Abstract
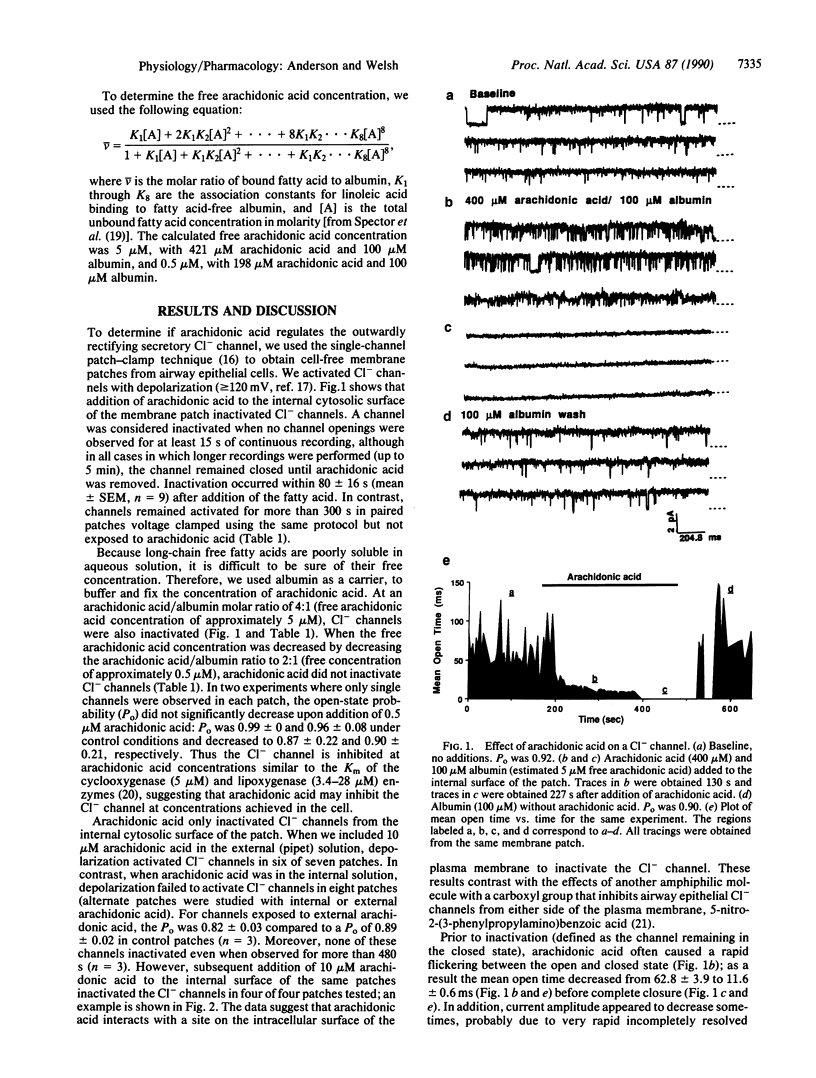
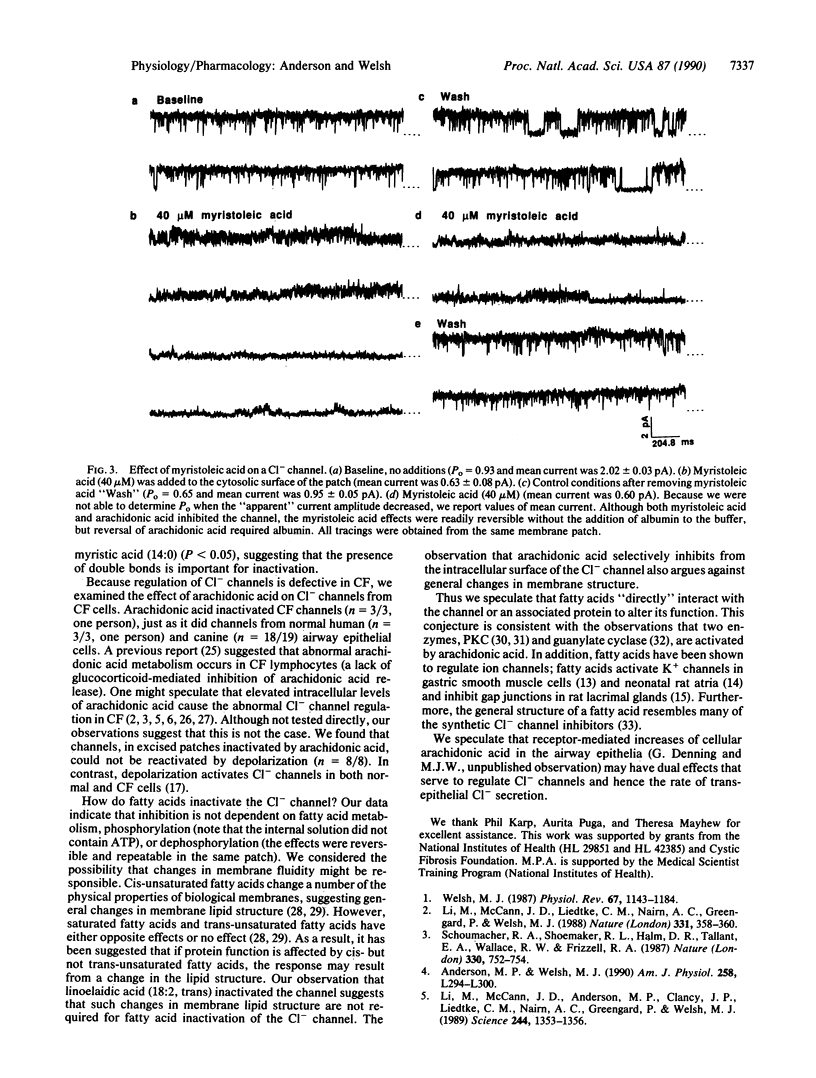
Apical membrane Cl- channels control the rate of transepithelial Cl- secretion in airway epithelia. cAMP-dependent protein kinase and protein kinase C regulate Cl- channels by phosphorylation; in cystic fibrosis cells, phosphorylation-dependent activation of Cl- channels is defective. Another important signaling system involves arachidonic acid, which is released from cell membranes during receptor-mediated stimulation. Here we report that arachidonic acid reversibly inhibited apical membrane Cl- channels in cell-free patches of membrane. Arachidonic acid itself inhibited the channel and not a cyclooxygenase or lipoxygenase metabolite because (i) inhibitors of these enzymes did not block the response, (ii) fatty acids that are not substrates for the enzymes had the same effect as arachidonic acid, and (iii) metabolites of arachidonic acid did not inhibit the channel. Inhibition occurred only when fatty acids were added to the cytosolic surface of the membrane patch. Unsaturated fatty acids were more potent than saturated fatty acids. Arachidonic acid inhibited Cl- channels from both normal and cystic fibrosis cells. These results suggest that fatty acids directly inhibit apical membrane Cl- channels in airway epithelial cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. P., Welsh M. J. Isoproterenol, cAMP, and bradykinin stimulate diacylglycerol production in airway epithelium. Am J Physiol. 1990 Jun;258(6 Pt 1):L294–L300. doi: 10.1152/ajplung.1990.258.6.L294. [DOI] [PubMed] [Google Scholar]
- Carlstedt-Duke J., Brönnegård M., Strandvik B. Pathological regulation of arachidonic acid release in cystic fibrosis: the putative basic defect. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9202–9206. doi: 10.1073/pnas.83.23.9202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill L., Chilton F. H., Resau J. H., Bascom R., Hubbard W. C., Proud D. Cyclooxygenase metabolism of endogenous arachidonic acid by cultured human tracheal epithelial cells. Am Rev Respir Dis. 1989 Aug;140(2):449–459. doi: 10.1164/ajrccm/140.2.449. [DOI] [PubMed] [Google Scholar]
- Clancy J. P., McCann J. D., Li M., Welsh M. J. Calcium-dependent regulation of airway epithelial chloride channels. Am J Physiol. 1990 Feb;258(2 Pt 1):L25–L32. doi: 10.1152/ajplung.1990.258.2.L25. [DOI] [PubMed] [Google Scholar]
- Eling T. E., Danilowicz R. M., Henke D. C., Sivarajah K., Yankaskas J. R., Boucher R. C. Arachidonic acid metabolism by canine tracheal epithelial cells. Product formation and relationship to chloride secretion. J Biol Chem. 1986 Sep 25;261(27):12841–12849. [PubMed] [Google Scholar]
- Frizzell R. A., Rechkemmer G., Shoemaker R. L. Altered regulation of airway epithelial cell chloride channels in cystic fibrosis. Science. 1986 Aug 1;233(4763):558–560. doi: 10.1126/science.2425436. [DOI] [PubMed] [Google Scholar]
- Giaume C., Randriamampita C., Trautmann A. Arachidonic acid closes gap junction channels in rat lacrimal glands. Pflugers Arch. 1989 Jan;413(3):273–279. doi: 10.1007/BF00583541. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Holtzman M. J., Aizawa H., Nadel J. A., Goetzl E. J. Selective generation of leukotriene B4 by tracheal epithelial cells from dogs. Biochem Biophys Res Commun. 1983 Aug 12;114(3):1071–1076. doi: 10.1016/0006-291x(83)90671-x. [DOI] [PubMed] [Google Scholar]
- Hunter J. A., Finkbeiner W. E., Nadel J. A., Goetzl E. J., Holtzman M. J. Predominant generation of 15-lipoxygenase metabolites of arachidonic acid by epithelial cells from human trachea. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4633–4637. doi: 10.1073/pnas.82.14.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang T. C., Lu L., Zeitlin P. L., Gruenert D. C., Huganir R., Guggino W. B. Cl- channels in CF: lack of activation by protein kinase C and cAMP-dependent protein kinase. Science. 1989 Jun 16;244(4910):1351–1353. doi: 10.1126/science.2472005. [DOI] [PubMed] [Google Scholar]
- Kim D., Clapham D. E. Potassium channels in cardiac cells activated by arachidonic acid and phospholipids. Science. 1989 Jun 9;244(4909):1174–1176. doi: 10.1126/science.2727703. [DOI] [PubMed] [Google Scholar]
- Kim D., Lewis D. L., Graziadei L., Neer E. J., Bar-Sagi D., Clapham D. E. G-protein beta gamma-subunits activate the cardiac muscarinic K+-channel via phospholipase A2. Nature. 1989 Feb 9;337(6207):557–560. doi: 10.1038/337557a0. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Kleinfeld A. M., Hoover R. L., Karnovsky M. J. Lipid domains in membranes. Evidence derived from structural perturbations induced by free fatty acids and lifetime heterogeneity analysis. J Biol Chem. 1980 Feb 25;255(4):1286–1295. [PubMed] [Google Scholar]
- Kurachi Y., Ito H., Sugimoto T., Shimizu T., Miki I., Ui M. Arachidonic acid metabolites as intracellular modulators of the G protein-gated cardiac K+ channel. Nature. 1989 Feb 9;337(6207):555–557. doi: 10.1038/337555a0. [DOI] [PubMed] [Google Scholar]
- Leikauf G. D., Ueki I. F., Nadel J. A., Widdicombe J. H. Bradykinin stimulates Cl secretion and prostaglandin E2 release by canine tracheal epithelium. Am J Physiol. 1985 Jan;248(1 Pt 2):F48–F55. doi: 10.1152/ajprenal.1985.248.1.F48. [DOI] [PubMed] [Google Scholar]
- Li M., McCann J. D., Anderson M. P., Clancy J. P., Liedtke C. M., Nairn A. C., Greengard P., Welsch M. J. Regulation of chloride channels by protein kinase C in normal and cystic fibrosis airway epithelia. Science. 1989 Jun 16;244(4910):1353–1356. doi: 10.1126/science.2472006. [DOI] [PubMed] [Google Scholar]
- Li M., McCann J. D., Liedtke C. M., Nairn A. C., Greengard P., Welsh M. J. Cyclic AMP-dependent protein kinase opens chloride channels in normal but not cystic fibrosis airway epithelium. Nature. 1988 Jan 28;331(6154):358–360. doi: 10.1038/331358a0. [DOI] [PubMed] [Google Scholar]
- Li M., McCann J. D., Welsh M. J. Apical membrane Cl- channels in airway epithelia: anion selectivity and effect of an inhibitor. Am J Physiol. 1990 Aug;259(2 Pt 1):C295–C301. doi: 10.1152/ajpcell.1990.259.2.C295. [DOI] [PubMed] [Google Scholar]
- McCann J. D., Bhalla R. C., Welsh M. J. Release of intracellular calcium by two different second messengers in airway epithelium. Am J Physiol. 1989 Aug;257(2 Pt 1):L116–L124. doi: 10.1152/ajplung.1989.257.2.L116. [DOI] [PubMed] [Google Scholar]
- McCann J. D., Welsh M. J. Neuroleptics antagonize a calcium-activated potassium channel in airway smooth muscle. J Gen Physiol. 1987 Feb;89(2):339–352. doi: 10.1085/jgp.89.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhail L. C., Clayton C. C., Snyderman R. A potential second messenger role for unsaturated fatty acids: activation of Ca2+-dependent protein kinase. Science. 1984 May 11;224(4649):622–625. doi: 10.1126/science.6231726. [DOI] [PubMed] [Google Scholar]
- Naor Z., Shearman M. S., Kishimoto A., Nishizuka Y. Calcium-independent activation of hypothalamic type I protein kinase C by unsaturated fatty acids. Mol Endocrinol. 1988 Nov;2(11):1043–1048. doi: 10.1210/mend-2-11-1043. [DOI] [PubMed] [Google Scholar]
- Needleman P., Turk J., Jakschik B. A., Morrison A. R., Lefkowith J. B. Arachidonic acid metabolism. Annu Rev Biochem. 1986;55:69–102. doi: 10.1146/annurev.bi.55.070186.000441. [DOI] [PubMed] [Google Scholar]
- Ordway R. W., Walsh J. V., Jr, Singer J. J. Arachidonic acid and other fatty acids directly activate potassium channels in smooth muscle cells. Science. 1989 Jun 9;244(4909):1176–1179. doi: 10.1126/science.2471269. [DOI] [PubMed] [Google Scholar]
- Schoumacher R. A., Shoemaker R. L., Halm D. R., Tallant E. A., Wallace R. W., Frizzell R. A. Phosphorylation fails to activate chloride channels from cystic fibrosis airway cells. Nature. 1987 Dec 24;330(6150):752–754. doi: 10.1038/330752a0. [DOI] [PubMed] [Google Scholar]
- Spector A. A., Fletcher J. E., Ashbrook J. D. Analysis of long-chain free fatty acid binding to bovine serum albumin by determination of stepwise equilibrium constants. Biochemistry. 1971 Aug 17;10(17):3229–3232. doi: 10.1021/bi00793a011. [DOI] [PubMed] [Google Scholar]
- Wallach D., Pastan I. Stimulation of guanylate cyclase of fibroblasts by free fatty acids. J Biol Chem. 1976 Sep 25;251(18):5802–5809. [PubMed] [Google Scholar]
- Wangemann P., Wittner M., Di Stefano A., Englert H. C., Lang H. J., Schlatter E., Greger R. Cl(-)-channel blockers in the thick ascending limb of the loop of Henle. Structure activity relationship. Pflugers Arch. 1986;407 (Suppl 2):S128–S141. doi: 10.1007/BF00584942. [DOI] [PubMed] [Google Scholar]
- Welsh M. J. Electrolyte transport by airway epithelia. Physiol Rev. 1987 Oct;67(4):1143–1184. doi: 10.1152/physrev.1987.67.4.1143. [DOI] [PubMed] [Google Scholar]
- Welsh M. J., Li M., McCann J. D. Activation of normal and cystic fibrosis Cl- channels by voltage, temperature, and trypsin. J Clin Invest. 1989 Dec;84(6):2002–2007. doi: 10.1172/JCI114391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh M. J., Liedtke C. M. Chloride and potassium channels in cystic fibrosis airway epithelia. 1986 Jul 31-Aug 6Nature. 322(6078):467–470. doi: 10.1038/322467a0. [DOI] [PubMed] [Google Scholar]


