Abstract
IMPORTANCE
Resection of the primary tumor with negative margins is the gold standard treatment for squamous cell carcinoma of the oral tongue (SCCOT). A microscopically positive surgical margin is clearly associated with a higher risk for local recurrence, whereas a negative margin has traditionally been defined as greater than 5.0 mm clearance from the tumor, with lesser margins arbitrarily designated as close. The precise cutoff at which the risk for local recurrence with a close margin approximates that of a microscopically positive margin remains unclear.
OBJECTIVE
To determine whether the arbitrarily defined close margin (<5.0 mm) would portend as high a risk for local recurrence as a positive margin after resection of SCCOT.
DESIGN, SETTING, AND PARTICIPANTS
In this retrospective study, head and neck pathologists reviewed archived tumor specimens from 381 patients with SCCOT who underwent primary surgical resection at a tertiary care center from January 1, 2000, through December 31, 2012. Data were analyzed from November 15, 2015, to January 5, 2016. Time-dependent receiver operating characteristic curve analysis was used in patients who did not have a microscopically positive margin to determine an optimal margin cutoff for local recurrence-free survival (LRFS). Pathologic factors were assessed for LRFS in a multivariate Cox proportional hazards regression model.
MAIN OUTCOMES AND MEASURES
The primary end point was evaluation of the margin distance associated with LRFS.
RESULTS
Among the 381 patients included in the analysis (222 men [58.3%] and 159 women [41.7%]; mean [SD] age, 58 [14.7] years), the optimal cutoff associated with LRFS was determined to be 2.2 mm. This cutoff was compared with the traditionally accepted cutoff of 5.0 mm. Patients with a margin of 2.3 to 5.0 mm had similar LRFS as patients with a margin of greater than 5.0 mm (hazard ratio [HR], 1.31; 95% CI, 0.58–2.96), and all other comparisons were significantly different (HR for positive margin, 9.03; 95% CI, 3.45–23.67; HR for 0.01-to 2.2-mm margin, 2.83; 95% CI, 1.32–6.07). Based on this result, negative margins were redefined as those with a clearance of greater than 2.2 mm. In a multivariate model adjusting for pathologic factors, positive margins (adjusted HR, 5.73; 95% CI, 2.45–13.41) and margins of 0.01 to 2.2 mm (adjusted HR, 2.00; 95% CI, 1.13–3.55) were the variables most significantly associated with LRFS.
CONCLUSIONS AND RELEVANCE
In this study, local recurrence-free survival was significantly affected only with surgical margins of less than or equal to 2.2 mm in patients with SCCOT. This new definition of close margins stratifies the risk for local recurrence better than the arbitrary 5.0-mm cutoff that has been used.
Surgical resection of the primary tumor with negative margins is the gold standard for treatment of squamous cell carcinoma (SCC) of the oral tongue (SCCOT). The primary goal of an oncologic resection is the complete excision of the tumor with no residual cancer cells left behind. A microscopically positive surgical margin is associated with a higher risk for local recurrence and a poor clinical outcome.1–3 Close margins or the appropriate margin clearance from tumor remain as a controversial debate in the literature. More importantly, the treatment of a patient with a close margin resection is often a matter of discussion among clinicians.
The most widely accepted definition of adequate margin distance for SCCOT is 5.0 mm.4 Some authors have postulated that a margin distance from the tumor of 1.0 to 2.0 mm should be the definition of a close margin.5 Others have found that pathologic margins of 7.0 mm or less are associated with local recurrence, disease-specific survival (DSS), and overall survival in cancer of the oral cavity.6 Dik et al7 found no significant difference in local recurrence when comparing patients with stage I or stage II oral SCC who did not receive postoperative radiotherapy (RT) and had a margin of at least 3.0 mm with no more than 2 unfavorable histologic variables besides margin status with patients with free margins. Barry et al8 also studied stage I or stage II oral SCC and found no association between the size of the resection margin and local control or survival. Others have also shown that close margins alone are not sufficient to estimate clinical outcome and should not be an indication for adjuvant RT.9 Therefore, the precise cutoff at which the risk for local recurrence with a close margin approximates that of a microscopically positive margin remains unclear. We hypothesized that the arbitrarily defined close margin (<5.0 mm) would not portend as high a risk for local recurrence as a positive margin after resection of SCCOT.
Methods
From our departmental database, we identified a cohort of 1027 patients who underwent primary surgery for SCC of the oral cavity from January 1, 2000, through December 31, 2012. We performed a retrospective review of medical records to obtain patient, tumor, treatment, and outcomes information. This study was approved by the institutional review board of Memorial Sloan Kettering Cancer Center, who waived the need for informed consent.
In this cohort, 546 patients had SCCOT. To be eligible for inclusion, patients were required to have histopathologic slides available for review. A total of 381 patients met this criterion. When comparing the excluded patients (n = 165) with the inclusion cohort, similar distributions in age, sex, tobacco use, alcohol use, comorbidity index, clinical T stage, and clinical N stage were found. However, more patients in the exclusion group received postoperative adjuvant therapy (86 [52.1%] vs 95 [24.9%]).
All archived tumor specimens were reviewed by 1 of 3 head and neck pathologists (B.X., N.K., and R.A.G.) for margin status and other histologic variables. The pathologists were blinded to the patient’s outcome. If microscopic invasive carcinoma was found at the inked margin of the resected specimen, the margin was labeled as positive. In all cases, margin clearance was calculated by measuring the closest distance between the invasive carcinoma and the inked margin of the resection specimen using an oculometer. Additional tumor bed (revision) margins were rare, accounting for 20 (5.2%) of the 381 cases examined microscopically. In 13 cases, the additional revision did not affect the clearance because it did not correspond to the closest margin or to a positive margin on final histopathologic examination of the main specimen. In 7 of 381 patients (1.8%), the margin clearance had to be adjusted based on the revision. In these cases, we calculated the final tumor clearance by adding the shortest dimension of the revised margin to the distance between the tumor and the closest margin in the resection specimen. The location of the closest margin (mucosal or deep) was recorded. Tumor size was defined as the greatest diameter of the invasive tumor based on a combination of gross and microscopic examination. Patterns of tumor invasion were assessed as previously described.10 The presence and number of positive nodes were recorded. The presence and extent of extranodal extension were assessed as previously reported by Wreesmann et al.11 Other variables reviewed were the histologic SCC subtype (keratinizing, nonkeratinizing, or other variants), perineural invasion, lymphovascular invasion, and tumor grade.
We assessed associations between variables by Pearson χ2 or Fisher exact test. The primary end point for this study was local recurrence-free survival (LRFS). We calculated LRFS from the date of surgery to the date of recurrence or last disease assessment by a member of our disease management team. All local recurrences consisted of biopsy-proven lesions at the same site as the primary tumor. Patients without a local recurrence were considered to be censored at the last disease assessment date. Although overall survival and DSS were not primary end points, we reported survival outcomes at the median follow-up. We calculated overall survival from the date of surgery to the date of death or the last date the patient was known to be alive. We calculated DSS from the date of surgery to the date of death or the last disease assessment. A death was considered to be an event for DSS if the patient had active disease at the time of last disease assessment.
Data were analyzed from November 15, 2015, to January 5, 2016. We used time-dependent receiver operating characteristic curve analysis in patients who did not have a microscopically positive margin to determine an optimal margin cut-off for LRFS. This optimal cutoff for LRFS was calculated for maximizing the sensitivity and specificity by taking the largest difference between true-positive and false-positive results.12
Pathologic factors, including tumor size, histologic grade, perineural invasion, lymphovascular invasion, nodal status, and margin status, were assessed for LRFS using the Cox proportional hazards regression model and Kaplan-Meier statistics. Tumor size was categorized as 2.0 cm or less, 2.1 to 4.0 cm, or greater than 4.0 cm. Log-rank tests determined the univariate significance of a factor. Factors found to be significant with univariate analysis were included in a multivariate Cox proportional hazards regression model. Effect size or hazard ratio (HR) and 95% CI were used to report the magnitude of difference and the strength of association and precision of estimates.
Results
A total of 381 patients were eligible for analysis (222 men [58.3%] and 159 women [41.7%]; mean [SD] age, 58 [14.7] years). Details of the study cohort are shown in Table 1. Most patients were younger than 60 years (212 [55.6%]). Most patients used alcohol (260 [68.2%]) and/or tobacco (206 [54.1%]) to some extent. More than three-quarters of patients presented clinically with stage T1 (193 [50.7%]) or T2 (135 [35.4%]) tumors. Two hundred seventy-five patients (72.2%) presented with cN0 neck findings. A neck dissection was performed in 281 patients (73.8%); 106 were therapeutic neck dissections for a clinically positive node in the neck, and 175 were elective neck dissections for management of cN0 findings. Adjuvant treatment was used in 95 patients (24.9%), including RT alone in 65 (17.1%) and chemotherapy and RT in 30 (7.9%). Three (13.0%) patients with positive margins received adjuvant RT and 8 (34.8%) received chemotherapy and RT compared with 62 (17.3%) and 22 (6.1%), respectively, for the rest of the group.
Table 1.
Patient Demographic Characteristics and Clinical Tumor Stage at Presentation
| Variable | No. (%) of Patientsa (n = 381) |
|---|---|
| Sex | |
| Male | 222 (58.3) |
| Female | 159 (41.7) |
| Tobacco use | |
| Never | 175 (45.9) |
| Ever | 206 (54.1) |
| Alcohol use | |
| Never | 121 (31.8) |
| Ever | 260 (68.2) |
| Clinical T stage | |
| cT1 | 193 (50.7) |
| cT2 | 135 (35.4) |
| cT3 | 34 (8.9) |
| cT4 | 15 (3.9) |
| cTX | 4 (1.0) |
| Clinical N stage | |
| cN0 | 275 (72.2) |
| cN1 | 40 (10.5) |
| cN2 | 64 (16.8) |
| cN3 | 2 (0.5) |
Percentages have been rounded and may not total 100.
On pathologic review, we found that most tumors (270 [70.9%]) were 2.0 cm or smaller. The median greatest tumor dimension was 1.5 cm (interquartile range, 0.8–2.3 cm; range, 0.01–7.0 cm). Eighty-five patients were found to have lymph node metastases, of whom half had extranodal extension of tumor. One hundred five patients (27.6%) in our cohort were found to have perineural invasion, and 38 (10.0%) had lymphovascular invasion. Although 298 tumors (78.2%) invaded the muscle, 83 (21.8%) invaded only the lamina propria.
Twenty-three patients (6.0%) had positive margins. For the remaining 358 patients, the median margin distance was 3.7 mm (interquartile range, 1.8–5.5 mm; range, 0.01–16.0 mm). To assess the optimal margin cutoff, we excluded the patients with positive margins from the analysis. Using time-dependent receiver operating characteristic curve analysis, the optimal cutoff associated with LRFS was determined to be 2.2 mm (area under the curve, 0.671). On Kaplan-Meier analysis, we compared this cutoff with the traditionally accepted cutoff of 5.0 mm. Figure 1 shows that the absolute 2-year LRFS in patients with a margin of 2.3 to 5.0 mm was 93.5% (95% CI, 87.3%–96.7%), similar to 91.8% (95% CI, 83.4%–96.0%) in patients with a margin of greater than 5.0 mm. The absolute 2-year LRFS difference between patients with a margin of 2.3 to 5.0 mm and those with a margin greater than 5.0 mm was 1.7% (95% CI,−2.0% to 5.4%).
Figure 1. Local Recurrence-Free Survival (LRFS) by Margin Status.
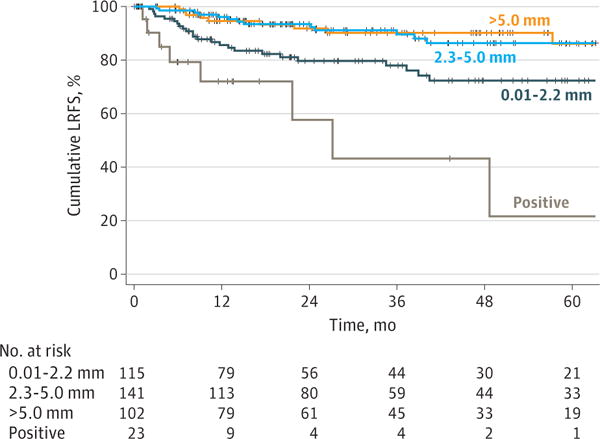
The absolute 2-year LRFS for the 141 patients with a margin of 2.3 to 5.0 mm was 93.5% (95% CI, 87.3%–96.7%) and for the 102 patients with a margin of greater than 5.0 mm was 91.8% (95% CI, 83.4%–96.0%). The absolute difference in 2-year LRFS was 1.7% (95% CI, −2.0% to 5.4%).
Using the Cox proportional hazards regression model, the HR for patients with a margin of 2.3 to 5.0 mm compared with that for patients with a margin of greater than 5.0 mm was 1.31 (95% CI, 0.58–2.96). All other comparisons were significantly different (HR for positive margin, 9.03; 95% CI, 3.45–23.67; HR for 0.01-to 2.2-mm margin, 2.83; 95% CI, 1.32–6.07). To address the possibility of treatment bias, we adjusted for adjuvant therapy and size (Figure 2). Patients in the group with 2.3-to 5.0-mm margins had similar findings to those of the patients with margins greater than 5.0 mm regardless of tumor size or adjuvant therapy (adjusted HR, 1.17; 95% CI, 0.51– 2.66) (Table 2). Based on this result, we redefined negative margins as those with a clearance of greater than 2.2 mm. Figure 3 shows the incidence of local recurrence by margin distance using the 2.2-mm cutoff.
Figure 2. Cox Proportional Hazards Regression Model for Local Recurrence-Free Survival (LRFS).

Local recurrence-free survival curves represent multivariate Cox proportional hazards regression analysis plotting margin status adjusted for tumor size and adjuvant therapy.
Table 2.
Multivariate Cox Proportional Hazards Regression Analysis for LRFS
| Variable | Adjusted HR (95% CI) |
|---|---|
| Margin, mm | |
| >5.0 | 1 [Reference] |
| 0.01–2.2 | 2.25 (1.03–4.92)a |
| 2.3–5.0 | 1.17 (0.51–2.66)a |
| Positive | 5.71 (2.08–15.65)a |
| PORT | 2.42 (1.22–4.79) |
| Tumor size, cm | |
| ≤2.0 | 1 [Reference] |
| 2.1–4.0 | 0.96 (0.44–2.09) |
| >4.0 | 2.99 (1.30–6.90) |
Abbreviations: HR, hazard ratio; LRFS, local recurrence-free survival; PORT, postoperative radiotherapy.
Adjusted for tumor size and adjuvant therapy.
Figure 3. Incidence of Local Recurrence.
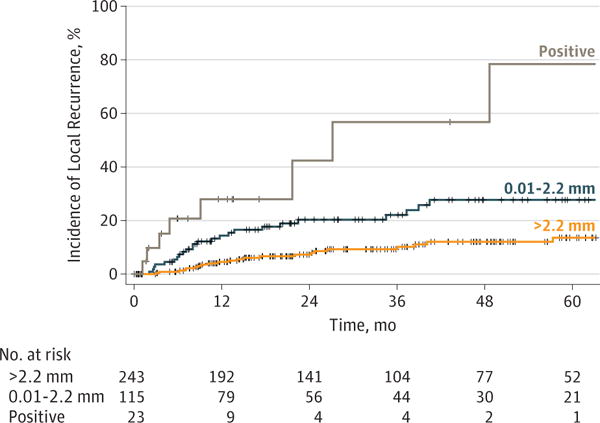
Analysis uses the margin cutoff of greater than 2.2 mm.
The median follow-up of surviving patients was 40 months (range, 0–150 months). At the median follow-up, overall survival among the entire group was 77.5%, DSS was 82.9%, and LRFS was 82.6%. Of the pathologic factors assessed, tumor size (HRfor 2.1–4.0 cm, 1.84; 95% CI, 0.97–3.51; HR for >4.0 cm, 6.17; 95% CI, 2.99–12.72), perineural invasion (HR, 2.24; 95% CI, 1.31– 3.85), nodal status (HR, 3.17; 95% CI, 1.82–5.55), and margin status (HR for 0.01–to 2.2-mm margin, 2.40; 95% CI, 1.38– 4.18; HR for positive margin, 7.67; 95% CI, 3.42–17.19) were significantly associated with local recurrence on univariate analysis (Table 3). When adjusting for these factors in a multivariate model, margin status was the variable most significantly associated with LRFS. A patient with a positive margin was approximately 6 times more likely to have a local recurrence when compared with a patient with a margin of greater than 2.2 mm, and the data suggest that patients with a positive margin could be as much as 13 times more likely to have a local recurrence (adjusted HR, 5.73; 95% CI, 2.45–13.41). Likewise, a patient with a margin clearance of 0.01 to 2.2 mm was approximately 2 times more likely to have a local recurrence when compared with a patient with a margin of greater than 2.2 mm, and the data suggest the increased risk could be greater than 3 times (adjusted HR, 2.00; 95% CI, 1.13–3.55).
Table 3.
Univariate Kaplan-Meier Analyses and Multivariate Cox Proportional Hazards Regression Analyses for LRFS
| Variablea | No. | HR (95% CI) | |
|---|---|---|---|
| Unadjusted | Adjustedb | ||
| Age, y | |||
| <60 | 212 | 1 [Reference] | NA |
| ≥60 | 169 | 1.20 (0.72–2.01) | NA |
| Tumor size, cmc | |||
| ≤2.0 | 270 | 1 [Reference] | NA |
| 2.1–4.0 | 79 | 1.84 (0.97–3.51) | 1.19 (0.59–2.43) |
| >4.0 | 31 | 6.17 (2.99–12.72) | 2.88 (1.19–6.97) |
| Grade | |||
| Well | 46 | 1 [Reference] | NA |
| Moderate | 303 | 1.89 (0.68–5.25) | NA |
| Poor | 32 | 3.78 (1.16–12.29) | NA |
| Perineural invasion | |||
| No | 276 | 1 [Reference] | 1 [Reference] |
| Yes | 105 | 2.24 (1.31–3.85) | 1.40 (0.76–2.58) |
| Vascular invasion | |||
| No | 343 | 1 [Reference] | NA |
| Yes | 38 | 1.46 (0.62–3.40) | NA |
| Pathologic N stage | |||
| pNO | 296 | 1 [Reference] | 1 [Reference] |
| pN + | 85 | 3.17 (1.82–5.55) | 1.89 (0.95–3.77) |
| Margin, mmd | |||
| >2.2 | 243 | 1 [Reference] | 1 [Reference] |
| 0.01–2.2 | 115 | 2.40 (1.38–4.18) | 2.00 (1.13–3.55) |
| Positive | 23 | 7.67 (3.42–17.19) | 5.73 (2.45–13.41) |
Abbreviations: HR, hazard ratio; LRFS, local recurrence-free survival; NA, not applicable.
Pattern of invasion, location of closest margin, and histologic subtype of carcinoma were not associated with local recurrence on univariate analysis.
Adjusted for tumor size, perineural invasion, pathologic N stage, and margin.
For 1 patient, tumor size was not assessable on pathologic review of the final resection (most of the tumor was removed with the initial biopsy).
On multivariate analysis, we found a statistically significant difference when comparing patients with a margin distance of greater than 2.2 mm with patients with a margin of 0.01 to 2.2 mm. Patients with a margin distance of 0.01 to 2.2 mm had a statistically significant difference compared with patients with positive margins. In addition, patients with positive margins had a statistically significant difference compared with patients with margins of greater than 2.2 mm.
Discussion
Our data show that the optimal margin cutoff associated with local recurrence in the group was 2.2 mm. The risk for local recurrence in the group of patients with a margin of 2.3 to 5.0 mm and the group with the traditionally accepted margin greater than 5.0 mm was not significantly different when looking at LRFS, even when adjusting for tumor size and adjuvant therapy. A positive margin or a margin ranging from 0.01 to 2.2 mm were strongly associated with a higher rate of local recurrence compared with a margin of greater than 2.2 mm.
Two prospective, randomized clinical trials13,14 that are used in the decision making about adjuvant therapy for SCC of the head and neck included margin status as their selection criteria. The Radiation Therapy Oncology Group (RTOG) 9501 study13 only included patients with positive margins, whereas the European Organization for Research and Treatment of Cancer Trial (EORTC) 22931 study14 also included patients with a close margin, defined as less than 5.0 mm. Although tumors originating at different sites behave very differently,15 both studies included patients with SCC of the oral cavity, oropharynx, hypopharynx, and larynx. Less than 30% of the patients enrolled in these trials had cancer of the oral cavity (RTOG 9501, 112 [26.9%] of the 416 patients; EORTC 22931, 87 [26.0%] of the 334 patients). Only 6% of the patients enrolled in the RTOG 9501 trial and 13% of the patients in the EORTC 22931 trial had margins alone as the high-risk feature for adjuvant therapy.16 The results of these 2 trials are, therefore, not generalizable to patients with cancer of the oral cavity who have close or positive margins as their only risk factor.
Although the distance between the edge of tumor and the closest resection margin is used as an important factor in deciding an adjuvant treatment of head and neck cancer, this number is a variable that is subject to various influences, including imprecise measurements. Our data showed that 23 (6.0%) of our patients with SCCOT primarily treated with surgical resection had positive margins, and 115 (30.2%) had a margin of 0.01 to 2.2 mm. This observation may be related to the influence of the extent of the surgical resection and to the way that the specimen is handled, oriented, and processed. Another factor that can influence the margin distance after resection is the shrinkage of normal tissues, and tissues from different anatomical sites can present different shrinkage rates.17 The instrument used to assess tumor clearance can also affect measurement and generate interobserver variability. In our experience, the oculometer is the most precise tool with the most reproducible measurements to assess tumor clearance. One of the major challenges in surgical oncology is to find a balance between the extent of resection to maximize local control and the maximal conservation of normal tissues to preserve function. Interpretation of the extent of normal tissue removal is therefore of significant interest, especially in management of SCCOT.
Limitations
Most patients included in the study had T1 or T2 tumors. Only 49 patients (12.9%) had T3 and T4 tumors. Therefore, we could argue that our observations regarding close margins are less relevant in patients with larger T stage tumors. However, most patients with T3 or T4 tumors would be treated with adjuvant RT irrespective of close margin status. Consequently, on a practical basis, a close margin diagnosis has more potential to influence therapy decisions in early-stage tumors and would, in any case, be less relevant for management of T3 or T4 tumors. This study is also subject to the other limitations of any retrospective study, and our goal is not to advocate for more conservative resections based on these data. Instead, our results highlight the need for a more rational definition and interpretation of surgical margins. A randomized prospective trial to test the hypothesis of close margins as prognostic of local recurrence in SCCOT would be the best way to answer this question. However, the results of such a trial would be difficult to interpret for many reasons, including the great variability in surgical technique and specimen processing. Current modalities for intraoperative assessment of margins, including frozen section analysis, are not reliable.18 Chang et al19 studied different methods of sampling the margins and their effect on local recurrence in patients with pT1 pNO and pT2 pNO SCCOT. Their group concluded that the status of the glossectomy specimen margins was prognostically more relevant than that of tumor bed margins and that relying on tumor bed margins can be associated with worse local control. The influence of technique of margin assessment was also reported in a recent study from Israel.20 That prospective, randomized clinical trial concluded that the rate of final negative margins using specimen-driven intraoperative margin assessment was significantly higher than that using patient-driven margin assessment.
With the development of new technologies, such as high-resolution microendoscopy,21 reflectance confocal microscopy,22 and targeted optical imaging agents,23 more accurate methods will be available to confirm that no cancer cells remain after the tumor resection. Real-time intraoperative delineation of tumor vs normal tissue will enable complete resection oftumors, with maximal function and aesthetic preservation. Until technological capabilities catchup to this ideal goal, the prognostic significance of a close margin needs to be interpreted cautiously and subjected to further scrutiny.
Conclusions
In this study cohort of patients with SCCOT, LRFS was significantly affected only with surgical margins of less than or equal to 2.2 mm. The 2.2-mm cutoff for margins stratifies the risk for local recurrence in our cohort better than the arbitrary cutoff of less than 5.0 mm that has been used.
Key Points.
Question Does the arbitrarily defined close resection margin of less than 5.0 mm portend as high a risk for recurrence as a positive margin after resection of squamous cell carcinoma of the oral tongue?
Findings In this study, patients with margins of 2.3 to 5.0 mm had similar local recurrence-free survival as patients with margins of greater than 5.0 mm. Based on this result, negative margins were redefined as those with a clearance of greater than 2.2 mm.
Meaning The optimal margin cutoff associated with local recurrence of squamous cell carcinoma of the oral tongue may be 2.2 mm.
Acknowledgments
Funding/Support: This study was supported in part through cancer center support grant P30 CA008748 from the National CancerInstitute, National Institutes of Health.
Role of the Funder/Sponsor: The study sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Drs Ghossein and Patel contributed equally to this work. Ms Migliacci had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Zanoni, Migliacci, Montero, Shah, Ghossein, Patel.
Acquisition, analysis, or interpretation of data: Zanoni, Migliacci, Xu, Katabi, Montero, Ganly, Wong, Ghossein, Patel.
Drafting of the manuscript: Zanoni, Migliacci, Montero, Ghossein, Patel.
Critical revision of the manuscript for important intellectual content: Zanoni, Migliacci, Xu, Katabi, Ganly, Shah, Wong, Patel.
Statistical analysis: Zanoni, Migliacci, Montero, Ganly.
Administrative, technical, or material support: Migliacci, Montero, Ghossein, Patel.
Study supervision: Ganly, Shah, Ghossein, Patel.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
Contributor Information
Daniella Karassawa Zanoni, Head and Neck Service, Department of Surgery, Memorial Sloan Kettering Cancer Center, New York, New York.
Jocelyn C. Migliacci, Head and Neck Service, Department of Surgery, Memorial Sloan Kettering Cancer Center, New York, New York.
Bin Xu, Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, New York.
Nora Katabi, Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, New York.
Pablo H. Montero, Head and Neck Service, Department of Surgery, Memorial Sloan Kettering Cancer Center, New York, New York.
Ian Ganly, Head and Neck Service, Department of Surgery, Memorial Sloan Kettering Cancer Center, New York, New York.
Jatin P Shah, Head and Neck Service, Department of Surgery, Memorial Sloan Kettering Cancer Center, New York, New York.
Richard J. Wong, Head and Neck Service, Department of Surgery, Memorial Sloan Kettering Cancer Center, New York, New York.
Ronald A. Ghossein, Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, New York.
Snehal G. Patel, Head and Neck Service, Department of Surgery, Memorial Sloan Kettering Cancer Center, New York, New York.
References
- 1.Looser KGSJ, Shah JP, Strong EW. The significance of “positive” margins in surgically resected epidermoid carcinomas. Head Neck Surg. 1978;1(2):107–111. doi: 10.1002/hed.2890010203. [DOI] [PubMed] [Google Scholar]
- 2.Sutton DN, Brown JS, Rogers SN, Vaughan ED, Woolgar JA. The prognostic implications of the surgical margin in oral squamous cell carcinoma. Int J Oral Maxillofac Surg. 2003;32(1):30–34. doi: 10.1054/ijom.2002.0313. [DOI] [PubMed] [Google Scholar]
- 3.Ganly I, Patel S, Shah J. Early stage squamous cell cancer of the oral tongue—clinicopathologic features affecting outcome. Cancer. 2012;118(1):101–111. doi: 10.1002/cncr.26229. [DOI] [PubMed] [Google Scholar]
- 4.Alicandri-Ciufelli M, Bonali M, Piccinini A, et al. Surgical margins in head and neck squamous cell carcinoma: what is ‘close’? Eur Arch Otorhinolaryngol. 2013;270(10):2603–2609. doi: 10.1007/s00405-012-2317-8. [DOI] [PubMed] [Google Scholar]
- 5.Wong LS, McMahon J, Devine J, et al. Influence of close resection margins on local recurrence and disease-specific survival in oral and oropharyngeal carcinoma. Br J Oral Maxillofac Surg. 2012;50(2):102–108. doi: 10.1016/j.bjoms.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Liao CT, Chang JT, Wang HM, et al. Analysis of risk factors of predictive local tumor control in oral cavity cancer. Ann Surg Oncol. 2008;15(3):915–922. doi: 10.1245/s10434-007-9761-5. [DOI] [PubMed] [Google Scholar]
- 7.Dik EA, Willems SM, Ipenburg NA, Adriaansens SO, Rosenberg AJ, van Es RJ. Resection of early oral squamous cell carcinoma with positive or close margins: relevance of adjuvant treatment in relation to local recurrence: margins of 3 mm as safe as 5 mm. Oral Oncol. 2014;50(6):611–615. doi: 10.1016/j.oraloncology.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 8.Barry CP, Ahmed F, Rogers SN, et al. Influence of surgical margins on local recurrence in T1/T2 oral squamous cell carcinoma. Head Neck. 2015;37(8):1176–1180. doi: 10.1002/hed.23729. [DOI] [PubMed] [Google Scholar]
- 9.Ch’ng S, Corbett-Burns S, Stanton N, et al. Close margin alone does not warrant postoperative adjuvant radiotherapy in oral squamous cell carcinoma. Cancer. 2013;119(13):2427–2437. doi: 10.1002/cncr.28081. [DOI] [PubMed] [Google Scholar]
- 10.Li Y, Bai S, Carroll W, et al. Validation of the risk model: high-risk classification and tumor pattern of invasion predict outcome for patients with low-stage oral cavity squamous cell carcinoma. Head Neck Pathol. 2013;7(3):211–223. doi: 10.1007/s12105-012-0412-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wreesmann VB, Katabi N, Palmer FL, et al. Influence of extracapsular nodal spread extent on prognosis of oral squamous cell carcinoma. Head Neck. 2016;38(suppl 1):E1192–E1199. doi: 10.1002/hed.24190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics. 2000;56(2):337–344. doi: 10.1111/j.0006-341x.2000.00337.x. [DOI] [PubMed] [Google Scholar]
- 13.Cooper JS, Pajak TF, Forastiere AA, et al. Radiation Therapy Oncology Group 9501/Intergroup Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350(19):1937–1944. doi: 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 14.Bernier J, Domenge C, Ozsahin M, et al. European Organization for Research and Treatment of Cancer Trial 22931 Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350(19):1945–1952. doi: 10.1056/NEJMoa032641. [DOI] [PubMed] [Google Scholar]
- 15.Berrino F, Gatta G, EUROCARE Working Group Variation in survival of patients with head and neck cancer in Europe by the site of origin of the tumours. Eur J Cancer. 1998;34(14 Spec):2154–2161. doi: 10.1016/s0959-8049(98)00328-1. [DOI] [PubMed] [Google Scholar]
- 16.Bernier J, Cooper JS, Pajak TF, et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (#9501) Head Neck. 2005;27(10):843–850. doi: 10.1002/hed.20279. [DOI] [PubMed] [Google Scholar]
- 17.Johnson RE, Sigman JD, Funk GF, Robinson RA, Hoffman HT. Quantification of surgical margin shrinkage in the oral cavity. Head Neck. 1997;19(4):281–286. doi: 10.1002/(sici)1097-0347(199707)19:4<281::aid-hed6>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 18.Black C, Marotti J, Zarovnaya E, Paydarfar J. Critical evaluation of frozen section margins in head and neck cancer resections. Cancer. 2006;107(12):2792–2800. doi: 10.1002/cncr.22347. [DOI] [PubMed] [Google Scholar]
- 19.Chang AM, Kim SW, Duvvuri U, et al. Early squamous cell carcinoma of the oral tongue: comparing margins obtained from the glossectomy specimen to margins from the tumor bed. Oral Oncol. 2013;49(11):1077–1082. doi: 10.1016/j.oraloncology.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 20.Amit M, Na’ara S, Leider-Trejo L, et al. Improving the rate of negative margins after surgery for oral cavity squamous cell carcinoma: a prospective randomized controlled study. Head Neck. 2016;38(suppl1):E1803–E1809. doi: 10.1002/hed.24320. [DOI] [PubMed] [Google Scholar]
- 21.Miles BA, Patsias A, Quang T, Polydorides AD, Richards-Kortum R, Sikora AG. Operative margin control with high-resolution optical microendoscopy for head and neck squamous cell carcinoma. Laryngoscope. 2015;125(10):2308–2316. doi: 10.1002/lary.25400. [DOI] [PubMed] [Google Scholar]
- 22.Contaldo M, Poh CF, Guillaud M, et al. Oral mucosa optical biopsy by a novel handheld fluorescent confocal microscope specifically developed: technologic improvements and future prospects. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116(6):752–758. doi: 10.1016/j.oooo.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Kossatz S, Brand C, Gutiontov S, et al. Detection and delineation of oral cancer with a PARP1 targeted optical imaging agent. Sci Rep. 2016;6:21371. doi: 10.1038/srep21371. [DOI] [PMC free article] [PubMed] [Google Scholar]


