Abstract
The opportunistic fish pathogen, Enterococcus faecalis has been reported to cause mass mortality in several fish species in different countries. The objectives of this study were to (i) identify E. faecalis from the diseased fishes through molecular techniques; (ii) assess the antibiotic susceptibility profile of E. faecalis isolates; and (iii) control disease in tilapia fish by treatment with medicinal plant extracts. A total of 48 isolates were phenotypically identified as Enterococcus species from tilapia, stinging catfish and walking catfish cultivated in several fish farms in Gazipur. Ten randomly selected isolates were identified as E. faecalis by 16S rRNA gene sequencing. Artificial infection revealed that most of the isolates caused moderate to high mortality in fishes with characteristic disease symptoms. These isolates exhibited resistance to multiple antibiotics in vitro. Bioassay revealed that organic extracts of Tamarindus indica and Emblica officinalis leaves, Allium sativum bulb, and Syzygium aromaticum bud inhibited the growth of E. faecalis. Methanol extracts of A. sativum and methanol and acetone extracts of S. aromaticum significantly reduced the mortality of fish artificially infected with E. faecalis as both preventive and therapeutic agents. This is the first report on molecular identification, and herbal control of fish pathogenic E. faecalis in Bangladesh.
Introduction
Bacteria are the leading causative agents of diseases in freshwater fishes all over the world1. Aeromonas, Edwardsiella, Pseudomonas, Flavobacterium, Vibrio and Streptococcus are major genera of fish pathogens causing diseases in different tropical freshwater fishes2. In recent years, some opportunistic bacterial fish pathogens have been identified as the causal agents for severe outbreaks in aquaculture facilities. Among them, Enterococcus sp. has emerged as one of the important fish pathogens, which severely impacts aquaculture practices worldwide3. The incidence of fish diseases caused by Enterococcus sp. was first reported in Yellow tail (Seriola quinqueradiata) in Japan4 and then in Turbot (Scophthalmus maximus)5 and tilapia(Oreochromis niloticus)6. E. faecalis has been reported as a pathogen causing streptococcal infection in tilapia in lakes of Egypt, and Thailand7–9. In Bangladesh, Enterococcus sp. is often isolated from both healthy and infected fish10–12. Bangladesh is ranked fifth among the inland aquaculture producing countries in the world13. However, no studies have so far been conducted on the pathological involvement of E. faecalis in aquaculture in Bangladesh.
Antibiotic resistance is a great concern in the management of bacterial diseases worldwide. Enterococcus shows resistance against a wide range of antibiotics14. However, no information is available on the antibiotic susceptibility profile of E. faecalis isolated from the diseased fish in Bangladesh. As antibiotic resistance is a growing concern for management of bacterial diseases, alternative disease management strategies are needed. Extracts of medicinal plants exhibit antibacterial activities against human, plant, and fish pathogens15. Bangladesh is rich in diverse traditional medicinal plants16. However, no study has so far been conducted on management of fish diseases caused by E. faecalis using medicinal plant extracts from Bangladesh. Therefore, the objectives of this study were to (i) identify E. faecalis from the diseased fishes through molecular techniques; (ii) assess the antibiotic susceptibility profile in isolated fish pathogenic E. faecalis; and (iii) control of disease in O. niloticus fish by treatment with medicinal plant extracts.
Results
Isolation and Identification of Bacteria from Infected Fish
A total of 48 bacterial strains were isolated from the infected tilapia and catfish. Morphological studies revealed that all of these isolates gave small to medium sized, circular, smooth and raised colonies on KF Streptococcal agar plates. All of them formed dark red colored colonies on KF Streptococcal agar media but formed creamy transparent colored colonies when grown on nutrient agar media. All isolates were Gram positive, cocci, non-motile, catalase and oxidase negative, D-glucose fermentative, and methyl red, Voges-Proskauer and indole positive. They grew in the presence of 6.5% NaCl, 40% bile salts, 0.1% methylene blue milk at pH 9.6 and at 10 °C and 45 °C (Table 1). Based on the morphological, physiological and biochemical characteristics, all the isolates were tentatively identified as Enterococcus species. Among them, 10 isolates were randomly selected for further molecular, pathological, antibiotic susceptibility and herbal disease control studies.
Table 1.
Morphological, physiological and biochemical characters of 48 E. faecalis isolates collected from infected fishes in Bangladesh.
| Test Type | Tests | Characteristics |
|---|---|---|
| Colony characters | Size | M |
| Type | R | |
| Color | Dark red | |
| Shape | C | |
| Morphological character | Shape | Cocci |
| Physiological characters | Motility | − |
| Growth at 10 °C | + | |
| Growth at 45 °C | + | |
| Growth in 40% bile salt | + | |
| Growth in 0.1% methylene blue milk at pH 9.6 | + | |
| Growth in 6.5% NaCl | + | |
| Biochemical characters | Gram’s staining | + |
| Catalase | − | |
| Oxidase | − | |
| Oxidative-Fermentative | F | |
| Methyl Red | + | |
| Voges-Proskauer | + | |
| Indole | + |
M: medium; R: round; C = convex; F: Fermentative; +: positive; − : negative.
The 16S rRNA gene sequence data of the ten selected isolates exhibited 99.62 to 99.93% homology with E. faecalis strain ATCC 19433. The sequences of the isolates have been deposited to the NCBI GenBank. The source of isolation, sequence homology with E. faecalis strain ATCC 19433 and NCBI GenBank accession number are presented in Supplementary Table S1.
Molecular Detection of E. faecalis by Specific Primer
Amplification of a 1022 bp E. faecalis specific PCR product was obtained in the present study by combination of the E. faecalis specific forward primer EfacF1 and a universal reverse primer 1492R (Fig. 1). The EfacF1 and universal 1492R primer set amplified E. faecalis isolates only.
Figure 1.
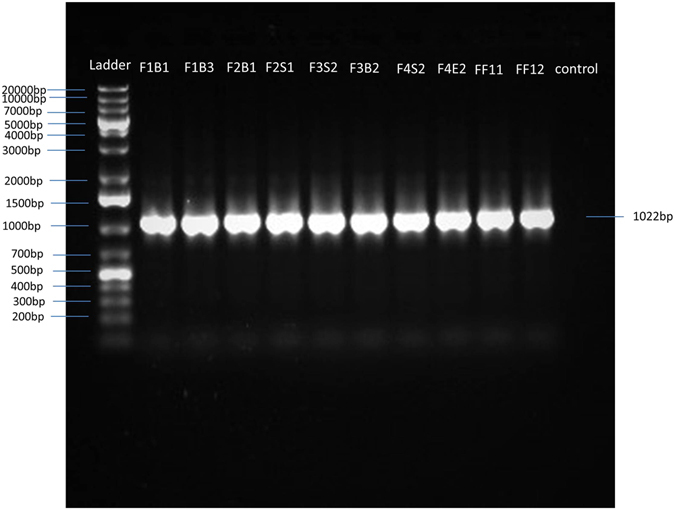
PCR amplification of E. faecalis isolates with specific forward (Efac F1) and universal reverse (1492R) primer. Ladder, 1 kb plus DNA ladder.
In vivo Infection Model of the Isolated E. faecalis
To observe whether E. faecalis isolates were pathogenic to fish, we conducted an artificial challenge study under laboratory conditions. All 10 tested E. faecalis isolates produced disease symptoms in artificially infected tilapia (O. niloticus) except for isolate F4S2. In O. niloticus, the first clinical sign appeared within 24 hours followed by the death of fish within 72 hours. Major clinical symptoms observed were corneal opacity combined with uni- or bi-lateral exophthalmia, erosion in tail followed by hemorrhage under pelvic fin and signs of asphyxiation (Fig. 2). Among the E. faecalis isolates, five (F1B1, F2B1, F2S1, F3S2 and FF11), two (F1B3 and F4E2), two (F3B2 and FF12) and one isolate (F4S2) were high, moderate, weak and avirulent, respectively (Fig. 3). We recovered the same bacterial isolates from the abdomen, brain, and tail of artificially challenged fish, and identified them as E. faecalis based on their phenotypic characteristics.
Figure 2.

Artificially infected fish with E. faecalis isolate expressing distinct disease symptoms. (a) Control; (b) Swollen abdomen and hemorrhages at the base of pelvic fins; (c) Bilateral opacity and sign of asphyxiation; (d) Erosion in caudal fin.
Figure 3.
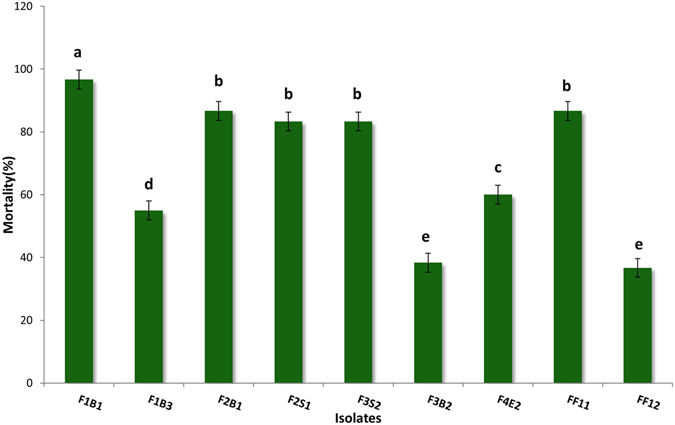
Mortality of tilapia fish (Oreochromis niloticus) exposed to fish pathogenic E. faecalis isolates in the laboratory conditions. One way ANOVA was performed for analyzing the data of three replicated experiment and data in column varies significantly in LSD at p ≤ 0.05. Different letter bars indicates significant variations in mortality of fish in different groups by the E. faecalis isolates at p ≤ 0.05.
Antibiotic Susceptibility Profile
To find out whether the fish pathogenic E. faecalis isolates had resistance against commercial antibiotics, we screened them against 11 antibiotics using disk diffusion assay. Surprisingly, all of the E. faecalis isolates displayed resistance to multiple antibiotics viz., amoxycillin, ampicillin, cefradine, cefuroxime, erythromycin and penicillin-G (Table 2). However, these isolates exhibited varying levels of susceptibility to nitrofurantoin, azithromycin, gentamycin, levofloxacin, and vancomycin (Fig. 4).
Table 2.
Antibiotic susceptibility profile of fish pathogenic E. faecalis isolates from infected fishes in Bangladesh.
| Inhibition zone ratio for tested antibiotics | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Isolates | Amoxycillin (AMX) | Ampicillin (AMP) | Azithromycin (AZM) | Cefradine (CH) | Cefuroxime (CXM) | Erythromycin (E) | Gentamicin (GEN) | Levofloxacin (LE) | Nitrofurantoin (NIT) | Penicillin-G (P) | Vancomycin (VA) |
| F1B1 | R | R | 2.3 ± 0.0 | 2.2 ± 0.1 | R | R | 3.3 ± 0.1 | 3.4 ± 0.0 | 3.1 ± 0.1 | R | 2.2 ± 0.1 |
| F1B3 | R | R | 2.3 ± 0.1 | 2.2 ± 0.1 | R | R | 3.3 ± 0.0 | 3.4 ± 0.1 | 3.1 ± 0.0 | R | 2.1 ± 0.0 |
| F2B1 | R | R | 2.5 ± 0.2 | 2.1 ± 0.1 | R | R | 3.2 ± 0.0 | 3.5 ± 0.0 | 3.1 ± 0.1 | R | 2.3 ± 0.1 |
| F2S1 | R | R | 2.7 ± 0.2 | 2.2 ± 0.1 | R | R | 3.1 ± 0.0 | 3.5 ± 0.1 | 3.2 ± 0.1 | R | 2.2 ± 0.1 |
| F3S2 | R | R | 2.3 ± 0.1 | 2.1 ± 0.1 | R | R | 3.4 ± 0.1 | 3.4 ± 0.1 | 3.2 ± 0.1 | R | 2.1 ± 0.0 |
| F3B2 | R | R | 2.2 ± 0.1 | 2.4 ± 0.0 | R | R | 3.3 ± 0.1 | 3.2 ± 0.1 | 3.0 ± 0.0 | R | 2.2 ± 0.1 |
| F4S2 | R | R | 2.3 ± 0.0 | 2.2 ± 0.1 | R | R | 3.2 ± 0.0 | 3.2 ± 0.1 | 3.1 ± 0.0 | R | 2.2 ± 0.1 |
| F4E2 | R | R | 2.0 ± 0.0 | 2.2 ± 0.1 | R | R | 3.1 ± 0.1 | 3.5 ± 0.0 | 3.0 ± 0.0 | R | 2.1 ± 0.0 |
| FF11 | R | R | 2.2 ± 0.1 | 2.2 ± 0.1 | R | R | 3.3 ± 0.1 | 3.5 ± 0.1 | 3.2 ± 0.0 | R | 2.3 ± 0.0 |
| FF12 | R | R | 2.2 ± 0.1 | 2.0 ± 0.0 | R | R | 3.2 ± 0.1 | 3.2 ± 0.0 | 3.2 ± 0.1 | R | 2.4 ± 0.0 |
Erythromycin (15 µg disk−1), Penicillin (10 µg disk−1), Amoxycillin (30 µg disk−1), Vancomycin (30 µg disk−1), Ampicillin (25 µg disk−1), Levofloxacin (5 µg disk−1), Cefuroxime (30 µg disk−1), Azithromycin (30 µg disk−1), Nitrofurantoin (30 µg disk−1), Cefradine (25 µg disk−1), Gentamicin (10 µg disk−1), R = Resistant. Disk diameter is 6.0 mm. Data are presented as Mean ± SE (n = 3).
Figure 4.
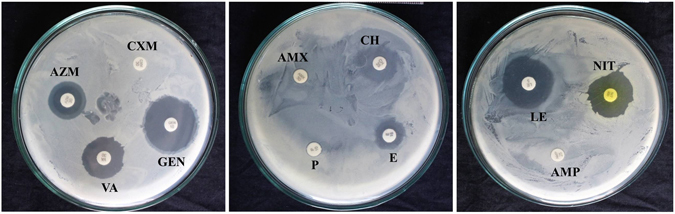
Antibiotic susceptibility profile of fish pathogenic E. faecalis FF11 against commercially available antibiotics.
In Vitro Inhibitory Effects of Plant Extracts
To see whether plant extracts can inhibit the growth of fish pathogenic E. faecalis isolates, both aqueous and polar extracts of 23 medicinal plants were screened in vitro. Out of the 23 aqueous extracts tested, the extracts of Tamarindus indica and Emblica officinalis leaves, Allium sativum bulb, and Syzygium aromaticum buds remarkably inhibited the growth of E. faecalis (Table 3). Among the active extracts, T. indica and E. officinalis exhibited low to moderate antibacterial activity with bacteriostatic effects against all E. faecalis isolates tested. A. sativum inhibited the bacterial growth with high zones of inhibition with a bacteriostatic effect. The crude aqueous extracts of S. aromaticum resulted moderate zone of inhibition in disk diffusion assay with bactericidal activity against all E. faecalis isolates.
Table 3.
In vitro inhibitory activities of disk containing aqueous extracts of medicinal plants of Bangladesh against fish pathogenic E. faecalis isolates.
| Name of plant species | Type of inhibition | Inhibition zone ratio for tested aqueous extracts of medicinal plant | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F1B1 | F1B3 | F2B1 | F2S1 | F3S2 | F3B2 | F4S2 | F4E2 | FF11 | FF12 | Avg. zone | ||
| Tamarindus indica | Bacteriostatic | 1.8 ± 0.1 | 1.7 ± 0.2 | 1.8 ± 0.1 | 1.8 ± 0.1 | 1.6 ± 0.2 | 1.8 ± 0.1 | 1.8 ± 0.2 | 1.6 ± 0.1 | 1.8 ± 0.0 | 1.7 ± 0.2 | 1.7 ± 0.02 |
| Emblica officinalis | Bacteriostatic | 1.2 ± 0.0 | 1.2 ± 0.1 | 1.3 ± 0.0 | 1.1 ± 0.1 | 1.2 ± 0.1 | 1.1 ± 0.2 | 1.2 ± 0.6 | 1.3 ± 0.1 | 1.3 ± 0.2 | 1.4 ± 0.2 | 1.2 ± 0.03 |
| Allium sativum | Bacteriostatic | 3.0 ± 0.2 | 2.7 ± 0.2 | 3.1 ± 0.3 | 2.9 ± 0.3 | 2.7 ± 0.0 | 2.9 ± 0.3 | 2.8 ± 0.1 | 3.0 ± 0.1 | 2.7 ± 0.3 | 2.8 ± 0.2 | 2.9 ± 0.04 |
| Syzygium aromaticum | Bactericidal | 2.6 ± 0.2 | 2.4 ± 0.1 | 2.6 ± 0.1 | 2.6 ± 0.1 | 2.4 ± 0.1 | 2.6 ± 0.1 | 2.6 ± 0.2 | 2.6 ± 0.0 | 2.3 ± 0.1 | 2.5 ± 0.1 | 2.5 ± 0.04 |
8 mm filter paper disc soaked with aqueous extracts of T. indica, E. officinalis, A. sativum and S. aromaticum (30 µl disk−1) were used. Data are presented as Mean ± SE (n = 3).
In case of the organic extracts of medicinal plants, the methanol extracts of A. sativum exhibited antibacterial activity against the E. faecalis isolates (Table 4). On the other hand, moderate and moderate to high zone of inhibition with bactericidal effects were recorded in n-hexane and methanol and acetone extracts of S. aromaticum, respectively (Fig. 5).
Table 4.
In vitro inhibitory activities of disk containing different organic extracts of Syzygium aromaticum and Allium sativum against fish pathogenic E. faecalis isolates.
| Treatment | Inhibition zone ratio for tested organic extracts of medicinal plants | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Plant Extract | F1B1 | F1B3 | F2B1 | F2S1 | F3B2 | F3S2 | F4E2 | FF11 | FF12 | Avg. zone | |
| A. sativum | Methanol extract* | 2.1 ± 0.0 | 2.2 ± 0.2 | 1.9 ± 0.0 | 1.9 ± 0.1 | 1.8 ± 0.2 | 2.2 ± 0.2 | 2.1 ± 0.0 | 2.2 ± 0.1 | 1.9 ± 0.2 | 2.0 ± 0.05 |
| S. aromaticum | Methanol extract* | 2.0 ± 0.1 | 2.1 ± 0.2 | 2.3 ± 0.0 | 2.2 ± 0.1 | 2.0 ± 0.0 | 2.1 ± 0.2 | 2.2 ± 0.1 | 2.4 ± 0.1 | 2.1 ± 0.1 | 2.2 ± 0.04 |
| Acetone extract* | 2.0 ± 0.1 | 2.0 ± 0.1 | 2.1 ± 0.2 | 2.1 ± 0.1 | 2.2 ± 0.2 | 2.0 ± 0.1 | 1.9 ± 0.0 | 2.1 ± 0.0 | 2.1 ± 0.2 | 2.1 ± 0.03 | |
| n-Hexane extract* | 1.4 ± 0.1 | 1.3 ± 0.1 | 1.4 ± 0.1 | 1.4 ± 0.0 | 1.2 ± 0.1 | 1.2 ± 0.0 | 1.4 ± 0.0 | 1.4 ± 0.1 | 1.2 ± 0.1 | 1.3 ± 0.03 | |
8 mm filter paper disk soaked with methanol extract of A. sativum, methanol extract of S. aromaticum, acetone extract of S. aromaticum and n-hexane extract of S. aromaticum (30 µl disk−1) were used. Data are presented as Mean ± SE (n = 3).
Figure 5.

In vitro antibacterial activity of disk containing solvent extract of Allium sativum and Syzygium aromaticum. Note: (i) methanol extract of S. aromaticum, (ii) acetone extract of S. aromaticum, (iii) Acetone extract of A. sativum, (iv) Blank control, (v) Azithromycin antibiotic disk.
Determination of Minimum Inhibitory Concentration for the Effective Extracts
For determining minimum inhibitory concentration (MIC) of the organic extracts of A. sativum and S. aromaticum, a quantitative bioassay was carried out against a highly virulent strain of E. faecalis FF11 using disk diffusion method. Bioassay revealed that the MIC against the most highly virulent pathogen for methanol extract of A. sativum was 62.5 µg ml−1. On the other hand, MICs for a methanol and acetone extracts of S. aromaticum were 62.5 µg ml−1, and n-hexane extract of S. aromaticum was 125 µg ml−1.
In Vivo Effects of Plant Extracts as Preventive Agents
To know whether the medicinal plant extracts are effective in prevention of disease caused by E. faecalis, an in vivo bioassay was carried out. The fish were fed with various extracts before exposing them to the highly virulent isolates of E. faecalis. As there were variation in the untreated control survival rate, so the data was analyzed using relative percentage of survival (RPS) method comparing the percentage of fish that survived in the treatment group against the control group without treatment. Moderate to high (66.7 to 75.0%) relative percentage of survival (RPS) of fish was recorded when a methanol extract of A. sativum or acetone and methanol extracts of S. aromaticum were applied as preventive agents against E. faecalis infection (Table 5). Although prevention of disease by plant extracts varied against different isolates of E. faecalis, the average relative percentage of survival of fish against high, moderate and weak virulent isolates for any of the plant extracts were very similar to each other.
Table 5.
Relative percentage of survival (RPS) of fish fed with medicinal plant extracts as preventive agents in artificial infection challenge with fish pathogenic E. faecalis.
| Treatment | RPS (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High Virulent Strains | Moderate Virulent Strains | Low Virulent Strains | ||||||||||
| F1B1 | F2B1 | FF11 | F2S1 | F3S2 | Avg. RPS | F1B3 | F4E2 | Avg. RPS | F3B2 | FF12 | Avg. RPS | |
| A. sativum Methanol extract | 80 | 70 | 70 | 75 | 75 | 74 | 67 | 75 | 71 | 100 | 50 | 75 |
| S. aromaticum Acetone extract | 60 | 80 | 80 | 100 | 50 | 74 | 75 | 75 | 75 | 100 | 50 | 75 |
| S. aromaticum Methanol extract | 40 | 60 | 80 | 100 | 75 | 71 | 67 | 67 | 67 | 75 | 75 | 75 |
| S. aromaticum n-Hexane extract | 60 | 60 | 80 | 50 | 50 | 65 | 50 | 67 | 58 | 50 | 50 | 50 |
In Vivo Effects of Plant Extracts as Therapeutic Agents
Effects of organic extracts of A. sativum and S. aromaticum as therapeutic agents were evaluated in laboratory conditions that compared them with two commercial antibiotics, azithromycin and levofloxacin. The fish treated with azithromycin and levofloxacin exhibited average survival of 75.8 ± 1.5% and 62.5 ± 1.2%, respectively. On the other hand, survival of fish treated with methanol extracts of A. sativum was 70.8 ± 4.2% (Fig. 6), which did not vary significantly compared to treatment with azithromycin. A moderate survival rate (60.0 ± 2.2 to 63.3 ± 1.6%) was recorded in fish treated with acetone, methanol or n-hexane extracts of S. aromaticum. No survival of fish in the untreated control group was found.
Figure 6.
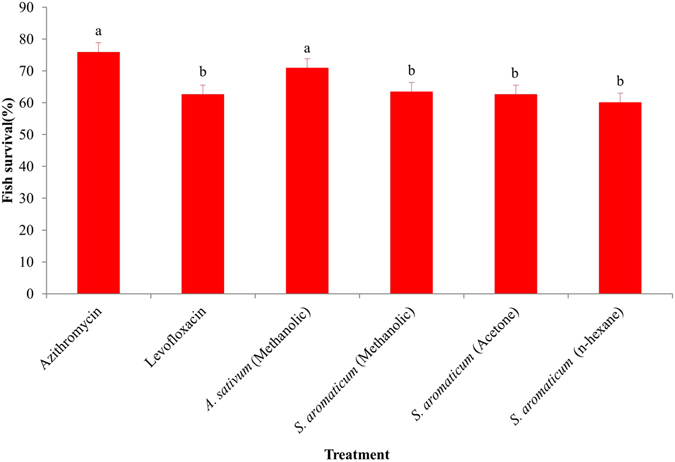
Average survival rate (therapeutic effects) of O. niloticus fed with medicinal plant extracts and commercial antibiotics after infection challenge by a high virulent strain of E. faecalis. One way ANOVA was performed for analysis of the data and mean values in the bars followed by the same letter(s) are not significantly different as assessed by LSD (least significance difference) at p ≤ 0.05.
Discussion
Enterococcus sp. has frequently been isolated from various disease-infected fishes in Bangladesh10, 11. We identified and characterized ten virulent strains of E. faecalis isolated from infected tilapia and catfish collected from local fish farms in Bangladesh. Our 16S rRNA gene sequencing data, PCR study with specific primer, and artificial challenge study confirmed that all of these 10 isolates are E. faecalis. Furthermore, we found that all of these E. faecalis were resistant to several antibiotics but highly susceptible to the crude extracts of two medicinal herbs, A. sativum and S. aromaticum. Methanol extracts of A. sativum and methanol and acetone extracts of S. aromaticum, when used as both therapeutic and preventive significantly reduced the mortality of tilapia fish artificially infected with E. faecalis. This study for the first time confirmed and identified E. faecalis as a virulent pathogen causing high mortality in tilapia and catfish in Bangladesh.
E. faecalis strains have been reported as antibiotic resistant bacteria in many countries14. In the present study, all of the E. faecalis isolates showed resistance to multiple antibiotics. Enterococci are able to acquire transposons, resistance plasmids, and sex pheromone plasmids from a wide range of recipients, which facilitate them to act as a reservoir of resistance genes17. Resistance of Enterococci to various antibiotics such as chloramphenicol, clindamycin, erythromycin, tetracycline, aminoglycosides, beta-lactamases and vancomycin has been reported18, 19. Various antibiotics and growth promoting agents are widely used in aquaculture, livestock and poultry rearing facilities in Bangladesh without proper awareness about their application. This may lead to the resistance of the fish pathogen E. faecalis to multiple antibiotics shown in this study. Resistance of E. faecalis to various cell wall degrading antibiotics such as penicillin-G, ampicillin and vancomycin has been reported19–21. In this study, all E. faecalis isolates were found to be resistant to penicillin-G and ampicillin but, sensitive to vancomycin, which is very rare for this pathogen. The antibiotic susceptibility profile of a fish pathogenic E. faecalis demonstrated in this article has not previously been reported.
One of the interesting findings of this study is that multiple antibiotic resistant strains of E. faecalis show high susceptibility to both aqueous and organic extracts of several herbal medicines including A. sativum, and S. aromaticum (Table 4). Both methanol and acetone extracts of these medicinal plants displayed higher inhibitory activity against E. faecalis compared to ethyl acetate and n-hexane extracts, indicating that the active compounds are relatively polar secondary metabolites. This hypothesis is supported by the inhibitory effects of aqueous extracts of the medicinal plants against the E. faecalis in vitro. A further bioassay-guided chromatographic fractionation and purification process would lead to the discovery of bioactive secondary metabolites from A. sativum, and S. aromaticum extracts against the multiple antibiotic resistant E. faecalis.
Another noticeable finding of this study is that organic extracts of A. sativum and S. aromaticum significantly increase the survival rate of tilapia from infection by E. faecalis in artificial infection. (Table 5). We also demonstrated that these plant extracts were equally effective as a therapeutic agent against the disease caused by E. faecalis. The methanol extract of A. sativum provided significant recovery of fish against the infection by the most virulent strains of E. faecalis, which exhibited equivalent efficacy to azithromycin. Moderate rates of survival were also obtained in this study in fish treated with acetone, methanol or n-hexane extracts of S. aromaticum against E. faecalis infection that were similar to the treatment of fish with levofloxacin. In this study, organic extracts of A. sativum and S. aromaticum provided significant prevention and survival of fish against E. faecalis infection. Several antibacterial secondary metabolites have been discovered from both A. sativum and S. aromaticum 22. Among the bioactive compounds, ajone and allicin present in A. sativum, are commonly known to be active against Gram positive pathogenic bacteria. The antibacterial effects of clove (S. aromaticum) essential oils have been reported23–27. This report for the first time demonstrated both preventive and therapeutic efficacies of the organic extracts of A. sativum and S. aromaticum against E. faecalis infection in tilapia. These findings indicate that medicinal plant extracts could be used as a natural alternative to the synthetic antibiotics to control enterococcal infection in fish. As crude extracts contains multiple secondary metabolites, chances of the development of resistance against crude plant extracts are likely to be lesser than those of pure antibiotics.
In summary, we isolated 10 strains of multiple antibiotic resistant E. faecalis with varying levels of virulence from naturally infected tilapia and catfish and identified them through phenotypic properties, 16S rRNA gene sequencing and specific PCR primers. Although the E. faecalis isolates demonstrated resistance to multiple antibiotics, they showed remarkable susceptibility to both aqueous and organic extracts of A. sativum and S. aromaticum. Application of methanol and acetone extracts of these herbal medicines through feed effectively prevented and suppressed infection in tilapia fish challenged with virulent strains of E. faecalis. Taken together, our results suggest that medicinal plant extracts could be used as a potential alternative to the synthetic antibiotics to control fish diseases cause by E. faecalis in aquaculture. A further study is required to elucidate the mode of action of the plant crude extracts in preventing and/or recovering fish disease caused by E. faecalis.
Methods
Collection of Fish Sample
A number of 17 tilapia (Oreochromis niloticus) and 8 catfish (Clarias batrachus and Heteropneustes fossilis) suspected of being infected with Enterococcus sp. were collected from several fish farms located at the Gazipur district of Bangladesh. The external symptoms observed in infected fish samples were excess secretion of slime, protruding opaque eyes, and swollen abdomen. Hemorrhages under the pelvic fin region and erosion in the tail were also observed in the infected fish. Decrease in appetite, lethargy, erratic swimming, and spinning movements before death were also noticed in infected and moribund fish.
Isolation of Pathogen and Phenotypic Identification
Bacteria were isolated from skin surface, tail, gut, eye and brain of the infected fish on KF streptococcal agar media (Himedia, India). The isolates were subcultured on nutrient agar (Micromaster, India) and maintained in nutrient broth (Micromaster, India) media. Preliminary phenotypic identification of the isolates was performed following the standard morphological, physiological and biochemical analyses28.
Molecular Identification of Bacterial Isolates
Ten randomly selected representative isolates were used for molecular identification through 16S rRNA gene sequencing. They were cultured in 10 ml test tubes containing nutrient broth at 28 °C and 120 rpm in a shaking incubator for 24 hours. Genomic DNA of the isolates was extracted by using sodium acetate for PCR analysis followed by sequencing. Briefly, bacterial cells were harvested up to 2 × 106 cell ml−1 into 1.5 ml microcentrifuge tubes for centrifugation for 10 min at 5000 × g. One milliliter of 95% ethanol was added to the harvested bacterial cells followed by the addition of 50 µl of 3 M sodium acetate before incubating the tube at −20 °C for 2 hours. The sample was centrifuged for 2 minutes at 13000 × g and the supernatant was discarded. One milliliter of 100% ethanol was added to the tube and incubated at 20 °C for 1 minute. The ethanol was removed after centrifugation at high speed and the precipitated DNA was allowed to dry for 5 minutes and then rehydrated in 50 µl of 1 × TE buffer. The extracted DNA was either freshly used for PCR or stored at −20 °C for further use. The PCR reaction mixture was prepared following standard protocols29. The 16S rRNA gene from the genomic DNA was amplified with universal primer set (27F and 1492R) in a PCR thermocycler (Eppendrof Master Cycler). The PCR amplification was done by an initial denaturation at 94 °C for 5 min; 35 cycles of a denaturation at 94 °C for 1 min, an annealing at 57 °C for 40 sec and an extension at 72 °C for 1 min and a final extension step at 72 °C for 10 min.
The PCR amplicons were purified using Gene JET PCR purification kit (Thermo Scientific Inc., USA) following the manufacturer’s purification protocol and sequenced at the Center for Advanced Research in Sciences (CARS) of University of Dhaka in Bangladesh. Analysis of the sequenced data was done using the BLAST program available in National Center for Biotechnology Information (NCBI) website (www.ncbi.nlm.nih.gov). The identified isolates were stored in freezer in 10% glycerol.
E. faecalis Specific Primer Based PCR
In order to develop a PCR protocol for specific detection of E. faecalis, sequences of the 16S rRNA genes of the ten E. faecalis isolates and sequences available in the NCBI GenBank database were aligned using the CLUSTAL W software program and forward and reverse primers were designed from the conserved regions of E. faecalis 16S rDNA sequences based on the alignment. In addition, the universal reverse primer 1492R was also used in this study. Sequences of the designed primers are given in Table 6. The theoretical specificity of each primer was determined by matching the primers to the Ribosomal Database Project II (RDP-II) using the CHECK-PROBE function30. The designed primers were purchased from Sigma Ltd.
Table 6.
Sequences, size and GC contents of primers used for PCR amplification.
| Primers | Sequence (5′-3′) | Nucleotide numbers in E. coli 16S rDNA sequence | Primer size (bp) | GC content (%) |
|---|---|---|---|---|
| EfacF1 | CGTTAGTAACTGAACGTC | 470–488 | 18 | 44.44 |
| Efac R1 | GACCGCGAGGTCATGCA | 1353–1370 | 17 | 64.7 |
| Universal 1492R | GGATACCTTGTTACGACTT | 1473–1492 | 19 | 42.1 |
PCR amplification was conducted in a PCR thermocycler (Eppendrof Master Cycler) which included an initial denaturation at 94 °C for 5 min and 35 cycles comprising denaturation at 94 °C for 1 min, annealing at 48 °C for 40 s, and extension at 72 °C for 1 min and a final extension at 72 °C for 10 minutes with a hold temperature of 4 °C. Amplified PCR products were separated by loading 5 μl of PCR products mixed with 1 μl of 6 × gel loading dye (Thermo Scientific Inc., USA) in 1.5% agarose gel and electrophoresis was run at 100 Volt for 40 minutes. DNA ladder (1 Kb DNA ladder, Bioneer Ltd.) was also loaded in the gel to determine size of the PCR amplicons. The amplicons were visualized in a gel documentation system (Kita G-1000, Germany) after ethidium bromide staining.
In vivo Infection Model
Young Nile tilapia (O. niloticus; average weight 15.0 ± 2.0 g) was collected from rearing pond of the Faculty of Fisheries, Bangabandhu Sheikh Mujibur Rahman Agricultural University, Gazipur, Bangladesh where no previous occurrence of Streptococcal or Enterococcal infection in fish was recorded. The fish were kept in aquarium in the laboratory at room temperature for two weeks, fed twice daily with commercial pellets and observed carefully for any sign of disease or abnormality in their behavior. Prior to challenge study, 10 randomly chosen tilapia fish were sacrificed and their kidneys, eye, skin and fins were aseptically collected and spread on KF Streptococcal agar medium (Himedia, India) and incubated at 28 °C for 72 hours to observe pre-existence or colonization of Enterococcal pathogens in the fish. The experimental fish were first anesthetized by using MS222 and then bathed in bacterial suspension for (2–4 × 105 cfu ml−1) for 15 minutes. The fish were transferred to an aquarium containing 20 L of fresh underground water. Microbiological evaluation of the water used in the aquarium was also done before initiation of the experiment. Twenty fish was kept in each aquarium and three replicated aquaria were used for each of the bacterial isolates used in the study. Continuous aeration was maintained in the aquarium. The fish were fed with commercial feed pellets at 5% of their body weight. Fifty percent of the aquarium water was exchanged every day. A control group fish (without immersion in bacterial suspension) was maintained in three aquaria under same condition. Disease incidence in the tilapia fish was recorded at 7 days duration.
In vitro Antibiogram Assay
Susceptibility profile of isolated 10 fish pathogenic E. faecalis isolates to various commercial antibiotic disks was determined by disk diffusion method31. The antibiotic disks used in this study were erythromycin (15 µg disk−1), penicillin (10 µg disk−1), amoxycillin (30 µg disk−1), vancomycin (30 µg disk−1), ampicillin (25 µg disk−1), levofloxacin (5 µg disk−1), cefuroxime (30 µg disk−1), azithromycin (30 µg disk−1), nitrofurantoin (30 µg disk−1), cefradine (25 µg disk−1), gentamicin (10 µg disk−1). Bacterial culture was spread on the Isosensei Test Agar plates (Micromaster, India), antibiotic disks were aseptically placed on the culture plate and incubated at 37 °C for 24 h in an incubator. After incubation, the diameter of zone of inhibition was measured, and the isolates were termed resistant according to CLSI-specified interpretive criteria32.
Extraction and Inhibitory Activity of Medicinal Plant Extract
The inhibitory activities of crude aqueous extracts of 23 medicinal plants selected based on their medicinal properties33, 34 were screened on E. faecalis isolates. For this purpose, desired parts of plants were rinsed with sterilized distilled water and cut into small pieces. Then the small pieces were weighed and grounded using a mortar and pastel. Sensitivity of the E. faecalis isolates to crude aqueous plant extracts was determined as described elsewhere33. Data were collected for three replicated plates for each of the crude medicinal plant extracts to individual isolates.
To know more details about the active principles in the active plant extracts, the plant extracts were successively fractionated with n-hexane, ethyl acetate, acetone, methanol and then bioassay was performed against the pathogenic isolates using the disk diffusion method. For fractionation, fresh plant materials were dried till all aqueous portions are removed. 25 g of dried plant materials were taken from each sample and ground to powder form using electric blender. The powdered plant material was added into 100 ml n-hexane, ethyl acetate, acetone, methanol for each sample. They were incubated 72 hours in orbital shaker at room temperature. The extracts were then evaporated in rotary evaporator at 50 °C. The dried extract samples were later dissolved in distilled water making 25 mg ml−1 solvent35. For performing disk diffusion method, sterilized filter paper disks (8 mm in size) were soaked with 30 µl extracts (25 mg ml−1 solvent) and kept overnight at room temperature and dried aseptically to ensure complete evaporation of the solvent. A suspension of fresh culture of experimental bacteria was prepared and 30 µl of bacterial inoculum was spread over the Isosensei Test Agar plate with a sterilized glass rod. Then the disks containing fraction of herbal extracts or untreated as control (without extract) were carefully dispensed at uniform distances over the surface of bacterial culture. All plates were incubated at 28 °C for 24 h. After incubation, plates were observed for formation of inhibitory zone on the microbial lawns. Diameter of the disks and diameter of the zone of inhibitions were measured and ratios between the diameters were calculated32. Each treatment was replicated for three times.
Determination of MIC
To determine the minimum inhibitory concentration (MIC) of four types of organic solvent extracts of medicinal plants, serial two-fold dilution technique was followed. Dilutions were adjusted at 1000 µg ml−1, 500 µg ml−1, 250 µg ml−1, 125 µg ml−1, 62.5 µg ml−1 and 31.25 µg ml−1 (w/v) and the disks were prepared as described earlier. The MIC was determined against a highly virulent strain (FF11) following the disk diffusion method. Three replications were used for each dilution. Thirty microliter of bacterial culture having a concentration of 105 cfu ml−1 was inoculated in each culture plate. The growth of bacteria that were decrease in the next dilution was considered as MIC value32.
In vivo Effects of Plant Extracts as Preventive Agents
In vivo effects of methanol extract of garlic (A. sativum) and methanol, acetone and n-hexane extracts of clove (S. aromaticum) as preventive agents against E. faecalis infection on fingerlings of O. niloticus were determined under laboratory condition. For this purpose, 25 mg ml−1 methanol extract of garlic and n-hexane, methanol and acetone extract of S. aromaticum was prepared. These extracts were mixed separately with commercial fish feed by spraying and dried overnight at room temperature. For this purpose, five groups of fish (270 fish in each group) was maintained in five separate aquarium of which one group was fed normal commercial fish feed while the other groups were fed any of the four plant extracts (375 mg kg−1 fish). We selected the dose of plant extracts from an earlier study for a highly virulent strain F1B112. Fish were fed the feed at a rate of 5% of their body weight twice a day for 14 days. After that, each group of fish was artificially infected with nine virulent strains of E. faecalis each of which had three replications (10 fish in each aquarium) following the bath challenge method as described earlier and observed for 7 days. After initiation of artificial infection the fish were fed normal commercial fish feed at the same rate and mortality of the fish in each aquarium was recorded. Continuous aeration and daily exchange of 50% water was maintained throughout the study period. At the end of the experiment the average mortality of fish for each treatment was calculated. Since, virulence level of E. faecalis isolates varied in the control group relative percent survival (RPS)36 and average relative percent survival of fish in each treatment was analyzed.
In Vivo Effects of Plant Extracts as Therapeutic Agents
Therapeutic potentials of methanol extract of garlic (A. sativum) and methanol, acetone and n- hexane extracts of clove (S. aromaticum) on the O. niloticus fingerlings against a highly virulent isolate F1B1 was evaluated in in vivo condition. Two commercial antibiotics, azithromycin and levofloxacin were also used to compare the property of plant extracts with the commercial antibiotics. The fish (O. niloticus) were exposed to bacterial suspension of the E. faecalis isolate F1B1 as described earlier and then seven groups of fish each of which have 3 replications (n = 10) were kept in separate aquarium. Different group of fish were fed commercial fish feed mixed with methanol extract of garlic (375 mg kg−1 fish), methanol extract of clove (375 mg kg−1 fish), acetone extract of clove (375 mg kg−1 fish), n-hexane extract of clove (375 mg kg−1 fish), azithromycin (10 mg kg−1 fish), levofloxacin (14 mg kg−1 fish) and normal fish feed (control group). The fish were feed twice a day and the experiment was continued for 7 days. External disease symptoms and abnormal behaviors in the treated fish were observed. Aeration was maintained throughout the experiment. Around 50% water of the aquarium was exchanged in two days interval.
Statistical Analysis of Data
Experiments for in vivo infection model of E. faecalis isolates and evaluation of biological activities of the crude extracts were carried out using a complete randomized design (CRD). Data were analyzed by one-way analysis of variance (ANOVA) and the mean values were separated by LSD (least significant difference) posthoc statistic. All the analyses were performed using SPSS (IBM SPSS statistics 21, Georgia, USA). Mean value ± standard error of 3 replications was used in Tables and Figures.
Ethical Statement
The animal experiment in this study were carried out following guidelines and recommendations of “Guidelines for the Use of Fishes in Research” published by American Fisheries Society (2014) since there is no specific guideline for use of fish in research in Bangladesh. However, the research works were strictly supervised by an advisory committee of research works of M. R. with the approval of Dean, Graduate studies, BSMRAU. The advisory committee monitored the research works considering the ethical issues.
Electronic supplementary material
Acknowledgements
The authors are thankful to the IDRS-BFRI for major financial support for this work under the project “Molecular Identification of the Pathogen Causing Streptococcal Infection in Tilapia and Control Measures of the Disease”, and World Bank for partial financial support through a sub- project CP #2071 of Higher Education Quality Enhancement Project (HEQEP) of University Grants Commission of Bangladesh. Sincere thanks are due to Tahsin Islam Sakif of Banani, Dhaka, Bangladesh for linguistic editing.
Author Contributions
M.M.R., initiated the research works; M.M.R., conceived the study; M.R., M.M.R. and M.T.I., drafted, edited and interpreted data; M.R., performed the experiments; M.M.R. and S.C.D., performed bioinformatics analysis; M.S.A. and M.J.A., contributed the materials and provided valuable suggestions.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-03673-1
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Md. Mahbubur Rahman, Email: mahbub-biotech@bsmrau.edu.bd.
Md. Tofazzal Islam, Email: tofazzalislam@yahoo.com.
References
- 1.Zorrilla I, et al. Bacteria recovered from diseased cultured gilthead sea bream (Sparusaurata L.) in southwestern Spain. Aquaculture. 2003;218:11–20. doi: 10.1016/S0044-8486(02)00309-5. [DOI] [Google Scholar]
- 2.Plumb, J. A. Infectious diseases of tilapia. In Tilapia aquaculture in the Americas 212–222 (Edited by Costapierce, B. A. & Rakocy, J. E.) (1997).
- 3.Martins, et al. Haematological changes in Nile tilapia. Braz. J. Biol. 2008;68(3):657–661. doi: 10.1590/S1519-69842008000300025. [DOI] [PubMed] [Google Scholar]
- 4.Kusuda R, Salati F. Major bacterial diseases affecting mariculture in Japan. Ann. Rev. Fish Dis. 1993;3:69–85. doi: 10.1016/0959-8030(93)90029-B. [DOI] [Google Scholar]
- 5.Nieto J, Devesa S, Quiroga I, Toranzo A. Pathology of Enterococcus sp. infection in farmed turbot, Scophthalmus maximus L. J. Fish Dise. 1995;18:21–30. doi: 10.1111/j.1365-2761.1995.tb01262.x. [DOI] [Google Scholar]
- 6.Plumb, J. & Hanson, L. Health Maintenance and Principal Microbial Diseases of Cultured Fishes, 3rd. (John Wiley & Sons, 2010).
- 7.Petersen A, Dalsgaard A. Antimicrobial resistance of intestinal Aeromonas spp. and Enterococcus spp. in fish cultured in integrated broiler-fish farms in Thailand. Aquaculture. 2003;219:71–82. doi: 10.1016/S0044-8486(03)00018-8. [DOI] [Google Scholar]
- 8.Ahmed ME, El-Refaey Studies on major bacterial diseases affecting fish; Tilapia Oreochromis niloticus, Catfish, Clarias gariepinus and mullets in Port Said, Egypt with special references to its pathological alterations. Researcher. 2013;5:5–14. [Google Scholar]
- 9.Abou El-Geit EN, Saad TT, Abdo MH, Mona SZ. Microbial infections among some fishes and crustacean species during blooming phenomenon in Qaroun Lake-Egypt. Life Sci. J. 2013;2:10. [Google Scholar]
- 10.Iqbal MM, Chowdhury MBR, Uddin MN, Rahman MM. Studies on the bacterial flora in the slime and kidney of a farmed fish, Cirrhinus mrigala. Bangladesh J Fish. 1996;19:87–93. [Google Scholar]
- 11.Chowdhury MBR, Nahiduzzaman M, Rahman MM, Uddin MN. Status of bacterial flora in a hybrid catfish, Clarias batrachus × Clarias gariepinus. Bangladesh J. Fish. 1998;21:49–54. [Google Scholar]
- 12.Rahman, M. M. Molecular Identification of the Pathogen Causing Streptococcal Infection in Tilapia and Control Measures of the Disease. 1–22 (IDRS-BFRI, 2015).
- 13.FAO. The State of World Fisheries and Aquaculture 2016. Contributing to Food Security and Nutrition For All (Rome, 2016).
- 14.Arias CA, Murray BE. The rise of the Enterococcus: beyond vancomycin resistance. Nat. Rev. Microbiol. 2012;10:266–278. doi: 10.1038/nrmicro2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reverter M, Bontemps N, Lecchini D, Banaigs B, Sasal P. Use of plant extracts in fish aquaculture as an alternative to chemotherapy: current status and future perspectives. Aquaculture. 2014;433:50–61. doi: 10.1016/j.aquaculture.2014.05.048. [DOI] [Google Scholar]
- 16.Yusuf, M., Begum, J., Hoque, M. N. & Chowdhury, J. U. Medicinal Plants of Bangladesh, 2nd. (Dhaka, 2009).
- 17.Gilmore M, Lebreton F, Van Schaik W. Genomic transition of enterococci from gut commensals to leading causes of multidrug-resistant hospital infection in the antibiotic era. Curr. Opin. Microbiol. 2013;16:10–16. doi: 10.1016/j.mib.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murray BE. The life and times of the Enterococcus. Clinic. Microbiol. Rev. 1990;3:46–65. doi: 10.1128/CMR.3.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rubinstein E, Keynan Y. Vancomycin-resistant Enterococci. Crit. Care Clinics. 2013;29:841–852. doi: 10.1016/j.ccc.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Fang C, Stiegeler E, Cook G, Mascher T, Gebhard S. Bacillus subtilis as a platform for molecular characterization of regulatory mechanisms of Enterococcus faecalis resistance against cell wall antibiotics. PLoS One. 2014;9:93169. doi: 10.1371/journal.pone.0093169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shepard B, Gilmore M. Antibiotic-resistant enterococci: the mechanisms and dynamics of drug introduction and resistance. Microb. Infect. 2002;4:215–224. doi: 10.1016/S1286-4579(01)01530-1. [DOI] [PubMed] [Google Scholar]
- 22.Shahidi, F. & Ho, C. Phytochemicals and Phytopharmaceuticals, 69–71 (AOCS Press, 2000).
- 23.Karuppiah P, Rajaram S. Antibacterial effect of Allium sativum cloves and Zingiber officinale rhizomes against multiple-drug resistant clinical pathogens. Asian Pac. J. Trop. Biomed. 2012;2:597–601. doi: 10.1016/S2221-1691(12)60104-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ankri S, Mirelman D. Antimicrobial properties of allicin from garlic. Microbes and Infect. 1999;1:125–129. doi: 10.1016/S1286-4579(99)80003-3. [DOI] [PubMed] [Google Scholar]
- 25.Naganawa R, et al. Inhibition of microbial growth by ajoene, a sulfur-containing compound derived from garlic. Appl. Environ. Microbiol. 1996;62:4238–4242. doi: 10.1128/aem.62.11.4238-4242.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fu, Y. et al. Antimicrobial activity of clove and rosemary essential oils alone and in combination. Phytother. Res. 21, 989–994 (2007). [DOI] [PubMed]
- 27.Abdullah B, Hatem S, Jumaa WA. A comparative study of the antibacterial activity of clove and rosemary essential oils on multidrug resistant bacteria. UK J. Pharm. Biosci. 2015;3:18–22. doi: 10.20510/ukjpb/3/i1/89220. [DOI] [Google Scholar]
- 28.Devriese LA, Pot B, Collins MD. Phenotypic identification of the genus Enterococcus and differentiation of phylogenetically distinct enterococcal species and species groups. J. Appl. Bacteriol. 1993;75:399–408. doi: 10.1111/j.1365-2672.1993.tb02794.x. [DOI] [PubMed] [Google Scholar]
- 29.Rahman MM, Somsiri T, Ezura Y, Tajima K. Distribution of Aeromonas spp. emphasizing on a newly identified species Aeromonas sp. T8 isolated from EUS-affected fish and aquatic animals in Southeast Asia. Pakistan J. Biol. Sci. 2004;7(2):258–268. doi: 10.3923/pjbs.2004.258.268. [DOI] [Google Scholar]
- 30.Cole JR, et al. The Ribosomal Database Project (RDP-II): Previewing a new autoaligner that allows regular updates and the new prokaryotic taxonomy. Nucleic Acids Res. 2003;31.1:442–443. doi: 10.1093/nar/gkg039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jorgensen JH, Ferraro M. Antimicrobial susceptibility testing: A review of general principles and contemporary practices. Clin. Infect. Dis. 2009;49:1749–1755. doi: 10.1086/647952. [DOI] [PubMed] [Google Scholar]
- 32.CLSI. Performance Standards for Antibacterial Susceptibility Testing: Fifteenth Informational Supplement (Wayne, PA, 2005).
- 33.Rahman MM, Hossain MN. Antibiotic and herbal sensitivity of some Aeromonas sp. Isolates collected from diseased carp fishes. Prog. Agric. 2010;21(1 & 2):117–129. [Google Scholar]
- 34.Muniruzzaman M, Chowdhury MBR. Sensitivity of fish pathogenic bacteria to various medicinal herbs. J. Ban. Vet. Med. 2004;2(1):75–82. [Google Scholar]
- 35.Gull I, et al. Inhibitory effect of Allium sativum and Zingiber officinale extracts on clinically important drug resistant pathogenic bacteria. Ann. Clin. Microbiol. Antimicrob. 2012;11(1):8. doi: 10.1186/1476-0711-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amend DF. Potency testing of fish vaccines. Dev. Biol. Standard. 1981;49:447–454. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


