Abstract
Purpose
To evaluate the risk factors, incidence, and rate of progression of geographic atrophy (GA) in eyes with neovascular age-related macular degeneration (nAMD) treated with ranibizumab.
Design
Post-hoc analysis of a prospective clinical study.
Participants
69 participants with nAMD in at least one eye.
Methods
Participants were prospectively treated in the study eye with 0.5 mg intravitreal ranibizumab. Study eyes received 4 monthly injections followed by pro re nata injections until a fluid-free macula was achieved on optical coherence tomography. Risk factors assessed included baseline demographics, treatment, and ocular characteristics on imaging. Eyes were evaluated on fundus autofluorescence (FAF) for GA. The rate of GA area growth in study and fellow eyes was analyzed by linear regression of square-root transformed areas.
Main Outcome Measures
Development of new-onset GA and rate of GA area growth measured on ocular imaging, including FAF images of the study eyes.
Results
Sixty-nine participants (mean age 78.8±7.8 years) with an average of 40.0±13.6 months of follow-up were analyzed. Twenty-two of 69 study eyes (32%) were treatment naïve. During their first year of the study, participants received an average of 9.2±3.3 injections in the study eye. Of 63 study eyes with quality baseline images, 22 (35%) had pre-existing GA. Of the remaining 41 eyes, 7 (17%) developed new-onset GA during study follow-up. Those who developed new GA were older (all ≥79 years old) and had received fewer study injections on average (6.9 vs. 10.4 injections at 1 year) compared to those who did not develop new GA. Of the 12 treatment naïve study eyes without GA at baseline, 1 (8.3%) developed new GA during the study. In 21 study eyes with quantifiable GA area, eyes with GA present at baseline (16/21) enlarged by 0.34±0.26 mm/year, compared to 0.19±0.12 mm/year in eyes developing new-onset GA (5/21).
Conclusions
While 17% of study eyes without GA present at baseline receiving ranibizumab developed new GA, the role of ranibizumab in the development of GA is unclear. Further prospective longitudinal studies are required to determine the eyes most at risk of developing GA in the setting of anti-VEGF treatment.
Age-related macular degeneration (AMD) is the leading cause of blindness in the United States.1, 2 Intravitreal injections of anti-vascular endothelial growth factor (VEGF) agents including ranibizumab have preserved vision in many patients affected by neovascular AMD (nAMD).3, 4 However, data from the Comparison of AMD Treatment Trials (CATT) showing that approximately 18% of participants treated with anti-VEGF agents for 2 years developed geography atrophy (GA) has led to increasing concern that anti-VEGF agents may increase the risk of GA development.5 At this time, the superior visual outcomes of eyes with nAMD treated with anti-VEGF in clinical trials outweigh the potentially increased risk of GA development, but this risk should be further evaluated.
We previously reported on a study designed to image choroidal neovascularization (CNV) with high-speed indocyanine green (ICG) angiography to follow therapy with intravitreal ranibizumab.6 In this post-hoc analysis of these data, we evaluate factors affecting new GA development, incidence of GA, and GA growth rate in eyes undergoing ranibizumab therapy for nAMD. We determine the extent and expansion of GA based on fundus autofluorescence (FAF). The increased contrast sensitivity provided by autofluorescence images enables detection of small areas of atrophy in early disease compared to traditional color fundus photography.7,8 This longitudinal, multimodal imaging study of participants with ICG-verified CNV provides a unique opportunity to evaluate the incidence and progression of GA in eyes with nAMD treated with ranibizumab.
Methods
This study was approved by the National Institutes of Health (NIH) Institutional Review Board and adhered to the tenets of the Declaration of Helsinki. All participants provided written informed consent. This work is compliant with the Health Insurance Portability and Accountability Act and is registered as clinical trial NCT00656903 on www.clinicaltrials.gov.
Study Participants
Participant selection and study intervention have been previously described.9 Between March 2009 and August 2012, 75 participants with nAMD at the NIH Clinical Center in Bethesda, MD, were enrolled. Participants were ≥50 years old without medical conditions that might prevent consistent follow-up or contraindications for undergoing fluorescein and ICG angiography.
Each participant contributed one study eye to the protocol. If both eyes met the inclusion criteria, the study eye was chosen at the investigator’s discretion. Inclusion criteria for the study eye included diagnosis of AMD defined by the presence of drusen ≥63 m in size, CNV with associated exudation secondary to AMD, and visual acuity (VA) >20/400. Exclusion criteria included: CNV or decreased vision not due to nAMD, subfoveal GA or fibrosis, myopic retinopathy, or a spherical equivalent refraction greater than −8.00 diopters. Eyes were also excluded for a history of CNV treatment using transpupillary thermotherapy, external beam radiation therapy, submacular surgery, and previous vitrectomy or scleral buckle. In this post-hoc analysis, we included eyes with ≥ 1 year of follow up and secondarily evaluated fellow eyes with ≥1 year of follow up.
Study Intervention
Each study eye received induction therapy with intravitreal 0.5 mg ranibizumab (Lucentis, Genentech, Inc, San Francisco, CA) at baseline, 1 month, 2 months, and 3 months, followed by pro re nata (PRN) treatment. The fellow eye received intravitreal ranibizumab at the investigator’s discretion.
All participants were evaluated monthly for retreatment in the PRN phase until the conclusion of this study in April 2014 and received monthly injections in the study eye until a fluid-free macula was achieved on Cirrus optical coherence tomography (OCT; Carl Zeiss Meditec, Jena, Germany). A fluid-free macula was defined as complete resolution of subretinal and/or intraretinal fluid, which allowed for persistence of a retinal pigment epithelial detachment (PED). If fluid or hemorrhage recurred, the study eye received intravitreal ranibizumab again following the PRN protocol without repeating the induction protocol. Best-corrected VA (BCVA) was evaluated with the Early Treatment Diabetic Retinopathy Study VA chart.
At each annual visit, participants underwent imaging with OCT, color fundus photography, FAF, FA (Topcon Medical Systems, Inc, Oakland, NJ), and high-speed ICG (Heidelberg Engineering, Heidelberg, Germany). ICG images were captured with a 30° view following injection with 1 mL of 8.3 mg/mL ICG dye. FA images were obtained with a 50° view following injection with 5 mL of 10% fluorescein sodium.
Determination of Geographic Atrophy
For each participant’s study eye, one photo each of color fundus photography, FAF, and late (10 min) FA from baseline and each subsequent year of enrollment were aligned to a color fundus photo using i2k Align software (DualAlign, Clifton, NY). This process created a stack of images for each eye across time and across the 3 imaging modalities aligned to a single photo. GA was defined on FAF as a region of hypo-autofluorescence ≥0.05 mm2 in area located within the vascular arcades that remained present across subsequent images and corresponded to at least one of the following criteria: (1) sharp margins and visible large choroidal vessels on color fundus photography; (2) sharp margins and uniform hyper-fluorescence on FA; and/or (3) retinal pigment epithelium (RPE) and outer retinal loss on OCT.
Two trained graders (A.T., J.K.) outlined the perimeter of all qualifying GA lesions on FAF images within stacks using the freehand drawing tool in ImageJ (NIH, Bethesda, MD). Homogenous contiguous areas of GA were manually traced. If multiple GA lesions existed, they were traced separately and summed. Pixel count was converted to area using the known 177 mm2 area covered by the 50° Topcon color fundus photo.9 Fellow eyes were similarly monitored and quantitated for GA excluding those with disciform lesions.
Potential Risk Factors for New Geographic Atrophy
Participant baseline information including age, sex, race, hypertension, and GA status in the fellow eye were evaluated and analyzed as potential risk factors for new GA development. BCVA, prior treatment for AMD, and number of intravitreal injections received during the study were also analyzed. Data on previous treatments were collected from a retrospective chart review.
Baseline FA and ICG imaging were used to characterize the CNV type (classic/minimally classic/occult/retinal angiomatous proliferation [RAP]/indeterminate) and the size of the lesion (mm2) in the study eye, as previously published.9 OCT was used to identify the presence of epiretinal membrane and foveal fluid in the study eye, defined as the presence of intraretinal fluid, sub-retinal fluid, or sub-RPE fluid within 500 μm of the foveal center. The presence of hemorrhage was identified on color fundus photography and the rate of GA development within an area of recorded hemorrhage was calculated.
For each study eye with quantifiable GA meeting the study definition of GA, we determined square root-transformed area (mm), shortest distance from lesion edge to fovea (mm), and whether the area of GA at any time point was completely outside the area of the baseline total CNV lesion on FA and ICG (GA location overlapping/non-overlapping/unknown). On FA, total CNV lesion included CNV, exudation, contiguous hemorrhage, and serous PED. On ICG, total CNV lesion included the entire area of hyper-fluorescence on late (30 min) frames.
Analysis
Participants were classified into three study groups based on progression of the study eye: did not have any GA while enrolled in the study (never developed GA), developed GA while enrolled in the study (developed new GA), or had existing GA at baseline (GA present at baseline). For analysis of potential characteristics contributing to new GA in the setting of ranibizumab therapy, study eyes that developed new GA were compared to those that never developed GA. Due to the small number of participants in these groups, descriptive statistics are presented.
For analysis of GA growth, linear regressions were fit to square root-transformed GA areas. A comparison of GA growth in study vs. fellow eyes was performed in participants with bilateral quantifiable GA.
Results
Of the 75 participants enrolled in the study, 69 participants were available for analysis (Figure 1). Four participants were excluded for having less than 1 year of follow-up and 2 participants were later diagnosed with other diseases (vitelliform dystrophy and macular telangiectasia type 2). The 69 included participants had mean ± standard deviation age of 78.8±7.8 years and 42 (60.9%) participants were women. Included participants completed an average of 40.0±13.6 months of follow-up and 24 participants completed at least 4 years of follow-up. Fifty (72.5%) of the 69 participants completed the study as per protocol at the study endpoint.
Figure 1.
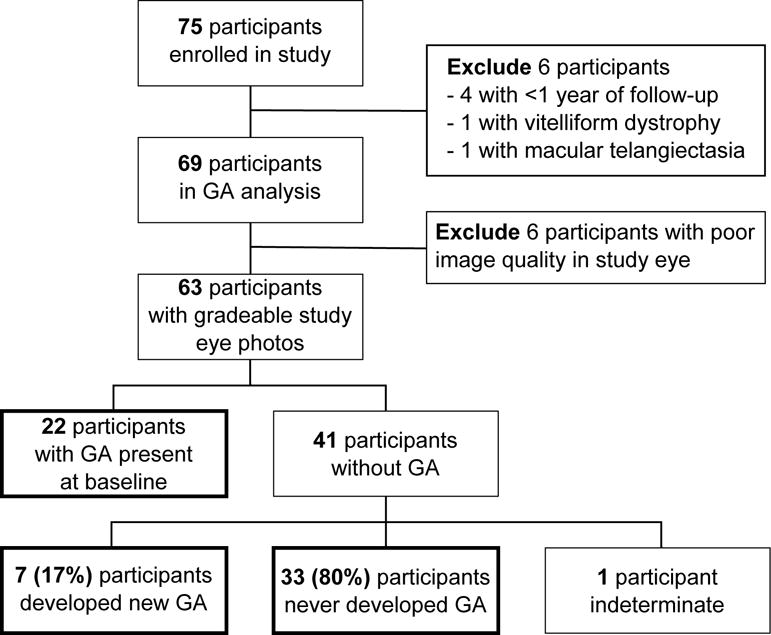
Study flow chart. Bolded boxes indicate study eye geographic atrophy (GA) analysis groups
Previous Treatments in Study Eyes
Of the 69 analyzed study eyes, 44 (63.8%) study eyes were previously treated, 22 (31.9%) were treatment naïve, and 3 (4.3%) had unknown previous treatment status. Previous treatments in the study eye included: intravitreal injections of bevacizumab (35 eyes/44 participants), ranibizumab (23/44), PDT (5/44), steroids (5/44), sirolimus (2/44), pegaptanib (1/44), and focal laser (1/44). Eyes that had received prior anti-VEGF therapy had received an average of 7.3±6.4 total anti-VEGF injections (43 eyes/44 participants), 6.5±5.5 injections of bevacizumab (35/44), and 3.4±3.3 injections of ranibizumab (23/44).
Study and Fellow Eye Treatment During the Study
Participants received more injections in the study eye during the first year of enrollment compared to subsequent years (Table 1 available at http://www.aaojournal.org). During their first year, participants received an average of 9.2±3.3 injections in the study eye, which was greater than the 5.0±3.6 injections that were given to 11 fellow eyes receiving treatment for nAMD. The 61 participants enrolled for at least 2 years received 6.2±4.7 injections and 7 (11.5%) required no further injections after the first year. The 39 participants enrolled for at least 3 years received 6.2±5.1 injections.
Geographic Atrophy Stratification
Of the 69 study eyes available for analysis, 6 (8.7%) were unable to be classified for GA due to poor image quality from cataracts. Thirty-three (47.8%) eyes never developed GA, 7 (10.1%) eyes developed new GA, 22 (31.9%) eyes had GA present at baseline, and 1 (1.5%) eye had indeterminate progression (Figure 1).
Incidence and Risk of New GA Development in Study Eyes
Seven (17.1%) of the 41 study eyes without GA at baseline developed GA during the study (Figure 1). Of these seven eyes, 6 (85.7%) eyes developed GA by 1 year and 1 (14.3%) eye developed GA by 2 years. Four (47.1%) of the 7 eyes developed GA within an area of prior heterogeneity on FAF and three (42.9%) developed GA within an area of prior hemorrhage (Figure 2).
Figure 2.
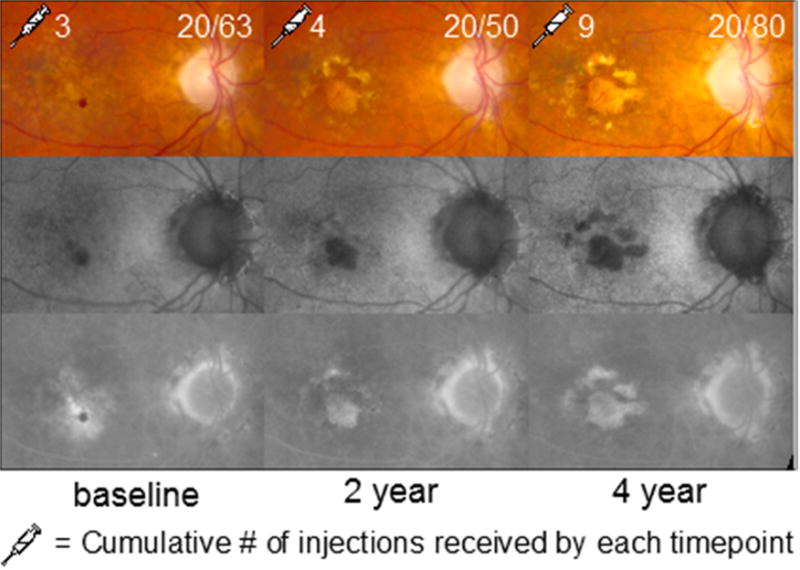
Study eye of an 82 year-old female participant who had previously been treated with 3 anti-VEGF injections. She had hemorrhage on color fundus photography (CFP) at baseline and developed progressive geographic atrophy on fundus autofluorescence (FAF) and fluorescein angiography (FA) within the area of prior hemorrhage and exudation.
Compared to the 33 participants who did not develop GA in the study eye, those 7 who developed new GA were older (all ≥79 years old; Table 2), had poorer baseline VA (57.1% vs. 15.1% had baseline VA 20/100-20/160; Table 3), and had received fewer study injections on average (6.9 vs. 10.4 injections at 1 year). They were also less likely to have been treatment naïve at enrollment and have occult CNV (1/7 [74%] vs. 11/33 [33%] were treatment naïve and 2/7 [28.6%] vs. 20/33 [60.6%] were occult; Table 3).
Table 2.
Comparison of participant characteristics by geographic atrophy status in the study eyea
| Participant Characteristic | Never developed GA N=33 N (%) |
Developed new GA N=7 N (%) |
GA present at baseline N=22 N (%) |
|---|---|---|---|
| Age at enrollment | |||
| 50–69 | 6 (18.2) | 0 | 3 (13.6) |
| 70–79 | 16 (48.5) | 1 (14.3) | 5 (22.7) |
| 80–89 | 10 (30.3) | 5 (71.4) | 13 (59.1) |
| ≥90 | 1 (3.0) | 1 (14.3) | 1 (4.6) |
| Sex | |||
| Female | 18 (54.5) | 6 (85.7) | 15 (68.2) |
| Male | 15 (45.5) | 1 (14.3) | 7 (31.8) |
| Race | |||
| White | 31 (94.0) | 7 (100) | 19 (86.5) |
| Black | 0 | 0 | 1 (4.5) |
| Other | 1 (3.0) | 0 | 1 (4.5) |
| Unknown | 1 (3.0) | 0 | 1 (4.5) |
| Hypertension | |||
| Absent | 11 (33.3) | 1 (14.3) | 10 (45.5) |
| Present | 22 (66.7) | 6 (85.7) | 12 (54.5) |
| Length of enrollment (months) | |||
| 12–23 | 5 (15.2) | 0 | 2 (9.1) |
| 24–35 | 10 (30.3) | 2 (28.6) | 7 (31.8) |
| 36–47 | 10 (30.3) | 1 (14.3) | 1 (4.5) |
| 48–59 | 7 (21.2) | 4 (57.1) | 10 (45.5) |
| 60 | 1 (3.0) | 0 | 2 (9.1) |
| GA status in fellow eye | |||
| Present | 1 (3.0) | 1 (14.3) | 9 (40.9) |
| Absent | 21 (63.7) | 4 (57.1) | 4 (18.2) |
| Disciform | 7 (21.2) | 1 (14.3) | 7 (31.8) |
| Prosthesis | 1 (3.0) | 0 | 0 |
| Indeterminate | 3 (9.1) | 1 (14.3) | 2 (9.1) |
GA: geographic atrophy
63 study eyes included in this analysis due to exclusion of 6 eyes for ungradeable baseline photos
Table 3.
Comparison of baseline ocular characteristics by geographic atrophy status in the study eyea
| Ocular Characteristic | Never developed GA N=33 N (%) |
Developed new GA N=7 N (%) |
GA present at baseline N=22 N (%) |
Ocular Characteristic | Never developed GA N=33 N (%) |
Developed new GA N=7 N (%) |
GA present at baseline N=22 N (%) |
|---|---|---|---|---|---|---|---|
| Visual Acuity | Previous Treatment | ||||||
| 20/25–20/40 | 15 (45.5) | 1 (14.3) | 9 (40.9) | Treatment naïve | 11 (33.3) | 1 (14.3) | 9 (40.9) |
| 20/50–20/80 | 10 (30.3) | 1 (14.3) | 7 (31.8) | PDT + injectionsb | 0 | 1 (14.3) | 3 (13.6) |
| 20/100–20/160 | 5 (15.1) | 4 (57.1) | 6 (27.3) | Injectionsb only | 20 (60.7) | 4 (57.1) | 10 (45.5) |
| 20/200–20/320 | 2 (6.1) | 1 (14.3) | 0 | Focal laser | 1 (3.0) | 0 | 0 |
| <20/400 | 1 (3.0) | 0 | 0 | Unknown | 1 (3.0) | 1 (14.3) | 0 |
| CNV Classification | CNV size on ICG (mm2) | ||||||
| Classic | 4 (12.1) | 0 | 5 (22.7) | 0–2.5 | 21 (63.6) | 3 (42.8) | 13 (59.1) |
| Minimally classic | 9 (27.3) | 3 (42.8) | 6 (27.3) | 2.6–5.0 | 5 (15.2) | 2 (28.6) | 6 (27.3) |
| Occult | 20 (60.6) | 2 (28.6) | 10 (45.5) | 5.1–7.5 | 2 (6.0) | 0 | 2 (9.1) |
| RAP | 0 | 1 (14.3) | 1 (4.5) | Indeterminate | 5 (15.2) | 2 (28.6) | 1 (4.5) |
| Indeterminate | 0 | 1 (14.3) | 0 | ||||
| Foveal fluid | Epiretinal membrane | ||||||
| Present | 29 (87.9) | 0 | 19 (86.4) | Present | 3 (9.1) | 0 | 4 (18.2) |
| Absent | 4 (12.1) | 7 (100) | 3 (13.6) | Absent | 29 (87.9) | 7 (100) | 18 (81.8) |
| Unknown | 1 (3.0) | 0 | 0 | ||||
GA: geographic atrophy; CNV: choroidal neovascularization; RAP: retinal angiomatous proliferation; PDT: photodynamic therapy; ICG: indocyanine green angiography
63 study eyes included in this analysis due to exclusion of 6 eyes for ungradeable baseline photos
Injections include intravitreal bevacizumab, ranibizumab, steroids, sirolimus, and pegaptanib
Of the 5 study eyes that had previously received PDT, 3 eyes entered the study with GA present at baseline, 1 eye had no GA at baseline (but we were unable to determine whether GA developed [Figure 3B]), and 1 eye developed new GA. One (8.3%) of the 12 treatment naïve study eyes without GA at baseline developed new GA during the study (Table 3).
Figure 3.
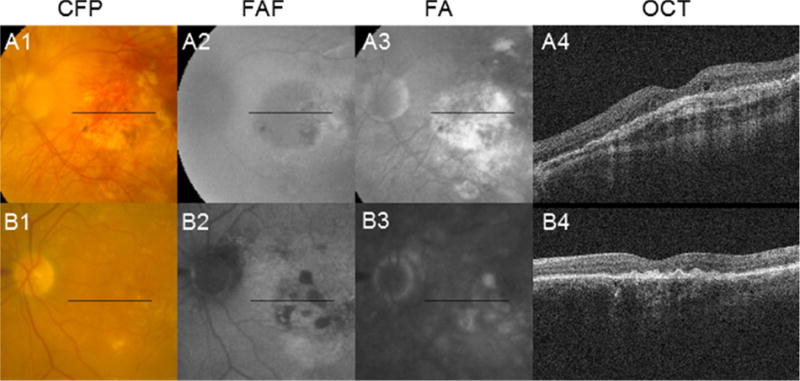
Cases that mimic geographic atrophy (GA). (A1–A4) The 4-year follow-up images of a 51 year-old male participant with active choroidal neovascularization are depicted. Findings of GA include: color fundus photography (CFP; A1) showing a central patch of what appears to be depigmentation with sharply demarcated borders and visibility of underlying choroidal vessels; fundus autofluorescence (FAF; A2) showing hypo-autofluorescence in an area of apparent depigmentation; and late fluorescein angiography (FA; A3) showing hyper-fluorescence bounded by sharp borders. However, optical coherence tomography (OCT; A4) shows fibrous proliferation at the level of the retinal pigment epithelium (RPE) with associated shadowing, making classifying the depigmented lesion as GA problematic. (B1–B4) The 3-year follow-up images of a 69 year-old male participant with active choroidal neovascularization are depicted. Findings suggestive of GA include: FA (B2) showing hypo-autofluorescence with sharp borders in areas corresponding to patchy hypopigmentation on CFP (B1); and late FA (B3) showing corresponding hyper-fluorescence bounded by sharp borders. However, OCT (B4) shows irregular thickening at the level of the RPE with associated shadowing, also making classifying the depigmented lesion as GA problematic.
Eyes with GA at Baseline
Twenty-two (31.9%) study eyes had GA at baseline. Compared to the study eyes that did not develop GA, eyes with baseline GA received fewer injections on average (8.1±2.7 vs. 10.4±3.3 injections during year 1, P Value <0.01), but were similarly distributed in previous treatment status, types of CNV, CNV size, and presence of sub-foveal fluid (Table 3).
Fellow Eyes
Of 13 fellow eyes with ≥1 year of neovascular disease treated with ranibizumab and without GA at baseline, 5 (38.5%) eyes developed new GA. The majority of these participants (4/5) also had GA in the study eye. Four (80%) of these 5 fellow eyes with new GA had converted to neovascular disease during the study and 1 (20%) eye had previous neovascularization treated with 3 injections of bevacizumab prior to enrollment. In all 4 fellow eyes that developed both new-onset GA and new neovascular disease, neovascular disease preceded GA development (by 2 years in 3 eyes and by 1 year in 1 eye). None of the 18 non-neovascular fellow eyes without GA present at baseline developed new GA during the study.
GA Progression
Twenty-nine (42.0%) study eyes had GA during the study. However, 8 (27.6%) of those eyes had unquantifiable GA due to fibrous changes within the GA area, poor quality of images secondary to cataract, or obscured area from cataract, pigment, or hemorrhage. In 14 (16.3%) of 86 FAF images, there was a >20% disagreement between the GA areas calculated by the two graders and a third grader (N.J.) measured the areas. Measurements from all 3 graders were averaged to determine the GA area in these photos for analysis.
The 16 study eyes with quantifiable GA at baseline enlarged by an average rate of 0.34±0.26 mm/year (square root-transformed areas), faster than 0.19±0.12 mm/year in the 5 study eyes that developed new GA (Table 4 available at http://www.aaojournal.org). Seven participants (6 with GA present at baseline, 1 with new GA) had bilateral quantifiable GA. Although GA growth rate was highly variable, a plot of the GA areas in 3 participants with non-neovascular fellow eyes and 4 participants with neovascular fellow eyes showed that the growth rates between these participants’ study and fellow eyes were similar regardless of the number of injections the study eye received (Figure 4 available at http://www.aaojournal.org). FAF revealed that in one treatment naïve participant who developed new GA lesions outside an area of prior hemorrhage, those lesions corresponded to areas of hypo-autofluorescence 2 years prior to onset of neovascular disease (Figure 5).
Figure 5.

An 85 year-old female participant developed new neovascular disease in her study eye at baseline and had rapid progression of geographic atrophy (GA) within and outside the area of prior hemorrhage. White arrows indicate seemingly new GA lesions following initiation of anti-vascular endothelial growth factor therapy when color fundus photography is examined alone, but fundus autofluorescence two years prior to development of neovascularization reveal the presence of hypo-autofluorescence in these areas (black arrows). The study eye follows the same pattern of progression as the fellow eye.
Hemorrhage and GA
Eleven study eyes (15.9%) had hemorrhage at baseline, two of which were due to RAP lesions. In the area of hemorrhage, 6 (54.5%) participants developed GA, whereas the remaining did not develop GA during the study. The two eyes with RAP lesions had hemorrhage and subsequent GA overlying the area of previous hemorrhage.
Discussion
We found that 17% of participants receiving ranibizumab treatment in this prospective study developed new GA in the study eye on FAF, which is similar to the 18% reported in the CATT study at 2 years.5 GA development in CATT was associated with older age, poorer baseline VA, RAP lesions, GA in the fellow eye, and more frequent dosing.5 Although these risk factors could not be thoroughly evaluated in the current study due to the small number of participants enrolled, age and baseline VA were similarly associated with GA development in our study. Our finding that study eyes that developed new GA were less likely to have occult CNV is consistent with evidence that neovascularization characteristics may affect GA development and progression.10,11
One of the strengths of the current study is longer follow-up, with an average of 3 years of follow up and some participants followed for up to 5 years. Interestingly, all the new GA found in the participants’ study and fellow eyes occurred within either 2 years of study enrollment or conversion to neovascular disease, suggesting that GA risk in the context of ranibizumab therapy might be highest during the first 2 years of treatment. Further studies with long-term follow-up are still needed to fully evaluate this potential risk because our results are confounded by the previous treatments for neovascular disease that over half of our study eyes had received.
We identified GA on FAF, whereas other studies assessing GA used traditional color fundus photography and/or FA.5, 12 Khanifar et al. showed that interobserver agreement was slightly higher for FAF when compared to color fundus photography and more GA lesions were identified on FAF than color fundus photography.4 FAF provides increased contrast sensitivity for detecting small GA lesions earlier in disease, which may have led to more participants being identified with GA present at baseline in this study. We found that eyes with >20% disagreements in GA areas between graders often had small lesions, where a 20% difference could be easily made by small discrepancies in manual tracings. FAF was valuable in characterizing eyes that had autofluorescence changes overlying areas that would later progress to GA. In these instances, these GA lesions were most likely not anti-VEGF related, as demonstrated in Figure 5. Many eyes that developed new GA had heterogeneous autofluorescence, suggesting that underlying RPE dysfunction may predispose those eyes to developing GA. Characterization of high-risk patterns of FAF, similar to that performed by Einbock et al.,13 may be useful in identifying eyes at highest risk for developing GA following anti-VEGF therapy in future studies.
In this study, we used multiple imaging modalities to identify GA areas. However, even with these modalities, we encountered cases that had characteristic fundus findings strongly suggestive of GA on one imaging modality, but did not meet all of the criteria for GA (Figure 3). These cases highlight the fact that in the anti-VEGF era, disciform scars that would have obscured the fundus anatomy generally do not develop, allowing for imaging of other lesions that may develop in eyes with AMD. With multimodal imaging including spectral domain OCT, we can capture concomitant and often confounding characteristics, such as fibrous proliferation, within areas of seemingly apparent RPE loss that make classifying lesions as GA more difficult.
Our average GA growth rate of 0.34 mm/year (N=16) in study eyes with GA present at baseline is slightly lower than the rate of 0.45 mm/year in 81 treatment naïve eyes with baseline GA and new neovascularization treated with anti-VEGF therapy reported in CATT.11 Our rate, is however, comparable to the rate of 0.28–0.30 mm/year reported in non-neovascular eyes with GA present at baseline in the Age-Related Eye Disease (AREDS; N=115)14 and the FAF in AMD (FAM; N=86) studies.15
Our average GA growth rate in eyes that developed new GA of 0.19 mm/year (N=5) is notably lower than that reported in both CATT (0.41 mm/year, N=113) and AREDS (0.3 mm/year, N=478). This finding is likely attributable to the small number of eyes that developed new GA in our study, but could also be explained by the modality used for grading GA. Our study and FAM graded GA using FAF, whereas CATT used FA and AREDS used stereoscopic color fundus photography. With color fundus photography, there is a tendency to measure larger GA areas, especially as lesion size increases, when compared to FAF.8
Our study found that while GA growth rates were highly variable between participants, they were similar between the study and fellow eye of the same participant. This finding is consistent with other studies demonstrating a high concordance rate in patients with bilateral GA.16 Our study also supports previous reports that GA in the fellow eye is a significant risk factor for GA development and growth.11, 15, 17 While genetic factors may play a role in GA progression,15 they were not evaluated in this study. The finding that ranibizumab treatment did not appear to accelerate rates of GA growth in the study eye compared to both non-neovascular and neovascular fellow eyes is consistent with findings from CATT showing that increased frequency of anti-VEGF dosing did not increase GA growth rate despite increasing the rate of new GA development.5, 11 These findings suggest that GA growth rate may be independent of frequency of ranibizumab therapy.
It remains unknown whether the eyes that developed new GA in this study did so as a consequence of ranibizumab therapy or if they were destined to develop GA regardless of anti-VEGF therapy. A retrospective review of 81 eyes by Tanaka et al.18 found that new GA was either CNV-related or an enlargement of pre-existing GA. In our study, only 3 of the 7 study eyes that developed new GA had overlapping CNV identified, which may have been due to differing definitions of CNV compared to that used by Tanaka et al. The remaining eyes developed GA in areas of heterogeneous FAF, indicating that multiple etiologies are responsible for GA development and progression in nAMD.
This study is limited by the small number of participants, signs of previous exudative disease in many participants rendering their photos difficult to grade for new GA, and previous treatment in many eyes limiting the attribution of new GA to ranibizumab therapy itself during the study. The strengths of this study include: its prospective nature, long duration of follow-up, and multiple imaging modalities used to evaluate for GA. This prospective study using ranibizumab for nAMD treatment shows that 17% of participants developed new GA. In addition, multimodal imaging, including FAF, may be beneficial in identifying eyes most likely to develop GA. Further studies are needed to determine the eyes most at risk of developing GA in the setting of anti-VEGF treatment.
Supplementary Material
Figure 4 (Supplement). Plots of geographic atrophy (GA) growth in 7 participants with bilateral GA. Participants in the top row had fellow eyes that had received anti-vascular endothelial growth factor (VEGF) treatment for neovascular disease during and/or before entering the study. Participants in the bottom row had fellow eyes that were non-neovascular and therefore never received anti-VEGF.
Acknowledgments
This work was funded by National Institutes of Health (NIH) Intramural Research Program. Alisa Thavikulwat and Jane Kim were supported by the NIH Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from Pfizer Inc., The Doris Duke Charitable Foundation, The Newport Foundation, The American Association for Dental Research, The Howard Hughes Medical Institute, and the Colgate-Palmolive Company, as well as other private donors.
Abbreviations
- AMD
age-related macular degeneration
- AREDS
Age-Related Eye Disease Study
- BCVA
best-corrected VA
- CATT
Comparison of AMD Treatment Trials
- FA
fluorescein angiography
- FAF
fundus autofluorescence
- FAM
Fundus Autofluorescence in AMD
- GA
geographic atrophy
- ICG
indocyanine green angiography
- nAMD
neovascular AMD
- NIH
National Institutes of Health
- OCT
optical coherence tomography
- PED
pigment epithelium detachment
- PRN
pro re nata
- RAP
retinal angiomatous proliferation
- RPE
retinal pigment epithelium
- VA
visual acuity
- VEGF
vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meeting Presentation: Paper presentation at the Association for Research in Vision and Ophthalmology (ARVO) Annual Meeting, Denver, CO, May 3, 2015
The study drug, ranibizumab, was provided by Genentech, Inc, San Francisco, CA. No funding was provided and the company was not involved with the design, conduct or analyses of the study.
Conflict of Interest: No conflicting relationship exists for any author.
References
- 1.Congdon N, O’Colmain B, Klaver CC, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122(4):477–85. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 2.Friedman DS, O’Colmain BJ, Munoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122(4):564–72. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 3.Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432–44. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 4.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–31. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 5.Grunwald JE, Daniel E, Huang J, et al. Risk of geographic atrophy in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2014;121(1):150–61. doi: 10.1016/j.ophtha.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yannuzzi LA, Slakter JS, Sorenson JA, et al. Digital indocyanine green videoangiography and choroidal neovascularization. 1992. Retina. 2012;32(Suppl 1):191. doi: 10.1097/iae.0b013e31823f98c7. [DOI] [PubMed] [Google Scholar]
- 7.Delori FC, Dorey CK, Staurenghi G, et al. In vivo fluorescence of the ocular fundus exhibits retinal pigment epithelium lipofuscin characteristics. Invest Ophthalmol Vis Sci. 1995;36(3):718–29. [PubMed] [Google Scholar]
- 8.Khanifar AA, Lederer DE, Ghodasra JH, et al. Comparison of color fundus photographs and fundus autofluorescence images in measuring geographic atrophy area. Retina. 2012;32(9):1884–91. doi: 10.1097/IAE.0b013e3182509778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicholson BP, Nigam D, Toy B, et al. Effect of ranibizumab on high-speed indocyanine green angiography and minimum intensity projection optical coherence tomography findings in neovascular age-related macular degeneration. Retina. 2015;35(1):58–68. doi: 10.1097/IAE.0000000000000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu L, Mrejen S, Jung JJ, et al. Geographic atrophy in patients receiving anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Retina. 2015;35(2):176–86. doi: 10.1097/IAE.0000000000000374. [DOI] [PubMed] [Google Scholar]
- 11.Grunwald JE, Pistilli M, Ying GS, et al. Growth of Geographic Atrophy in the Comparison of Age-related Macular Degeneration Treatments Trials. Ophthalmology. 2015;122(4):809–16. doi: 10.1016/j.ophtha.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindblad AS, Lloyd PC, Clemons TE, et al. Change in area of geographic atrophy in the Age-Related Eye Disease Study: AREDS report number 26. Arch Ophthalmol. 2009;127(9):1168–74. doi: 10.1001/archophthalmol.2009.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Einbock W, Moessner A, Schnurrbusch UE, et al. Changes in fundus autofluorescence in patients with age-related maculopathy. Correlation to visual function: a prospective study. Graefes Arch Clin Exp Ophthalmol. 2005;243(4):300–5. doi: 10.1007/s00417-004-1027-3. [DOI] [PubMed] [Google Scholar]
- 14.Domalpally A, Danis RP, White J, et al. Circularity index as a risk factor for progression of geographic atrophy. Ophthalmology. 2013;120(12):2666–71. doi: 10.1016/j.ophtha.2013.07.047. [DOI] [PubMed] [Google Scholar]
- 15.Grassmann F, Fleckenstein M, Chew EY, et al. Clinical and genetic factors associated with progression of geographic atrophy lesions in age-related macular degeneration. PLoS One. 2015;10(5):e0126636. doi: 10.1371/journal.pone.0126636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fleckenstein M, Adrion C, Schmitz-Valckenberg S, et al. Concordance of disease progression in bilateral geographic atrophy due to AMD. Invest Ophthalmol Vis Sci. 2010;51(2):637–42. doi: 10.1167/iovs.09-3547. [DOI] [PubMed] [Google Scholar]
- 17.Fleckenstein M, Schmitz-Valckenberg S, Adrion C, et al. Progression of age-related geographic atrophy: role of the fellow eye. Invest Ophthalmol Vis Sci. 2011;52(9):6552–7. doi: 10.1167/iovs.11-7298. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka E, Chaikitmongkol V, Bressler SB, Bressler NM. Vision-threatening lesions developing with longer-term follow-up after treatment of neovascular age-related macular degeneration. Ophthalmology. 2015;122(1):153–61. doi: 10.1016/j.ophtha.2014.07.046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 4 (Supplement). Plots of geographic atrophy (GA) growth in 7 participants with bilateral GA. Participants in the top row had fellow eyes that had received anti-vascular endothelial growth factor (VEGF) treatment for neovascular disease during and/or before entering the study. Participants in the bottom row had fellow eyes that were non-neovascular and therefore never received anti-VEGF.


