Abstract
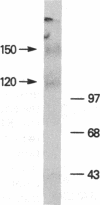
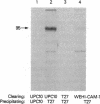
Previously, using flow cytometric resonance energy transfer and lateral diffusion measurements, we demonstrated that a 95-kDa protein identified by two monoclonal antibodies (OKT27 and OKT27b) interacts physically with the 55-kDa alpha protein of the high-affinity interleukin 2 (IL-2) receptor. In the present study, this 95-kDa protein (p95) was purified and amino acid sequence data were obtained that showed strong homology to the human intercellular adhesion molecule 1 (ICAM-1). The identity of the p95 protein with ICAM-1 was confirmed by sequential immunoprecipitations using OKT27 and an antibody, WEHI-CAM-1, that is directed toward ICAM-1. We confirmed the physical proximity of p95/ICAM-1 to the IL-2 receptor alpha subunit by demonstrating that radiolabeled IL-2 could be cross-linked to this protein expressed on activated T cells. In functional studies, the antibodies OKT27 and OKT27b inhibited T-cell proliferative responses to OKT3, to soluble antigen, and to heterologous cells (mixed lymphocyte reaction). However, these antibodies did not inhibit IL-2-induced proliferation of an IL-2-dependent T-cell line. Taken together with our previous observations, the present studies suggest that ICAM-1 is in proximity and interacts physically with the high-affinity IL-2 receptor. The association of ICAM-1 with the IL-2 receptor may facilitate the paracrine IL-2-mediated stimulation of T cells expressing IL-2 receptors by augmenting homotypic T-T-cell interaction, by receptor-directed focusing of IL-2 release by helper T cells, and by focusing IL-2 receptors of the physically linked cells to the site of lymphocyte function-associated antigen 1-ICAM-1-IL-2 receptor interaction.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyd A. W., Wawryk S. O., Burns G. F., Fecondo J. V. Intercellular adhesion molecule 1 (ICAM-1) has a central role in cell-cell contact-mediated immune mechanisms. Proc Natl Acad Sci U S A. 1988 May;85(9):3095–3099. doi: 10.1073/pnas.85.9.3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty G. J., Murdoch S., Hogg N. The function of human intercellular adhesion molecule-1 (ICAM-1) in the generation of an immune response. Eur J Immunol. 1988 Jan;18(1):35–39. doi: 10.1002/eji.1830180107. [DOI] [PubMed] [Google Scholar]
- Dustin M. L., Rothlein R., Bhan A. K., Dinarello C. A., Springer T. A. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol. 1986 Jul 1;137(1):245–254. [PubMed] [Google Scholar]
- Edidin M., Aszalos A., Damjanovich S., Waldmann T. A. Lateral diffusion measurements give evidence for association of the Tac peptide of the IL-2 receptor with the T27 peptide in the plasma membrane of HUT-102-B2 T cells. J Immunol. 1988 Aug 15;141(4):1206–1210. [PubMed] [Google Scholar]
- Goldman N. D., Liu T. Y. Biosynthesis of human C-reactive protein in cultured hepatoma cells is induced by a monocyte factor(s) other than interleukin-1. J Biol Chem. 1987 Feb 15;262(5):2363–2368. [PubMed] [Google Scholar]
- Herrmann T., Diamantstein T. The mouse high affinity IL 2 receptor complex. I. Evidence for a third molecule, the putative gamma-chain, associated with the alpha- and/or beta-chain of the receptor. Immunobiology. 1987 Sep;175(3):145–158. doi: 10.1016/s0171-2985(87)80024-4. [DOI] [PubMed] [Google Scholar]
- Hori T., Uchiyama T., Tsudo M., Umadome H., Ohno H., Fukuhara S., Kita K., Uchino H. Establishment of an interleukin 2-dependent human T cell line from a patient with T cell chronic lymphocytic leukemia who is not infected with human T cell leukemia/lymphoma virus. Blood. 1987 Oct;70(4):1069–1072. [PubMed] [Google Scholar]
- Kirkman R. L., Shapiro M. E., Carpenter C. B., Milford E. L., Ramos E. L., Tilney N. L., Waldmann T. A., Zimmerman C. E., Strom T. B. Early experience with anti-Tac in clinical renal transplantation. Transplant Proc. 1989 Feb;21(1 Pt 2):1766–1768. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larson R. S., Springer T. A. Structure and function of leukocyte integrins. Immunol Rev. 1990 Apr;114:181–217. doi: 10.1111/j.1600-065x.1990.tb00565.x. [DOI] [PubMed] [Google Scholar]
- Makgoba M. W., Sanders M. E., Ginther Luce G. E., Dustin M. L., Springer T. A., Clark E. A., Mannoni P., Shaw S. ICAM-1 a ligand for LFA-1-dependent adhesion of B, T and myeloid cells. Nature. 1988 Jan 7;331(6151):86–88. doi: 10.1038/331086a0. [DOI] [PubMed] [Google Scholar]
- Moos M., Jr, Nguyen N. Y., Liu T. Y. Reproducible high yield sequencing of proteins electrophoretically separated and transferred to an inert support. J Biol Chem. 1988 May 5;263(13):6005–6008. [PubMed] [Google Scholar]
- Morgan D. A., Ruscetti F. W., Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976 Sep 10;193(4257):1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Inamoto T., Sugie K., Masutani H., Shindo T., Tagaya Y., Yamauchi A., Ozawa K., Yodoi J. Mitogenicity and down-regulation of high-affinity interleukin 2 receptor by YTA-1 and YTA-2, monoclonal antibodies that recognize 75-kDa molecules on human large granular lymphocytes. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1318–1322. doi: 10.1073/pnas.86.4.1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo W. J., Conrad L., Janeway C. A., Jr Receptor-directed focusing of lymphokine release by helper T cells. Nature. 1988 Mar 24;332(6162):378–380. doi: 10.1038/332378a0. [DOI] [PubMed] [Google Scholar]
- Robb R. J., Greene W. C., Rusk C. M. Low and high affinity cellular receptors for interleukin 2. Implications for the level of Tac antigen. J Exp Med. 1984 Oct 1;160(4):1126–1146. doi: 10.1084/jem.160.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb R. J., Munck A., Smith K. A. T cell growth factor receptors. Quantitation, specificity, and biological relevance. J Exp Med. 1981 Nov 1;154(5):1455–1474. doi: 10.1084/jem.154.5.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothlein R., Springer T. A. The requirement for lymphocyte function-associated antigen 1 in homotypic leukocyte adhesion stimulated by phorbol ester. J Exp Med. 1986 May 1;163(5):1132–1149. doi: 10.1084/jem.163.5.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothlein R., Springer T. A. The requirement for lymphocyte function-associated antigen 1 in homotypic leukocyte adhesion stimulated by phorbol ester. J Exp Med. 1986 May 1;163(5):1132–1149. doi: 10.1084/jem.163.5.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saragovi H., Malek T. R. Evidence for additional subunits associated to the mouse interleukin 2 receptor p55/p75 complex. Proc Natl Acad Sci U S A. 1990 Jan;87(1):11–15. doi: 10.1073/pnas.87.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C., Newman R. A., Sutherland D. R., Asser U., Greaves M. F. A one-step purification of membrane proteins using a high efficiency immunomatrix. J Biol Chem. 1982 Sep 25;257(18):10766–10769. [PubMed] [Google Scholar]
- Sharon M., Gnarra J. R., Baniyash M., Leonard W. J. Possible association between IL-2 receptors and class I HLA molecules on T cells. J Immunol. 1988 Nov 15;141(10):3512–3515. [PubMed] [Google Scholar]
- Sharon M., Klausner R. D., Cullen B. R., Chizzonite R., Leonard W. J. Novel interleukin-2 receptor subunit detected by cross-linking under high-affinity conditions. Science. 1986 Nov 14;234(4778):859–863. doi: 10.1126/science.3095922. [DOI] [PubMed] [Google Scholar]
- Simmons D., Makgoba M. W., Seed B. ICAM, an adhesion ligand of LFA-1, is homologous to the neural cell adhesion molecule NCAM. Nature. 1988 Feb 18;331(6157):624–627. doi: 10.1038/331624a0. [DOI] [PubMed] [Google Scholar]
- Smith K. A. T-cell growth factor. Immunol Rev. 1980;51:337–357. doi: 10.1111/j.1600-065x.1980.tb00327.x. [DOI] [PubMed] [Google Scholar]
- Soulillou J. P., Peyronnet P., Le Mauff B., Hourmant M., Olive D., Mawas C., Delaage M., Hirn M., Jacques Y. Prevention of rejection of kidney transplants by monoclonal antibody directed against interleukin 2. Lancet. 1987 Jun 13;1(8546):1339–1342. doi: 10.1016/s0140-6736(87)90648-9. [DOI] [PubMed] [Google Scholar]
- Springer T. A., Dustin M. L., Kishimoto T. K., Marlin S. D. The lymphocyte function-associated LFA-1, CD2, and LFA-3 molecules: cell adhesion receptors of the immune system. Annu Rev Immunol. 1987;5:223–252. doi: 10.1146/annurev.iy.05.040187.001255. [DOI] [PubMed] [Google Scholar]
- Staunton D. E., Marlin S. D., Stratowa C., Dustin M. L., Springer T. A. Primary structure of ICAM-1 demonstrates interaction between members of the immunoglobulin and integrin supergene families. Cell. 1988 Mar 25;52(6):925–933. doi: 10.1016/0092-8674(88)90434-5. [DOI] [PubMed] [Google Scholar]
- Szöllösi J., Damjanovich S., Goldman C. K., Fulwyler M. J., Aszalos A. A., Goldstein G., Rao P., Talle M. A., Waldmann T. A. Flow cytometric resonance energy transfer measurements support the association of a 95-kDa peptide termed T27 with the 55-kDa Tac peptide. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7246–7250. doi: 10.1073/pnas.84.20.7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsudo M., Kozak R. W., Goldman C. K., Waldmann T. A. Demonstration of a non-Tac peptide that binds interleukin 2: a potential participant in a multichain interleukin 2 receptor complex. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9694–9698. doi: 10.1073/pnas.83.24.9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama T., Broder S., Waldmann T. A. A monoclonal antibody (anti-Tac) reactive with activated and functionally mature human T cells. I. Production of anti-Tac monoclonal antibody and distribution of Tac (+) cells. J Immunol. 1981 Apr;126(4):1393–1397. [PubMed] [Google Scholar]
- Waldmann T. A. The multi-subunit interleukin-2 receptor. Annu Rev Biochem. 1989;58:875–911. doi: 10.1146/annurev.bi.58.070189.004303. [DOI] [PubMed] [Google Scholar]
- Yodoi J., Teshigawara K., Nikaido T., Fukui K., Noma T., Honjo T., Takigawa M., Sasaki M., Minato N., Tsudo M. TCGF (IL 2)-receptor inducing factor(s). I. Regulation of IL 2 receptor on a natural killer-like cell line (YT cells). J Immunol. 1985 Mar;134(3):1623–1630. [PubMed] [Google Scholar]