Abstract
Our study represents the first report demonstrating the antileishmanial activity of nicotinamide (NAm), a form of vitamin B3. A 5 mM concentration of NAm significantly inhibited the intracellular growth of Leishmania amastigotes and the NAD-dependent deacetylase activity carried by parasites overexpressing Leishmania major SIR2 (LmSIR2). However, the transgenic parasites were as susceptible as the wild-type parasites to NAm-induced cell growth arrest. Therefore, we conclude that NAm inhibits leishmanial growth and that overexpression of LmSIR2 does not overcome this inhibition. The mechanism of the inhibition is not defined but may include other in vivo targets. NAm may thus represent a new antileishmanial agent which could potentially be used in combination with other drugs during therapy.
Niacin is the generic name for two compounds: nicotinamide (NAm) and nicotinic acid (NicotAc). Both were first used clinically in 1937, when these compounds were each shown to act as a “pellagra-preventive” factor. NAm may also have a role in the treatment of osteoarthritis; it has been shown to improve the global impact of osteoarthritis, and it also improved joint flexibility by reducing inflammation (5). Currently, NAm is in trials as a therapy to prevent cancer recurrence and insulin-dependent (type I) diabetes (6). Besides this, the activity of NAm has been evaluated in anti-Mycobacterium tuberculosis studies performed from 1945 to 1961 and in anti-human immunodeficiency virus studies performed from 1991 to the present (reviewed in reference 9).
Interest in the class III NAD-dependent deacetylase family of proteins (the silent information regulatory 2 [SIR2] protein family) is expanding rapidly. We have recently reported that overexpression of a Leishmania cytoplasmic SIR2-related protein promotes the survival of amastigotes, the vertebrate stage of the parasites, by preventing programmed cell death (14). Taking into account the fact that NAm is a physiological inhibitor of certain deacetylase SIR2 proteins, such as Saccharomyces cerevisiae SIR2 and human SIRT1 (2), we reasoned that NAm may have an impact on parasite growth by interfering with metabolic processes involving deacetylase activities linked to SIR2-like proteins.
In order to evaluate this concept, we monitored the growth of Leishmania amastigotes and promastigotes under axenic culture conditions in medium with or without NAm. A cloned line of Leishmania infantum (MHOM/MA/67/ITMAP-263) was used in all experiments. Each subculture was initiated at 5 × 105 parasites/ml of medium. Axenically grown amastigote forms of L. infantum were maintained at 37°C with 5% CO2 by weekly subpassages in a cell-free medium called MAA/20 (medium for axenically grown amastigotes) in 25-ml flasks, as previously described (13). Promastigote forms were maintained at 26°C by weekly subpassage in SDM 79 medium (3a) supplemented with 10% fetal calf serum, 100 U of penicillin per ml, and 100 μg of streptomycin per ml. NAm (Sigma, St. Louis, Mo.) was added at the appropriate concentration, and the mean number of viable parasites was determined by fluorescence-activated cell sorter analysis, as previously described (12).
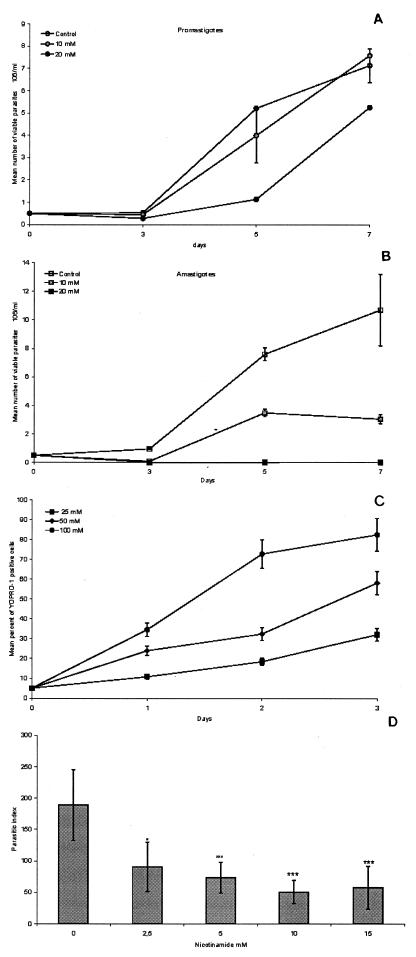
As shown in Fig. 1A, NAm was not able to inhibit the proliferation of promastigote parasites regardless of the amount of NAm added to the medium (50% inhibitory concentration [IC50] of 13.9 ± 4.2 mM). By contrast, amastigote proliferation was strongly affected (IC50 of 5.5 ± 0.5 mM) (Fig. 2B). In fact, adding 20 mM to the culture medium completely abolished the proliferative capacity of axenic amastigotes, whereas a delay in the growth of promastigotes occurred. The growth-inhibitory activity of NAm was not restricted to L. infantum since Leishmania amazonensis (MHOM/BR/76/LTB-012) amastigotes were also found to be sensitive to the activity of NAm (IC50 of 11.3 ± 2.1 mM). Furthermore, we found that the acid derivative of NAm, NicotAc, exerted a growth-inhibitory activity towards Leishmania parasites, although at higher concentrations (IC50 of 12.7 ± 2.0 mM). Indeed, 25 mM NicotAc was unable to abolish amastigote proliferation, thus suggesting that the inhibitory effect of NicotAc was only transient (data not shown). As a control, we have monitored the effect of NAm against Trypanosoma brucei gambiense (MHOM/RCA/1999/BAT32) parasites and have found that, even at concentration as high as 80 mM, NAm does not kill parasites but transiently inhibits parasite growth; its IC50 evaluated after 3 days of culture was found to be close to 40 mM (data not shown).
FIG. 1.
(A and B) Leishmaniostatic activity of NAm against promastigotes (A) and axenically grown amastigotes (B). (C) Leishmanicidal activity of a high concentration of NAm against axenically grown amastigotes. Results are expressed as means of results from triplicate experiments. (D) Inhibition of intracellular amastigote growth mediated by NAm. Results are representative of one of two experiments carried out six times. *, P < 0.05; **, P < 0.005; ***, P < 0.001.
FIG. 2.
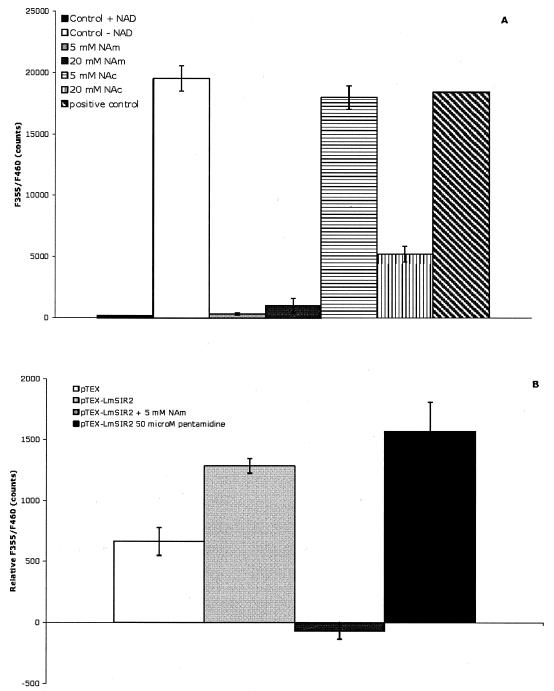
Activity of NAm against the NAD-dependent deacetylase activity of SIRT1 (A) and the NAD-dependent deacetylase activity detected in parasites carrying extra copies of LmSIR2 (pTEX-LmSIR2) (B). Results are given as means of results of two experiments carried out in duplicate.
These observations prompted us to examine the nature of NAm-induced amastigote growth arrest. Cells were seeded at 5 × 105 parasites/ml, and NAm was added at various concentrations ranging from 25 to 100 mM. After 24, 48, and 72 h of incubation, aliquots (106 parasites) were collected, washed, and incubated for 10 min with 10 μM YOPRO-1 (Molecular Probes). The mean percentage of YOPRO-1-positive cells was determined as previously described (12). At concentrations higher than 25 mM, NAm exerted a strong dose-dependent leishmanicidal activity against axenic amastigotes, as demonstrated by the occurrence of YOPRO-1-positive cells. Maximal effect was observed after 3 days of culture in the presence of 100 mM NAm (Fig. 1C).
Having observed that NAm induced axenic amastigote death, it was of interest to examine its effect on intracellular amastigote proliferation. In a first series of experiments, THP-1 monocytes were incubated for 3 days with various concentrations of NAm and the growth and viability of cells were recorded. With up to 10 mM NAm, no effect on cell growth and viability was observed (data not shown). In contrast, 20 mM NAm inhibited the proliferation of THP-1 monocytes by about 45%, in agreement with the values recorded for other cell types, namely, SupT1 and PBLs cells (8).
Thus, THP-1-differentiated macrophages were infected with stationary-phase amastigotes at a host cell/parasite ratio of 5:1. After 4 h, nonadherent parasites were removed and NAm was added to the medium at the appropriate concentration. After 3 days of incubation time, cells were fixed with methanol and stained with Giemsa stain. The parasitic index (mean percentage of infected macrophages times the number of amastigotes per macrophage) was determined. As shown in Fig. 1D, NAm significantly inhibited the in vitro proliferation of intracellular amastigotes. Maximal activity was observed with 10 mM NAm. At this concentration, a reduction of almost 70% of the parasitic index was observed. Interestingly, at a low dosage (2.5 mM), NAm is also able to significantly inhibit intracellular amastigote proliferation compared to the proliferation of control, nontreated cultures (P < 0.05).
The sir2 family of protein deacetylase has emerged as important regulators of seemingly diverse cellular processes, such as gene silencing, apoptosis, metabolism, and aging (3). Five SIR2-related proteins are present in yeast (SIR2 and HST1 to -4), and seven such proteins are found in humans (SIRT1 to -7). Diverse subcellular localizations among SIR2 homologs have been described previously (3). All have a common NAD-dependent deacetylase activity. The deacetylation reaction generates three products: acetyl-ADP-ribose, NAm, and a deacetylated peptide substrate (10). It has recently been demonstrated that NAm is a strong noncompetitive inhibitor of sir2-like enzymes in vitro (2). Therefore, complementary experiments were conducted in order to examine whether NAm could interfere with Leishmania deacetylase activity in vitro. To test this possibility, we used a commercially available Cyclex SIR2 assay kit and SIRT1 as a standard enzyme (CycLex Co., Ltd., Nagano, Japan). As shown in Fig. 2A, the deacetylase activity of SIRT1 is strictly dependent on the presence of 200 μM NAD. A low level of fluorescence was detected when NAD was absent from the reaction buffer (Fig. 2A [control without NAD]). Addition of 5 or 20 mM NAm to the assay mixture almost completely abrogated the enzymatic activity of SIRT1. In contrast, 5 mM NicotAc had no significant effect, in agreement with the data reported by other investigators (2). We have also determined the capacity of NAm to inhibit the activity of the lysyl-endopeptidase. The reaction was carried out with a fluorodeacetylated substrate and is referred as the positive control in Fig. 2A. NAm did not significantly inhibit the activity of the endopeptidase, since 20 mM NAm did not inhibit the reaction (Fig. 2A).
Having established a standard inhibitory assay, we then examined the effect of NAm on the NAD-dependent deacetylase activity contained in Leishmania extracts from mutant parasites carrying extra copies of the Leishmania major SIR2 (LmSIR2) gene (pTEX-LmSIR2) or empty plasmid DNA (pTEX) already established in our laboratory (14). Briefly, 2 × 105 parasites were collected, washed two times with phosphate-buffered saline (0.01 M, pH 7.2), and incubated in a lysis solution (100 mM Tris-HCl, 150 mM NaCl, 1 mM EDTA, 1% NP-40, 5 μM Trichostatin A, pH 8.8); cells were then centrifuged for 20 min at 10,000 rpm (Biofresco Heraeus) at 4°C, and the supernatant was collected. Then, deacetylase activity in the supernatant in the presence or absence of NAD (200 μM) and in the presence or absence of NAm (5 mM) or pentamidine (50 μM) was measured. Results are expressed as relative F355/F460 counts (fluorescence F355/F460 counts in the presence of NAD minus fluorescence F355/F460 counts in the absence of NAD). This process allowed us to discriminate between fluorescence due to the action of SIR2 proteins and fluorescence due to the presence of compounds which could interfere with the test. As shown in Fig. 2B, parasites overexpressing LmSIR2 had more NAD-dependent deacetylase activity than parasites carrying the empty pTEX vector. A 5 mM concentration of NAm significantly inhibited the NAD-dependent deacetylase activity detected in parasites overexpressing LmSIR2 (Fig. 2B). As a control, we monitored the inhibitory capacity of an irrelevant molecule, pentamidine (Fig. 2B). Pentamidine has no effect on the deacetylase activity carried out by parasites overexpressing LmSIR2 and was not able to inhibit the activity of SIRT1 under our experimental conditions (data not shown). In order to see if pentamidine, which is itself a fluorescence molecule, could interfere with our protocol, we recorded the fluorescence of pentamidine in the Sir2 deacetylation buffer; no significant fluorescence was detected at the wavelength used to detect the deacetylase activity (data not shown).
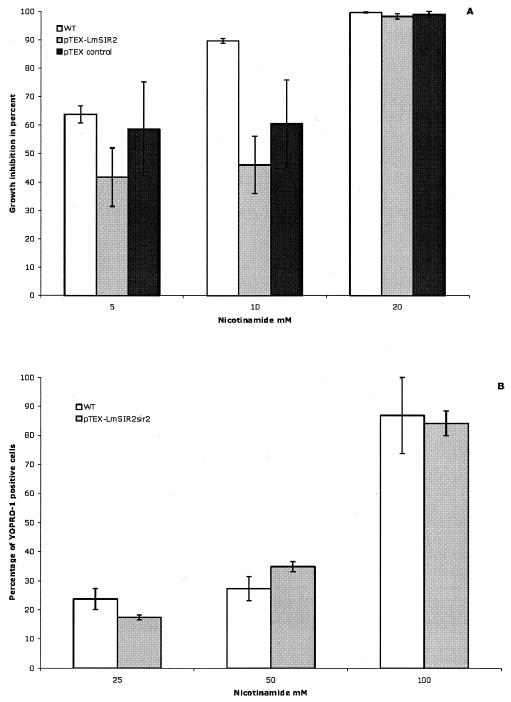
In yeast and Caenorhabdis elegans, SIR2 is a limiting component of longevity (reviewed in reference 4) and NAm is able to accelerate yeast aging by inhibiting SIR2 in vivo (2). In the protozoan parasite L. infantum, amastigotes carrying extra copies of the SIR2 gene, when maintained under normal axenic culture conditions, showed a striking increase in survival due to an inherent resistance to apoptosis-like death, leading to a longer stationary phase of growth (14). To further examine the possible correlation between the level of SIR2 expression and the sensitivity or resistance to NAm-induced Leishmania amastigote death, NAm was added to cultures of mutant L. infantum amastigotes which overexpress LmSIR2 or carry the empty pTEX plasmid as controls. As shown in Fig. 3A and B, adding extra copies of LmSIR2 to amastigotes did not confer resistance to NAm-induced death. Thus, even if the NAD-dependent deacetylase activity of LmSIR2 is readily inhibited by NAm and LmSIR2 plays a role in the survival of Leishmania amastigotes, LmSIR2 should represent one of the targets of NAm-mediated cell growth arrest.
FIG. 3.
Leishmaniostatic (A) and leishmanicidal (B) activities of NAm against amastigote parasites carrying extra copies of LmSIR2 (pTEX-LmSIR2) or the empty pTEX vector or against wild-type (WT) parasites. Results are mean values from quadruplicate experiments.
The microbicidal mechanism of action of NAm is not currently known. Its activity may come to be understood as that of an indirect antimicrobial that has primarily a beneficial effect on the host. Among the reasons to suggest an effect is the body of literature that reports an immunomodulatory role for NAm in a wide variety of experimental systems (9, 11). Moreover, antioxidant and cryoprotective effects of NAm are documented (15). Thus, our study represents the first report showing the antiparasitic activity of NAm. Although NAm inhibits the NAD-dependent deacetylase activity of SIR2-like enzymes, LmSIR2 seems not to be the sole target in Leishmania. In fact, Leishmania possesses two other SIR2-related members whose function and localization are currently unknown. Implication of this protein family in the survival of Leishmania parasites has to be investigated. We can hypothesize that one or all of them are essential for the survival of the parasite and that their inhibition leads to the death of the parasite. Alternatively, other essential physiological functions would be the targets for NAm. SIR2 genes are not known to be essential for the survival or multiplication of mammalian cells (7). Thus, knowing whether or not the antileishmanial activity of NAm is mediated through the inhibition of SIR2 protein in Leishmania may provide some information for the determination of new therapeutic targets. These studies imply that we should carry out systematic inactivations of genes coding for members of the SIR2 family in order to determine to what extent these genes are essential for the survival of the parasites. Such studies are in progress. Finally, since NAm is able to inhibit both mammalian and leishmanial SIR2 enzymes, it will be of interest to determine the inhibitory constant of NAm against leishmanial SIR2 enzymes. The concentrations of NAm and NicotAc found to inhibit the intracellular growth of L. infantum (IC50s of less than 2.5 mM) are far higher than those found in whole blood (about 45 μM) but are close to the plasmatic concentration of NAm achievable (0.7 to 2.3 mM) in patients treated with accelerated radiotherapy for head and neck cancer (1). In conclusion, NAm is an inexpensive agent that can be administered orally without significant side effects. Since NAm and its derivatives are potentially beneficial components, it will be of interest to investigate whether leishmaniasis might benefit from the therapeutic use of such components in combination with antiparasitic drugs.
Acknowledgments
This work was supported in part by a grant from the IRD and INSERM. R.S. was supported by an FCT (Portugal) grant, and A.M.A. was supported by a DSF IRD (France) grant. B.V. was supported by an ANRS (France) grant.
We are grateful to P. Grebault and G. Cuny for providing the T. brucei strain.
REFERENCES
- 1.Bernier, J., M. R. L. Stratford, J. Deneckamp, M. F. Dennis, S. Bieri, F. Hagen, O. Kocagöncü, M. Bolla, and A. Rojas. 1998. Pharmacokinetics of nicotinamide in cancer patients treated with accelerated radiotherapy: the experience of the Co-operative Group of Radiotherapy of the European Organization for Research and Treatment of Cancer. Radiother. Oncol. 48:123-133. [DOI] [PubMed] [Google Scholar]
- 2.Bitterman, K. J., R. M. Anderson, H. Y. Cohen, M. Latorre-Esteves, and D. A. Sinclair. 2002. Inhibition of silencing and accelerated aging by nicotinamide, a putative negative regulator of yeast sir2 and human SIRT1. J. Biol. Chem. 277:45099-45107. [DOI] [PubMed] [Google Scholar]
- 3.Blander, G., and L. Guarente. 2004. The sir2 family of protein deacetylases. Annu. Rev. Biochem. 73:417-435. [DOI] [PubMed] [Google Scholar]
- 3a.Brun, R., and M. Schönenberger. 1979. Cultivation and in vitro cloning or procyclic culture forms of Trypanosoma brucei in a semi-defined medium. Acta Trop. 36:289-292. [PubMed]
- 4.Imai, S., F. B. Johnson, R. A. Marciniak, M. McVey, P. U. Park, and L. Garante. 2000. Sir2: an NAD-dependent histone deacetylase that connects chromatin silencing, metabolism, and aging. Cold Spring Harbor Symp. Quant. Biol. 65:297-302. [DOI] [PubMed] [Google Scholar]
- 5.Jonas, W. B., C. P. Rapoza, and W. F. Blair. 1996. The effect of niacinamide on osteoarthritis: a pilot study. Inflamm. Res. 45:330-334. [DOI] [PubMed] [Google Scholar]
- 6.Kaanders, J. H., L. A. Pop, H. A. Marres, I. Bruaset, F. J. van den Hoogen, M. A. Merkx, and A. J. van der Kogel. 2002. ARCON: experience in 215 patients with advanced head-and-neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 52:769-778. [DOI] [PubMed] [Google Scholar]
- 7.McBurney, M. W., X. Yang, K. Jardine, M. Bieman, J. Th'ng, and M. Lemieux. 2003. The absence of SIR2a protein has no effect on global gene silencing in mouse embryonic stem cells. Mol. Cancer Res. 1:402-409. [PubMed] [Google Scholar]
- 8.Murray, M. F., and A. Srinivasan. 1995. Nicotinamide inhibits HIV-1 in both acute and chronic in vitro infection. Biochem. Biophys. Res. Commun. 210:954-959. [DOI] [PubMed] [Google Scholar]
- 9.Murray, M. F. 2003. Nicotinamide: an oral antimicrobial agent with activity against both Mycobacterium tuberculosis and human immunodeficiency virus. Clin. Infect. Dis. 36:453-460. [DOI] [PubMed] [Google Scholar]
- 10.North, B. J., and E. Verdin. 2004. Sirtuins: Sir2-related NAD-dependent protein deacetylases. Genome Biol. 5:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Otsuka, A., T. Hanafusa, J. Miyagawa, N. Kono, and S. Tarui. 1991. Nicotinamide and 3-aminobenzamide reduce interferon-gamma-induced class II MHC (HLA-DR and -DP) molecule expression on cultured human endothelial cells and fibroblasts. Immunopharmacol. Immunotoxicol. 13:263-280. [DOI] [PubMed] [Google Scholar]
- 12.Sereno, D., P. Holzmuller, I. Mangot, G. Cuny, A. Ouaissi, and J. L. Lemesre. 2001. Antimonial-mediated DNA fragmentation in Leishmania infantum amastigotes. Antimicrob. Agents Chemother. 45:2064-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sereno, D., and J. L. Lemesre. 1997. Axenically cultured amastigote forms as an in vitro model for investigation of antileishmanial agents. Antimicrob. Agents Chemother. 41:972-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vergnes, B., D. Sereno, N. Madjidian-Sereno, J. L. Lemesre, and A. Ouaissi. 2002. Cytoplasmic SIR2 homologue overexpression promotes survival of Leishmania parasites by preventing programmed cell death. Gene 21:139-150. [DOI] [PubMed] [Google Scholar]
- 15.Yang, J., and J. D. Adams. 2004. Nicotinamide and its pharmacological properties for clinical therapy. Drug Design Rev. 1:43-52. [Google Scholar]