Abstract
Seabirds concentrate nutrients from large marine areas on their nesting islands playing an important ecological role in nutrient transfer between marine and terrestrial ecosystems. Here we investigate the role of guano on corals reefs across scales by analyzing the stable nitrogen isotopic (δ15N) values of the scleractinian coral Pocillopora damicornis on fringing reefs around two Pacific remote islets with large seabird colonies. Marine stations closest to the seabird colonies had higher nitrate + nitrite concentrations compared to more distant stations. Coral and zooxanthellae δ15N values were also higher at these sites, suggesting that guano-derived nitrogen is assimilated into corals and contributes to their nitrogen requirements. The spatial extent of guano influence was however restricted to a local scale. Our results demonstrate that seabird-derived nutrients not only spread across the terrestrial ecosystem, but also affect components of the adjacent marine ecosystem. Further studies are now needed to assess if this nutrient input has a positive or negative effect for corals. Such studies on remote islets also open fresh perspectives to understand how nutrients affect coral reefs isolated from other anthropogenic stressors.
Introduction
Ecosystems, even those with seemingly distinct borders, rarely function independently of other adjacent systems1. Ecologists are increasingly recognizing the important effects that cross-ecosystem transport of energy and nutrients have on plant and animal populations and communities2, 3. A well known example of this is how seabirds concentrate marine-derived nutrients on breeding islands in the form of feces (guano) which contains ~15–20% nitrogen (N), as well as 10% phosphorus4–6. These nutrients dramatically alter terrestrial ecosystem functioning and dynamics and can support increased primary and secondary productivity7, 8. However, although many studies have demonstrated nitrogen enrichment of terrestrial components due to guano deposition across various taxonomic groups7, 9–11, only a few have studied its retroaction on marine ecosystems and most of these studies were restricted to temperate regions and high nutrient waters4, 12–14. In the tropics, coral reefs can be found adjacent to islands with large populations of breeding seabirds, and could be potentially affected by local nutrient enrichment due to the transport of seabird-derived nutrients in surrounding waters. While two studies have explored the influence of guano on these tropical marine ecosystems12, 15 and suggested that nitrogen from guano enriched seawater and reef primary producers, none have focused on its impact upon corals.
Reef building corals have essential nitrogen needs and, thriving in nutrient-poor tropical waters16 where nitrogen is a major limiting nutrient for primary productivity17, they have developed specific adaptations for conserving this element. Their establishment and maintenance are partly due to their symbiosis with unicellular dinoflagellates, Symbiodinium spp. (zooxanthellae), that can take up and retain dissolved inorganic nitrogen (ammonium and nitrate) from the surrounding waters18–20. These zooxanthellae can also recycle the animal wastes and subsequently transfer them back to the coral host as amino acids21, ammonium or urea22. Corals are also able to ingest nitrogen-rich sediment particles23, 24 and plankton25, 26. While it is widely admitted that coastal eutrophication and excess nutrient supply have a strong impact on corals, leading essentially to a decrease in skeletal growth19, 27–30, the potential effects of nutrients from the breeding sites of seabirds on coral reefs has never been studied.
The Chesterfield islands and D’Entrecasteaux Reefs (Coral Sea, Western Pacific Ocean) are remote reef complexes located at 550 and 230 km respectively from the New Caledonia main land31 (Fig. 1); they are uninhabited and isolated from anthropogenic nitrogen influences and their surrounding seawaters have typically low nutrient concentrations32. Both reef systems have been listed as conservation priority sites for New Caledonia and are part of the Natural Marine Park of the Coral Sea spanning 1.3 million km2 of marine ecosystems33. Reynard and Surprise, two islets in Chesterfield and D’Entrecasteaux Reefs, respectively, provide refuge for large seabird colonies (up to 40,000 breeding pairs)34, 35 and represent an ideal study system to evaluate seabird guano influence on adjacent coral reefs without any anthropogenic impacts. Seabird guano is enriched in 15N relative to 14N, partly due to the birds’ high trophic position36 and to preferential volatilization of 14N from guano37. Thus, nitrogen stable isotope ratios can be used as a useful tool to trace guano incorporation in marine food webs7, 12, with high δ15N values in animal tissues being a proxy for seabird derived nitrogen.
Figure 1.
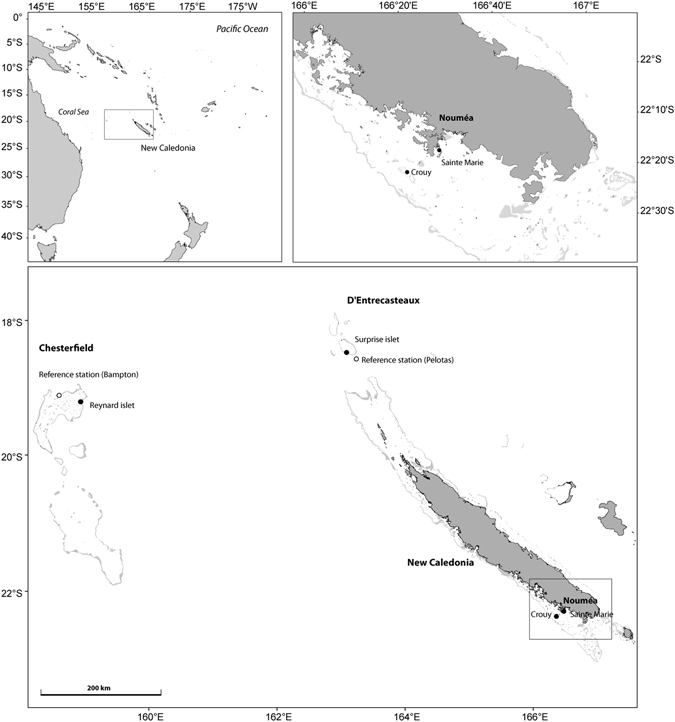
Sampling sites in New Caledonia. The map was generated with ArcGIS 10.2.2 (http://www.esri.com/arcgis/aboutarcgis) and customized in Adobe Illustrator CS3 (version 13, www.adobe.com).
The main aim of this study was to assess if seabird guano was a potential nitrogen source for reef building corals and to evaluate the spatial extent of such influence on adjacent coral reefs. We selected reefs adjacent to two islets in the Chesterfield Islands and D’Entrecasteaux Reefs with thousands of breeding seabirds34, 35 and compared them to a reference site without nesting seabirds to assess whether seabird presence was associated with higher concentrations of nutrients (phosphate and nitrate) and higher coral δ15N values. Pocillopora damicornis is a common and locally abundant zooxanthellate scleractinian coral in the Chesterfield and D’Entrecasteaux Reefs38, 39. It was thus targeted for sampling to perform nitrogen stable isotope analyses in the animal and zooxanthellae tissue separately, as both compartments use nitrogen40. Guano was sampled from different seabirds on their nests, and seawater and coral samples were collected along a gradient from the shore (from 10 to 800 m, Fig. 2). We hypothesized that coral reef waters closest to seabird colonies would have highest concentrations of dissolved nutrients and that corals would have higher δ15N values compared to the farthest and reference sites. Results were also compared to those from a site subjected to high levels of anthropogenic inputs in the southern lagoon of New Caledonia (Nouméa, Fig. 1) to evaluate the importance of seabird-derived vs. anthropogenic-derived nitrogen on corals and their surrounding waters.
Figure 2.
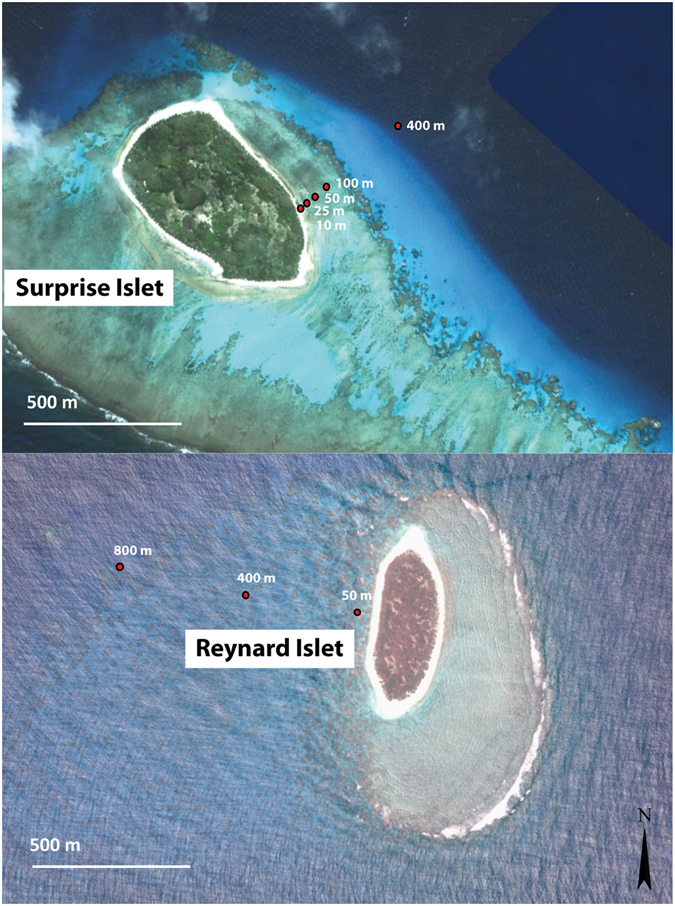
Geographic location of coral and nutrient sampling stations for Surprise and Reynard islets. The satellite images for the map were obtained from Government of New Caledonia (via the opensource portal https://explorateur-carto.georep.nc), and site locations were overlaid in Adobe Illustrator CS3 (version 13, www.adobe.com).
Results
Nutrients
At both islets, a clear NOx (nitrate + nitrite) spatial gradient was observed, with higher NOx concentrations closer to the shore (Fig. 3). At Reynard islet, we found significantly higher NOx values at 50 m and 400 m from the shore (2.44 µM and 0.38 µM, respectively) compared to the reference station (0.14 µM, Table 1 and Supplementary Table S1). NOx concentrations at 800 m were not significantly different from the reference station. At Surprise, NOx concentrations were similar and high at 10 and 25 m (~21 µM, Table 1 and Supplementary Table S1), then decreased to 2.76 µM at 50 m and 0.69 µM at 100 m. NOx concentrations were significantly higher from the reference station at all sites but 400 m. Comparing islets, NOx concentrations at 50 m (2.44 vs. 2.76 µM) and 400 m (0.38 vs 0.33 µM) were similar at Reynard and Surprise, respectively. In Nouméa, NOx values at Sainte Marie station were 0.93 µM and 0.10 µM at Crouy (Table 1).
Figure 3.
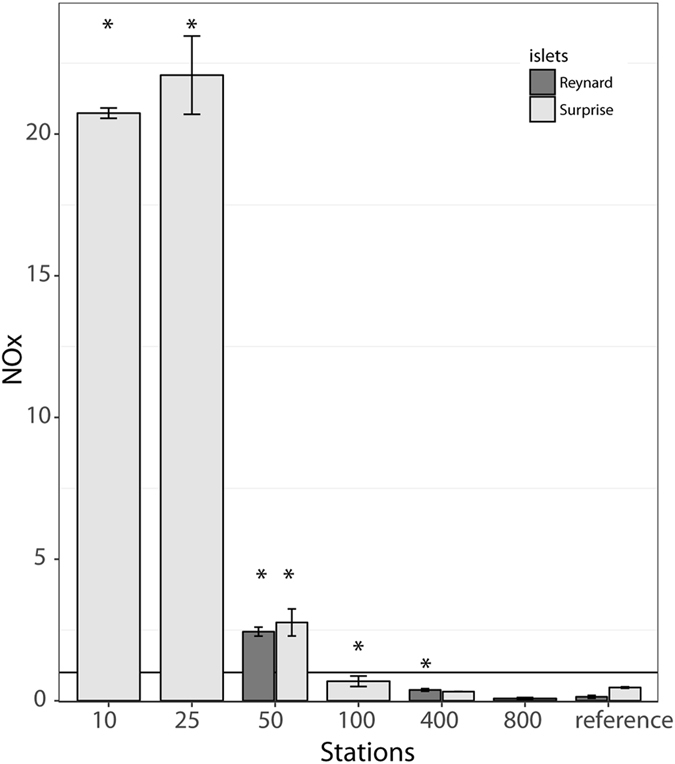
NOx concentrations (nitrates + nitrites) from Chesterfield (Reynard islet) and D’Entrecasteaux reefs (Surprise islet) in µmol/L. The black line indicates the average NOx concentration at Sainte Marie (Nouméa), a station with high anthropogenic inputs. Stars denote significantly higher values compared to the reference station at a 0.05 significance level.
Table 1.
NOx (nitrates + nitrites) at the different stations in Nouméa and at Reynard and Surprise (Mean ± SD, N = 3 per station).
| Date | Site | Distance from the shore | NOx (µmol.L−1) |
|---|---|---|---|
| 10/11/2015 | Reynard | 50 m | 2.44 ± 0.13 |
| 10/11/2015 | Reynard | 400 m | 0.38 ± 0.04 |
| 10/11/2015 | Reynard | 800 m | 0.08 ± 0.03 |
| 22/11/2015 | Bampton | Reference station | 0.14 ± 0.04 |
| 05/10/2016 | Surprise | 10 m | 20.74 ± 0.18 |
| 05/10/2016 | Surprise | 25 m | 22.08 ± 1.38 |
| 05/10/2016 | Surprise | 50 m | 2.76 ± 0.48 |
| 05/10/2016 | Surprise | 100 m | 0.69 ± 0.18 |
| 05/10/2016 | Surprise | 400 m | 0.33 ± 0.01 |
| 02/03/2017 | Pelotas | Reference station | 0.47 ± 0.02 |
| 31/10/2014 | Sainte Marie | urbanized | 0.93 ± 0.25 |
| 31/10/2014 | Crouy | Reference station | 0.10 ± 0.02 |
Reference stations were sampled at 50 m from the reef crest.
Stable isotopes in corals and guano
Guano δ15N values at Reynard islet were 10.3 ± 0.3‰, 11.4 ± 0.1‰ and 18.3 ± 1.3‰ for the red-footed booby, the great frigatebird and the brown booby, respectively (N = 3 per seabird species). At Surprise islet, they were 11.5 ± 1.2‰ and 11.5 ± 0.8‰ for the red-footed booby and the great frigatebird, respectively (N = 3 per species).
Coral tissues and zooxanthellae showed similar spatial patterns with significantly higher δ15N values at the closest sites (25 m, 50 m and, when applicable, 100 m from the shore at Surprise and Reynard islets, Fig. 4 and Supplementary Table S2) compared to the reference stations. Coral tissue and zooxanthellae δ15N values decreased with increasing distance from the shore. More specifically, at Reynard islet, δ15N values in coral tissue and zooxanthellae were significantly higher at the 50 m site compared to the reference station (p < 0.001). Stations at 400 and 800 m were either significantly lower or showed no difference from the reference station. At Surprise islet, the 25 m, 50 m and 100 m sites were all significantly higher than the reference station (p < 0.001), with the 25 m showing the highest δ15N values for both coral tissue and zooxanthellae. When comparing the values at the two islets at matching 50 m stations, higher values were observed at Reynard islet for both coral and zooxanthellae (Table 2).
Figure 4.
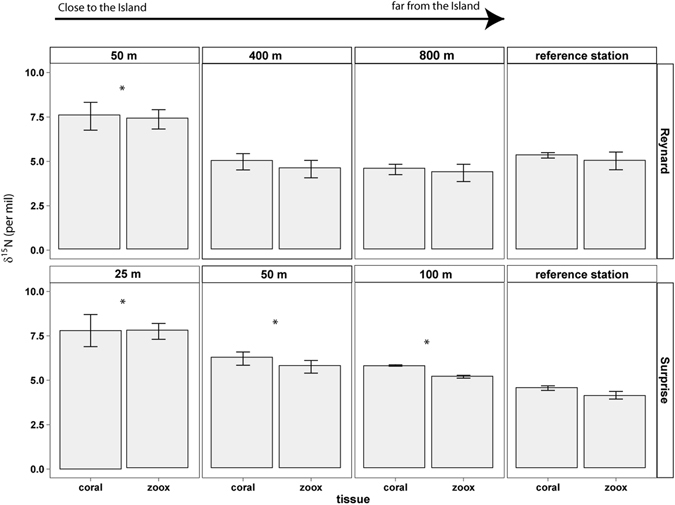
δ15N values from Reynard islet and Surprise islets in coral tissues and zooxanthellae. Stars denote significantly higher values compared to the reference station (0.05 significance level, statistical results for coral and zooxanthellae are the same). Note that the distances of sampled sites from the shore are different between islets.
Table 2.
δ15N values (mean ± SD, N = 5) in coral tissue (coral) and zooxanthellae (zoox) at reference stations (50 m from the reef crest), Reynard and Surprise islets.
| site | sampling distance (m) | tissue | δ15N (‰) |
|---|---|---|---|
| Reynard | 50 | coral | 7.5 ± 0.8 |
| Reynard | 50 | zoox | 7.4 ± 0.5 |
| Reynard | 400 | coral | 5.0 ± 0.5 |
| Reynard | 400 | zoox | 4.6 ± 0.5 |
| Reynard | 800 | coral | 4.5 ± 0.3 |
| Reynard | 800 | zoox | 4.3 ± 0.5 |
| Bampton | Reference station | coral | 5.3 ± 0.2 |
| Bampton | Reference station | zoox | 5.0 ± 0.5 |
| Surprise | 25 | coral | 7.8 ± 0.9 |
| Surprise | 25 | zoox | 7.8 ± 0.4 |
| Surprise | 50 | coral | 6.2 ± 0.4 |
| Surprise | 50 | zoox | 5.8 ± 0.4 |
| Surprise | 100 | coral | 5.7 ± 0.0 |
| Surprise | 100 | zoox | 5.2 ± 0.1 |
| Pelotas | Reference station | coral | 4.5 ± 0.1 |
| Pelotas | Reference station | zoox | 4.1 ± 0.2 |
| Sainte Marie | urbanized | coral | 5.7 ± 0.3 |
| Sainte Marie | urbanized | zoox | 4.8 ± 0.3 |
| Crouy | Reference station | coral | 3.7 ± 0.1 |
| Crouy | Reference station | zoox | 2.7 ± 0.4 |
Values in Nouméa stations (Sainte Marie and Crouy) are also indicated.
In Nouméa, at the Sainte Marie station, δ15N values in coral tissues were 5.7 ± 0.3‰ and 4.8 ± 0.3‰ in zooxanthellae (Table 2). At Crouy, far from urbanized areas, δ15N values were 3.7 ± 0.1‰ and 2.7 ± 0.4‰ in coral and zooxanthellae, respectively.
Discussion
The high δ15N values found in both zooxanthellae and tissues of corals collected close to the seabird colonies suggest that guano is a significant source of nitrogen for them. The high coral δ15N values and high nutrient concentrations at sites closest to shore provide further evidence that seabirds can influence coral reefs via cross-ecosystem nutrient subsidies. However, the spatial extent of seabird influence seemed restricted to reefs close to the island within 100 to 400 m from the shore.
Guano-derived nitrogen and coral nutrition
Seabird guano showed high δ15N values (on average 11‰ for the red-footed booby and the great frigatebird and 18‰ for the brown booby), in the range of values found by other studies14, 41, 42. These δ15N values not only depend on the trophic level or the diet of the seabird but also on the state of mineralization of the guano (e.g., ammonia volatilization), which can have large isotope effects and increase its isotopic values43. Although we have not measured the δ15N values of dissolved inorganic nitrogen (hereafter DIN) or particulate organic matter in the surrounding waters, we have found significantly higher NOx concentrations at sites close to seabird colonies compared to more distant sites. This guano has most probably 15N-enriched the base of the marine ecosystem (both dissolved and particulate phases) and caused 15N enrichment in the coral tissues. Such 15N enrichment is indeed reflected in corals as δ15N values found in coral tissues and zooxanthellae sampled close to the seabird colonies were ~2.5 to 3‰ higher than at farthest sites and reference sites (~8‰ vs. 5‰). This result, together with the high NOx concentrations in seawater at the examined sites, demonstrates significant local enrichment in nitrogen from seabird guano and, most importantly, uptake and assimilation of this seabird-derived nitrogen in coral tissues and their symbiotic zooxanthellae. Corals use multiple ways to supply their nitrogen needs: they can efficiently take up and retain DIN (such as ammonium and nitrate44–46), dissolved organic nitrogen (DON, under the form of amino acids and even urea21, 47), organic matter and planktonic preys40. Several possibilities might thus explain this coral 15N enrichment but our study did not allow us to determine which pathway was predominant.
To estimate the importance of guano derived N to these corals we use a simple mathematical mixing model to determine the proportions of various sources in a mixture (a common approach in isotope geochemistry48–50). Using such simple mixing model (%Nguano = (δ15Nnearshore coral − δ15Nreference coral)/(δ15Nguano − δ15Nreference coral) × 100) we estimate coral tissues obtain 15 to 50% of their N from seabird guano in these near-shore ecosystems. Using the reference site coral tissue and seabird guano as end-members, with no trophic enrichment from guano to coral tissue (i.e., direct uptake from DIN/DON) we calculate ~45% of N is derived from guano in near-shore corals using 11‰ as the guano value, and ~20% when using 18‰ as the guano value (i.e., range of guano δ15N values measured in this study). If, instead, the assimilation occurs via the planktonic preys, these values fall to about 30% and 15%, respectively, when using a traditional 3.4‰ trophic enrichment. While the end-members used here are rough approximations (i.e., guano likely undergoes several transformations before corals utilize guano derived N), it does provide a first assessment of the importance of guano derived N in these coral communities.
Such assimilation of guano-derived nitrogen in tropical waters has already been observed in macroalgae around Hawaii12 with δ15N values 1.5‰ higher in macroalgae at high seabird density sites compared to low seabird density sites, but our study provides the first evidence of its assimilation by a coral, both in tissue and its zooxanthellae. These nutrients will probably impact higher trophic level consumers such as parrot fish feeding on corals51, 52 and even higher trophic level organisms, since baseline isotopic values have been shown to propagate up to top predators in marine and terrestrial systems7, 53. Assimilation of seabird-derived nitrogen by coral and zooxanthellae could have important unanticipated effects on coral health even if it is still subject to debate whether high levels of nitrogen are beneficial or not to corals54. High levels of added nutrients (up to 15 µM of NH4+) have been shown to have direct toxic effects on corals by hindering calcification19, 27–30 and reducing their resistance to thermal stress55, 56. These studies suggest that enhanced DIN availability would promote excessive zooxanthellae growth rates, which ultimately disrupts the stability and functioning of the coral-zooxanthellae endosymbiosis55, 57. The combination of elevated temperature and nitrate enrichment would produce an even more pronounced reduction of coral photosynthetic production56. However, other studies showed that an addition of nitrates could increase coral resilience to bleaching by increasing the potential for energy storage within the coral58–60. Assimilation of seabird-derived nitrogen by coral could then be particularly significant in terms of resilience to climate change, especially as some studies predict that increasing sea surface temperatures will decrease the availability of nitrates61.
Further studies are then needed to investigate if the nitrogen enrichment from seabird guano would positively or negatively affect coral metabolism (e.g., photosynthetic efficiency, calcification rates) and their potential to recover from bleaching. Nutrient enrichment can also directly or indirectly mediate changes in community structure (e.g., from coral- to algal-dominated)62–65 and it would be interesting to assess such changes in these remote environments where the effect of nutrient inputs are not confounded by multiple anthropogenic stressors.
Spatial and temporal influence of seabird-derived guano on corals and seawater
The influence of seabird guano on corals and their surrounding seawaters can occur through both percolation of accumulated guano on the islands via precipitation, and direct guano excretion into the water during foraging flights and return to islands15, 66 (Fig. 5). Increased nutrient concentrations (NH4 +, NO3 −, NO2 − and PO4 −) in adjacent waters have already been reported from seabird islands in marine waters12, 15. However, compared to other studies4, 12, the advantage of these remote Pacific low carbonate islets as models is that the observed stable isotope enrichment is not masked by anthropogenic eutrophication. Surprise and Reynard islets, at 230 and 550 km North West of the New Caledonia mainland, respectively, are far from anthropogenic inputs, such that the observed enrichment can only be due to the transport of seabird guano into surrounding waters. As a comparison, waters in the south-west lagoon of New Caledonia were in general below 0.04 µM for NOx with values up to 1.6 µM at urbanized embayment stations67–69 where nutrient loading from the city is known to be elevated68, 69.
Figure 5.
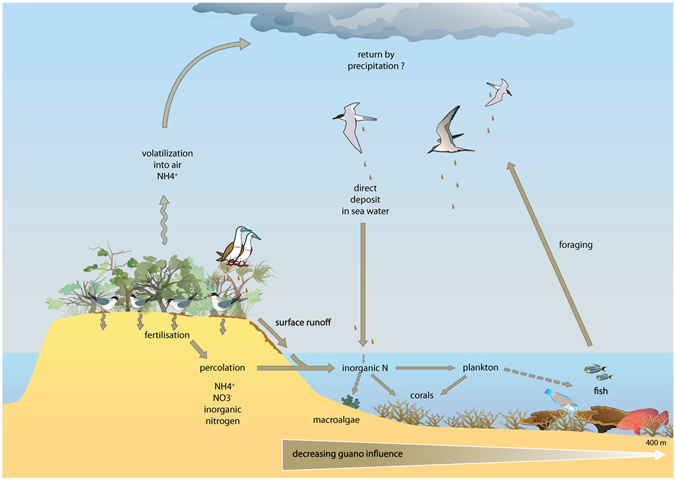
Conceptual diagram showing the different pathways for guano-derived nitrogen to enter seawater and marine food webs (brown arrows) with hypothetical transfers to fish and macroalgae in broken lines, generated with Adobe illustrator CS3 (version 13, www.adobe.com).
Local nutrient enrichment from seabird guano at remote islets can then be quite substantial, since NOx concentrations at Surprise and Reynard were much higher even than the ones found in the Sainte-Marie urban site close to Nouméa city (×10 at 10 m and 25 m, and ×2 at 50 m). Coral δ15N values were also higher by 2 to 3‰ compared to Saint-Marie corals, where nutrient loading from Nouméa city is known to be elevated68, 69. The comparison of those absolute values remains however, not very informative given the drastically different origin for the nitrogen pollution. Knowledge of the δ15N isotopic value of the base of the food web (particulate organic matter or DIN) would be required for an in-depth comparison.
Guano deposition could potentially have far-reaching effects70. Our results, however, showed the markedly localized effect of isotopic enrichment by birds, with significant differences in isotopic nitrogen values and NOx concentrations recorded at sites only 25–400 m distant from the shore. At 400 m from the shore, coral and zooxanthellae δ15N values were similar to the reference sites, but NOx concentrations were still higher than the reference site, suggesting that guano could still have an influence there. Therefore, while we cannot exactly assess the extent of this spatial influence, our study suggests that seawater enrichment from seabird guano might be limited to ~100 to 400 m from the shore.
The nutrient concentrations measured in our study only provide a snapshot in time in contrast to isotopic results which capture a longer time period. Coral tissue turnover rates are not known, but their tissues are unlikely to integrate more than a few months of data. For example, bivalve muscle turnover is expected to be 3 to 4 months71. Since our results were collected during a single season, the austral summer, we cannot rule out that the extent of enrichment may vary seasonally due to, for instance, seasonal changes in guano accumulation or rainfall. However, while a portion of the seabird species in the Chesterfield area (namely, wedge-tailed shearwater, black and brown noddies, and black-naped terns) breed in the summer, as do most seabird species south of the Coral Sea35, other species (masked booby, red-footed booby, frigatebirds, and fairy tern) breed in the winter34. Therefore, guano deposition should occur all year round at both islands, as is often the case in tropical ecosystems72 and its influence on coral reefs should not be restricted to a particular time or season of the year. It should be noted, however, that additional δ15N variability could be introduced if levels of guano deposition and 15N enrichment vary amongst the different seabird species that co-occur in this area. Studies at different time of the year on corals or sequential sampling of the organic matter embedded in the skeleton of bivalve shells73 or corals74 would allow to further assess the temporal importance of this cross-ecosystem nutrient exchange.
Nutrient concentration in waters surrounding seabird islands might depend on nest density, seabird species but also on island structure and vegetation, wave exposure, and weather conditions13, 72. The comparison between sites is difficult in our study as different distances from the shore were selected for the sampling in Reynard and Surprise islets. This comparison can only be done at 50 m from the shore with significantly higher coral and zooxanthellae δ15N values (~1.5‰ higher), but similar nutrient concentrations at Reynard compared to Surprise islet. This would suggest higher importance of guano-derived nitrogen on corals at Reynard islet. This result might be expected as the seabird colony is larger at Reynard compared to Surprise (40,000 vs. 5,000 breeding pairs34, 35) and on a smaller surface area (8 vs. 24 ha). Surprise also has more complex and stratified vegetation cover, which could impede guano spreading into surrounding waters, as well as promote N uptake by the vegetation. Further analyses with similar sampled gradients between the two islets would be needed to confirm this pattern.
Conclusion
Our study suggests that seabird guano can be a significant source of nitrogen for corals on remote islets. To further test the importance of guano on coral nutrition, it would be interesting to analyze carbon, nitrogen and phosphorus concentrations of coral and zooxanthellae tissues to determine if seabird guano also represents a non-negligible source of phosphorus for corals, and to confirm higher nitrogen incorporation at stations close to seabird colonies. Seabirds, obtaining their food from the ocean but exerting their reproductive activities on islands, would thus not only be important vectors of nitrogen to terrestrial systems but also to the near-shore marine ecosystem. The effects found in this study are spatially restricted to reefs close to the island within ~100 to 400 m from the shore, which, to the best of our knowledge, is the first estimate for the spatial extent of guano influence on corals.
As it is widely known that corals, which regularly experience elevated concentrations of dissolved inorganic nitrogen, are undergoing important physiological modifications, it is essential to determine whether this guano input also affect their physiological state and their resistance to climate change. Further studies on isotopic variations over time and space in a broader range of taxonomic groups (e.g., invertebrates, fish, and macroalgae) would contribute to our understanding of the seabirds’ role in near-shore ecosystem communities. This is particularly true in a context of global change and the accompanying shifts in seabird populations worldwide75–77. Finally, given the challenges of studying the impact of nutrients on corals in isolation of other anthropogenic stressors (e.g., heavy metals, human eutrophication), remote islets with nesting seabird populations provide an ideal study system to investigate how nutrient enrichment in general can affect marine environments.
Materials and Methods
Study sites
This study was conducted at two remote coral reef islets in the Coral Sea (South Pacific, New-Caledonia): Surprise in the D’Entrecasteaux Reefs, and Reynard in the Chesterfield Islands (Fig. 1). Surprise Islet is part of the Surprise atoll located 230 km north of the main island of New Caledonia. It is a 24 ha carbonate islet reaching a maximum of 9 m in elevation. Terrestrial habitats include a central open area with bare ground and patches of various herbaceous plant species surrounded by woody vegetation78. Surprise provides refuge for at least 10 breeding seabird species (mostly wedge-tailed shearwater, brown and red-footed booby, great and lesser frigatebirds, black noddy, and sooty tern), totaling ca. 5,000 pairs35. Reynard islet belongs to the Bampton reefs in the north part of the Chesterfield Islands (central Coral Sea, at ca. 550 km north-west from New-Caledonia’s main island). It is a 8.1 ha low-lying carbonate islet, with low and grassy vegetation, housing 8 breeding seabird species for a total of >40,000 breeding pairs34. Both islets are surrounded by coral reefs supporting hard coral dominated benthic assemblages38, 39. As all islands in the region are hosting seabirds, it was not possible to get control samples at corresponding distances from a seabird-free island. For this reason, a reference station was chosen far from any seabird nesting island and with similar benthic assemblages at 50 m off the reef crest towards the lagoon for both sites (Fig. 1). As a comparison, two sites in the southern lagoon of Nouméa characterized by different levels of anthropogenic impact were sampled: Sainte Marie receiving urban nutrient inputs from the nearby city, and Crouy far from nutrient inputs (15 km from the city, Fig. 1).
Coral and guano sampling and analysis
Coral collection (sampling license issued by Government of New Caledonia) took place in November 2015 (austral summer), during the breeding season of most of the seabird species present in the study area34, 35. The stations were chosen to be gradually farther away from the islets and followed that of a co-occurring and more extensive coral survey, which is why the site distances differed between islets. At Surprise, P. damicornis was collected by SCUBA at 25, 50 and 100 m from the shore. At Reynard, samples were collected at 50, 400 and 800 m from the shore (Fig. 2). In Nouméa, corals were sampled in December 2014. Five branch portions (5 cm) were cut from five parent colonies at each sampling station (~5 m depth) and frozen until analysis. The tissue was removed from the skeleton using an air-pick79 and homogenized with a Potter tissue grinder. Zooxanthellae and coral tissue were separated by centrifugation80 and tissue was filtered through pre-combusted 25mm GF/F filters to concentrate coral tissues. Both zooxanthellae and filters were frozen, dried at 60 °C, and encapsulated in tin/silver cups for δ15N analysis (zooxanthellae were ground). Guano was also sampled from the nests of different seabird species at Surprise and Reynard islets, dried, homogeneized and prepared as described above for δ15N analysis (i.e., the red-footed booby Sula sula, the great frigatebird Fregata minor and the brown booby Sula leucogaster plotus). All samples were analyzed using a Thermo Delta Advantage mass spectrometer in continuous flow mode connected to a Costech Elemental Analyzer via a ConFlo IV at Union College (Schenectady, NY, USA). The combined uncertainty (analytical uncertainty and average correction factor) for δ15N (Air) is ±0.15‰, based on an in-house acetanilide standard.
Nutrient sampling and analysis
Unfiltered triplicates of surface seawater 40 ml samples were collected at Reynard islet (i.e., on November 10th and 22nd 2015) and Surprise islet (October 10th 2016 and March 2nd 2017) at different distances from the islets (Table 1). Nutrients were sampled in both reference sites at 50 m from the reef crest. The reference site for Surprise was also sampled at 400 m from the reef crest, and showed similar NOx values than at 50 m (0.47 vs 0.46 µM). This supports our assumption that any distance from the reef crest at the reference site is representative of waters without seabird influence. Samples were also collected in Nouméa, at Sainte Marie and Crouy, on October 31st 2014. All water samples were fixed with HgCl2 pending nitrate + nitrite (NO3 + NO2) analysis. Nitrate + nitrite (reported as NOx) concentrations were determined according to Raimbault et al.81 on a Bran-Luebbe III continuous flow autoanalyzer.
Statistical analyses
The δ15N values of coral tissues and zooxanthellae and nutrient concentrations sampled at different distances from the shore were tested for difference against the reference sites with Generalized Linear Models (GLM) in R82. For each GLM, the chosen islet reference site (see above) was defined as the model’s intercept, which allowed to directly test for a significant difference in the values observed at each station against that of the reference site for that islet. Due to the small sample size, model performance was evaluated by a visual examination of the distribution of the quantile residuals as implemented in the R package ‘statmod’83. A Gamma error distribution with an inverse link was selected for the final models as its properties matched those of the response variables and it resulted in improved residual diagnostics without the need for transformation. A p-value of <0.05 was considered as statistically significant. Reported values throughout were expressed as mean ± SD.
Electronic supplementary material
Acknowledgements
Coral and seawater were sampled during the cruise CHEST (http://dx.doi.org/10.17600/15004500, coral sampling authorization N°2015-2303/GNC from the Government of New Caledonia) and Post-Blanco1 (sampling authorization N°2017-575/GNC from the Government of New Caledonia) on board of the RV Alis (IRD). For Surprise, we thank the Government of New Caledonia and the Affaires Maritimes for providing the RV Amborella and permission to access to the reef. We thank the LAMA laboratory, Philippe Gérard and the US IMAGO for nutrient analyses, Jean Louis Menou for guano sampling, Sarah Katz and Mason King for assistance in the isotope lab and Boris Colas from SPC for help in producing Figure 5. We also thank the US National Science Foundation for funding Union College’s isotope ratio mass spectrometer and peripherals (NSF-MRI #1229258).
Author Contributions
Conceived and designed the experiments: A.L., C.M., E.V. Realized the sampling: F.B., F.H., G.B., C.P., H.J. Performed the analyses: L.B., D.P.G. and A.V., Analyzed the data: A.L., L.B. L.T.B. Wrote the paper: A.L., F.H., D.P.G., E.V., C.M., L.T.B. and F.B. All authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-03781-y
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Barrett K, et al. Marine subsidies alter the diet and abundance of insular and coastal lizard populations. Oikos. 2005;109:145–153. doi: 10.1111/j.0030-1299.2005.13728.x. [DOI] [Google Scholar]
- 2.Polis GA, Hurd SD. Linking Marine and Terrestrial Food Webs: Allochthonous Input from the Ocean Supports High Secondary Productivity on Small Islands and Coastal Land Communities. Am. Nat. 1996;147:396–423. doi: 10.1086/285858. [DOI] [Google Scholar]
- 3.Gende SM, Edwards RT, Willson MF, Wipfli MS. Pacific Salmon in Aquatic and Terrestrial Ecosystems. BioScience. 2002;52:917–928. doi: 10.1641/0006-3568(2002)052[0917:PSIAAT]2.0.CO;2. [DOI] [Google Scholar]
- 4.Gagnon K, Rothäusler E, Syrjänen A, Yli-Renko M, Jormalainen V. Seabird Guano Fertilizes Baltic Sea Littoral Food Webs. Plos One. 2013;8:e61284. doi: 10.1371/journal.pone.0061284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mizota C, Noborio K, Mori Y. The Great Cormorant (Phalacrocorax carbo) colony as a ‘hot spot’ of nitrous oxide (N2O) emission in central Japan. Atmos. Environ. 2012;57:29–34. doi: 10.1016/j.atmosenv.2012.02.007. [DOI] [Google Scholar]
- 6.Bird MI, Tait E, Wurster CM, Furness RW. Stable carbon and nitrogen isotope analysis of avian uric acid. Rapid Commun. Mass Spectrom. 2008;22:3393–3400. doi: 10.1002/rcm.3739. [DOI] [PubMed] [Google Scholar]
- 7.Caut S, et al. Seabird Modulations of Isotopic Nitrogen on Islands. PLOS ONE. 2012;7:e39125. doi: 10.1371/journal.pone.0039125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mulder, C. P., Anderson, W. B., Towns, D. R. & Bellingham, P. J. Seabird islands: ecology, invasion, and restoration (Oxford University Press, 2011).
- 9.McFadden TN, Kauffman JB, Bhomia RK. Effects of nesting waterbirds on nutrient levels in mangroves, Gulf of Fonseca, Honduras. Wetl. Ecol. Manag. 2016;24:217–229. doi: 10.1007/s11273-016-9480-4. [DOI] [Google Scholar]
- 10.Zwolicki A, Zmudczyńska-Skarbek KM, Iliszko L, Stempniewicz L. Guano deposition and nutrient enrichment in the vicinity of planktivorous and piscivorous seabird colonies in Spitsbergen. Polar Biol. 2013;36:363–372. doi: 10.1007/s00300-012-1265-5. [DOI] [Google Scholar]
- 11.Doughty CE, et al. Global nutrient transport in a world of giants. Proc. Natl. Acad. Sci. 2016;113:868–873. doi: 10.1073/pnas.1502549112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Honig SE, Mahoney B. Evidence of seabird guano enrichment on a coral reef in Oahu, Hawaii. Mar. Biol. 2016;163:1–7. doi: 10.1007/s00227-015-2808-4. [DOI] [Google Scholar]
- 13.Kolb GS, Ekholm J, Hambck PA. Effects of seabird nesting colonies on algae and aquatic invertebrates in coastal waters. Mar. Ecol. Prog. Ser. 2010;417:287–300. doi: 10.3354/meps08791. [DOI] [Google Scholar]
- 14.Wainright SC, Haney JC, Kerr C, Golovkin AN, Flint MV. Utilization of nitrogen derived from seabird guano by terrestrial and marine plants at St. Paul, Pribilof Islands, Bering Sea, Alaska. Mar. Biol. 1998;131:63–71. doi: 10.1007/s002270050297. [DOI] [Google Scholar]
- 15.Staunton Smith J, Johnson C. Nutrient inputs from seabirds and humans on a populated coral cay. Mar. Ecol. Prog. Ser. 1995;124:189–200. doi: 10.3354/meps124189. [DOI] [Google Scholar]
- 16.Furnas MJ. The behavior of nutrients in tropical aquatic ecosystems. Pollut. Trop. Aquat. Syst. 1992;1:30–65. [Google Scholar]
- 17.Hatcher BG. Coral reef primary productivity. A hierarchy of pattern and process. Trends Ecol. Evol. 1990;5:149–155. doi: 10.1016/0169-5347(90)90221-X. [DOI] [PubMed] [Google Scholar]
- 18.Falkowski PG, Dubinsky Z, Muscatine L, McCloskey L. Population Control in Symbiotic Corals. BioScience. 1993;43:606–611. doi: 10.2307/1312147. [DOI] [Google Scholar]
- 19.Marubini F, Davies PS. Nitrate increases zooxanthellae population density and reduces skeletogenesis in corals. Mar. Biol. 1996;127:319–328. doi: 10.1007/BF00942117. [DOI] [Google Scholar]
- 20.Muscatine L. The role of symbiotic algae in carbon and energy flux in reef corals. Ecosyst. World. 1990;25:75–87. [Google Scholar]
- 21.Ferrier MD. Net uptake of dissolved free amino acids by four scleractinian corals. Coral Reefs. 1991;10:183–187. doi: 10.1007/BF00336772. [DOI] [Google Scholar]
- 22.Furla P, et al. The Symbiotic Anthozoan: A Physiological Chimera between Alga and Animal. Integr. Comp. Biol. 2005;45:595–604. doi: 10.1093/icb/45.4.595. [DOI] [PubMed] [Google Scholar]
- 23.Mills MM, Lipschultz F, Sebens KP. Particulate matter ingestion and associated nitrogen uptake by four species of scleractinian corals. Coral Reefs. 2004;23:311–323. doi: 10.1007/s00338-004-0380-3. [DOI] [Google Scholar]
- 24.Mills MM, Sebens KP. Ingestion and assimilation of nitrogen from benthic sediments by three species of coral. Mar. Biol. 2004;145:1097–1106. doi: 10.1007/s00227-004-1398-3. [DOI] [Google Scholar]
- 25.Houlbrèque F, Tambutt E, Richard C, FerrierPags C. Importance of a micro-diet for scleractinian corals. Mar. Ecol. Prog. Ser. 2004;282:151–160. doi: 10.3354/meps282151. [DOI] [Google Scholar]
- 26.Ferrier-Pagès C, Witting J, Tambutté E, Sebens KP. Effect of natural zooplankton feeding on the tissue and skeletal growth of the scleractinian coral Stylophora pistillata. Coral Reefs. 2003;22:229–240. doi: 10.1007/s00338-003-0312-7. [DOI] [Google Scholar]
- 27.Stambler N, Popper N, Dubinsky Z, Stimson J. Effects of Nutrient Enrichment and Water Motion on the Coral Pocillopora damicornis. Pac. Sci. 1991;45:299–407. [Google Scholar]
- 28.Marubini F, Thake B. Bicarbonate addition promotes coral growth. Limnol. Oceanogr. 1999;44:716–720. doi: 10.4319/lo.1999.44.3.0716. [DOI] [Google Scholar]
- 29.Ferrier-Pagès C, Leclercq N, Jaubert J, Pelegr SP. Enhancement of pico- and nanoplankton growth by coral exudates. Aquat. Microb. Ecol. 2000;21:203–209. doi: 10.3354/ame021203. [DOI] [Google Scholar]
- 30.Renegar DA, Riegl BM. Effect of nutrient enrichment and elevated CO2 partial pressure on growth rate of Atlantic scleractinian coral Acropora cervicornis. Mar. Ecol. Prog. Ser. 2005;293:69–76. doi: 10.3354/meps293069. [DOI] [Google Scholar]
- 31.Maire E, et al. How accessible are coral reefs to people ? A global assessment based on travel time. Ecol. Lett. 2016;19:351–360. doi: 10.1111/ele.12577. [DOI] [PubMed] [Google Scholar]
- 32.Bonnet S, et al. Contrasted geographical distribution of N2 fixation rates and nifH phylotypes in the Coral and Solomon Seas (southwestern Pacific) during austral winter conditions. Glob. Biogeochem. Cycles. 2015;29:2015GB005117. doi: 10.1002/2015GB005117. [DOI] [Google Scholar]
- 33.Gardes, L. et al. Analyse stratégique de l’Espace maritime de la Nouvelle-Calédonie – vers une gestion intégrée (2014).
- 34.Borsa P, Pandolfi M, Andréfouët S, Bretagnolle V. Breeding Avifauna of the Chesterfield Islands, Coral Sea: Current Population Sizes, Trends, and Threats. Pac. Sci. 2010;64:297–314. doi: 10.2984/64.2.297. [DOI] [Google Scholar]
- 35.Robinet O, Sirgouant S, Bretagnolle V. Marine Birds of d’Entrecasteaux Reefs (New Caledonia, Southwestern Pacific): Diversity, Abundance, Trends and Threats. Colon. Waterbirds. 1997;20:282–290. doi: 10.2307/1521694. [DOI] [Google Scholar]
- 36.Anderson WB, Polis GA. Nutrient fluxes from water to land: seabirds affect plant nutrient status on Gulf of California islands. Oecologia. 1999;118:324–332. doi: 10.1007/s004420050733. [DOI] [PubMed] [Google Scholar]
- 37.Mizutani H, Kabaya Y, Wada E. Ammonia volatilization and high 15N/14N ratio in a penguin rookery in Antarctica. Geochem. J. 1985;19:323–327. doi: 10.2343/geochemj.19.323. [DOI] [Google Scholar]
- 38.Lasne, G. In Contribution to the biological inventory and resource assessment of the Chesterfield reefs (Clua, E., Gardes, L., McKenna, S., Vieux, C., 2011).
- 39.Wantiez, L., Frolla, P., Goroparawa, D. & Keller, F. Communautés biologiques et habitats coralliens des atolls d’Entrecasteaux. Etat des lieux 2012. Maintien de l’intégrité du bien (2013).
- 40.Houlbrèque F, Ferrier-Pagès C. Heterotrophy in Tropical Scleractinian Corals. Biol. Rev. 2009;84:1–17. doi: 10.1111/j.1469-185X.2008.00058.x. [DOI] [PubMed] [Google Scholar]
- 41.Blais JM, et al. Arctic Seabirds Transport Marine-Derived Contaminants. Science. 2005;309:445–445. doi: 10.1126/science.1112658. [DOI] [PubMed] [Google Scholar]
- 42.Mizutani H, Wada E. Nitrogen and Carbon Isotope Ratios in Seabird Rookeries and their Ecological Implications. Ecology. 1988;69:340–349. doi: 10.2307/1940432. [DOI] [Google Scholar]
- 43.Szpak P, Millaire J-F, White CD, Longstaffe FJ. Influence of seabird guano and camelid dung fertilization on the nitrogen isotopic composition of field-grown maize (Zea mays) J. Archaeol. Sci. 2012;39:3721–3740. doi: 10.1016/j.jas.2012.06.035. [DOI] [Google Scholar]
- 44.Bythell JC. Nutrient uptake in the reef-building coral Acropora Palmata at natural environmental concentrations. Mar Ecol Prog Ser. 1990;68:65–69. doi: 10.3354/meps068065. [DOI] [Google Scholar]
- 45.Grover R, Maguer J-F, Reynaud-Vaganay S, Ferrier-Pages C. Uptake of ammonium by the scleractinian coral Stylophora pistillata: effect of feeding, light, and ammonium concentrations. Limnol. Oceanogr. 2002;47:782–790. doi: 10.4319/lo.2002.47.3.0782. [DOI] [Google Scholar]
- 46.Grover R, Maguer J-F, Allemand D, Ferrier-Pages C. Nitrate uptake in the scleractinian coral Stylophora pistillata. Limnol. Oceanogr. 2003;48:2266–2274. doi: 10.4319/lo.2003.48.6.2266. [DOI] [Google Scholar]
- 47.Hoegh-Guldberg O, Williamson J. Availability of two forms of dissolved nitrogen to the coral Pocillopora damicornis and its symbiotic zooxanthellae. Mar. Biol. 1999;133:561–570. doi: 10.1007/s002270050496. [DOI] [Google Scholar]
- 48.Ward EJ, Semmens BX, Phillips DL, Moore JW, Bouwes N. A quantitative approach to combine sources in stable isotope mixing models. Ecosphere. 2011;2:1–11. doi: 10.1890/ES10-00190.1. [DOI] [Google Scholar]
- 49.Phillips DL. Converting isotope values to diet composition: the use of mixing models. J. Mammal. 2012;93:342–352. doi: 10.1644/11-MAMM-S-158.1. [DOI] [Google Scholar]
- 50.Fry, B. Stable Isotope Ecology (Springer, 2006).
- 51.Mumby PJ. Herbivory versus corallivory: are parrotfish good or bad for Caribbean coral reefs? Coral Reefs. 2009;28:683–690. doi: 10.1007/s00338-009-0501-0. [DOI] [Google Scholar]
- 52.Mumby PJ, et al. Fishing, trophic cascades, and the process of grazing on coral reefs. Science. 2006;311:98–101. doi: 10.1126/science.1121129. [DOI] [PubMed] [Google Scholar]
- 53.Lorrain A, et al. Nitrogen isotopic baselines and implications for estimating foraging habitat and trophic position of yellowfin tuna in the Indian and Pacific Oceans. Deep Sea Res. Part II Top. Stud. Oceanogr. 2015;113:188–198. doi: 10.1016/j.dsr2.2014.02.003. [DOI] [Google Scholar]
- 54.Fabricius KE, Cséke S, Humphrey C, De’ath G. Does Trophic Status Enhance or Reduce the Thermal Tolerance of Scleractinian Corals? A Review, Experiment and Conceptual Framework. Plos One. 2013;8:e54399. doi: 10.1371/journal.pone.0054399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wooldridge SA, Done TJ. Improved water quality can ameliorate effects of climate change on corals. Ecol. Appl. 2009;19:1492–1499. doi: 10.1890/08-0963.1. [DOI] [PubMed] [Google Scholar]
- 56.Nordemar I, Nyström M, Dizon R. Effects of elevated seawater temperature and nitrate enrichment on the branching coral Porites cylindrica in the absence of particulate food. Mar. Biol. 2003;142:669–677. doi: 10.1007/s00227-002-0989-0. [DOI] [Google Scholar]
- 57.Wooldridge SA. A new conceptual model for the warm-water breakdown of the coral–algae endosymbiosis. Mar. Freshw. Res. 2009;60:483–496. doi: 10.1071/MF08251. [DOI] [Google Scholar]
- 58.Béraud E, Gevaert F, Rottier C, Ferrier-Pagès C. The response of the scleractinian coral Turbinaria reniformis to thermal stress depends on the nitrogen status of the coral holobiont. J. Exp. Biol. 2013;216:2665–2674. doi: 10.1242/jeb.085183. [DOI] [PubMed] [Google Scholar]
- 59.Chauvin A, Denis V, Cuet P. Is the response of coral calcification to seawater acidification related to nutrient loading? Coral Reefs. 2011;30:911. doi: 10.1007/s00338-011-0786-7. [DOI] [Google Scholar]
- 60.Tanaka Y, et al. Nutrient availability affects the response of juvenile corals and the endosymbionts to ocean acidification. Limnol Ocean. 2014;59:1468–1476. doi: 10.4319/lo.2014.59.5.1468. [DOI] [Google Scholar]
- 61.Beman JM, et al. Global declines in oceanic nitrification rates as a consequence of ocean acidification. Proc. Natl. Acad. Sci. USA. 2011;108:208–213. doi: 10.1073/pnas.1011053108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vermeij MJA, et al. The Effects of Nutrient Enrichment and Herbivore Abundance on the Ability of Turf Algae to Overgrow Coral in the Caribbean. Plos One. 2010;5:e14312. doi: 10.1371/journal.pone.0014312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.De’ath G, Fabricius K. Water quality as a regional driver of coral biodiversity and macroalgae on the Great Barrier Reef. Ecol. Appl. 2010;20:840–850. doi: 10.1890/08-2023.1. [DOI] [PubMed] [Google Scholar]
- 64.McCook LJ. Macroalgae, nutrients and phase shifts on coral reefs: scientific issues and management consequences for the Great Barrier Reef. Coral Reefs. 1999;18:357–367. doi: 10.1007/s003380050213. [DOI] [Google Scholar]
- 65.Nyström M, Folke C, Moberg F. Coral reef disturbance and resilience in a human-dominated environment. Trends Ecol. Evol. 2000;15:413–417. doi: 10.1016/S0169-5347(00)01948-0. [DOI] [PubMed] [Google Scholar]
- 66.Young, H. S., Hurrey, L. & Kolb, G. S. In Seabird islands: Ecology, invasion, and restoration 243–260 (Mulder, C. P. H., Anderson, W. B., Towns, D. R. & Bellingham, P. J., 2011).
- 67.Fichez R, et al. Biogeochemical typology and temporal variability of lagoon waters in a coral reef ecosystem subject to terrigeneous and anthropogenic inputs (New Caledonia) Mar. Pollut. Bull. 2010;61:309–322. doi: 10.1016/j.marpolbul.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 68.Le Borgne R, Douillet P, Fichez R, Torréton J-P. Hydrography and plankton temporal variabilities at different time scales in the southwest lagoon of New Caledonia: A review. Mar. Pollut. Bull. 2010;61:297–308. doi: 10.1016/j.marpolbul.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 69.Torréton J-P, et al. Variability of primary and bacterial production in a coral reef lagoon (New Caledonia) Mar. Pollut. Bull. 2010;61:335–348. doi: 10.1016/j.marpolbul.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 70.Riddick SN, et al. Measurement of ammonia emissions from tropical seabird colonies. Atmos. Environ. 2014;89:35–42. doi: 10.1016/j.atmosenv.2014.02.012. [DOI] [Google Scholar]
- 71.Lorrain A, et al. Differential δ13C and δ15N signatures among scallop tissues: implications for ecology and physiology. J. Exp. Mar. Biol. Ecol. 2002;275:47–61. doi: 10.1016/S0022-0981(02)00220-4. [DOI] [Google Scholar]
- 72.Smith, J. L., Mulder, C. P. H. & Ellis, J. C. Seabirds as ecosystem engineers: nutrient inputs and physical disturbance. Seab. Isl. Ecol. Invasion Restor. Oxf. Univ. Press N. Y. 27–55 (2011).
- 73.Gillikin DP, et al. High-resolution nitrogen stable isotope sclerochronology of bivalve shell carbonate-bound organics. Geochim. Cosmochim. Acta. 2017;200:55–66. doi: 10.1016/j.gca.2016.12.008. [DOI] [Google Scholar]
- 74.Marion GS, et al. Coral skeletal δ15N reveals isotopic traces of an agricultural revolution. Mar. Pollut. Bull. 2005;50:931–944. doi: 10.1016/j.marpolbul.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 75.Paleczny M, Hammill E, Karpouzi V, Pauly D. Population Trend of the World’s Monitored Seabirds, 1950–2010. Plos One. 2015;10:e0129342. doi: 10.1371/journal.pone.0129342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sekercioglu CH. Increasing awareness of avian ecological function. Trends Ecol. Evol. 2006;21:464–471. doi: 10.1016/j.tree.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 77.Sekercioglu ÇH, Daily GC, Ehrlich PR. Ecosystem consequences of bird declines. Proc. Natl. Acad. Sci. 2004;101:18042–18047. doi: 10.1073/pnas.0408049101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Caut S, Angulo E, Courchamp F. Dietary shift of an invasive predator: rats, seabirds and sea turtles. J. Appl. Ecol. 2008;45:428–437. doi: 10.1111/j.1365-2664.2007.01438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rodolfo-Metalpa R, Martin S, Ferrier-Pagès C, Gattuso J-P. Response of the temperate coral Cladocora caespitosa to mid- and long-term exposure to pCO2 and temperature levels projected for the year 2100 AD. Biogeosciences. 2010;7:289–300. doi: 10.5194/bg-7-289-2010. [DOI] [Google Scholar]
- 80.Houlbrèque F, Tambutté E, Ferrier-Pagès C. Effect of zooplankton availability on the rates of photosynthesis, and tissue and skeletal growth in the scleractinian coral Stylophora pistillata. J. Exp. Mar. Biol. Ecol. 2003;296:145–166. doi: 10.1016/S0022-0981(03)00259-4. [DOI] [Google Scholar]
- 81.Raimbault P, Slawyk G, Coste B, Fry J. Feasibility of using an automated colorimetric procedure for the determination of seawater nitrate in the 0 to 100 nM range: Examples from field and culture. Mar. Biol. 1990;104:347–351. doi: 10.1007/BF01313277. [DOI] [Google Scholar]
- 82.R Development Core Team. R: A language and environment for statistical computing (R Foundation for Statistical Computing, 2016).
- 83.Dunn PK, Smyth GK. Randomized quantile residuals. J. Comput. Graph. Stat. 1996;5:236–244. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


