Abstract
The bone metastasis-derived PC3 and the lymph node metastasis-derived LNCaP prostate cancer cell lines are widely studied, having been described in thousands of publications over the last four decades. Here, we report short-read whole-genome sequencing (WGS) and de novo assembly of PC3 (ATCC CRL-1435) and LNCaP (clone FGC; ATCC CRL-1740) at ∼70 × coverage. A known homozygous mutation in TP53 and homozygous loss of PTEN were robustly identified in the PC3 cell line, whereas the LNCaP cell line exhibited a larger number of putative inactivating somatic point and indel mutations (and in particular a loss of stop codon events). This study also provides preliminary evidence that loss of one or both copies of the tumor suppressor Capicua (CIC) contributes to primary tumor relapse and metastatic progression, potentially offering a treatment target for castration-resistant prostate cancer (CRPC). Our work provides a resource for genetic, genomic, and biological studies employing two commonly-used prostate cancer cell lines.
Keywords: prostate cancer, human, cell line, WGS, sequencing, genomics, Genome Report
Cultured cancer cell lines, such as the human-derived PC3 and LNCaP, are critical for prostate cancer research. The androgen-dependent LNCaP cell line (clone FGC) is derived from a lymph node metastasis (Horoszewicz 1980; Horoszewicz et al. 1983), and the androgen-independent PC3 cell line is derived from a bone metastasis (Kaighn et al. 1979). Since their development, almost 40 yr ago, they have emerged as major tools in prostate cancer research (with PubMed searches 15.01.17 for “PC3 AND prostate” and “LNCaP AND prostate” returning 3266 and 7080 hits, respectively). While these cell lines have been interrogated using array- and sequencing-based technologies (Liu et al. 2008; Barretina et al. 2012; Spans et al. 2012; Klijn et al. 2015), whole-genome sequences for the PC3 and LNCaP cell lines have not been published. Albeit currently relatively costly, WGS offers better coverage than exome sequencing, and improved detection of single nucleotide and small indel mutations and structural variants such as copy number alterations (Meynert et al. 2014; Belkadi et al. 2015; Warr et al. 2015).
Materials and Methods
Cell lines
PC3 (ATCC CRL-1435) and LNCaP clone FGC (ATCC CRL-1740; hereafter termed LNCaP) prostate cancer cell lines were obtained from the American Type Culture Collection (ATCC, Rockville, MD), and maintained in Roswell Park Memorial Institute RPMI 1640 medium (Invitrogen, Carlsbad, CA) with 10% Fetal Calf Serum (Thermo Fisher Scientific, Waltham, MA), supplemented with 100 U/ml penicillin G and 100 ng/ml streptomycin (Invitrogen). All cell lines were passaged at 2–3-d intervals on reaching 70% confluency using TrypLE Select (Invitrogen). Cell morphology and viability were monitored by microscopic observation and regular Mycoplasma testing was performed (Universal Mycoplasma Detection Kit; ATCC).
Sequencing
DNA was extracted using a QIAamp DNA mini kit (QIAGEN, Hilden, Germany) from low passage (passage four) PC3 and LNCaP cell lines, cultivated from frozen stocks obtained directly from the ATCC. Sequencing was performed by Macrogen (Seoul, South Korea). Briefly, library preparation was performed using a TruSeq Nano DNA kit (Illumina, San Diego, CA) with a target insert size of 350 bp. Paired-end libraries (150 bp) were sequenced using a HiSeqX sequencer (Illumina). Base calls were converted into FASTQ files using bcl2fastq v2.15.0 and provided to our laboratory.
Normal prostate data
Raw data from normal human prostate samples were obtained from the National Institutes of Health’s Cancer Genome Atlas (The Cancer Genome Atlas Research Network 2015) (NCBI dbGaP: phs000178.v9.p8). These included a WGS sample (PCAWG.e22e63de-c436-43c0-a595-022622c1fe06) and three RNA-seq samples (120215-UNC10-SN254-0327-AC0CMCACXX-ACTTGA-L005, 120502-UNC14-SN744-0235-BD0YUTACXX-ACTTGA-L005, and 130221-UNC9-SN296-0338-BC1PYCACXX-TGACCA-L008). The WGS file (101 bp paired-end library; 950 M reads) was provided as an unaligned BAM (uBAM) file and converted to FASTQ files using bamUtils v1.0.14 genome.sph.umich.edu/wiki/BamUtil.
Data processing
Raw reads (FASTQ) were trimmed using scythe v0.994 github.com/vsbuffalo/scythe, with default settings, to remove low quality bases and read-pairs, and contaminating adapter sequences.
Mapping of genome reads to reference genome:
FASTQ files were mapped to human reference genome GRCh38 build 82 (the reference genome in all subsequent analyses) using BWA-MEM (Li 2013), available in v0.7.12-r1039, and a sorted BAM file was generated by SAMtools v1.3.1 (Li et al. 2009). Genome coverage was estimated using QualiMap v2.2.1 (García-Alcalde et al. 2012; Okonechnikov et al. 2016).
de novo genome assembly:
PC3 and LNCaP genomes were assembled de novo using SGA v0.10.15 (Simpson and Durbin 2012) (available at github.com/jts/sga), as outlined in the user manual, except that assembled contigs were indexed using BWA-MEM (Li 2013) instead of the bundled Python script sga-align (calls BWA sample: bwa mem -t $CPU final-contigs.fa $READ1 $READ2 | samtools view -F2304 -b -o reads.bam -).
Resulting scaffolds were gap filled using “sga gapfill” and error-corrected FASTQ reads. The genome assemblies (gapfilled scaffolds) were evaluated using QUAST v4.3 (Gurevich et al. 2013) and the human reference genome. Genes of interest were interrogated in the assembled genomes using BLAST, via a local instance of SequenceServer v1.0.9 (Priyam et al. 2015), and GMAP v2016-11-07 (a genomic mapping and alignment program for mRNA and EST sequences) (Wu and Watanabe 2005).
Single nucleotide variant (SNV) and short indel calling:
Samtools v1.3.1 mpileup and bcftools (Li et al. 2009) were used to interrogate indexed BAM files of reads aligned to the reference genome and generate a VCF (Variant Call Format) file of SNVs and short indel variants. Variants (likely to be common germline variants) present in HapMap (Gibbs et al. 2003), 1000 genomes phase 3 (2504 human genomes) (Sudmant et al. 2015), and the National Heart Lung and Blood Institutes Exome Sequencing Project (Tennessen et al. 2012) (bundled variant data file available at https://goo.gl/mEogvD) were excluded.
Next, variant files (VCF) were filtered using SnpSift (Cingolani et al. 2012a) with the following parameters: QUAL ≥ 200 && DP ≥ 30; where QUAL denotes minimum variance confidence and DP total depth threshold. Filtered variants were annotated using SnpEff v4.3g (Cingolani et al. 2012b).
Copy number variation (CNV) calling:
To screen PC3 and LNCaP genomes for CNV, we employed the R package “cn.mops” (Copy Number estimation by a Mixture Of PoissonS) (Klambauer et al. 2012). Briefly, paired-end genome reads from PC3 and LNCaP were aligned to the reference genome and compared with normal prostate reads to obtain genome-wide read-depth profiles. Custom R scripts were used to parse the output.
Gene expression potential analysis:
We interrogated publicly available transcriptome data from PC3 (Wang et al. 2015) (NCBI GEO: GSE65112) and LNCaP cells (Metzger et al. 2016) (NCBI GEO: GSE64529). In addition, transcriptome data from normal prostate samples were obtained from TCGA (see above). Briefly, paired-end reads were trimmed using scythe, and aligned to human reference genome GRCh38 build 82 using the spliced-read mapper TopHat (v2.0.9) (Kim et al. 2013) and reference gene annotations to guide the alignment. Raw gene counts were computed from the generated BAM files by featureCounts v1.4.5-p1 (Liao et al. 2014), counting exon features of the gene annotation file (gtf) in order to include noncoding RNA genes. FeatureCounts output files were analyzed using the R programming language (v.3.2.2) (R Core Team 2013). Raw counts were normalized by Trimmed Mean of M-values (TMM) correction (Robinson and Oshlack 2010; Robinson et al. 2010). The expression of genes in normal prostate, LNCaP, and PC3 was assessed using the Universal exPression Code (UPC) method (Piccolo et al. 2013), available in the R package “SCAN.UPC”. This method estimates the active/inactive state of genes in a sample, where a UPC value > 0.5 indicates that a gene is actively transcribed.
cBioPortal analysis:
Data on copy number alterations in prostate cancer tumor tissue were obtained using the cBioPortal tool (www.cbioportal.org) (Cerami et al. 2012; Gao et al. 2013) with the following parameters: “GENE: HOMDEL HETLOSS;”, where “GENE” denotes a gene symbol. Clinical information was also downloaded and the data further analyzed using custom R scripts.
The following cBioPortal prostate cancer data sets were interrogated: ‘NEPC (Trento/Cornell/Broad 2016)’ (34 primary and 73 metastatic tumors) (Beltran et al. 2016), ‘Prostate (Broad/Cornell 2013)’ (55 primary tumors and 1 metastatic tumor) (Baca et al. 2013), ‘Prostate (FHCRC, 2016)’ (19 primary and 130 metastatic tumors) (Kumar et al. 2016), ‘Prostate (MICH), (11 primary and 47 metastatic tumors) (Grasso et al. 2012), ‘Prostate (MSKCC 2010)’ (157 primary and 37 metastatic tumors) (Taylor et al. 2010), ‘Prostate (MSKCC 2014)’ (101 primary and 3 metastatic tumors), (Hieronymus et al. 2014), ‘Prostate (SU2C)’ (150 metastatic tumors) (Robinson et al. 2015), and ‘Prostate (TCGA)’ (492 primary and 1 metastatic tumors) (The Cancer Genome Atlas Research Network 2015).
Kaplan–Meier survival analysis:
Kaplan–Meier survival analysis was performed to compare disease-free survival (DFS) in patient groups stratified by CNVs. DFS is defined as the time to either recurrence or relapse, second cancer, or death (Gill and Sargent 2006). In the context of prostate cancer, DFS is a suitable surrogate for overall survival (OS), given that metastatic disease is not curable and recurrence of disease would be expected to contribute significantly to mortality.
‘The Prostate (MSKCC 2010)’, ‘Prostate (MSKCC 2014)’, and ‘Prostate (TCGA)’ cBioPortal data sets were interrogated. Kaplan–Meier survival analysis (Rich et al. 2010) was performed using the R package “survival” (Therneau 2013), fitting survival curves (survfit) and computing log-rank P-values using the survdiff function, with ρ = 0 (equivalent to the method employed by UCSC Xena; see goo.gl/4knf62). Survival curves were plotted where survival was significantly different between two groups (log-rank P ≤ 0.05). Groups with <10 samples with recorded events were considered unreliable (Mallett et al. 2010).
Gene ontology term enrichment analysis
Gene enrichment analyses were performed using DAVID (Database for Annotation, Visualization and Integrated Discovery) (Huang et al. 2009a). All gene groups are potentially informative, despite lower rankings, and serve to guide biological interpretation (Huang et al. 2009b).
Data availability
The genome reads reported in this paper have been deposited in the BioProject database as PRJNA361315 (PC3) and PRJNA361316 (LNCaP). Code used to generate the data and CNV analysis output files (tabulated text files) are available at github.com/sciseim/PCaWGS. Genome assemblies (FASTA format) (Seim 2017a,b), and filtered and annotated single-nucleotide and indel variation data files (VCF format) (Seim 2017c), have been deposited at Zenodo. A BLAST server is available at http://ghrelinlab.org.
Results and Discussion
WGS
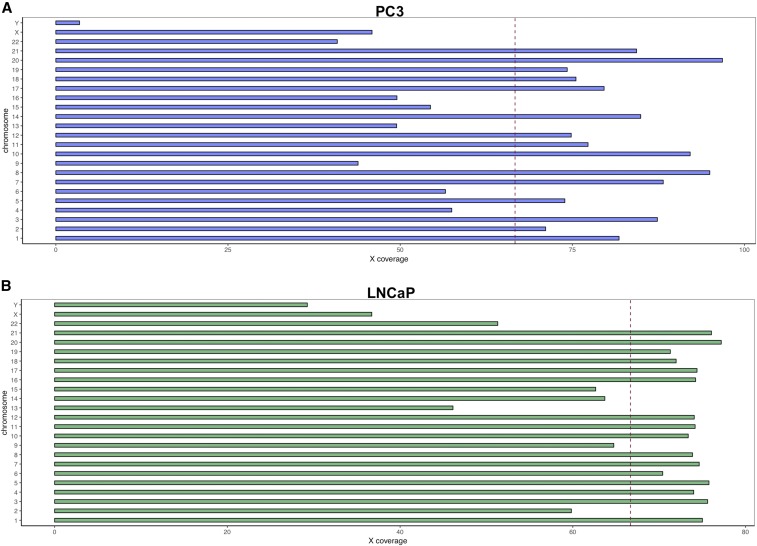
PC3 and LNCaP prostate cancer cells were obtained directly from ATCC, cultured for four passages, and 150 bp paired-end reads obtained using an Illumina HiSeqX sequencer. Following read trimming, 1.53 billion reads from PC3 were retained, of which 99.9% could be aligned to the Ensembl GRCh38.82 human reference genome at ∼71 × mean coverage (Figure 1A). Similarly, we obtained 1.49 billion trimmed reads from LNCaP with a 99.9% alignment rate and mean coverage of ∼69 × (Figure 1B).
Figure 1.
Read-depth across chromosomes in the (A) PC3 and (B) LNCaP prostate cancer cell lines. The red dotted line indicates mean genome-wide sequencing coverage (X).
We also performed de novo genome assembly to allow characterization of whole-gene loci by BLAST and other mappers. The final, gap-filled PC3 genome assembly consisted of 1.66 M scaffolds (largest scaffold 692.4 kb) with an N50 of 23.3 kb and an NG50 (number of sequences with lengths equal to or larger than N50) of 22.4 kb. The LNCaP assembly consisted of 1.70 M scaffolds (largest scaffold 536.0 kb) at an N50 value of 44.4 kb and NG50 of 45.0 kb.
Single-nucleotide and indel variation
After excluding common germline sequence variants (SNVs and short indels), filtering by SnpSift (Cingolani et al. 2012a), and annotation by SnpEff (Cingolani et al. 2012b), we identified in LNCaP 0.94 M and in PC3 0.56 M sequence variants (SNVs and short indels) that were private or unique to the particular cell line (Table 1). As expected, the majority of variants were found in noncoding regions.
Table 1. SNV and indel variant-calling statistics of the prostate cancer cell lines PC3 and LNCaP.
| PC3 Private | LNCaP Private | Shared | ||||
|---|---|---|---|---|---|---|
| Number and percentage of variants by type | ||||||
| SNVs | 318,380 | 34.0% | 404,282 | 72.1% | 166,912 | 65.0% |
| Indels | 618,149 | 66.0% | 156,182 | 27.9% | 89,919 | 35.0% |
| Number of events by type | ||||||
| 3′-UTR | 15,572 | 10,500 | 3938 | |||
| 5′-UTR premature start codon | 211 | 289 | 49 | |||
| 5′-UTR | 2613 | 1868 | 692 | |||
| Conservative_inframe_deletion | 39 | 22 | 7 | |||
| Conservative_inframe_insertion | 468 | 119 | 72 | |||
| Disruptive_inframe_deletion | 62 | 19 | 7 | |||
| Disruptive_inframe_insertion | 172 | 64 | 44 | |||
| Downstream_gene | 107,761 | 56,728 | 29,770 | |||
| Frameshift | 276 | 167 | 44 | |||
| Intergenic_region | 563,630 | 326,261 | 175,014 | |||
| Intron | 916,272 | 576,268 | 191,182 | |||
| Missense | 3520 | 5717 | 1667 | |||
| Non_coding_transcript_exon | 5848 | 3846 | 2091 | |||
| Non_coding_transcript | 18 | 10 | 1 | |||
| Protein_protein_contact | 120 | 17 | 5 | |||
| Sequence_feature | 7978 | 5930 | 1457 | |||
| Splice_acceptor | 80 | 131 | 21 | |||
| Splice_donor | 56 | 138 | 17 | |||
| Splice_region | 1313 | 1174 | 437 | |||
| Start_lost | 16 | 14 | 3 | |||
| Stop_gained | 58 | 378 | 29 | |||
| Stop_lost | 25 | 4 | 9 | |||
| Structural_interaction | 1160 | 808 | 1 | |||
| Synonymous | 2402 | 2727 | 81 | |||
| Upstream_gene | 107,281 | 57,447 | 1301 | |||
Common germline variants were excluded and variants were further filtered using SnpSift, with a total depth threshold at 30 (DP ≥ 30) and a minimum variance confidence of 200 (QUAL ≥ 200), and annotated by SnpEff. SNV, single nucleotide variant; UTR, untranslated region.
In particular, we noted that LNCaP had a larger number of stop_gained events, which are changes predicted to confer nonsense mutations and result in nonfunctional proteins or proteins with reduced function (Table 1). In LNCaP, SNVs and indel variants contributed 378 stop_gained events in 209 genes. We next identified biological processes overrepresented in this gene set (Table 2). This included a C→T transition at amino acid position 318 of menin (MEN1) (c.T954A in NCBI RefSeq NM_000244). Somatic inactivating mutations of menin are found in endocrine cancers (Falchetti et al. 2009), suggesting that MEN1 is a tumor suppressor gene. However, it has recently been reported that MEN1 is an oncogene in prostate cancer. Menin interacts with the androgen receptor and patients with overexpression of MEN1 show poor OS (Malik et al. 2015). The MEN1 SNV is present in an LNCaP sample interrogated by whole-exome sequencing (Taylor et al. 2010). Therefore, it is not likely to be a sequencing or data processing artifact. The functional regions of menin are currently not known, thus, the effect of the LNCaP premature stop codon event cannot be predicted. Interrogation of eight cBioPortal data sets suggests that inactivating mutations in the coding sequence of MEN1 in prostate cancer is unique to LNCaP (data not shown); however, it is possible that distinct patient populations possess this variant (e.g., see Lindquist et al. (2016)).
Table 2. Significantly overrepresented biological processes associated with sequence variants contributing stop_gained events in the PC3 and LNCaP prostate cancer cell lines.
| GO Term | Genes | Fisher’s Exact P |
|---|---|---|
| PC3 private sequence variants | ||
| Detection of bacterium | HLA-DRB1, HLA-A, HLA-DRB5, HLA-B | 5.1E−10 |
| Antigen processing and presentation | HLA-DRB1, HLA-A, HLA-DRB5, HLA-B | 2.4E−07 |
| Interferon-γ-mediated signaling pathway | HLA-DRB1, HLA-A, HLA-DRB5, HLA-B | 6.7E−07 |
| Immune response | HLA-DRB1, HLA-A, HLA-DRB5, HLA-B, IL1A | 4.7E−05 |
| Antigen processing and presentation of endogenous peptide antigen via MHC class I via ER pathway, TAP-independent | HLA-A, HLA-B | 2.9E−06 |
| Regulation of interleukin-10 secretion | HLA-DRB1, HLA-DRB5 | 2.9E−06 |
| Regulation of interleukin-4 production | HLA-DRB1, HLA-DRB5 | 5.8E−06 |
| Protection from natural killer cell mediated cytotoxicity | HLA-A, HLA-B | 9.6E−06 |
| Immunoglobulin production involved in immunoglobulin mediated immune response | HLA-DRB1, HLA-DRB5 | 9.6E−06 |
| Humoral immune response mediated by circulating immunoglobulin | HLA-DRB1, HLA-DRB5 | 2E−05 |
| Antigen processing and presentation of exogenous peptide antigen via MHC class I, TAP-independent | HLA-A, HLA-B | 3.5E−05 |
| T-helper 1-type immune response | HLA-DRB1, HLA-DRB5 | 6.3E−05 |
| Positive regulation of T cell mediated cytotoxicity | HLA-A, HLA-B | 7.5E−05 |
| Inflammatory response to antigenic stimulus | HLA-DRB1, HLA-DRB5 | 0.0001 |
| Antigen processing and presentation of peptide or polysaccharide antigen via MHC class II | HLA-DRB1, HLA-DRB5 | 0.00013 |
| Negative regulation of interferon-γ production | HLA-DRB1, HLA-DRB5 | 0.00036 |
| Positive regulation of insulin secretion involved in cellular response to glucose stimulus | HLA-DRB1, HLA-DRB5 | 0.00039 |
| Antigen processing and presentation of peptide antigen via MHC class I | HLA-A, HLA-B | 0.00041 |
| Negative regulation of T cell proliferation | HLA-DRB1, HLA-DRB5 | 0.00063 |
| Protein tetramerization | HLA-DRB1, HLA-DRB5 | 0.00074 |
| Antigen processing and presentation of exogenous peptide antigen via MHC class I, TAP-dependent | HLA-A, HLA-B | 0.0018 |
| Type I interferon signaling pathway | HLA-A, HLA-B | 0.0019 |
| T cell costimulation | HLA-DRB1, HLA-DRB5 | 0.0028 |
| Antigen processing and presentation of exogenous peptide antigen via MHC class II | HLA-DRB1, HLA-DRB5 | 0.0038 |
| Protein deubiquitination | USP17L18, USP17L11 | 0.0044 |
| LNCaP private sequence variants | ||
| Bundle of His cell to Purkinje myocyte communication | GPR155, GNAS, CBR3, CHL1 | 0.00012 |
| Response to nitrosative stress | PRKCQ, CD3E, NLRP3 | 0.00068 |
| Cognition | FPGT-TNNI3K, TNNI3K | 0.0008 |
| Interleukin-1 β production | MEN1, NTRK3, PAX6, PRKDC | 0.0011 |
| Positive regulation of interleukin-4 production | LAMA2, PRKCQ, ROBO1, PAX6, SPTBN1, CHL1 | 0.0012 |
| Humoral immune response mediated by circulating immunoglobulin | GCLC, DUSP6 | 0.0023 |
| Type B pancreatic cell differentiation | NTRK3, AP1B1, FREM2, ROBO1, PRKDC, TBC1D32 | 0.004 |
| Negative regulation of protein phosphorylation | LRP1, STAB1, VTN, SSC4D, AMN, DMBT1 | 0.0042 |
| Axon guidance | NLRP3, CASP1 | 0.0076 |
| Heart development | LAMA2, FREM2, ROBO1, STAB1, ITGB4, VTN, CERCAM, COL16A1, CHL1, AOC3 | 0.014 |
| Receptor-mediated endocytosis | EXO1, HLA-DQB1 | 0.016 |
| Cell adhesion | MEN1, PAX6 | 0.027 |
| Shared sequence variants (PC3 and LNCaP) | ||
| Sensory perception of taste | TAS2R43, TAS2R31 | 0.00048 |
| Detection of chemical stimulus involved in sensory perception of bitter taste | TAS2R43, TAS2R31 | 0.00092 |
| O-glycan processing | MUC3A, MUC6 | 0.0021 |
| Digestion | PRSS2, PRSS1 | 0.0023 |
| Extracellular matrix disassembly | PRSS2, PRSS1 | 0.0033 |
Stop_gained events are denoted changes predicted to confer nonsense mutations and result in nonfunctional proteins or proteins with reduced function. Gene enrichment analysis was performed using DAVID (Database for Annotation, Visualization and Integrated Discovery). MHC, major histocompatibility complex; ER, endoplasmic reticulum; TAP, transporter associated with antigen processing.
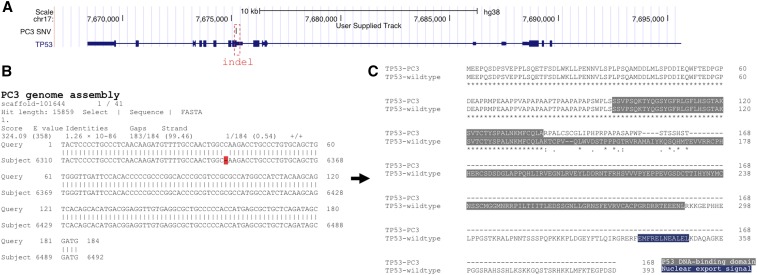
In PC3, 58 stop_lost events (Table 1) in 20 genes, (AHNAK2, DNAH6, FAT3, GOLGA6L3, GOLGA6L9, HLA-A, HLA-B, HLA-DRB1, HLA-DRB5, HOXA9, IL1A, ITPR2, MEGF6, MUC19, OR8K3, PRPF3, PRSS1, PTPRD, USP17L11, and USP17L18) were observed. There was a significant enrichment for HLA class II antigen presenting genes associated with the immune response (Table 3; Fisher’s exact P ∼ 0.05). It has recently been shown that the PC3, LNCaP, and DuPro (but not the DU145) prostate cancer cell lines and prostate cancer tissues express HLA class II molecules (Younger et al. 2008; Doonan and Haque 2015). However, we could not identify any prostate cancer patients with stop_lost events in these genes using the cBioPortal tool (Cerami et al. 2012; Gao et al. 2013) (data not shown). While evasion of the antitumour immune response is an emerging research area (Drake 2010; Corrales et al. 2016), caution should be exercised when considering the use of PC3 cells in these studies. Sequence variant analysis and interrogation of the PC3 de novo genome assembly by BLAST and GMAP confirmed that the tumor suppressor p53 (TP53) is inactivated by a single frameshift event (p.A138fs; indel; c.*4955A in NCBI RefSeq NM_000546) (Carroll et al. 1993) (Figure 2A).
Table 3. Putative deleted genes and their expression in the LNCaP and PC3 prostate cancer cell lines.
| Symbol | Description | CNV Region | Gene Start | Gene End | NP UPC | LNCaP UPC | PC3 UPC |
|---|---|---|---|---|---|---|---|
| LNCaP | |||||||
| PWRN1 | Prader-Willi region nonprotein coding RNA 1 | 15:24430001–24520000 | 24493137 | 24652130 | 0.0 | 0.0 | 0.0 |
| PC3 | |||||||
| ATP6V0A1 | ATPase H+ transporting V0 subunit a1 | 17:42110001–42520000 | 42458844 | 42522611 | 1 | 1 | 0 |
| CDH18 | Cadherin 18 | 5:19850001–19960000 | 19472951 | 20575873 | 0 | 0 | 0 |
| CIC | Capicua transcriptional repressor | 19:42280001–42320000 | 42268537 | 42295797 | 1 | 1 | 0 |
| CTNNA1 | Catenin α 1 | 5:138770001–138980000 | 138610967 | 138935034 | 1 | 1 | 0 |
| DDX3Y | DEAD-box helicase 3, Y-linked | Y:11530001–16450000 | 12904108 | 12920478 | 1 | 1 | 0 |
| DHX58 | DExH-box helicase 58 | 17:42110001–42520000 | 42101404 | 42112733 | 1 | 0 | 0 |
| DYDC1 | DPY30 domain containing 1 | 10:80250001–80560000 | 80336105 | 80356755 | 0 | 0 | 0 |
| DYDC2 | DPY30 domain containing 2 | 10:80250001–80560000 | 80344745 | 80368073 | 0 | 0 | 0 |
| FAM213A | Family with sequence similarity 213 member A | 10:80250001–80560000 | 80407829 | 80437115 | 1 | 0 | 0 |
| GHDC | GH3 domain containing | 17:42110001–42520000 | 42188799 | 42194532 | 1 | 1 | 0 |
| HCRT | Hypocretin neuropeptide precursor | 17:42110001–42520000 | 42184060 | 42185452 | 0 | 0 | 0 |
| HSPB9 | Heat shock protein family B (small) member 9 | 17:42110001–42520000 | 42121431 | 42123352 | 0 | 0 | 0 |
| KAT2A | Lysine acetyltransferase 2A | 17:42110001–42520000 | 42113108 | 42121358 | 1 | 1 | 0 |
| KCNH4 | Potassium voltage-gated channel Subfamily H member 4 | 17:42110001–42520000 | 42156891 | 42181278 | 0 | 0 | 0 |
| LIPJ | Lipase family member J | 10:87910001–88750000 | 88586753 | 88606976 | 0 | 0 | 0 |
| LRRTM2 | Leucine rich repeat transmembrane neuronal 2 | 5:138770001–138980000 | 138868923 | 138875368 | 0 | 0 | 0 |
| MAT1A | Methionine adenosyltransferase 1A | 10:80250001–80560000 | 80271820 | 80289684 | 0 | 0 | 0 |
| MIR548AT | MicroRNA 548at | 17:42110001–42520000 | 42494773 | 42494830 | 0 | 0 | 0 |
| NLGN4Y | Neuroligin 4, Y-linked | Y:11530001–16450000 | 14522638 | 14845650 | 1 | 0.2 | 0 |
| PAFAH1B3 | Platelet activating factor acetylhydrolase 1b catalytic subunit 3 | 19:42280001–42320000 | 42297033 | 42303546 | 1 | 0.7 | 0 |
| PRR19 | Proline rich 19 | 19:42280001–42320000 | 42302098 | 42310821 | 0 | 0 | 0 |
| PTEN | Phosphatase and tensin homolog | 10:87910001–88750000 | 87863113 | 87971930 | 1 | 1 | 0 |
| PTRF | Polymerase I and transcript release factor | 17:42110001–42520000 | 42402452 | 42423517 | 1 | 0 | 0 |
| RAB5C | RAB5C, member RAS oncogene family | 17:42110001–42520000 | 42124976 | 42155044 | 1 | 1 | 0 |
| RNLS | Renalase, FAD dependent amine oxidase | 10:87910001–88750000 | 88273864 | 88584530 | 0.6 | 0 | 0 |
| SH2D4B | SH2 domain containing 4B | 10:80250001–80560000 | 80537902 | 80646560 | 0 | 0 | 0 |
| SIL1 | SIL1 nucleotide exchange factor | 5:138770001–138980000 | 138946720 | 139293557 | 1 | 1 | 0 |
| SIRPB1 | Signal regulatory protein β 1 | 20:1580001–1620000 | 1563521 | 1620061 | 0 | 0 | 0 |
| STAT3 | Signal transducer and activator of transcription 3 | 17:42110001–42520000 | 42313324 | 42388568 | 1 | 1 | 0 |
| STAT5A | Signal transducer and activator of transcription 5A | 17:42110001–42520000 | 42287547 | 42311943 | 1 | 0 | 0 |
| STAT5B | Signal transducer and activator of transcription 5B | 17:42110001–42520000 | 42199168 | 42276707 | 1 | 1 | 0 |
| MEM145 | Transmembrane protein 145 | 19:42280001–42320000 | 42313325 | 42325062 | 0 | 0 | 0 |
| TMSB4Y | Thymosin β 4, Y-linked | Y:11530001–16450000 | 13703567 | 13706024 | 0 | 0 | 0 |
| TSPAN14 | Tetraspanin 14 | 10:80250001–80560000 | 80454166 | 80533123 | 1 | 1 | 0 |
| TSPY1 | Testis-specific protein, Y-linked 1 | Y:9450001–10200000 | 9466955 | 9490081 | 0 | 0 | 0 |
| TTTY13 | Testis-specific transcript, Y-linked 13 (nonprotein coding) | Y:21420001–21630000 | 21583600 | 21594666 | 0 | 0 | 0 |
| TTTY15 | Testis-specific transcript, Y-linked 15 (nonprotein coding) | Y:11530001–16450000 | 12662334 | 12692233 | 1 | 1 | 0 |
| USP9Y | Ubiquitin specific peptidase 9, Y-linked | Y:11530001–16450000 | 12701231 | 12860839 | 1 | 1 | 0 |
| UTY | Ubiquitously transcribed tetratricopeptide repeat containing, Y-linked | Y:11530001–16450000 | 13248379 | 13480673 | 1 | 1 | 0 |
CNV regions are listed for putative homozygous deletion events (CNV = 0). UPC refers to Universal exPression Code score, where UPC value > 0.5 indicates that a gene is actively transcribed. CNV, copy number variation; NP, normal prostate tissue; RAB5C, Ras-related protein Rab-5C; FAD, flavin adenine dinucleotide.
Figure 2.
Overview of a p53 (TP53) sequence variant in the PC3 prostate cancer cell line. (A) Genome browser display showing an indel event in the PC3 p53 gene (TP53). (B) Sequence alignment of TP53 in the PC3 genome and the reference genome assembly (Ensembl GRCh38 build 82). An indel is indicated in red. (C) Sequence alignment of TP53 protein products encoded by PC3 and the reference transcript NM_000546. An indel results in a frameshift (p.A138fs) and a truncated protein in PC3. chr, chromosome; SNV, single nucleotide variation.
PC3 shared 0.26 M sequence variants (166,912 SNVs and 89,919 indels) with LNCaP, and 21 of these constituted stop_lost events (Table 1). Overrepresented biological processes in PC3 and LNCaP included “O-glycan processing” (the mucins MUC3A and MUC6) and “extracellular matrix disassembly” (the trypsinogens PRSS1 and PRSS2) (Table 2). Interestingly, while we have identified MUC3A stop_gained events in PC3 and LNCaP, cell lines generated from Caucasian patients, a recent study suggests that MUC3A protein-changing variants are rare in Caucasians and predominant in African Americans, the subpopulation with the highest prevalence of prostate cancer, where MUC3A changes are observed in 88% of patients (Lindquist et al. 2016).
Taken together, these data indicate that protein-coding genes in LNCaP are perturbed extensively by point and indel mutations. Even after filtering steps, our LNCaP data (at passage four from the ATCC stock) reveal a clear difference in the number of particular variant events compared to PC3. However, previous exome sequencing work suggests that the genome of the parental LNCaP strain sequenced here (clone FGC) and its derived strains are inherently unstable (Spans et al. 2012, 2014), and this could give rise to the apparently high mutation rate in protein-coding sequences. As with studies of the HeLa genome (Adey et al. 2013; Landry et al. 2013), further genome sequencing efforts are warranted to investigate whether the variants reported here are somatic mutations found in particular LNCaP strains, or if they represent preexisting subpopulations within the parental LNCaP strain. In the future, single-cell WGS is likely to resolve this issue. Nevertheless, LNCaP and PC3 appear to have distinct SNV and indel profiles.
Putative gene loss
Most human cancers have CNVs, which impact upon gene dosage through loss or gain of whole chromosomes or chromosome segments (Hanahan and Weinberg 2011). Previous studies have described CNVs in PC3 and LNCaP using targeted techniques, such as exome sequencing. However, WGS, together with continuously updated gene annotations, offers improved detection of copy number changes (Meynert et al. 2014; Belkadi et al. 2015; Warr et al. 2015).
CNVs were identified using the R package cn.mops (Klambauer et al. 2012). In particular, we wished to identify genes that are lost in PC3 and LNCaP. The absence of this information can misinform even the most well-designed in vitro or cell line xenograft experiment (e.g., where a gene in an important pathway is lost). In the context of CNV analysis, we were interested in identifying putative homozygous deletions (CNV = 0; CNV0 events), i.e., genes that are inactivated by partial or complete gene deletion. To inform this analysis, we also considered the transcriptional potential of each gene by analyzing publicly available transcriptome (RNA-seq) data from normal prostate, LNCaP, and PC3. Genes with a UPC value of ∼0.5 were considered inactive (Piccolo et al. 2013).
Although a large number of SNVs and indel variations were observed in LNCaP, only a single homozygous deletion event (CNV0) was observed in this cell line. In the complex Prader-Willi gene locus there was a putative loss of PWRN1, a gene associated with epigenetic reprogramming during spermatogenesis (Wawrzik et al. 2009) (Table 3).
In contrast to LNCaP, 39 CNV0 events were found in PC3 (Table 3). CNV of the Y chromosome was evident from the PC3 sequence coverage (Figure 1A). In agreement with previous studies employing cDNA microarrays (Clark et al. 2003) and multicolor fluorescence in situ hybridization (Aurich-Costa et al. 2001), our CNV analysis revealed that large regions of the Y chromosome (including eight genes) were deleted in PC3 (Table 3). Several genes on chromosome 5 (CDH18, CTNNA1, LRRTM2, and SIL1), chromosome 10 (DYDC1, DYDC2, FAM213A, LIPJ, MAT1A, PTEN, RNLS, SH2D4B, and TSPAN14), and chromosome 17 (ATP6V0A1, DHX58, GHDC, HCRT, HSPB9, KAT2A, KCNH4, MIR548AT, PTRF, RAB5C, STAT3, STAT5A, and STAT5B) have also previously been reported to be deleted in PC3 (Liu et al. 2008; Krohn et al. 2012; The Cancer Genome Atlas Research Network 2015; Ibeawuchi et al. 2015).
Clinical observations and experimental studies indicate that the growth hormone receptor (GHR) mediates the development and progression of cancer (Brooks and Waters 2010), and GHR expression is elevated in prostate cancer cell lines and tissues (Chopin et al. 2002; Weiss-Messer et al. 2004). Interestingly, we noted that the genes encoding the classical growth hormone receptor signaling molecules STAT3 (STAT3) and STAT5 (STAT5A and STAT5B) were lost in PC3 cells. Thus, autocrine GHR actions are likely to be associated with alternative signaling pathways (Barclay et al. 2010) in PC3. Loss of STAT3 in PC3 has been firmly established experimentally (Yuan et al. 2005; Pencik et al. 2015), and there is evidence to suggest that STAT3 suppresses prostate cancer metastasis and confers a good prognosis (Pencik et al. 2015).
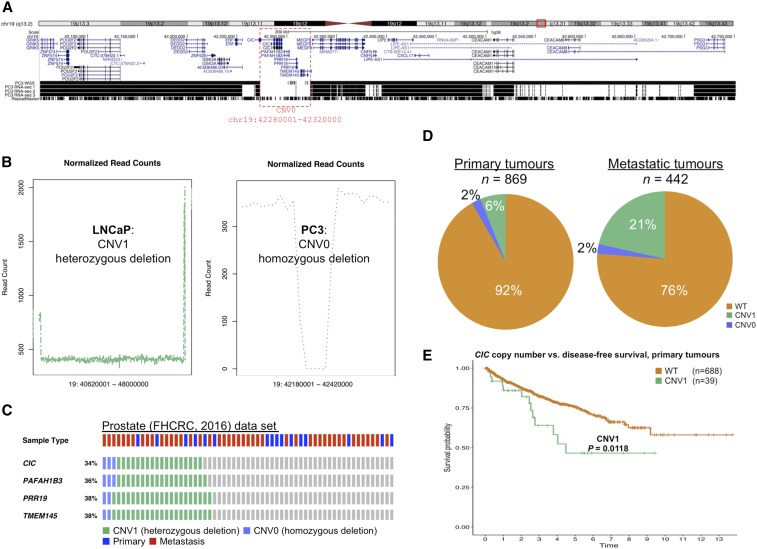
We identified a homozygous deletion event spanning four genes (CIC, PAFAH1B3, PRR19, and TMEM145) on chromosome 19 in PC3 (Figure 3A). In LNCaP, a genome coverage plot of reads flanking this region revealed a putative heterozygous event (CNV1; loss of a single copy of the same genes) (Figure 3B). Of these four genes, the mammalian homolog of Drosophila CIC (Jiménez et al. 2012) is particularly interesting. Capicua is a transcriptional repressor of cancer metastasis in a number of cancers (Choi et al. 2015; Okimoto et al. 2017). Recent WGS data also suggests that CIC is lost in PC3 cells (Iorio et al. 2016). Homozygous deletions of CIC have been reported in neuroblastoma (Nagaishi et al. 2014; Fransson et al. 2016), and a homozygous deletion of CIC in a subpopulation of H1975 human nonsmall cell lung cancer cell line xenografts rendered them highly metastatic (Okimoto et al. 2017). We interrogated 75 cBioPortal data sets from diverse tumors, confirming that one or two copies of CIC are lost in many cancer types (see Supplemental Material, Figure S1).
Figure 3.
Deletion of the tumor suppressor Capicua (CIC) in prostate cancer (A) Genome browser display showing a CNV0 event (red dotted line) on chromosome 19 that spans CIC, PAFAH1B3, PRR19, and TMEM145 in the PC3 prostate cancer cell line. (B) Plot of putative chromosomal loss spanning the four-gene region in LNCaP (left panel) and PC3 (right panel). The x-axis represents the genomic position and the y-axis the number of normalized counts. (C) Loss of the chromosome 19 region encompassing CIC, PAFAH1B3, PRR19, and TMEM145 in the ‘Prostate FHCRC 2016’ cBioPortal data set. Individual tumor samples are shown in columns and genes in rows. (D) CIC copy number alterations in primary and metastatic prostate cancer samples from eight clinical data sets interrogated using cBioPortal. (E) Disease-free survival in primary prostate cancer patients with loss of a single CIC gene copy (n = 13 relapse events) is significantly decreased compared to those without any CIC deletion events. ‘The Prostate (MSKCC 2010)’, ‘Prostate (MSKCC 2014)’, and ‘Prostate (TCGA)’ cBioPortal data sets were interrogated. P-values were calculated by Kaplan–Meier analysis (log-rank test). Time denotes years. CNV0, homozygous deletion; CNV1, heterozygous deletion; WT, wild-type.
CIC is abundantly expressed in normal prostate tissue, whereas its expression is reduced in primary tumors and ablated in metastatic prostate cancer (Choi et al. 2015). To characterize the potential clinical significance of CIC deletions in prostate cancer, we further examined 1311 tumors from eight data sets using the cBioPortal tool. While homozygous deletion events of the four genes deleted in PC3 cells were rare, a substantial fraction of prostate tumors harbored heterozygous deletions of these genes (Figure 3C). Approximately 6% of primary prostate tumors had heterozygous deletions and 2% had homozygous deletions of CIC, whereas 21% of metastatic tumors had homozygous CIC deletions and 2% heterozygous deletions (Figure 3D).
Prostate cancer relapse or recurrence frequently results in incurable metastasis, ultimately causing patient death (Wu et al. 2014; Weiner et al. 2016). As CIC deletions were more frequent in metastatic tumors, we reasoned that deletion of one or both copies of CIC is a means by which primary tumors in patients that eventually develop metastatic lesions achieve increased fitness and survival. The association between CIC homozygous deletion events and DFS in primary tumors could not be reliably assessed due to the low number (n = 2) of patients with recorded relapses; however, patients with primary prostate tumors with one lost copy of CIC (heterozygous deletion events) had a significantly worse outcome (P = 0.018, log-rank test) (Figure 3E). Similarly, OS is significantly worse in advanced-stage gastric cancer patients with low CIC expression (Okimoto et al. 2017).
A recent study comparing PC3 and LNCaP reported that the long form of the CIC protein (CIC-L) was not expressed and that the short form (CIC-S) was expressed at extremely low levels in PC3 cells (Choi et al. 2015). Our CNV analysis, employing WGS reads, interrogation of the de novo PC3 assembly using BLAST and GMAP, and analysis of RNA-seq reads mapped to the reference genome, failed to detect an intact CIC gene in PC3. We sequenced low-passage PC3 cells sourced directly from ATCC and speculate that the previous study (Choi et al. 2015) detected low-level gene expression by PC3 subpopulations with intact CIC resulting from genetic drift during prolonged subculture (passaging; see Festuccia et al. (1999); Li et al. (2008)).
Taken together, these data suggest that although a rare event in prostate tumors, homozygous deletion of CIC is not an idiosyncrasy of the PC3 cell line. Moreover, loss of a single gene copy of CIC is relatively common in prostate cancer. We speculate that disruption of one or both copies of CIC renders prostate cancer patients susceptible to an adverse disease outcome. A previous study employing forced overexpression of CIC in PC3 and LNCaP demonstrated that CIC is repressed by a trio of microRNAs (Choi et al. 2015). Altered MAPK signaling through the ERK pathway also suppresses endogenous CIC in lung cancer (Okimoto et al. 2017). Collectively, our data raise the possibility that the combination of microRNA repression, altered ERK signaling, and somatic events in the CIC locus promote tumorigenesis and confer a poor disease outcome.
Relevance of findings
In summary, we provide genome sequence data for PC3 and LNCaP, prostate cancer cell lines commonly employed in cancer research.
These data contribute to a catalog of cancer genomes, adding to recent whole-transcriptome sequencing, pharmacological profiling, and whole-exome sequencing efforts (Barretina et al. 2012; Klijn et al. 2015; Iorio et al. 2016) aimed at enhancing our understanding of human disease. For example, the phenomenon of androgen independence in prostate cancer has intrigued scientists for decades. Of the two cell lines interrogated in our study, PC3 is androgen-independent, whereas the LNCaP strain sequenced (LNCaP-FGC) is androgen-dependent. Recent work, including an investigation of 150 patients with metastatic CRPC (Robinson et al. 2015), suggests that anomalies (mutations, amplifications, and deletions) in a number of genes in the androgen receptor pathway play a role in the transition to androgen independence. We speculate that future work—employing WGS, RNA-sequencing, epigenetic profiling, and similar high-throughput methods—on a large number of cell lines and clinical samples is likely to identify genes critical for androgen independence. For instance, an androgen-independent strain of LNCaP (LNCaP-LNO) has been developed from cultures of an early passage of the LNCaP cells sequenced in our study (LNCaP-FGC) (van Steenbrugge et al. 1991). LNCaP-LNO and LNCaP-FGC were compared at the gene expression level (Oosterhoff et al. 2005); hinting that specific gene mutations or copy number events render LNCaP-LNO cells androgen-insensitive.
Raw reads (see Data availability in Materials and Methods) and sequence (SNV and indel) and CNV data are made available. We have generated de novo genome assemblies of both cell lines, allowing genes of interest to be investigated further, enabling, for example, the validation of gene loci associated with novel transcripts obtained from Trinity de novo transcriptome analysis (Grabherr et al. 2011; Haas et al. 2013). In addition, the genomes can be interrogated using a BLAST server, available at http://ghrelinlab.org. We acknowledge the limitations of short-insert (350 bp) genome sequencing, particularly when resolving complex repetitive or heterozygous regions (Rhoads and Au 2015; Merker et al. 2016). However, we anticipate that as sequencing becomes increasingly affordable, our sequencing efforts will complement future long-read genome assembly work and prove useful when correcting for errors (sequence polishing).
Finally, we reveal that one or both copies of CIC, a tumor metastasis suppressor gene, are frequently lost in prostate cancer and could drive metastatic CRPC. We anticipate that further biological insights into the role of Capicua in prostate cancer will shortly be gained by the research community, in line with the ethos of G3: Genes, Genomes, Genetics Genome Reports.
Supplementary Material
Supplemental material is available online at www.g3journal.org/lookup/suppl/doi:10.1534/g3.117.039909/-/DC1.
Acknowledgments
We acknowledge the use of the high-performance computational facilities at the Queensland University of Technology (QUT). This work was supported by the National Health and Medical Research Council of Australia (to I.S., P.L.J., and L.K.C.), the Cancer Council Queensland (to I.S. and L.K.C.), a QUT Vice-Chancellor’s Senior Research Fellowship (to I.S.), the Movember Foundation and the Prostate Cancer Foundation of Australia through a Movember Revolutionary Team Award (to L.K.C. and C.C.N.), and the Australian Government Department of Health.
Footnotes
Communicating editor: B. J. Andrews
Literature Cited
- Adey A., Burton J. N., Kitzman J. O., Hiatt J. B., Lewis A. P., et al. , 2013. The haplotype-resolved genome and epigenome of the aneuploid HeLa cancer cell line. Nature 500: 207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurich-Costa J., Vannier A., Gregoire E., Nowak F., Cherif D., 2001. IPM-FISH, a new M-FISH approach using IRS-PCR painting probes: application to the analysis of seven human prostate cell lines. Genes Chromosomes Cancer 30: 143–160. [PubMed] [Google Scholar]
- Baca S. C., Prandi D., Lawrence M. S., Mosquera J. M., Romanel A., et al. , 2013. Punctuated evolution of prostate cancer genomes. Cell 153: 666–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay J. L., Kerr L. M., Arthur L., Rowland J. E., Nelson C. N., et al. , 2010. In vivo targeting of the growth hormone receptor (GHR) Box1 sequence demonstrates that the GHR does not signal exclusively through JAK2. Mol. Endocrinol. 24: 204–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barretina J., Caponigro G., Stransky N., Venkatesan K., Margolin A. A., et al. , 2012. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 483: 603–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkadi A., Bolze A., Itan Y., Cobat A., Vincent Q. B., et al. , 2015. Whole-genome sequencing is more powerful than whole-exome sequencing for detecting exome variants. Proc. Natl. Acad. Sci. USA 112: 5473–5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltran H., Prandi D., Mosquera J. M., Benelli M., Puca L., et al. , 2016. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat. Med. 22: 298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks A. J., Waters M. J., 2010. The growth hormone receptor: mechanism of activation and clinical implications. Nat. Rev. Endocrinol. 6: 515–525. [DOI] [PubMed] [Google Scholar]
- Carroll A. G., Voeller H. J., Sugars L., Gelmann E. P., 1993. p53 oncogene mutations in three human prostate cancer cell lines. Prostate 23: 123–134. [DOI] [PubMed] [Google Scholar]
- Cerami E., Gao J., Dogrusoz U., Gross B. E., Sumer S. O., et al. , 2012. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2: 401–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi N., Park J., Lee J.-S., Yoe J., Park G. Y., et al. , 2015. miR-93/miR-106b/miR-375-CIC-CRABP1: a novel regulatory axis in prostate cancer progression. Oncotarget 6: 23533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopin L., Veveris-Lowe T., Philipps A. F., Herington A., 2002. Co-expression of GH and GHR isoforms in prostate cancer cell lines. Growth Horm. IGF Res. 12: 126–136. [DOI] [PubMed] [Google Scholar]
- Cingolani P., Patel V., Coon M., Nguyen T., Land S., et al. , 2012a Using Drosophila melanogaster as a model for genotoxic chemical mutational studies with a new program, Snpsift. Front. Genet. 3: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cingolani P., Platts A., Coon M., Nguyen T., Wang L., et al. , 2012b A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly (Austin) 6: 80–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J., Edwards S., Feber A., Flohr P., John M., et al. , 2003. Genome-wide screening for complete genetic loss in prostate cancer by comparative hybridization onto cDNA microarrays. Oncogene 22: 1247–1252. [DOI] [PubMed] [Google Scholar]
- Corrales L., Matson V., Flood B., Spranger S., Gajewski T. F., 2016. Innate immune signaling and regulation in cancer immunotherapy. Cell Res. 27: 96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doonan B. P., Haque A., 2015. Prostate cancer immunotherapy: exploiting the HLA class II pathway in vaccine design. J. Clin. Cell. Immunol. 6: 351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake C. G., 2010. Prostate cancer as a model for tumour immunotherapy. Nat. Rev. Immunol. 10: 580–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falchetti A., Marini F., Luzi E., Giusti F., Cavalli L., et al. , 2009. Multiple endocrine neoplasia type 1 (MEN1): not only inherited endocrine tumors. Genet. Med. 11: 825–835. [DOI] [PubMed] [Google Scholar]
- Festuccia C., Gravina G. L., Angelucci A., Millimaggi D., Bologna M., 1999. Culture conditions modulate cell phenotype and cause selection of subpopulations in PC3 prostate cancer cell line. Anticancer Res. 20: 4367–4371. [PubMed] [Google Scholar]
- Fransson S., Östensson M., Djos A., Javanmardi N., Kogner P., et al. , 2016. Estimation of copy number aberrations: comparison of exome sequencing data with SNP microarrays identifies homozygous deletions of 19q13. 2 and CIC in neuroblastoma. Int. J. Oncol. 48: 1103–1116. [DOI] [PubMed] [Google Scholar]
- Gao J., Aksoy B. A., Dogrusoz U., Dresdner G., Gross B., et al. , 2013. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 6: pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Alcalde F., Okonechnikov K., Carbonell J., Cruz L. M., Götz S., et al. , 2012. Qualimap: evaluating next-generation sequencing alignment data. Bioinformatics 28: 2678–2679. [DOI] [PubMed] [Google Scholar]
- Gibbs R. A., Belmont J. W., Hardenbol P., Willis T. D., Yu F., et al. , 2003. The international HapMap project. Nature 426: 789–796. [DOI] [PubMed] [Google Scholar]
- Gill S., Sargent D., 2006. End points for adjuvant therapy trials: has the time come to accept disease-free survival as a surrogate end point for overall survival? Oncologist 11: 624–629. [DOI] [PubMed] [Google Scholar]
- Grabherr M. G., Haas B. J., Yassour M., Levin J. Z., Thompson D. A., et al. , 2011. Full-length transcriptome assembly from RNA-seq data without a reference genome. Nat. Biotechnol. 29: 644–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasso C. S., Wu Y.-M., Robinson D. R., Cao X., Dhanasekaran S. M., et al. , 2012. The mutational landscape of lethal castration-resistant prostate cancer. Nature 487: 239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich A., Saveliev V., Vyahhi N., Tesler G., 2013. QUAST: quality assessment tool for genome assemblies. Bioinformatics 29: 1072–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas B. J., Papanicolaou A., Yassour M., Grabherr M., Blood P. D., et al. , 2013. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat. Protoc. 8: 1494–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D., Weinberg R. A., 2011. Hallmarks of cancer: the next generation. Cell 144: 646–674. [DOI] [PubMed] [Google Scholar]
- Hieronymus H., Schultz N., Gopalan A., Carver B. S., Chang M. T., et al. , 2014. Copy number alteration burden predicts prostate cancer relapse. Proc. Natl. Acad. Sci. USA 111: 11139–11144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horoszewicz J., 1980. The LNCaP cell line-a new model for studies on human prostatic carcinoma. Prog. Clin. Biol. Res. 37: 115–132. [PubMed] [Google Scholar]
- Horoszewicz J. S., Leong S. S., Kawinski E., Karr J. P., Rosenthal H., et al. , 1983. LNCaP model of human prostatic carcinoma. Cancer Res. 43: 1809–1818. [PubMed] [Google Scholar]
- Huang D. W., Sherman B. T., Lempicki R. A., 2009a Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 37: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D. W., Sherman B. T., Lempicki R. A., 2009b Systematic and integrative analysis of large gene lists using (DAVID) bioinformatics resources. Nat. Protoc. 4: 44–57. [DOI] [PubMed] [Google Scholar]
- Ibeawuchi C., Schmidt H., Voss R., Titze U., Abbas M., et al. , 2015. Exploring prostate cancer genome reveals simultaneous losses of PTEN, FAS and PAPSS2 in patients with PSA recurrence after radical prostatectomy. Int. J. Mol. Sci. 16: 3856–3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iorio F., Knijnenburg T. A., Vis D. J., Bignell G. R., Menden M. P., et al. , 2016. A landscape of pharmacogenomic interactions in cancer. Cell 166: 740–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez G., Shvartsman S. Y., Paroush Z., 2012. The Capicua repressor–a general sensor of RTK signaling in development and disease. J. Cell Sci. 125: 1383–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaighn M., Narayan K. S., Ohnuki Y., Lechner J., Jones L., 1979. Establishment and characterization of a human prostatic carcinoma cell line (PC-3). Invest. Urol. 17: 16–23. [PubMed] [Google Scholar]
- Kim D., Pertea G., Trapnell C., Pimentel H., Kelley R., et al. , 2013. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 14: R36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klambauer G., Schwarzbauer K., Mayr A., Clevert D.-A., Mitterecker A., et al. , 2012. cn.MOPS: mixture of Poissons for discovering copy number variations in next-generation sequencing data with a low false discovery rate. Nucleic Acids Res. 40: e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klijn C., Durinck S., Stawiski E. W., Haverty P. M., Jiang Z., et al. , 2015. A comprehensive transcriptional portrait of human cancer cell lines. Nat. Biotechnol. 33: 306–312. [DOI] [PubMed] [Google Scholar]
- Krohn A., Diedler T., Burkhardt L., Mayer P.-S., De Silva C., et al. , 2012. Genomic deletion of PTEN is associated with tumor progression and early PSA recurrence in ERG fusion-positive and fusion-negative prostate cancer. Am. J. Pathol. 181: 401–412. [DOI] [PubMed] [Google Scholar]
- Kumar A., Coleman I., Morrissey C., Zhang X., True L. D., et al. , 2016. Substantial interindividual and limited intraindividual genomic diversity among tumors from men with metastatic prostate cancer. Nat. Med. 22: 369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry J. J., Pyl P. T., Rausch T., Zichner T., Tekkedil M. M., et al. , 2013. The genomic and transcriptomic landscape of a HeLa cell line. G3 3: 1213–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H., 2013 Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv Available at: https://arxiv.org/abs/1303.3997.
- Li H., Chen X., Calhoun-Davis T., Claypool K., Tang D. G., 2008. PC3 human prostate carcinoma cell holoclones contain self-renewing tumor-initiating cells. Cancer Res. 68: 1820–1825. [DOI] [PubMed] [Google Scholar]
- Li H., Handsaker B., Wysoker A., Fennell T., Ruan J., et al. , 2009. The sequence alignment/map format and SAMtools. Bioinformatics 25: 2078–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y., Smyth G. K., Shi W., 2014. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30: 923–930. [DOI] [PubMed] [Google Scholar]
- Lindquist K. J., Paris P. L., Hoffmann T. J., Cardin N. J., Kazma R., et al. , 2016. Mutational landscape of aggressive prostate tumors in African American men. Cancer Res. 76: 1860–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Xie C. C., Zhu Y., Li T., Sun J., et al. , 2008. Homozygous deletions and recurrent amplifications implicate new genes involved in prostate cancer. Neoplasia 10: 897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik R., Khan A. P., Asangani I. A., Cieślik M., Prensner J. R., et al. , 2015. Targeting the MLL complex in castration-resistant prostate cancer. Nat. Med. 21: 344–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallett S., Royston P., Waters R., Dutton S., Altman D. G., 2010. Reporting performance of prognostic models in cancer: a review. BMC Med. 8: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merker, J., A. M. Wenger, T. Sneddon, M. Grove, D. Waggott et al., 2016 Long-read whole genome sequencing identifies causal structural variation in a Mendelian disease. bioRxiv Available at: https://doi.org/10.1101/090985. [DOI] [PMC free article] [PubMed]
- Metzger E., Willmann D., McMillan J., Forne I., Metzger P., et al. , 2016. Assembly of methylated KDM1A and CHD1 drives androgen receptor-dependent transcription and translocation. Nat. Struct. Mol. Biol. 23: 132–139. [DOI] [PubMed] [Google Scholar]
- Meynert A. M., Ansari M., FitzPatrick D. R., Taylor M. S., 2014. Variant detection sensitivity and biases in whole genome and exome sequencing. BMC Bioinformatics 15: 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaishi M., Suzuki A., Nobusawa S., Yokoo H., Nakazato Y., 2014. Alpha-internexin and altered CIC expression as a supportive diagnostic marker for oligodendroglial tumors with the 1p/19q co-deletion. Brain Tumor Pathol. 31: 257–264. [DOI] [PubMed] [Google Scholar]
- Okimoto R. A., Breitenbuecher F., Olivas V. R., Wu W., Gini B., et al. , 2017. Inactivation of Capicua drives cancer metastasis. Nat. Genet. 49: 87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okonechnikov K., Conesa A., García-Alcalde F., 2016. Qualimap 2: advanced multi-sample quality control for high-throughput sequencing data. Bioinformatics 32: 292–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosterhoff J. K., Grootegoed J. A., Blok L. J., 2005. Expression profiling of androgen-dependent and-independent LNCaP cells: EGF vs. androgen signalling. Endocr. Relat. Cancer 12: 135–148. [DOI] [PubMed] [Google Scholar]
- Pencik J., Schlederer M., Gruber W., Unger C., Walker S. M., et al. , 2015. STAT3 regulated ARF expression suppresses prostate cancer metastasis. Nat. Commun. 6: 7736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo S. R., Withers M. R., Francis O. E., Bild A. H., Johnson W. E., 2013. Multiplatform single-sample estimates of transcriptional activation. Proc. Natl. Acad. Sci. USA 110: 17778–17783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priyam, A., B. J. Woodcroft, V. Rai, A. Munagala, I. Moghul et al., 2015 Sequenceserver: a modern graphical user interface for custom BLAST databases. bioRxiv Available at: https://doi.org/10.1101/033142. [DOI] [PMC free article] [PubMed]
- R Core Team, 2013 R: A Language and Environment for Statistical Computing R Foundation for Statistical Computing, Vienna, Austria. Available at: http://www.r-project.org.
- Rhoads A., Au K. F., 2015. PacBio sequencing and its applications. Genomics Proteomics Bioinformatics 13: 278–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich J. T., Neely J. G., Paniello R. C., Voelker C. C., Nussenbaum B., et al. , 2010. A practical guide to understanding Kaplan-Meier curves. Otolaryngol. Head Neck Surg. 143: 331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D., Van Allen E. M., Wu Y.-M., Schultz N., Lonigro R. J., et al. , 2015. Integrative clinical genomics of advanced prostate cancer. Cell 161: 1215–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M. D., Oshlack A., 2010. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 11: R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M. D., McCarthy D. J., Smyth G. K., 2010. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26: 139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seim, I., 2017a de novo genome assembly of the LNCaP human prostate cancer cell line. Zenodo DOI: 10.5281/zenodo.245173.
- Seim, I., 2017b de novo genome assembly of the PC3 human prostate cancer cell line. Zenodo DOI: 10.5281/zenodo.244912.
- Seim, I., 2017c Filtered and annotated SNV and indel variants in the PC3 and LNCaP human prostate cancer cell lines. Zenodo DOI: 10.5281/zenodo.245431. [DOI] [PMC free article] [PubMed]
- Simpson J. T., Durbin R., 2012. Efficient de novo assembly of large genomes using compressed data structures. Genome Res. 22: 549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spans L., Atak Z. K., Van Nieuwerburgh F., Deforce D., Lerut E., et al. , 2012. Variations in the exome of the LNCaP prostate cancer cell line. Prostate 72: 1317–1327. [DOI] [PubMed] [Google Scholar]
- Spans L., Helsen C., Clinckemalie L., Van den Broeck T., Prekovic S., et al. , 2014. Comparative genomic and transcriptomic analyses of LNCaP and C4–2B prostate cancer cell lines. PLoS One 9: e90002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudmant P. H., Rausch T., Gardner E. J., Handsaker R. E., Abyzov A., et al. , 2015. An integrated map of structural variation in 2,504 human genomes. Nature 526: 75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor B. S., Schultz N., Hieronymus H., Gopalan A., Xiao Y., et al. , 2010. Integrative genomic profiling of human prostate cancer. Cancer Cell 18: 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennessen J. A., Bigham A. W., O’Connor T. D., Fu W., Kenny E. E., et al. , 2012. Evolution and functional impact of rare coding variation from deep sequencing of human exomes. Science 337: 64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Cancer Genome Atlas Research Network , 2015. The molecular taxonomy of primary prostate cancer. Cell 163: 1011–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therneau, T., 2013 A package for survival analysis in S. R package version 2.37–4. Available at: http://CRAN.R-project.org/package=survival. Accessed: January 9, 2017.
- van Steenbrugge G. J., Van Uffelen C., Bolt J., Schröder F., 1991. The human prostatic cancer cell line LNCaP and its derived sublines: an in vitro model for the study of androgen sensitivity. J. Steroid Biochem. Mol. Biol. 40: 207–214. [DOI] [PubMed] [Google Scholar]
- Wang Q., Hardie R.-A., Hoy A. J., van Geldermalsen M., Gao D., et al. , 2015. Targeting ASCT2-mediated glutamine uptake blocks prostate cancer growth and tumour development. J. Pathol. 236: 278–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warr A., Robert C., Hume D., Archibald A., Deeb N., et al. , 2015. Exome sequencing: current and future perspectives. G3 5: 1543–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wawrzik M., Spiess A.-N., Herrmann R., Buiting K., Horsthemke B., 2009. Expression of SNURF–SNRPN upstream transcripts and epigenetic regulatory genes during human spermatogenesis. Eur. J. Hum. Genet. 17: 1463–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner A., Matulewicz R., Eggener S., Schaeffer E., 2016. Increasing incidence of metastatic prostate cancer in the United States (2004–2013). Prostate Cancer Prostatic Dis. 19: 395–397. [DOI] [PubMed] [Google Scholar]
- Weiss-Messer E., Merom O., Adi A., Karry R., Bidosee M., et al. , 2004. Growth hormone (GH) receptors in prostate cancer: gene expression in human tissues and cell lines and characterization, GH signaling and androgen receptor regulation in LNCaP cells. Mol. Cell. Endocrinol. 220: 109–123. [DOI] [PubMed] [Google Scholar]
- Wu J. N., Fish K. M., Evans C. P., deVere White R. W., Dall’Era M. A., 2014. No improvement noted in overall or cause-specific survival for men presenting with metastatic prostate cancer over a 20-year period. Cancer 120: 818–823. [DOI] [PubMed] [Google Scholar]
- Wu T. D., Watanabe C. K., 2005. GMAP: a genomic mapping and alignment program for mRNA and EST sequences. Bioinformatics 21: 1859–1875. [DOI] [PubMed] [Google Scholar]
- Younger A., Amria S., Jeffrey W., Mahdy A., Goldstein O., et al. , 2008. HLA class II antigen presentation by prostate cancer cells. Prostate Cancer Prostatic Dis. 11: 334–341. [DOI] [PubMed] [Google Scholar]
- Yuan Z.-l., Guan Y.-j., Chatterjee D., Chin Y. E., 2005. Stat3 dimerization regulated by reversible acetylation of a single lysine residue. Science 307: 269–273. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The genome reads reported in this paper have been deposited in the BioProject database as PRJNA361315 (PC3) and PRJNA361316 (LNCaP). Code used to generate the data and CNV analysis output files (tabulated text files) are available at github.com/sciseim/PCaWGS. Genome assemblies (FASTA format) (Seim 2017a,b), and filtered and annotated single-nucleotide and indel variation data files (VCF format) (Seim 2017c), have been deposited at Zenodo. A BLAST server is available at http://ghrelinlab.org.