizophrenia (Adamczyk et al., 2016, Bozikas et al., 2007, Corcoran et al., 1997, Davenport, 2008, Falkenberg et al., 2007, Marjoram et al., 2005, Polimeni and Reiss, 2006b, Polimeni et al., 2010, Tsoi et al., 2008). Similarly, but only as a non-significant trend, we found that schizophrenia outpatients were more likely to indicate nonsensical endings as more funny, and that they found funny endings less funny than the controls did. Importantly, these disturbances in the joke comprehension process were accompanied by an increased response time of joke ratings. However, this increased response time may be assumed as a nonspecific effect in schizophrenia (i.e. antipsychotic side-effects and cognitive impairments).
4.2. Neural substrates of impaired incongruity detection in schizophrenia
The semantic incongruity detection impairment in schizophrenia is related to temporal lobe alteration (e.g. right posterior temporal cortex hypofunction in schizophrenia) as assessed by between-group comparisons (ABS vs NEU). This region is recognized as being involved in social cognition and higher-order language processing (Bigler et al., 2007, Goel and Dolan, 2001, Thermenos et al., 2013). Recent studies indicate that schizophrenia is related to morphological disturbances in the V layer of the STG with GABA-ergic hypofunction in this region (Steiner et al., 2016), with a lower volume of posterior STG (Narayanaswamy et al., 2015), and decreased fronto-temporal connectivity from the right STG to the dorsal ACC (Yoon et al., 2015) or IFG and STG/MTG (Straube et al., 2014). These findings support our data and suggest that diminished incongruity detection in schizophrenia subjects is related specifically to structural and functional alterations in the fronto-temporal connections. Altogether, this may be considered a primary source of the impairments in humor processing (differences in processing absurd semantic meaning in schizophrenia).
Additionally, within-group contrasts in healthy controls reveal that the incongruity detection process is related to the activation of the ventromedial IFG, which may be the result of surprising nonsensical endings. Enhanced activity of frontal regions is associated with language comprehension and semantic knowledge, i.e. controlled retrieval of semantic information (Devlin et al., 2003, Gough, 2005, Mollo et al., 2016). Activation in the dorsal ACC observed in healthy controls may be considered to be the executive center of the error detection and conflict monitoring system (Gauvin et al., 2016, Swick and Turken, 2002). Such activations have not been observed in schizophrenia outpatients, which may further underlie difficulties in understanding conflicting content. Thus, our results from healthy controls (but not subjects with schizophrenia) indicate that incongruity detection is related to specific bilateral hyperactivation in the ventromedial IFG and in the right dorsal ACC, possibly acting as a ‘warning system’ activated during processing of a non-executable task. At the same time, we note that processing the irresolvable incongruity of nonsensical punchlines in healthy controls is related to a high degree of deactivations in frontal, temporal, parietal and occipital cortices in both hemispheres. Most of these regions were found to be activated in other contrasts, resembling further steps in humor comprehension processing: incongruity resolution and elaboration. For example, the hypofunction of the dorsolateral PFC (dlPFC) may be related to unexecutable tasks, e.g. irresolvable semantic incongruities included in nonsensical condition (ABS). When not employed, the dlPFC may lead to a reduced comprehensibility, or vice versa. This is further supported by inhibition of the higher-level language comprehension process which is manifested by suppression of right MTG and the left IPL activity as parts of ventral and dorsal streams of speech processing, respectively (Hickok and Poeppel, 2015). It should be noted that observed deactivation in occipital cortices (e.g. middle occipital gyrus - MOG) may be related to the concept of the extended-TPJ, e.g. the temporo-occipito-parietal-junction (TOPJ), which represents regions of multimodal sensory processing (Neely et al., 2012). It is also relevant to social behavior (e.g. ToM) and detection and processing of unexpected stimuli, such as the process of incongruity detection and resolution (Vrticka et al., 2013).
The ABS vs NEU contrast reflecting incongruity detection suggests that people with schizophrenia experience difficulties in recognizing nonsensical content as mismatched to the semantic context of the story. This could be the result of disturbances within an error conflict monitoring system function employed in lexical and semantic reorganization. This interpretation is supported by other research into the role of the right temporal lobe (Bigler et al., 2007, Gainotti, 2013, Miozzo et al., 2016, Price, 2010), prefrontal cortices (Chou et al., 2009, Gough, 2005, Ligeza et al., 2016, Miyake et al., 2000) and dorsal ACC (Gauvin et al., 2016, Orr and Hester, 2012, Sohn et al., 2007, Swick and Jovanovic, 2002, Swick and Turken, 2002) function and its structural and functional alterations in schizophrenia (Callicott et al., 2000, Carter et al., 1998, Ferrarelli et al., 2015, Fornito et al., 2009, Minzenberg et al., 2009, Neuhaus et al., 2007, Polli et al., 2008, White et al., 2010, Yan et al., 2012, Yoon et al., 2015).
4.3. Neural substrates of impaired incongruity resolution and elaboration processing in schizophrenia
In the second contrast of verbal humor processing - the incongruity resolution and elaboration (FUN vs ABS) - the between-group analysis revealed significant suppression of activity in the left dorsomedial MFG/SFG in schizophrenia. Furthermore, findings from parametric modulation analysis concerning subjective evaluation of the comprehensibility of stories/punchlines seem to indicate the importance of the PFC in incongruity resolution process rather than in elaboration or funniness. This is generally in line with the literature data on the role of the PFC for incongruity resolution (Campbell et al., 2015, Chan et al., 2013, Kohn et al., 2011, Samson et al., 2008, Samson et al., 2009, Wild et al., 2006). However, our findings and other research vary substantially in the PFC location, with the exception of the unequivocal dominance of the left hemisphere in successful humor comprehension process and reported activations within the MFG/SFG in the various parts of BA 8/9/10 areas. Importantly, it should be considered, that a wide spectrum of the cognitive abilities identified with the PFC may reflex the complex nature of humor processing.
Moreover, the observed hypoactivation in the dorsomedial MFG/SFG (BA 8/9) in our clinical group seems to partially support a previous study on relatives of schizophrenia patients and observed hypoactivation in the middle PFC (BA 8/9) during ToM humor processing (Marjoram et al., 2006). However, differences in reported locations (medial PFC vs middle PFC) and examined functions (written joke comprehension vs ToM humor processing) should be emphasized.
In general, our results from humor comprehension processing in schizophrenia are in line with other research revealing suppressed engagement of the PFC in executive functioning, problem reasoning, and decision making in schizophrenia (Carter et al., 1998, Ferrarelli et al., 2015, Minzenberg et al., 2009, Nielsen, 2011). Thus, we may assume that the compromised process of resolving funny punchlines in schizophrenia is primarily associated with hypofunction in the PFC, which may be the principal cause of this deficit. Finally, considering the role of PFC deficiencies in schizophrenia during humor comprehension process, it should be emphasized that similar to the increase of the RT's or basic cognitive skills deficiencies (e.g. MoCA assessment), all these effects seem to be unspecific and related to hypofrontality, characteristics of schizophrenia.
Furthermore, our results from within-group analysis reveal that patterns of activation within the schizophrenia group are characterized by similar but smaller volumes of activations in the left SFG, bilateral TPJ and left precuneus. We also observed different lateralization patterns of activation in temporal cortices (bilateral activations of the posterior parts of temporal cortices in schizophrenia vs the right temporal pole in healthy controls) and a lack of specific humor-related activation of the left cerebellum and the left caudate nucleus in schizophrenia.
It should be noted that the brain activity pattern in healthy controls during the resolution and elaboration of incongruities in funny punchlines was mainly manifested by unspecific activations which contained cognitive and the emotional components, what may be simply associated with the overlapping of the incongruity resolution and elaboration stages as assessed in this contrast (FUN vs ABS). This is commonly reported in the literature as the correlates of subjective ratings of funniness also frequently activate cognitive components such as the dorsomedial PFC, TPJ, TP, ACC or precuneus (Vrticka et al., 2013). In line with previous studies (Bartolo et al., 2006, Chan et al., 2013, Marjoram et al., 2006, Samson et al., 2008, Samson et al., 2009), our results suggest that the proposed cognitive component of incongruity resolution for funny endings may be related to specific hyperactivation, particularly in the left hemisphere, and includes the dorsomedial MFG/SFG, TPJ and precuneus. However, it should be emphasized that these activations were related to both types of ratings. Importantly, the within-group analysis confirms that the volumes of activations of these regions were significantly smaller in the schizophrenia group. Furthermore, in healthy controls, the analysis including subjective ratings found just one activation of the subcortical region specifically dedicated to better understanding of jokes seemingly related to incongruity resolution: the left caudate nucleus. The activation within this region is a part of the brain reward system response following a successful resolution of semantic incongruities in funny endings. In previous research, activation of the caudate nuclei was recognized as being correlated with the mesocorticolimbic system activation during successful processing of jokes (Franklin and Adams, 2011, Mobbs et al., 2005). In other words, ‘getting a joke’ is rewarding and pleasurable in itself. However, it should be noted that in this study we did not find any activation of the left caudate nuclei during incongruity resolution and elaboration in the schizophrenia group, but we found a specific schizophrenia-related activation of the right caudate nuclei during funniness ratings.
Similarly, we found only one specific activation seemingly associated with emotional processing resulting from elaboration - the left posterior cerebellum - which was related to higher subjective funniness ratings. This supports previous studies into the role of the cerebellum in humor perception and appreciation as well as its relationship to laughter (Bartolo et al., 2006, Frank et al., 2012, Frank et al., 2013, Franklin and Adams, 2011, Goel and Dolan, 2007, Hutcherson et al., 2005, Wild et al., 2003). However, it should be emphasized that we did not find such cerebellum activation in subjects with schizophrenia.
4.4. Neural substrates in the diminished complete humor processing in schizophrenia
Lastly, in the contrast reflecting the complete humor processing (FUN vs NEU), between-group analysis revealed differences in interhemispheric (L > R) dorsal ACC hypofunction. This supports findings from the analysis including funniness ratings. Thus, it may be seen as a neural substrate of the tendency to indicate funny endings as less funny and/or indicating nonsensical content as more funny, in contrast to the control group.
Additionally, the comparison of patterns of within-group contrast reveal that schizophrenia is characterized by smaller volumes of activation in the bilateral TPJ (with preserved dominance of the left hemisphere) simultaneously with a specific absence of temporal cortex responses. Importantly, the above mentioned findings were supported by more precise conjunction analysis (FUN – ABS masked by FUN - NEU) results. This revealed that in healthy controls the neural substrates engaged in the quintessence of the successful humor comprehension process (e.g. incongruity resolution and elaboration of the funny puns) were present within the temporal and parietal lobes of the both hemispheres (e.g. temporal lobe L < R; TPJ L > R). What is most important, in the schizophrenia group we found the sole essential activation for funny puns processing in the left posterior STG and Angular Gyrus, considered as a part of TPJ. Apart from lack of both temporal lobe activations, the most intriguing fact is that we did not find any such activation in the right hemisphere. This seems to be an essential difference in the pattern of the activation evoked by the funny jokes between healthy controls and schizophrenia outpatients. Furthermore, it may provide clear evidence of disturbances in the process of differentiation between neutral and funny content and problems with semantic change of incongruities within funny endings. Finally, the temporo-parietal processing alterations in the right hemisphere seems to be the most probable and evident cause of diminished humor processing abilities in schizophrenic population.
On the other hand, when including a subjective value of funniness, the most prominent differences between the groups indicate suppressed activation in the dorsal ACC in the schizophrenia group. The within-group pattern comparison also reveals a significantly lower number of activated regions in schizophrenia, especially in the frontal, temporal and parietal regions, and an absence of activation in the left cerebellum. Together, these findings indicate that the difficulty in assessing funniness is associated with hypofrontality (especially in the prefrontal and cingulate cortex). These disturbances may be also related to the observed tendency to indicate nonsense as funny, which may be interpreted as a deficiency in the conflict monitoring system (Neuhaus et al., 2007, Yan et al., 2012) resulting in detecting absurd content as semi-coherent. This is in accordance with our findings on the role of the dorsal ACC in the incongruity detection process. Finally, as we found no specific activation within the cerebellum in the SCH group, which can be associated with the observed tendency to find funny endings less amusing than the control group. Apart from the exceptional study by Marjoram et al. (2006) into high-risk relatives, we did not find any publications which investigate this issue in chronic schizophrenia outpatients.
Otherwise, the pattern of brain responses in healthy controls during the complete humor processing in the within-group contrast indicated that funny punchlines differed from neutral content in a greater temporo-parietal activation of both hemispheres (with left parietal and right temporal dominance). This is consistent with previous findings on humor (Bartolo et al., 2006, Campbell et al., 2015, Goel and Dolan, 2001, Kohn et al., 2011, Mobbs et al., 2003, Samson et al., 2008, Wild et al., 2006). Our research revealed unique results since, except the left cerebellum, we did not find brain activations believed to be neural correlates of the feeling of amusement such as the rectus gyri in the orbitofrontal/ventromedial PFC, ventral ACC, insula, amygdala or parahippocampal gyri (Chan et al., 2012, Franklin and Adams, 2011, Goel and Dolan, 2001, Mobbs et al., 2003, Samson et al., 2008, Wild et al., 2006).
One possible explanation is that the jokes included in our procedures were not funny as such, but rather they were almost-funny-jokes which were able to induce humor-related responses (i.e. ‘not funny, but intended to be funny’; Campbell et al., 2015). This may be because most of the ‘really funny’ jokes were rejected during pre-selection because of vulgar and/or offensive content, which would be used in a natural social environment (as opposed to our laboratory conditions). Significantly, this interpretation would be in accordance with the social violations theory of humor originated by Thomas Veatch (1998). Again, our jokes (with the funny punchlines) were correctly assessed as logical as the semantic transition was maintained in our experimental stimuli. This was previously confirmed by a panel of judges indicating that these jokes were considered funny. However, our procedures did not distinguish between the understanding of the jokes and being amused by the jokes. Thus, we can only consider the regions within the neural circuit involved in the humor comprehension process and not the state of amusement. In other words, our funny stimuli were able to induce brain responses as a result of specific processes associated with humor comprehension but without a strong emotional amusement reaction. We were only able to testify how this cognitive process runs, without gaining insight into its essential emotional responses. Otherwise, when analyzed by parametric methods (data not shown), our results reveal certain between-group differences in comparison to the funniness rating process, e.g. in the interhemispheric ventromedial prefrontal cortex, the rectus gyrus (BA11) or in the right amygdala; however, these activations did not survive the threshold in non-parametric tests.
Furthermore, in reference to the literature, we occasionally found contrary activations, i.e. we did not find activations in regions suggested by other authors for the incongruity detection process, e.g. the right MFG and MTG (Chan et al., 2013). More specifically, neuroimaging studies by Chan et al., 2012, Chan et al., 2013 reveal that each stage of humor comprehension involves different brain regions. They identified clear divisions between brain regions activated during incongruity detection (e.g. MTG and MFG in the right hemisphere), incongruity resolution (e.g. the left SFG and IPL) and elaboration (e.g. the left ventromedial PFC, the right ventral ACC, the amygdala and the bilateral parahippocampal gyrus). It should be noted that activations were found to be different and/or more or less extensive in different studies. In general, previous studies on humor indicated fronto-temporal and temporo-parietal activation in association with the cognitive component of joke processing, as well as ventromedial PFC activation in association with its emotional component (Bartolo et al., 2006, Goel and Dolan, 2001, Goel and Dolan, 2007, Mobbs et al., 2003, Samson et al., 2008, Samson et al., 2009; for an extended review see Vrticka et al., 2013). However, this differentiation between brain regions engaged in the humor-related neural circuit needs to be evaluated further, as suggested by our results and other recent findings (Campbell et al., 2015). Some authors suggest that fMRI methods may not be optimal for disentangling the incongruity detection and resolution processes because they occur almost instantaneously. This means that other methods which can provide high time resolution are more suitable (e.g. EEG or MEG), and need to be used in the evaluation of the dynamic time components related to this rapid neural network activation (Vrticka et al., 2013). Recent research (Du et al., 2013, Shibata et al., 2016) using EEG ERP methods investigated the locations and time course of incongruity detection and resolution. Du et al. (2013) found that incongruity detection of funny content results in a more negative ERP deflection generated in the left MFG and temporal lobe at a 400 ms time window. They also found a negative/positive ERP deflection change generated in the ACC between 600 and 800 ms, which reflects incongruity resolution and the later positives generated in the MFG and fusiform gyrus during the elaboration/appreciation stage at 1400 ms. Furthermore, other research shows that incongruity detection activates frontal regions (e.g. SFG and medial PFC) approx. 200 ms after the punchline and that incongruity resolution activates temporal-parietal regions at a time window of approx. 600 ms (Shibata et al., 2016). Thus, the right temporal and left fronto-parietal circuit seems to be a critical neural loop engaged in humor processing. We should note that in verbal humor, other stimuli modalities of jokes (e.g. cartoons) need to be considered in the three-step humor comprehension model to understand the specificity of the brain activations involved in the process of incongruity detection and resolution. Until then, the phenomenon of ‘getting a joke’ remains insufficiently well understood, therefore further studies into the dissociation of incongruity detection from the resolution process need to be conducted.
4.5. On the role of the right hemisphere in verbal humor processing: double hemispheric dissociation in humor processing
Our results support the critical role of the right hemisphere in the integration of the humorous content of funny punchlines. In particular, within-group analyses reveal specific activation in the right TPJ in response to funny conditions. We conclude that these activations may reflect the specificity of this region in the evaluation of the humorous component of funny punchlines. Moreover, this was confirmed by a positive correlation of subjective ratings and the activation of this region. In other words, the right hemisphere seems to be engaged specifically in the processing of incongruity resolution and elaboration (e.g. funny content processing), but not when resolution of semantic incongruities is impossible (e.g. nonsensical content processing). Similarly, we also argue that the activation of the right temporal pole (or more extensively, the right anterior MTG/STG) during the comprehension of funny punchlines reflects the process of obtaining coherence between distant semantic meanings within the figurative meaning of humorous stimuli. We found that the left anterior MTG/TP activation, indicated by the contrast reflecting the complete humor processing of funny punchlines, was specifically related to a better comprehensibility of the story but not its funniness. Thus, these results suggest a double dissociation of the right and left hemispheres during processing different aspects of humor. It is in line with previous studies indicating the engagement of the right temporal cortices in processing surprising, unexpected or less probable word meanings and its integration within semantic context (Federmeier and Kutas, 1999, Gainotti, 2013, Goel and Dolan, 2001, St George et al., 1999). Data from patients with right-hemisphere brain damage strongly supports this opinion. Damaged right-hemisphere language functions are manifested in an impaired ability to recognize, comprehend and use humorous stimuli (Benton and Bryan, 1996, Brownell et al., 1983, Bryan, 1988, Heath and Blonder, 2005, Lehman Blake, 2003, Winner et al., 1998). Importantly for this issue, it should be pointed out again, that our results from conjunction analysis strongly suggest, that neural network alterations of the right hemisphere, especially in the temporal and parietal lobes, may be the most prominent neural substrates of impaired verbal humor comprehension processing in schizophrenia.
5. Limitations
The results provide the first evidence concerning the neural substrate of diminished humor comprehension processing in schizophrenia. However, since our examinations are pioneering in their nature, some issues need to be discussed.
First of all, it must be pointed out again that the incongruity model of humor processing, commonly applied and assessed in humor research field, reflects only the theoretical approaches of Suls (1972) and Wyer and Collins (1992). Hence, investigation of the theoretical concept of incongruity detection and resolution may in future bring somewhat confusing results. Furthermore, considering complex nature of humor processing in the wide spectrum of human social interactions (Veatch, 1998) it must be pointed out that each experimental assessment may investigate only a small part of such a highly complex phenomenon as humor. Noteworthily, a conflict between a phenomenology of humor and methodological approach in MRI assessment should be discussed. Emotional cognitive process such as humor comprehension (i.e. incongruity detection and resolution) and emotional amusement (i.e. elaboration) should be considered in close relation with the biological reaction of laughter (which is a final element, different from pure elaboration). Hence, laughter is one of the most important elements of humor in its natural social conditions, but investigation of this aspect of humor is in their nature very hard under fMRI methodological conditions. The reason is obvious, the more the subject is laughing the more uncontrolled additional movements appear. Such motor artifacts were basically undesirable in the neuroimaging investigations (e.g. fMRI BOLD signal analysis exclusion criteria). Moreover, considering the above, we can be not sure of what in fact funniness rating means. In other words, it is unknown in which part funniness rating reflects the subjective emotional amusement, or rather the cognitive abilities to appreciate the processes of ‘getting a joke’.
Secondly, when considering differences between healthy controls and schizophrenia outpatients, we need to raise two issues. First, the effects in healthy controls are generally conserved under the FDR correction, while in schizophrenia subjects they are only significant when uncorrected. This may be related to the fact that the SCH group may be internally less homogeneous in terms of brain activity and deviation from the healthy brain. Moreover, this could be also affected by long-term antipsychotic pharmacotherapy on an individual basis. Other possible confounding factor, e.g. morphological differences between brains of schizophrenic subjects and controls (Gong et al., 2016, Rubinov and Bullmore, 2013, Van Haren et al., 2013, Zhang et al., 2014) may be also related to this issue. However, there are still no unequivocal findings concerning anatomical brain differences between schizophrenia and neurotypical. Moreover, concerning between-group differences, it should be noted that psychosocial functioning and psychopathological condition of the recruited schizophrenic subjects were relatively good. Conversely, the patients whose psychopathological symptoms increased (e.g. clinical deterioration such as paranoid thoughts or high anxiety levels) were automatically rejected from the MRI scanning procedure. It should be stressed that the clinical subjects were not coerced into enrolling in the study and they were all in relatively stable remission of the psychopathological symptoms.
Thus, our results concerning differences in brain responses during processing of jokes in the healthy controls and schizophrenia outpatients are preliminary guidelines to the humor comprehension neural circuit and its deficit. Further neuroimaging studies of clinical subjects need to be conducted to extend the knowledge about the neural basis of diminished humor comprehension in schizophrenia.
6. Conclusions
In conclusion, our results suggest that there are differences in verbal humor processing between individuals with schizophrenia and healthy controls. The differences are mostly clearly visible in longer response times and finding jokes less comprehensible, as compared to the control group. Neural substrates of these humor processing alterations in people with schizophrenia were indicated by attenuated brain activations in the right posterior temporal cortices (STG, BA 41) at the time when irresolvable incongruity in nonsensical content was being processed, the left dorsomedial frontal cortex (MFG/SFG, BA 8/9) when processing resolvable and funny incongruity in funny puns, and the interhemispheric dorsal anterior cingulate cortex (ACC, BA 24) during complete humor processing. Based on these conclusions, our pioneering work on humor processing in schizophrenia suggests there are differences throughout entire humor comprehension process, which is accompanied by fronto-temporal hypoactivation.
Funding sources
This study was supported by the National Science Centre, Poland (grant no 2014/13/B/HS6/03091) and the statutory activity of the Association for the Development of Psychiatry and Community Care, Krakow, Poland. Open access was sponsored by ‘Leading National Research Centre’ programme of the Ministry of Science and Higher Education, Poland.
Conflict of interest
All authors declare no conflicts of interest.
Acknowledgments
We are cordially grateful to all who agreed to participate in this study. Our thanks go also to the staff at “U Pana Cogito” and Occupational Therapy Workshops in Krakow for their assistance in coordinating the participants from the clinical group. We express our appreciation to Joanna Wieczorek, who significantly contributed to data analysis in this work.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2017.01.010.
Appendix A. Supplementary data
Fig. 1.
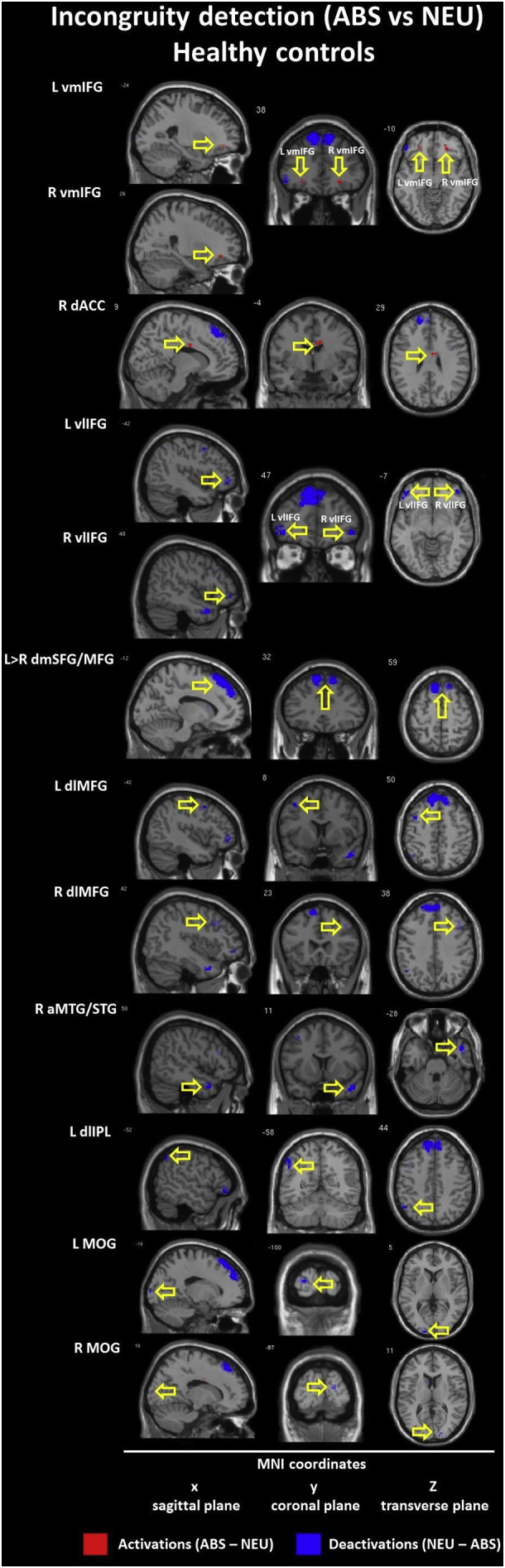
Pattern of brain activations during verbal humor processing - incongruity detection in healthy controls.
Fig. 1 Localizations of brain regions revealed by within-group contrasts during incongruity detection in healthy controls (n = 20). Images of the localizations of brain regions activations (left: sagittal; middle: coronal; right: transverse planes) were obtained by XjView toolbox (http://www.alivelearn.net/xjview). Statistical analysis utilized a non-parametric whole-brain voxel-wise Pseudo-t-test. Localizations are reported as local maximum threshold with k ≥ 10 voxels extent threshold and with False Discovery Rate (FDR) correction at alpha = 0.0500, or uncorrected at alpha = 0.0010. L – left hemisphere; R – right hemisphere; MNI - Montreal Neurological Institute coordinates; vmIFG, ventromedial Inferior Frontal Gyrus; dACC, dorsal Anterior Cingulate Cortex; vlIFG, ventrolateral Inferior Frontal Gyrus; dmSFG/MFG, dorsomedial Superior Frontal Gyrus/Middle Frontal Gyrus; dlMFG, dorsolateral Middle Frontal Gyrus; aMTG/STG, anterior Middle Temporal Gyrus/Superior Temporal Gyrus; dlIPL, dorsolateral Inferior Parietal Lobe; MOG, Middle Occipital Gyrus.
Fig. 2.
Pattern of brain activations during verbal humor processing - incongruity detection in schizophrenia outpatients.
Fig. 2 Localizations of brain regions revealed by within-group contrasts during incongruity detection in schizophrenia outpatients (n = 20). Images of the localizations of brain regions activations (left: sagittal; middle: coronal; right: transverse planes) were obtained by XjView toolbox (http://www.alivelearn.net/xjview). Statistical analysis utilized a non-parametric whole-brain voxel-wise Pseudo-t-test. Localizations are reported as local maximum threshold with k ≥ 10 voxels extent threshold and with False Discovery Rate (FDR) correction at alpha = 0.0500, or uncorrected at alpha = 0.0010. L – left hemisphere; R – right hemisphere; MNI - Montreal Neurological Institute coordinates; dmMFG/SFG, dorsomedial Middle Frontal Gyrus/Superior Frontal Gyrus; pMTG/STG, posterior Middle Temporal Gyrus/Superior Temporal Gyrus; IPL, Inferior Parietal Lobe; MOG, Middle Occipital Gyrus.
Fig. 3.
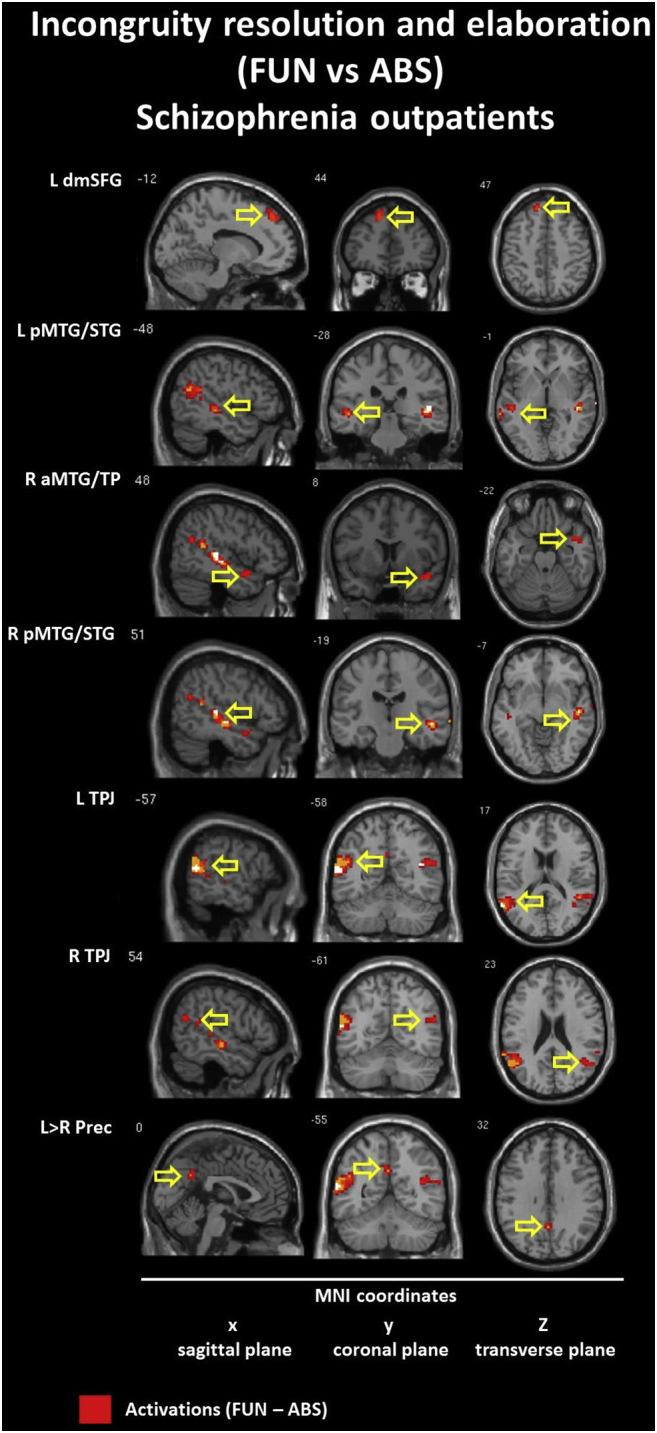
Pattern of brain activations during verbal humor processing - incongruity resolution and elaboration in healthy controls.
Fig. 3 Localizations of brain regions revealed by within-group contrasts during incongruity resolution and elaboration in healthy controls (n = 20). Images of the localizations of brain regions activations (left: sagittal; middle: coronal; right: transverse planes) were obtained by XjView toolbox (http://www.alivelearn.net/xjview). Statistical analysis utilized a non-parametric whole-brain voxel-wise Pseudo-t-test. Localizations are reported as local maximum threshold with k ≥ 10 voxels extent threshold and with False Discovery Rate (FDR) correction at alpha = 0.0500, or uncorrected at alpha = 0.0010. L – left hemisphere; R – right hemisphere; MNI - Montreal Neurological Institute coordinates; dmSFG, dorsomedial Superior Frontal Gyrus; aMTG/STG, anterior Middle Temporal Gyrus/Superior Temporal Gyrus; TPJ, Temporo-Parietal Junction; Prec, Precuneus; pCer, posterior Cerebellum; Caud, Caudate nucleus; dlIFG, dorsolateral Inferior Frontal Gyrus; vmIFG, ventromedial Inferior Frontal Gyrus.
Fig. 4.
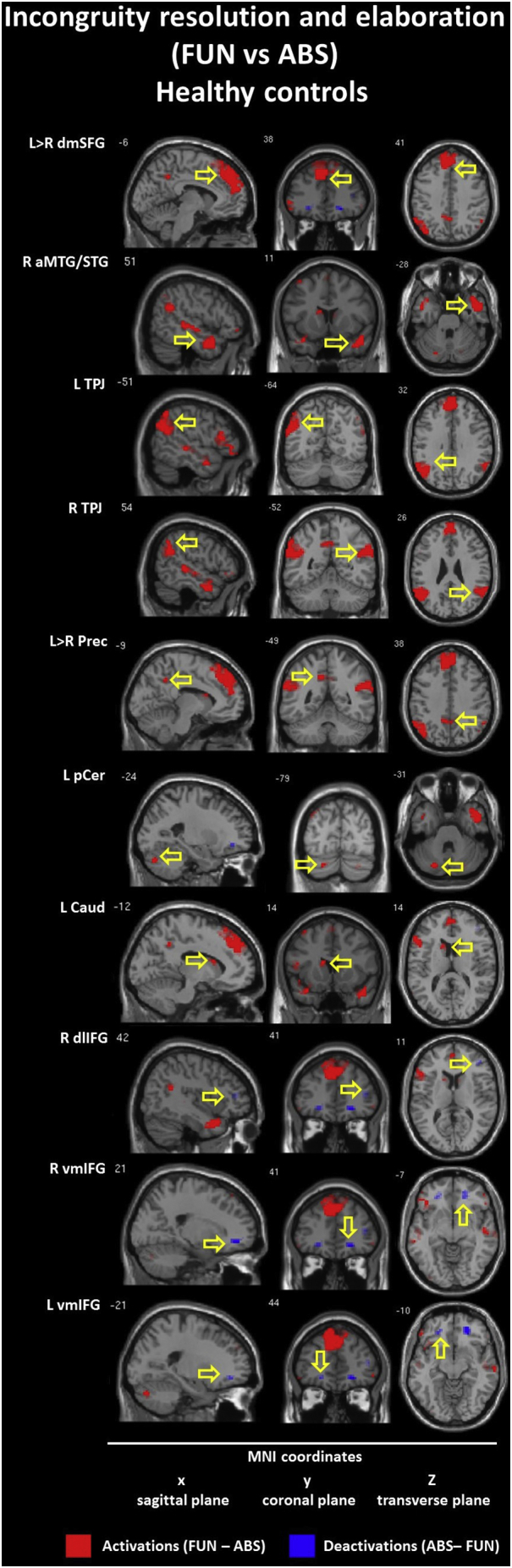
Pattern of brain activations during verbal humor processing - incongruity resolution and elaboration in schizophrenia outpatients.
Fig. 4 Localizations of brain regions revealed by within-group contrasts during incongruity resolution and elaboration in schizophrenia outpatients (n = 20). Images of the localizations of brain regions activations (left: sagittal; middle: coronal; right: transverse planes) were obtained by XjView toolbox (http://www.alivelearn.net/xjview). Statistical analysis utilized a non-parametric whole-brain voxel-wise Pseudo-t-test. Localizations are reported as local maximum threshold with k ≥ 10 voxels extent threshold and with False Discovery Rate (FDR) correction at alpha = 0.0500, or uncorrected at alpha = 0.0010. L – left hemisphere; R – right hemisphere; MNI - Montreal Neurological Institute coordinates; dmSFG, dorsomedial Superior Frontal Gyrus; pMTG/STG, posterior Middle Temporal Gyrus/Superior Temporal Gyrus; aMTG/TP, anterior Middle Temporal Gyrus/Temporal Pole; pMTG/STG, posterior Middle Temporal Gyrus/Superior Temporal Gyrus; TPJ, Temporo-Parietal Junction; Prec, Precuneus.
Fig. 5.
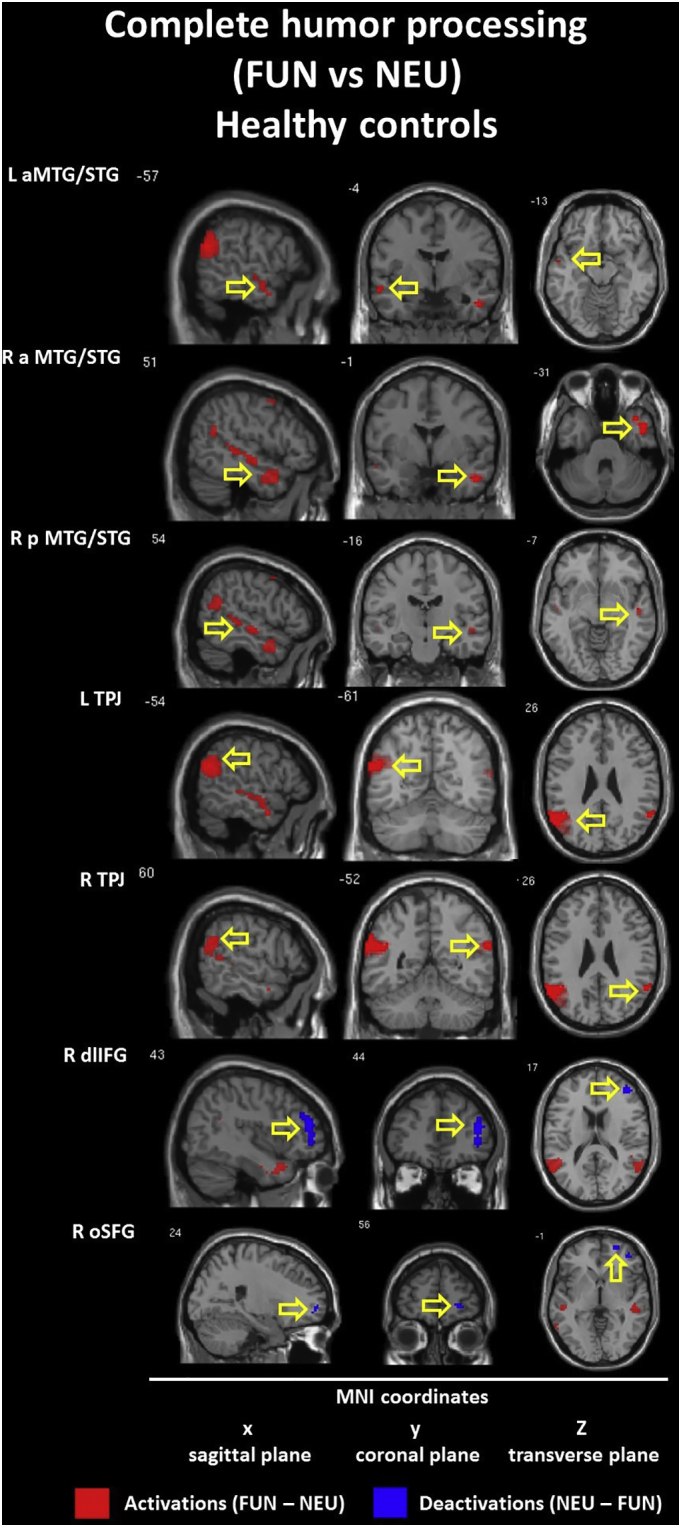
Pattern of brain activations during verbal humor processing – complete humor processing in healthy controls.
Fig. 5 Localizations of brain regions revealed by within-group contrasts during complete humor processing in healthy controls (n = 20). Images of the localizations of brain regions activations (left: sagittal; middle: coronal; right: transverse planes) were obtained by XjView toolbox (http://www.alivelearn.net/xjview). Statistical analysis utilized a non-parametric whole-brain voxel-wise Pseudo-t-test. Localizations are reported as local maximum threshold with k ≥ 10 voxels extent threshold and with False Discovery Rate (FDR) correction at alpha = 0.0500, or uncorrected at alpha = 0.0010. L – left hemisphere; R – right hemisphere; MNI - Montreal Neurological Institute coordinates; aMTG/STG, anterior Middle Temporal Gyrus/Superior Temporal Gyrus; pMTG/STG, posterior Middle Temporal Gyrus/Superior Temporal Gyrus; TPJ, Temporo-Parietal Junction; dlIFG, dorsolateral Inferior Frontal Gyrus; oSFG, orbital Superior Frontal Gyrus.
Fig. 6.
Pattern of brain activations during verbal humor processing - complete humor processing in schizophrenia outpatients.
Fig. 6 Localizations of brain regions revealed by within-group contrasts during complete humor processing in schizophrenia outpatients (n = 20). Images of the localizations of brain regions activations (left: sagittal; middle: coronal; right: transverse planes) were obtained by XjView toolbox (http://www.alivelearn.net/xjview). Statistical analysis utilized a non-parametric whole-brain voxel-wise Pseudo-t-test. Localizations are reported as local maximum threshold with k ≥ 10 voxels extent threshold and with False Discovery Rate (FDR) correction at alpha = 0.0500, or uncorrected at alpha = 0.0010. L – left hemisphere; R – right hemisphere; MNI - Montreal Neurological Institute coordinates; vlIFG, ventrolateral Inferior Frontal Gyrus; TPJ, Temporo-Parietal Junction.
Fig. 7.
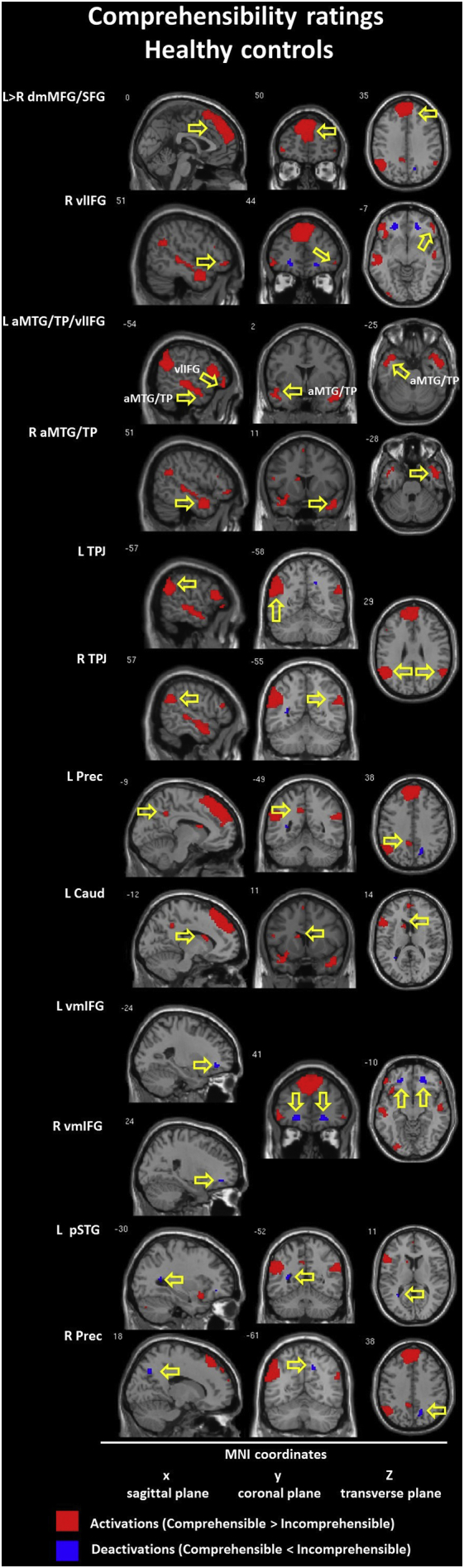
Pattern of brain activations during subjective ratings on comprehensibility in healthy controls.
Fig. 7 Localizations of brain regions revealed by within-group contrasts during comprehensibility ratings analyzed as parametric modulators in healthy controls (n = 20). Images of the localizations of brain regions activations (left: sagittal; middle: coronal; right: transverse planes) were obtained by XjView toolbox (http://www.alivelearn.net/xjview). Statistical analysis utilized a non-parametric whole-brain voxel-wise Pseudo-t-test. Localizations are reported as local maximum threshold with k ≥ 10 voxels extent threshold and with False Discovery Rate (FDR) correction at alpha = 0.0500, or uncorrected at alpha = 0.0010. L – left hemisphere; R – right hemisphere; MNI - Montreal Neurological Institute coordinates; dmMFG/SFG, dorsomedial Middle Frontal Gyrus/Superior Frontal Gyrus; vlIFG, ventrolateral Inferior Frontal Gyrus; aMTG/TP/vlIFG, anterior Middle Temporal Gyrus/Temporal Pole/Inferior Frontal Gyrus; TPJ, Temporo-Parietal Junction; Prec, Precuneus; Caud, Caudate nucleus; vmIFG, ventromedial Inferior Frontal Gyrus; pSTG, posterior Superior Temporal Gyrus.
Fig. 8.
Pattern of brain activations during subjective ratings on comprehensibility in schizophrenia outpatients.
Fig. 8 Localizations of brain regions revealed by within-group contrasts during comprehensibility ratings analyzed as parametric modulators in schizophrenia outpatients (n = 20). Images of the localizations of brain regions activations (left: sagittal; middle: coronal; right: transverse planes) were obtained by XjView toolbox (http://www.alivelearn.net/xjview). Statistical analysis utilized a non-parametric whole-brain voxel-wise Pseudo-t-test. Localizations are reported as local maximum threshold with k ≥ 10 voxels extent threshold and with False Discovery Rate (FDR) correction at alpha = 0.0500, or uncorrected at alpha = 0.0010. L – left hemisphere; R – right hemisphere; MNI - Montreal Neurological Institute coordinates; dmSFG, dorsomedial Superior Frontal Gyrus; pMTG/STG, posterior Middle Temporal Gyrus/Superior Temporal Gyrus; TPJ, Temporo-Parietal Junction; Prec, Precuneus.
Fig. 9.
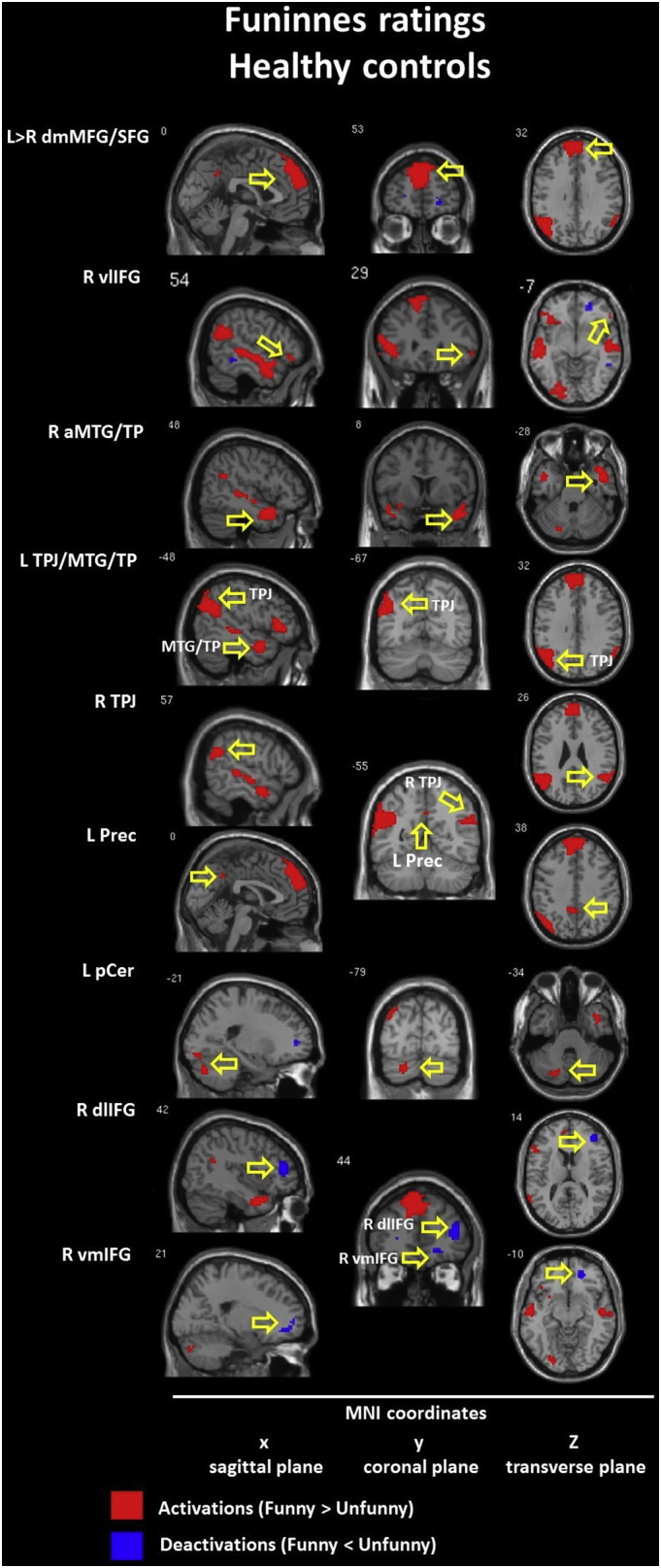
Pattern of brain activations during subjective ratings on funniness in healthy controls.
Fig. 9 Localizations of brain regions revealed by within-group contrasts during funniness ratings analyzed as parametric modulators in healthy controls (n = 20). Images of the localizations of brain regions activations (left: sagittal; middle: coronal; right: transverse planes) were obtained by XjView toolbox (http://www.alivelearn.net/xjview). Statistical analysis utilized a non-parametric whole-brain voxel-wise Pseudo-t-test. Localizations are reported as local maximum threshold with k ≥ 10 voxels extent threshold and with False Discovery Rate (FDR) correction at alpha = 0.0500, or uncorrected at alpha = 0.0010. L – left hemisphere; R – right hemisphere; MNI - Montreal Neurological Institute coordinates; dmMFG/SFG, dorsomedial Middle Frontal Gyrus/Superior Frontal Gyrus; vlIFG, ventrolateral Inferior Frontal Gyrus; aMTG/TP, anterior Middle Temporal Gyrus/Temporal Pole; TPJ, Temporo-Parietal Junction; Prec, Precuneus; pCer, posterior Cerebellum; dlIFG, dorsolateral Inferior Frontal Gyrus; vmIFG, ventromedial Inferior Frontal Gyrus.
Fig. 10.
Pattern of brain activations during subjective ratings on funniness in schizophrenia outpatients.
Fig. 10 Localizations of brain regions revealed by within-group contrasts during funniness ratings analyzed as parametric modulators in schizophrenia outpatients (n = 20). Images of the localizations of brain regions activations (left: sagittal; middle: coronal; right: transverse planes) were obtained by XjView toolbox (http://www.alivelearn.net/xjview). Statistical analysis utilized a non-parametric whole-brain voxel-wise Pseudo-t-test. Localizations are reported as local maximum threshold with k ≥ 10 voxels extent threshold and with False Discovery Rate (FDR) correction at alpha = 0.0500, or uncorrected at alpha = 0.0010. L – left hemisphere; R – right hemisphere; MNI - Montreal Neurological Institute coordinates; pMTG/STG, posterior Middle Temporal Gyrus/Superior Temporal Gyrus; TPJ, Temporo-Parietal Junction; Prec, Precuneus; Caud, Caudate nucleus.
Fig. 11.
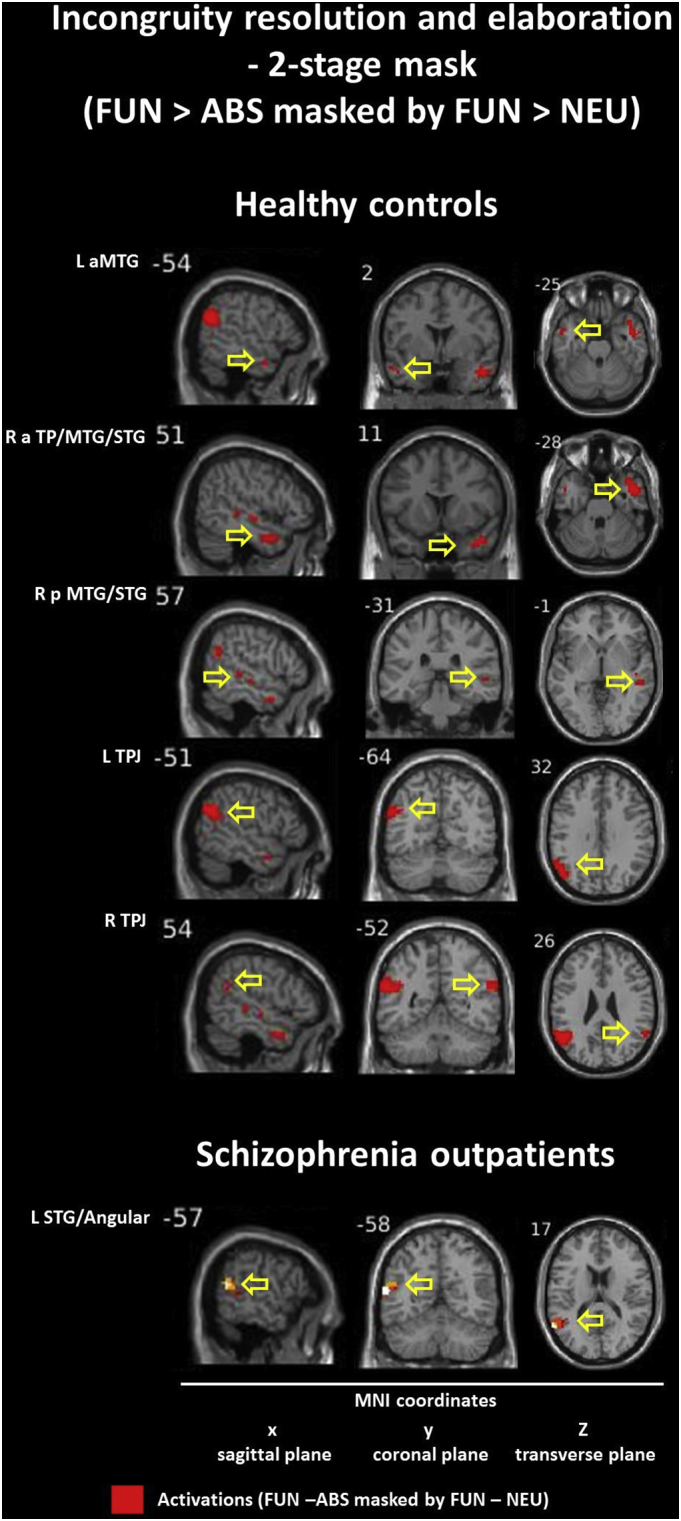
Pattern of brain activations during verbal humor processing – 2-stage masking for incongruity resolution and elaboration processing in healthy controls and schizophrenia outpatients.
Fig. 11 Localizations of brain regions revealed by within-group 2-stage mask contrasts during incongruity resolution and elaboration processing in healthy controls (n = 20) and schizophrenia outpatients (n = 20). Images of the localizations of brain regions activations (left: sagittal; middle: coronal; right: transverse planes) were obtained by XjView toolbox (http://www.alivelearn.net/xjview). Statistical analysis utilized a non-parametric whole-brain voxel-wise Pseudo-t-test. Localizations are reported as local maximum threshold with k ≥ 10 voxels extent threshold and with uncorrected significance level at alpha = 0.0010. L – left hemisphere; R – right hemisphere; MNI - Montreal Neurological Institute coordinates; aTP/MTG/STG, anterior Temporal Pole/Middle Temporal Gyrus/Superior Temporal Gyrus; pMTG/STG, posterior Middle Temporal Gyrus/Superior Temporal Gyrus; TPJ, Temporo-Parietal Junction.
References
- Adamczyk P., Daren A., Sułecka A., Błądziński P., Cichocki Ł., Kalisz A., Gawęda Ł., Cechnicki A. Do better communication skills promote sheltered employment in schizophrenia? Schizophr. Res. 2016;176:331–339. doi: 10.1016/j.schres.2016.08.015. [DOI] [PubMed] [Google Scholar]
- Atkins M., Burgess A., Bottomley C., Riccio M. Chlorpromazine equivalents: a consensus of opinion for both clinical and research applications. Psychiatr. Bull. 1997;21:224–226. [Google Scholar]
- Bartolo A., Benuzzi F., Nocetti L., Baraldi P., Nichelli P. Humor comprehension and appreciation: an FMRI study. J. Cogn. Neurosci. 2006;18:1789–1798. doi: 10.1162/jocn.2006.18.11.1789. [DOI] [PubMed] [Google Scholar]
- Bekinschtein T.A., Davis M.H., Rodd J.M., Owen A.M. Why clowns taste funny: the relationship between humor and semantic ambiguity. J. Neurosci. 2011;31:9665–9671. doi: 10.1523/JNEUROSCI.5058-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton E., Bryan K. Right cerebral hemisphere damage: incidence of language problems. Int. J. Rehabil. Res. Int. Z. Rehabil. Rev. Int. Rech. Readaptation. 1996;19:47–54. [PubMed] [Google Scholar]
- Bigler E.D., Mortensen S., Neeley E.S., Ozonoff S., Krasny L., Johnson M., Lu J., Provencal S.L., McMahon W., Lainhart J.E. Superior temporal gyrus, language function, and autism. Dev. Neuropsychol. 2007;31:217–238. doi: 10.1080/87565640701190841. [DOI] [PubMed] [Google Scholar]
- Bozikas V.P., Kosmidis M.H., Giannakou M., Anezoulaki D., Petrikis P., Fokas K., Karavatos A. Humor appreciation deficit in schizophrenia: the relevance of basic neurocognitive functioning. J. Nerv. Ment. Dis. 2007;195:325–331. doi: 10.1097/01.nmd.0000243798.10242.e2. [DOI] [PubMed] [Google Scholar]
- Brownell H.H., Michel D., Powelson J., Gardner H. Surprise but not coherence: sensitivity to verbal humor in right-hemisphere patients. Brain Lang. 1983;18:20–27. doi: 10.1016/0093-934x(83)90002-0. [DOI] [PubMed] [Google Scholar]
- Bryan K.L. Assessment of language disorders after right hemisphere damage. Br. J. Disord. Commun. 1988;23:111–125. doi: 10.3109/13682828809019881. [DOI] [PubMed] [Google Scholar]
- Bryan K. Whurr; London: 1995. The Right Hemisphere Language Battery (2nd ed.) [Google Scholar]
- Cai C., Yu L., Rong L., Zhong H. Effectiveness of humor intervention for patients with schizophrenia: a randomized controlled trial. J. Psychiatr. Res. 2014;59:174–178. doi: 10.1016/j.jpsychires.2014.09.010. [DOI] [PubMed] [Google Scholar]
- Callicott J.H., Bertolino A., Mattay V.S., Langheim F.J., Duyn J., Coppola R., Goldberg T.E., Weinberger D.R. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb. Cortex N. Y. N. 2000;1991(10):1078–1092. doi: 10.1093/cercor/10.11.1078. [DOI] [PubMed] [Google Scholar]
- Campbell D.W., Wallace M.G., Modirrousta M., Polimeni J.O., McKeen N.A., Reiss J.P. The neural basis of humour comprehension and humour appreciation: the roles of the temporoparietal junction and superior frontal gyrus. Neuropsychologia. 2015;79:10–20. doi: 10.1016/j.neuropsychologia.2015.10.013. [DOI] [PubMed] [Google Scholar]
- Carter C.S., Perlstein W., Ganguli R., Brar J., Mintun M., Cohen J.D. Functional hypofrontality and working memory dysfunction in schizophrenia. Am. J. Psychiatry. 1998;155:1285–1287. doi: 10.1176/ajp.155.9.1285. [DOI] [PubMed] [Google Scholar]
- Cechnicki A. Institute of Psychiatry and Neurology; Warsaw: 2011. Schizophrenia – a Multidimensional Process. An Evaluation of its Course, Prognosis and Long-term Treatment Results. [Google Scholar]
- Chan Y.-C., Chou T.-L., Chen H.-C., Liang K.-C. Segregating the comprehension and elaboration processing of verbal jokes: an fMRI study. NeuroImage. 2012;61:899–906. doi: 10.1016/j.neuroimage.2012.03.052. [DOI] [PubMed] [Google Scholar]
- Chan Y.-C., Chou T.-L., Chen H.-C., Yeh Y.-C., Lavallee J.P., Liang K.-C., Chang K.-E. Towards a neural circuit model of verbal humor processing: an fMRI study of the neural substrates of incongruity detection and resolution. NeuroImage. 2013;66:169–176. doi: 10.1016/j.neuroimage.2012.10.019. [DOI] [PubMed] [Google Scholar]
- Chou T.-L., Chen C.-W., Wu M.-Y., Booth J.R. The role of inferior frontal gyrus and inferior parietal lobule in semantic processing of Chinese characters. Exp. Brain Res. 2009;198:465–475. doi: 10.1007/s00221-009-1942-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran R., Cahill C., Frith C.D. The appreciation of visual jokes in people with schizophrenia: a study of “mentalizing” ability. Schizophr. Res. 1997;24:319–327. doi: 10.1016/s0920-9964(96)00117-x. [DOI] [PubMed] [Google Scholar]
- Davenport L. Humor recognition, but not appreciation, reduced in schizophrenia patients. Psychol. Med. 2008;38:801–810. [Google Scholar]
- Devlin J.T., Matthews P.M., Rushworth M.F.S. Semantic processing in the left inferior prefrontal cortex: a combined functional magnetic resonance imaging and transcranial magnetic stimulation study. J. Cogn. Neurosci. 2003;15:71–84. doi: 10.1162/089892903321107837. [DOI] [PubMed] [Google Scholar]
- Du X., Qin Y., Tu S., Yin H., Wang T., Yu C., Qiu J. Differentiation of stages in joke comprehension: evidence from an ERP study. Int. J. Psychol. 2013;48:149–157. doi: 10.1080/00207594.2012.665162. [DOI] [PubMed] [Google Scholar]
- Falkenberg I., Klügel K., Bartels M., Wild B. Sense of humor in patients with schizophrenia. Schizophr. Res. 2007;95:259–261. doi: 10.1016/j.schres.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Federmeier K.D., Kutas M. Right words and left words: electrophysiological evidence for hemispheric differences in meaning processing. Brain Res. Cogn. Brain Res. 1999;8:373–392. doi: 10.1016/s0926-6410(99)00036-1. [DOI] [PubMed] [Google Scholar]
- Ferrarelli F., Riedner B.A., Peterson M.J., Tononi G. Altered prefrontal activity and connectivity predict different cognitive deficits in schizophrenia. Hum. Brain Mapp. 2015;36:4539–4552. doi: 10.1002/hbm.22935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisekovic S., Memic A., Pasalic A. Correlation between moca and mmse for the assessment of cognition in schizophrenia. Acta Inform. Medica AIM J. Soc. Med. Inform. Bosnia Herzeg. Časopis Druš. Za Med. Inform. BiH. 2012;20:186–189. doi: 10.5455/aim.2012.20.186-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A., Yücel M., Dean B., Wood S.J., Pantelis C. Anatomical abnormalities of the anterior cingulate cortex in schizophrenia: bridging the gap between neuroimaging and neuropathology. Schizophr. Bull. 2009;35:973–993. doi: 10.1093/schbul/sbn025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank B., Propson B., Göricke S., Jacobi H., Wild B., Timmann D. Humor and laughter in patients with cerebellar degeneration. Cerebellum Lond. Engl. 2012;11:564–573. doi: 10.1007/s12311-011-0320-z. [DOI] [PubMed] [Google Scholar]
- Frank B., Andrzejewski K., Göricke S., Wondzinski E., Siebler M., Wild B., Timmann D. Humor, laughter, and the cerebellum: insights from patients with acute cerebellar stroke. Cerebellum Lond. Engl. 2013;12:802–811. doi: 10.1007/s12311-013-0488-5. [DOI] [PubMed] [Google Scholar]
- Franklin R.G., Adams R.B. The reward of a good joke: neural correlates of viewing dynamic displays of stand-up comedy. Cogn. Affect. Behav. Neurosci. 2011;11:508–515. doi: 10.3758/s13415-011-0049-7. [DOI] [PubMed] [Google Scholar]
- Gainotti G. The contribution of language to the right-hemisphere conceptual representations: a selective survey. J. Clin. Exp. Neuropsychol. 2013;35:563–572. doi: 10.1080/13803395.2013.798399. [DOI] [PubMed] [Google Scholar]
- Gardner D.M., Murphy A.L., O'Donnell H., Centorrino F., Baldessarini R.J. International consensus study of antipsychotic dosing. Am. J. Psychiatry. 2010;167:686–693. doi: 10.1176/appi.ajp.2009.09060802. [DOI] [PubMed] [Google Scholar]
- Gauvin H.S., De Baene W., Brass M., Hartsuiker R.J. Conflict monitoring in speech processing: an fMRI study of error detection in speech production and perception. NeuroImage. 2016;126:96–105. doi: 10.1016/j.neuroimage.2015.11.037. [DOI] [PubMed] [Google Scholar]
- Gelkopf M., Kreitler S., Sigal M. Laughter in a psychiatric ward. Somatic, emotional, social, and clinical influences on schizophrenic patients. J. Nerv. Ment. Dis. 1993;181:283–289. [PubMed] [Google Scholar]
- Gelkopf M., Sigal M., Kramer R. Therapeutic use of humor to improve social support in an institutionalized schizophrenic inpatient community. J. Soc. Psychol. 1994;134:175–182. doi: 10.1080/00224545.1994.9711380. [DOI] [PubMed] [Google Scholar]
- Gelkopf M., Gonen B., Kurs R., Melamed Y., Bleich A. The effect of humorous movies on inpatients with chronic schizophrenia. J. Nerv. Ment. Dis. 2006;194:880–883. doi: 10.1097/01.nmd.0000243811.29997.f7. [DOI] [PubMed] [Google Scholar]
- Goel V., Dolan R.J. The functional anatomy of humor: segregating cognitive and affective components. Nat. Neurosci. 2001;4:237–238. doi: 10.1038/85076. [DOI] [PubMed] [Google Scholar]
- Goel V., Dolan R.J. Social regulation of affective experience of humor. J. Cogn. Neurosci. 2007;19:1574–1580. doi: 10.1162/jocn.2007.19.9.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Q., Lui S., Sweeney J.A. A selective review of cerebral abnormalities in patients with first-episode schizophrenia before and after treatment. Am. J. Psychiatry. 2016;173:232–243. doi: 10.1176/appi.ajp.2015.15050641. [DOI] [PubMed] [Google Scholar]
- Gough P.M. Dissociating linguistic processes in the left inferior frontal cortex with transcranial magnetic stimulation. J. Neurosci. 2005;25:8010–8016. doi: 10.1523/JNEUROSCI.2307-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath R.L., Blonder L.X. Spontaneous humor among right hemisphere stroke survivors. Brain Lang. 2005;93:267–276. doi: 10.1016/j.bandl.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Hickok G., Poeppel D. Neural basis of speech perception. In: Celesia G.G., Hickok G., editors. Handbook of Clinical Neurology. Vol. 129. 2015. pp. 149–160. (3rd series). [DOI] [PubMed] [Google Scholar]
- Howes O.D., Murray R.M. Schizophrenia: an integrated socio-developmental-cognitive model. Lancet. 2014;383:1677–1687. doi: 10.1016/S0140-6736(13)62036-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcherson C.A., Goldin P.R., Ochsner K.N., Gabrieli J.D., Barrett L.F., Gross J.J. Attention and emotion: does rating emotion alter neural responses to amusing and sad films? NeuroImage. 2005;27:656–668. doi: 10.1016/j.neuroimage.2005.04.028. [DOI] [PubMed] [Google Scholar]
- Kay S.R., Fiszbein A., Opler L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kircher T.T.J., Leube D.T., Erb M., Grodd W., Rapp A.M. Neural correlates of metaphor processing in schizophrenia. NeuroImage. 2007;34:281–289. doi: 10.1016/j.neuroimage.2006.08.044. [DOI] [PubMed] [Google Scholar]
- Kohn N., Kellermann T., Gur R.C., Schneider F., Habel U. Gender differences in the neural correlates of humor processing: implications for different processing modes. Neuropsychologia. 2011;49:888–897. doi: 10.1016/j.neuropsychologia.2011.02.010. [DOI] [PubMed] [Google Scholar]
- Kuperberg G.R., Caplan D. Language dysfunction in schizophrenia. In: Schiffer R.B., Rao S.M., Fogel B.S., editors. Neuropsychiatry. Lippincott Williams and Wilkins; Philadelphia: 2003. pp. 444–466. [Google Scholar]
- Kuperberg G.R., McGuire P.K., David A.S. Reduced sensitivity to linguistic context in schizophrenic thought disorder: evidence from on-line monitoring for words in linguistically anomalous sentences. J. Abnorm. Psychol. 1998;107:423–434. doi: 10.1037//0021-843x.107.3.423. [DOI] [PubMed] [Google Scholar]
- Lehman Blake M. Affective language and humor appreciation after right hemisphere brain damage. Semin. Speech Lang. 2003;24:107–119. doi: 10.1055/s-2003-38902. [DOI] [PubMed] [Google Scholar]
- Levy B., Celen-Demirtas S., Surguladze T., Eranio S., Ellison J. Neuropsychological screening as a standard of care during discharge from psychiatric hospitalization: the preliminary psychometrics of the CNS screen. Psychiatry Res. 2014;215:790–796. doi: 10.1016/j.psychres.2014.01.010. [DOI] [PubMed] [Google Scholar]
- Ligeza T.S., Wyczesany M., Tymorek A.D., Kamiński M. Interactions between the prefrontal cortex and attentional systems during volitional affective regulation: an effective connectivity reappraisal study. Brain Topogr. 2016;29:253–261. doi: 10.1007/s10548-015-0454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Łojek E. Pracownia Testów Psychologicznych; Warsaw: 2007. The Right Hemisphere Language Battery: RHLB-PL: The Manual. [Google Scholar]
- Marjoram D., Tansley H., Miller P., MacIntyre D., Owens D.G.C., Johnstone E.C., Lawrie S. A theory of mind investigation into the appreciation of visual jokes in schizophrenia. BMC Psychiatry. 2005;5:12. doi: 10.1186/1471-244X-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marjoram D., Job D.E., Whalley H.C., Gountouna V.-E., McIntosh A.M., Simonotto E., Cunningham-Owens D., Johnstone E.C., Lawrie S. A visual joke fMRI investigation into theory of mind and enhanced risk of schizophrenia. NeuroImage. 2006;31:1850–1858. doi: 10.1016/j.neuroimage.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Mashal N., Vishne T., Laor N., Titone D. Enhanced left frontal involvement during novel metaphor comprehension in schizophrenia: evidence from functional neuroimaging. Brain Lang. 2013;124:66–74. doi: 10.1016/j.bandl.2012.11.012. [DOI] [PubMed] [Google Scholar]
- Mashal N., Vishne T., Laor N. The role of the precuneus in metaphor comprehension: evidence from an fMRI study in people with schizophrenia and healthy participants. Front. Hum. Neurosci. 2014;8:818. doi: 10.3389/fnhum.2014.00818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minzenberg M.J., Laird A.R., Thelen S., Carter C.S., Glahn D.C. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch. Gen. Psychiatry. 2009;66:811–822. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miozzo M., Williams A.C., McKhann G.M., Hamberger M.J. Topographical gradients of semantics and phonology revealed by temporal lobe stimulation. Hum. Brain Mapp. 2016 doi: 10.1002/hbm.23409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A., Friedman N.P., Emerson M.J., Witzki A.H., Howerter A., Wager T.D. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: a latent variable analysis. Cogn. Psychol. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Mobbs D., Greicius M.D., Abdel-Azim E., Menon V., Reiss A.L. Humor modulates the mesolimbic reward centers. Neuron. 2003;40:1041–1048. doi: 10.1016/s0896-6273(03)00751-7. [DOI] [PubMed] [Google Scholar]
- Mobbs D., Hagan C.C., Azim E., Menon V., Reiss A.L. Personality predicts activity in reward and emotional regions associated with humor. Proc. Natl. Acad. Sci. U. S. A. 2005;102:16502–16506. doi: 10.1073/pnas.0408457102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollo G., Karapanagiotidis T., Bernhardt B.C., Murphy C.E., Smallwood J., Jefferies E. An individual differences analysis of the neurocognitive architecture of the semantic system at rest. Brain Cogn. 2016;109:112–123. doi: 10.1016/j.bandc.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran J.M., Wig G.S., Adams R.B., Janata P., Kelley W.M. Neural correlates of humor detection and appreciation. NeuroImage. 2004;21:1055–1060. doi: 10.1016/j.neuroimage.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Narayanaswamy J.C., Kalmady S.V., Venkatasubramanian G., Gangadhar B.N. Clinical correlates of superior temporal gyrus volume abnormalities in antipsychotic-naïve schizophrenia. J. Neuropsychiatr. Clin. Neurosci. 2015;27:e128–e133. doi: 10.1176/appi.neuropsych.14030049. [DOI] [PubMed] [Google Scholar]
- Nasreddine Z.S., Phillips N.A., Bédirian V., Charbonneau S., Whitehead V., Collin I., Cummings J.L., Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- Neely M.N., Walter E., Black J.M., Reiss A.L. Neural correlates of humor detection and appreciation in children. J. Neurosci. 2012;32:1784–1790. doi: 10.1523/JNEUROSCI.4172-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus A.H., Koehler S., Opgen-Rhein C., Urbanek C., Hahn E., Dettling M. Selective anterior cingulate cortex deficit during conflict solution in schizophrenia: an event-related potential study. J. Psychiatr. Res. 2007;41:635–644. doi: 10.1016/j.jpsychires.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Nielsen R.E. Cognition in schizophrenia – a systematic review. Drug Discov. Today Ther. Strateg. 2011;8:43–48. [Google Scholar]
- Niznikiewicz M.A., Kubicki M., Mulert C., Condray R. Schizophrenia as a disorder of communication. Schizophr. Res. Treat. 2013;2013:952034. doi: 10.1155/2013/952034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt D.J., Need A.C. Where now for schizophrenia research? Eur. Neuropsychopharmacol. 2014;24:1181–1187. doi: 10.1016/j.euroneuro.2014.05.012. [DOI] [PubMed] [Google Scholar]
- Orr C., Hester R. Error-related anterior cingulate cortex activity and the prediction of conscious error awareness. Front. Hum. Neurosci. 2012;6:177. doi: 10.3389/fnhum.2012.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirce J.W. Generating stimuli for neuroscience using PsychoPy. Front. Neuroinformatics. 2009;2:10. doi: 10.3389/neuro.11.010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polimeni J., Reiss J.P. The first joke: exploring the evolutionary origins of humor. Evol. Psychol. 2006;4:347–366. [Google Scholar]
- Polimeni J., Reiss J.P. Humor perception deficits in schizophrenia. Psychiatry Res. 2006;141:229–232. doi: 10.1016/j.psychres.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Polimeni J.O., Campbell D.W., Gill D., Sawatzky B.L., Reiss J.P. Diminished humour perception in schizophrenia: relationship to social and cognitive functioning. J. Psychiatr. Res. 2010;44:434–440. doi: 10.1016/j.jpsychires.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Polli F.E., Barton J.J.S., Thakkar K.N., Greve D.N., Goff D.C., Rauch S.L., Manoach D.S. Reduced error-related activation in two anterior cingulate circuits is related to impaired performance in schizophrenia. Brain J. Neurol. 2008;131:971–986. doi: 10.1093/brain/awm307. [DOI] [PubMed] [Google Scholar]
- Price C.J. The anatomy of language: a review of 100 fMRI studies published in 2009. Ann. N. Y. Acad. Sci. 2010;1191:62–88. doi: 10.1111/j.1749-6632.2010.05444.x. [DOI] [PubMed] [Google Scholar]
- Rapp A.M., Langohr K., Mutschler D.E., Klingberg S., Wild B., Erb M. Isn't it ironic? Neural correlates of irony comprehension in schizophrenia. PLoS One. 2013;8:e74224. doi: 10.1371/journal.pone.0074224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Bores Ramírez L., Saracco-Álvarez R., Escamilla-Orozco R., Fresán Orellana A. Validity of the Montreal Cognitive Assessment Scale (MoCA) for the detection of cognitive impairment in schizophrenia. Salud Mental. 2014;37(6):485–490. [Google Scholar]
- Rubinov M., Bullmore E. Schizophrenia and abnormal brain network hubs. Dialogues Clin. Neurosci. 2013;15:339–349. doi: 10.31887/DCNS.2013.15.3/mrubinov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson A.C., Zysset S., Huber O. Cognitive humor processing: different logical mechanisms in nonverbal cartoons--an fMRI study. Soc. Neurosci. 2008;3:125–140. doi: 10.1080/17470910701745858. [DOI] [PubMed] [Google Scholar]
- Samson A.C., Hempelmann C.F., Huber O., Zysset S. Neural substrates of incongruity-resolution and nonsense humor. Neuropsychologia. 2009;47:1023–1033. doi: 10.1016/j.neuropsychologia.2008.10.028. [DOI] [PubMed] [Google Scholar]
- Schneider S., Wagels L., Haeussinger F.B., Fallgatter A.J., Ehlis A.-C., Rapp A.M. Haemodynamic and electrophysiological markers of pragmatic language comprehension in schizophrenia. World J. Biol. Psychiatry Off. J. World Fed. Soc. Biol. Psychiatry. 2015:1–13. doi: 10.3109/15622975.2015.1019359. [DOI] [PubMed] [Google Scholar]
- Shibata M., Terasawa Y., Osumi T., Masui K., Ito Y., Sato A., Umeda S. Time course and localization of brain activity in humor comprehension: an ERP/sLORETA study. Brain Res. 2016;1657:215–222. doi: 10.1016/j.brainres.2016.12.010. http://dx.doi.org/10.1016/j.brainres.2016.12.010 [DOI] [PubMed] [Google Scholar]
- Sohn M.-H., Albert M.V., Jung K., Carter C.S., Anderson J.R. Anticipation of conflict monitoring in the anterior cingulate cortex and the prefrontal cortex. Proc. Natl. Acad. Sci. U. S. A. 2007;104:10330–10334. doi: 10.1073/pnas.0703225104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St George M., Kutas M., Martinez A., Sereno M.I. Semantic integration in reading: engagement of the right hemisphere during discourse processing. Brain J. Neurol. 1999;122(7):1317–1325. doi: 10.1093/brain/122.7.1317. [DOI] [PubMed] [Google Scholar]
- Steiner J., Brisch R., Schiltz K., Dobrowolny H., Mawrin C., Krzyżanowska M., Bernstein H.-G., Jankowski Z., Braun K., Schmitt A. GABAergic system impairment in the hippocampus and superior temporal gyrus of patients with paranoid schizophrenia: a post-mortem study. Schizophr. Res. 2016;177:10–17. doi: 10.1016/j.schres.2016.02.018. [DOI] [PubMed] [Google Scholar]
- Straube B., Green A., Sass K., Kirner-Veselinovic A., Kircher T. Neural integration of speech and gesture in schizophrenia: evidence for differential processing of metaphoric gestures. Hum. Brain Mapp. 2013;34:1696–1712. doi: 10.1002/hbm.22015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube B., Green A., Sass K., Kircher T. Superior temporal sulcus disconnectivity during processing of metaphoric gestures in schizophrenia. Schizophr. Bull. 2014;40:936–944. doi: 10.1093/schbul/sbt110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suls J.M. A two-stage model for the appreciation of jokes and cartoons: an information-processing analysis. In: Goldstein J., McGhee P., editors. The Psychology of Humor: Theoretical Perspectives and Empirical Issues. Academic Press; New York: 1972. pp. 81–100. [Google Scholar]
- Swick D., Jovanovic J. Anterior cingulate cortex and the Stroop task: neuropsychological evidence for topographic specificity. Neuropsychologia. 2002;40:1240–1253. doi: 10.1016/s0028-3932(01)00226-3. [DOI] [PubMed] [Google Scholar]
- Swick D., Turken A.U. Dissociation between conflict detection and error monitoring in the human anterior cingulate cortex. Proc. Natl. Acad. Sci. U. S. A. 2002;99:16354–16359. doi: 10.1073/pnas.252521499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thermenos H.W., Whitfield-Gabrieli S., Seidman L.J., Kuperberg G., Juelich R.J., Divatia S., Riley C., Jabbar G.A., Shenton M.E., Kubicki M. Altered language network activity in young people at familial high-risk for schizophrenia. Schizophr. Res. 2013;151:229–237. doi: 10.1016/j.schres.2013.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titone D. Language, communication, & schizophrenia. J. Neurolinguistics. 2010;23:173–175. doi: 10.1016/j.jneuroling.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoi D.T.-Y., Lee K.-H., Gee K.A., Holden K.L., Parks R.W., Woodruff P.W.R. Humour experience in schizophrenia: relationship with executive dysfunction and psychosocial impairment. Psychol. Med. 2008;38(6):801–810. doi: 10.1017/S0033291707002528. [DOI] [PubMed] [Google Scholar]
- van der Gaag M., Hoffman T., Remijsen M., Hijman R., de Haan L., van Meijel B., van Harten P.N., Valmaggia L., de Hert M., Cuijpers A. The five-factor model of the Positive and Negative Syndrome Scale II: a ten-fold cross-validation of a revised model. Schizophr. Res. 2006;85:280–287. doi: 10.1016/j.schres.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Van Haren N.E., Cahn W., Hulshoff Pol H.E., Kahn R.S. Confounders of excessive brain volume loss in schizophrenia. Neurosci. Biobehav. Rev. 2013;37:2418–2423. doi: 10.1016/j.neubiorev.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Veatch T.C. A theory of humor. Humor: Int. J. Humor Res. 1998;11(2):161–215. [Google Scholar]
- Vrticka P., Black J.M., Reiss A.L. The neural basis of humour processing. Nat. Rev. Neurosci. 2013;14:860–868. doi: 10.1038/nrn3566. [DOI] [PubMed] [Google Scholar]
- White T.P., Joseph V., Francis S.T., Liddle P.F. Aberrant salience network (bilateral insula and anterior cingulate cortex) connectivity during information processing in schizophrenia. Schizophr. Res. 2010;123:105–115. doi: 10.1016/j.schres.2010.07.020. [DOI] [PubMed] [Google Scholar]
- Wible C.G. Schizophrenia as a disorder of social communication. Schizophr. Res. Treat. 2012;2012:920485. doi: 10.1155/2012/920485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild B., Rodden F.A., Grodd W., Ruch W. Neural correlates of laughter and humour. Brain J. Neurol. 2003;126:2121–2138. doi: 10.1093/brain/awg226. [DOI] [PubMed] [Google Scholar]
- Wild B., Rodden F.A., Rapp A., Erb M., Grodd W., Ruch W. Humor and smiling: cortical regions selective for cognitive, affective, and volitional components. Neurology. 2006;66:887–893. doi: 10.1212/01.wnl.0000203123.68747.02. [DOI] [PubMed] [Google Scholar]
- Winner E., Brownell H., Happé F., Blum A., Pincus D. Distinguishing lies from jokes: theory of mind deficits and discourse interpretation in right hemisphere brain-damaged patients. Brain Lang. 1998;62:89–106. doi: 10.1006/brln.1997.1889. [DOI] [PubMed] [Google Scholar]
- Witztum E., Briskin S., Lerner V. The use of humor with chronic schizophrenic patients. J. Contemp. Psychother. 1999;29(3):223–234. [Google Scholar]
- Woods S.W. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J. Clin. Psychiatry. 2003;64:663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- World Health Organization . World Health Organization; Geneva: 2011. ICD-10: International Statistical Classification of Diseases and Related Health Problems. [Google Scholar]
- World Medical Association Declaration of Helsinki Ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- Wyer R.S., Collins J.E. A theory of humor elicitation. Psychol. Rev. 1992;99(4):663–688. doi: 10.1037/0033-295x.99.4.663. [DOI] [PubMed] [Google Scholar]
- Yan H., Tian L., Yan J., Sun W., Liu Q., Zhang Y.-B., Li X.-M., Zang Y.-F., Zhang D. Functional and anatomical connectivity abnormalities in cognitive division of anterior cingulate cortex in schizophrenia. PLoS One. 2012;7(9):e45659. doi: 10.1371/journal.pone.0045659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon Y.B., Yun J.-Y., Jung W.H., Cho K.I.K., Kim S.N., Lee T.Y., Park H.Y., Kwon J.S. Altered fronto-temporal functional connectivity in individuals at ultra-high-risk of developing psychosis. PLoS One. 2015;10(8):e0135347. doi: 10.1371/journal.pone.0135347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F., Qiu L., Yuan L., Ma H., Ye R., Yu F., Hu P., Dong Y., Wang K. Evidence for progressive brain abnormalities in early schizophrenia: a cross-sectional structural and functional connectivity study. Schizophr. Res. 2014;159:31–35. doi: 10.1016/j.schres.2014.07.050. [DOI] [PubMed] [Google Scholar]