Abstract
Importance
Cancer treatments are associated with subsequent neoplasms in childhood cancer survivors. It is unknown whether temporal changes in therapy are associated with changes in subsequent neoplasm risk.
Objective
Quantify the association between temporal treatment dosing changes and subsequent neoplasm risk.
Design, Setting, Participants
Retrospective, multicenter cohort of five-year cancer survivors diagnosed before age 21 years from pediatric tertiary hospitals in the United States and Canada between 1970-1999, with follow-up through December 2015.
Exposures
Radiation and chemotherapy dose changes over time.
Main Outcomes and Measures
Subsequent neoplasm 15-year cumulative incidence, cumulative burden, and standardized incidence ratios (SIRs) for subsequent malignancies were compared by treatment decade. Multivariable models assessed relative rates (RRs) of subsequent neoplasms by 5-year increments, adjusting for demographic and clinical characteristics. Mediation analyses assessed whether changes in subsequent neoplasm rates over time were mediated by treatment variable modifications.
Results
Among 23,603 childhood cancer survivors (mean age at diagnosis, 7.7 years; 46% female) the most common initial diagnoses were acute lymphoblastic leukemia, Hodgkin lymphoma and astrocytoma. During a mean follow up of 20.5 years (374,638 person-years at risk), 1,639 survivors experienced 3,115 subsequent neoplasms, including 1,026 malignancies, 233 benign meningiomas, and 1,856 non-melanoma skin cancers. The most common subsequent malignancies were breast and thyroid cancers. Individuals receiving radiation decreased (1970s, 77% vs. 1990s, 33%), as did median dose (1970s, 30 Gy [IQR 24-44] vs. 1990s, 26 Gy [IQR 18-45]). Fifteen-year cumulative incidence of subsequent malignancies decreased by decade of diagnosis (1990s: 1.3%, 95%CI 1.1-1.5, 1980s: 1.7%, 95%CI 1.5-2.0, 1970s: 2.1%, 95%CI 1.7-2.4). Reference absolute rates per 1,000 person-years for subsequent malignancies, meningiomas and non-melanoma skin cancers were 1.12 (95%CI 0.84-1.57), 0.16 (95%CI 0.06-0.41), and 1.71 (95%CI 0.88-3.33), respectively, for survivors with reference characteristics (no chemotherapy, splenectomy or radiation therapy, male, attained age of 28). SIRs declined for subsequent malignancies over treatment decades, with advancing attained age. Relative rates declined with each 5-year increment for subsequent malignancies (RR=0.87, 95%CI 0.82-0.93, p<0.001), meningiomas (RR=0.85, 95%CI 0.75-0.97, p=0.034), and non-melanoma skin cancers (RR=0.75, 95%CI 0.67-0.84, p<0.001). Radiation dose changes were associated with reduced risk for subsequent malignancies, meningiomas, and non-melanoma skin cancers.
Conclusions and Relevance
Among childhood cancer survivors, the risk of subsequent malignancies at 15 years after initial cancer diagnosis remained increased for those diagnosed in the 1990s, although the risk was lower compared with those diagnosed in the 1970s. This lower risk was associated with reduction in therapeutic radiation dose.
Introduction
As cure rates for childhood cancers have improved, there has been an increasing awareness of the late health consequences of childhood cancer therapies. One outcome associated with significant morbidity and mortality for these survivors is the development of subsequent neoplasms, unique from recurrence of the original childhood malignancy 1. Survivors with a subsequent neoplasm are more likely to report adverse general and mental health outcomes 2, and have increased hospitalization rates 3 compared to survivors without a subsequent neoplasm. Subsequent malignant neoplasms are the most common non-relapse cause of late mortality, accounting for approximately half of all non-relapse deaths among five-year survivors 1,4.
The Childhood Cancer Survivor Study (CCSS) 5 and other cohorts of childhood cancer survivors 6 have reported extensively on the incidence of and risk factors for subsequent neoplasms 7-9. Therapeutic radiation has been strongly associated with development of subsequent neoplasms 10; however, dose-response relationships have also been identified for specific chemotherapeutic agents, including alkylating agents and epipodophyllotoxins 11-13. With this knowledge, childhood cancer treatment has been modified over time with the hope of reducing subsequent neoplasm risk, while maintaining or improving 5-year survival 14,15.
The CCSS cohort was recently expanded to include survivors diagnosed and treated across three decades (1970-1999). The aim of the present analysis was to assess temporal changes in subsequent neoplasms among individuals diagnosed during this period. We hypothesized that historical modifications in radiotherapy and chemotherapy dosing would be associated with changes in the incidence of subsequent neoplasms among five-year childhood cancer survivors, based on their decade of diagnosis, throughout the course of follow-up.
Materials and Methods
CCSS Cohort
The CCSS is a retrospective cohort study with longitudinal follow-up of 5-year survivors of childhood cancer diagnosed at one of 27 participating institutions in the United States or Canada between January 1, 1970 and December 31, 1999. Participants were <21 years of age at initial diagnosis of leukemia, Hodgkin lymphoma, non-Hodgkin lymphoma, central nervous system cancer, Wilms tumor, neuroblastoma, rhabdomyosarcoma, or bone cancer. Human subjects committee approval was granted at participating institutions prior to recruitment. Participants, or parents of children <18 years of age, provided informed consent. Minor participants were re-consented once they reached 18 years. Participants completed a baseline and up to four follow-up questionnaires. Race/ethnicity data was obtained through self-report. Participants were asked to select: White, Black, American Indian or Alaska Native, Asian or Pacific Islander, or Other, with the option to write in their race. Hispanic ethnicity was ascertained through a yes/no question. Race and ethnicity were included in the analysis for descriptive purposes. The final date of follow up was December 31, 2015. Study design and methods have been previously described 5,16.
Subsequent Neoplasm Ascertainment and Therapeutic Agents
Subsequent neoplasms were identified via self- or next-of-kin proxy-report or death certificate and confirmed by pathology report, or when unavailable, death certificate and/or medical records. Only subsequent neoplasms occurring ≥5 years following initial cancer diagnosis were evaluated. Subsequent neoplasms were classified into three mutually exclusive groups, based on historical experience with frequently occurring neoplasm sub-types: 1) subsequent malignant neoplasms, which include invasive neoplasms classified as International Classification of Disease for Oncology (ICD-O, 3rd version), behavior code of 3 17, excluding non-melanoma skin cancers; 2) benign meningiomas; and 3) non-melanoma skin cancers (including ICD-O morphology codes 8070, 8071, 8081, 8090 and 8094). Cancer therapies, including surgery, chemotherapy and radiation, were ascertained through medical record abstraction, as previously described 16,18. Cumulative alkylating agent dose was reported as a cyclophosphamide equivalent dose 19. Maximum radiation treatment dose was calculated for eight body regions (brain, other head, neck, chest, abdomen, pelvis, arm and leg) for each patient. For this analysis, we considered any radiation treatment (yes/no) for cumulative incidence and cumulative burden estimates, and maximum doses for multivariable models.
Statistical Methods
Cohort follow-up started at five years from diagnosis and ended upon death or date of last completed questionnaire. Cumulative burden, assessed using the mean cumulative count, 20,21 and cumulative incidence were estimated using time from initial diagnosis as the time scale, treating death as a competing risk event. Cumulative burden is an estimate of the average number of subsequent neoplasms per 100 survivors by a given time, in the presence of competing risk events, and accounts for multiple events in individuals, whereas cumulative incidence only accounts for the first event. Cumulative incidence and cumulative burden at 15 years from diagnosis were compared across treatment decades using permutation tests.
For subsequent malignant neoplasms, standardized incidence ratios (SIRs) (ratio of the observed to expected number of events) and absolute excess risk per 1,000 person years were calculated using age-sex-calendar-year-specific U.S. cancer incidence rates from the Surveillance, Epidemiology, and End Results program to determine expected numbers of events 22. Because comparison by treatment era is subject to confounding by attained age, SIRs were calculated stratifying on 10-year age intervals. Multivariable piecewise-exponential models were used to assess the incidence rate of subsequent neoplasm types, in association with demographic variables and childhood cancer diagnosis, adjusting for attained age, treatment doses and 5-year treatment eras. Reference absolute rates per 1,000 person-years were calculated using the fitted model for survivors with reference characteristics (no chemotherapy, splenectomy or radiation therapy, male, attained age of 28). Multiple subsequent neoplasm occurrences within individual survivors were included and accounted for in the models by modifications of the models using generalized estimating equations. Adjusted relative rates and 95% confidence intervals were estimated. In addition, using mediation analysis methods previously described 4,23-25, changes in subsequent neoplasm rates in 5-year treatment era increments were estimated with and without adjustment for treatment variables in the same model, to assess whether changes in subsequent neoplasm rates over time were mediated by treatment modifications. Specifically, as shown in the causal diagram that depicts the assumptions of the mediation analysis (eFigure 1), a multivariable piecewise-exponential model was fit to assess the association of treatment era with subsequent neoplasm rates, adjusting for attained age, sex, age at initial cancer diagnosis, and treatment variables (the full model), followed by removal of treatment variables from the model. Attenuation and the statistical significance of the treatment era regression coefficient, the parameter representing the adjusted log rate ratio of subsequent neoplasms by 5-year treatment increments, by the inclusion of treatment variables in the model, constitute the key step of establishing the mediator role of treatment variables, along with the associations of treatment era and treatment variables and those of treatment variables and subsequent neoplasm rates in the full model. Nonparametric bootstrap was used to test statistical significance of the changes in the regression coefficient associated with the 5-year treatment era with and without adjustment for treatment variables. For analyses examining treatment doses, only individuals with available treatment data were included. All tests were two-sided with p<0.05 considered statistically significant. SAS (version 9.4) was used for all statistical analyses including the mediation analysis and R (version 3.2.4) was used for statistical graphics.
Results
Cohort Characteristics
Among the 23,603 eligible, consented survivors (Figure 1), 46% were female, the mean age at primary diagnosis was 7.7 years, and the most common initial diagnoses were acute lymphoblastic leukemia (ALL), Hodgkin lymphoma and astrocytoma. Mean follow-up ranged from 15.7 years for those diagnosed in the 1990s to 27.6 years for survivors diagnosed in the 1970s. Over the course of 374,638 person-years at risk, 1,639 survivors experienced 3,115 subsequent neoplasms including 1,026 subsequent malignancies, 233 benign meningiomas, and 1,856 non-melanoma skin cancers (Table 1). The distribution of subsequent neoplasms by primary cancer diagnosis is shown in eTable 1 and the distribution of observed and expected subsequent malignancies, by decade of initial cancer diagnosis, is provided in eTable 2. The most frequently observed subsequent malignancies were breast and thyroid cancer.
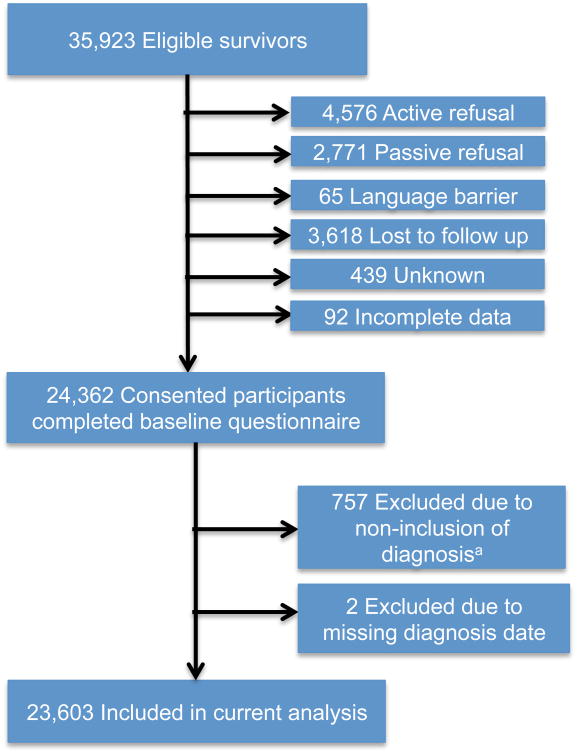
Figure 1. Cohort composition diagram for eligible and enrolled childhood cancer survivors.
aFrom 1970-1986 all types of soft tissue sarcomas (as initial childhood cancer diagnosis) were included in the Childhood Cancer Survivor Study cohort. However, for the period 1987-1999 rhabdomyosarcoma was the only type of soft tissue sarcoma included; thus, in order to have a homogeneous population across decades, we excluded non-rhabdomyosarcoma diagnoses.
Table 1. Demographic and treatment characteristics of survivors of childhood cancer, overall and by treatment era.
| Characteristics | Overall Cohort N=23,603 | 1970-79 N=6,223 | 1980-89 N=9,430 | 1990-99 N=7,950 |
|---|---|---|---|---|
| Mean age at primary diagnosis, years (SD) | 7.7 (6.0) | 8.4 (5.8) | 7.6 (5.8) | 7.4 (6.2) |
| Sex of patient, No. (%) | ||||
| Male | 12,656 (53.7) | 3,323 (53.4) | 5,105 (54.1) | 4,228 (53.5) |
| Female | 10,947 (46.3) | 2,900 (46.6) | 4,325 (45.9) | 3,722 (46.5) |
| Race/Ethnicity | ||||
| White, Non-Hispanic | 19,269 (80.8) | 5,533 (88.9) | 7,795 (82.4) | 5,941 (74.7) |
| Black, Non-Hispanic | 1,485 (6.4) | 241 (3.9) | 574 (6.0) | 670 (8.3) |
| Hispanic/Latino | 1,783 (8.1) | 291 (4.7) | 616 (6.8) | 876 (11.4) |
| Other | 1066 (4.7) | 158 (2.5) | 445 (4.9) | 463 (5.9) |
| Primary diagnosis, No. (%) | ||||
| Leukemia | 7,319 (39.4) | 2,029 (32.6) | 3,333 (40.1) | 1,957 (42.9) |
| Acute lymphoblastic leukemiaa | 6,148 (35.1) | 1,824 (29.3) | 2,894 (35.8) | 1,430 (37.9) |
| Acute myeloid leukemia | 868 (3.2) | 131 (2.1) | 334 (3.3) | 403 (3.8) |
| Other leukemia | 303 (1.1) | 74 (1.2) | 105 (1.0) | 124 (1.2) |
| Lymphoma | 4,928 (18.3) | 1,550 (24.9) | 1,834 (18.0) | 1,544 (14.7) |
| Hodgkin lymphoma | 2,996 (11.1) | 1,097 (17.6) | 1,059 (10.4) | 840 (8.0) |
| Non-Hodgkin lymphoma | 1,932 (7.2) | 453 (7.3) | 775 (7.6) | 704 (6.7) |
| Central nervous system | 4,236 (15.7) | 736 (11.9) | 1,503 (14.7) | 1,997 (19.0) |
| Astrocytoma | 2,594 (9.6) | 509 (8.2) | 946 (9.3) | 1,139 (10.8) |
| Medulloblastoma/Primitive neuroectodermal tumor | 997 (3.7) | 148 (2.4) | 350 (3.4) | 499 (4.8) |
| Other central nervous system cancer | 645 (2.4) | 79 (1.3) | 207 (2.0) | 359 (3.4) |
| Wilms tumor | 2,148 (8.0) | 534 (8.6) | 877 (8.6) | 737 (7.0) |
| Bone cancer | 1,972 (7.4) | 566 (9.1) | 760 (7.5) | 646 (6.1) |
| Osteosarcoma | 1,205 (4.5) | 360 (5.8) | 474 (4.7) | 371 (3.5) |
| Ewing sarcoma | 714 (2.7) | 203 (3.3) | 277 (2.7) | 234 (2.2) |
| Other bone cancers | 53 (0.2) | 3 (0.0) | 9 (0.1) | 41 (0.4) |
| Neuroblastoma | 1,838 (6.8) | 443 (7.1) | 675 (6.6) | 720 (6.9) |
| Rhabdomyosarcoma | 1,162 (4.3) | 365 (5.9) | 448 (4.4) | 349 (3.3) |
| Treatmentb, No. (%) | ||||
| Chemotherapy only | 2,334 (17.2) | 228 (4.2) | 931 (14.0) | 1,175 (26.9) |
| Radiation therapy only | 72 (0.3) | 20 (0.4) | 31 (0.4) | 21 (0.2) |
| Surgery only | 1,867 (7.5) | 273 (5.1) | 629 (6.8) | 965 (9.5) |
| Chemotherapy & Radiation | 2,307 (11.7) | 871 (16.2) | 993 (13.3) | 443 (7.8) |
| Chemotherapy & Surgery | 4,998 (22.1) | 705 (13.1) | 2,011 (22.6) | 2,282 (26.3) |
| Radiation & Surgery | 1,873 (7.6) | 774 (14.4) | 770 (8.4) | 329 (3.2) |
| All 3 treatments | 7,979 (33.3) | 2,483 (46.3) | 3,067 (34.3) | 2,429 (25.6) |
| Any chemotherapy | 17,978 (84.3) | 4,323 (79.8) | 7,089 (84.2) | 6,566 (86.8) |
| Any radiation therapy | 12,400 (53.1) | 4,243 (77.7) | 4,935 (56.7) | 3,222 (36.8) |
| Any surgery | 16,902 (70.0) | 4,269 (78.9) | 6,540 (72.1) | 6,093 (63.6) |
| Maximum radiation treatment dose to any body region, Gy | ||||
| None | 9,369 (49.3) | 1,232 (23.3) | 3,649 (45.6) | 4,488 (66.9) |
| 0.1-10 | 308 (1.3) | 58 (1.1) | 140 (1.6) | 110 (1.2) |
| 10.1-20 | 2,995 (14.7) | 602 (11.4) | 1,642 (20.6) | 751 (11.1) |
| 20.1-30 | 2,833 (12.5) | 1,396 (26.4) | 852 (10.2) | 585 (6.9) |
| 30.1-40 | 1,489 (6.3) | 779 (14.7) | 493 (5.5) | 217 (2.3) |
| 40.1-50 | 1,738 (7.3) | 749 (14.2) | 698 (7.8) | 291 (3.0) |
| >50 | 2,066 (8.6) | 467 (8.8) | 767 (8.6) | 832 (8.6) |
| Median dose (IQR) | 26.0 (18.0-45.0) | 30.0 (24.0-44.0) | 24.0 (18.0-45.0) | 26.0 (18.0-52.0) |
| Median dose for chemotherapy agents (mg/m2) (IQR) | ||||
| Cyclophosphamide equivalents | 7,395 (3,218-12,105) | 10,527 (5,702-16,629) | 7,359 (3,165-11,926) | 6,758 (2,958-10,268) |
| Anthracyclines | 186 (105-320) | 323 (212-436) | 232 (124-351) | 151 (101-247) |
| Epipodophyllotoxins | 2,000 (1,000-4,688) | 968 (640-1,944) | 2,645 (900-7,665) | 1,868 (1,087-4,026) |
| Platinum Agents | 503 (340-1,255) | 418 (294-689) | 444 (313-620) | 600 (360-2,177) |
| Cyclophosphamide equivalent dose (mg/m2) | ||||
| None | 9,743 (47.8) | 2,793 (58.5) | 3,668 (46.6) | 3,282 (43.7) |
| 1-3,999 | 2,560 (15.2) | 341 (7.1) | 1,192 (15.6) | 1,027 (18.8) |
| 4,000-7,999 | 2,640 (12.7) | 406 (8.5) | 1,047 (12.7) | 1,187 (14.6) |
| 8,000+ | 5,077 (24.3) | 1,231 (25.8) | 1,998 (25.1) | 1,848 (22.8) |
| Anthracycline (mg/m2) | ||||
| None | 11,192 (48.9) | 3,720 (72.0) | 4,267 (49.9) | 3,205 (36.1) |
| 0-100 | 1,392 (10.8) | 118 (2.3) | 552 (10.0) | 722 (15.8) |
| 101-300 | 4,994 (25.7) | 529 (10.2) | 1,788 (21.5) | 2,677 (37.5) |
| >300 | 3,278 (14.6) | 798 (15.5) | 1,565 (18.5) | 915 (10.6) |
| Epipodophyllotoxin (mg/m2) | ||||
| None | 17,577 (80.0) | 5,251 (97.9) | 7,191 (84.0) | 5,135 (66.6) |
| 1-1,000 | 1,013 (5.0) | 60 (1.1) | 351 (4.4) | 602 (7.7) |
| 1,001-4,000 | 1,771 (9.4) | 34 (0.6) | 412 (5.9) | 1,325 (17.3) |
| >4,000 | 829 (5.6) | 21 (0.4) | 373 (5.6) | 435 (8.4) |
| Platinum Agent (mg/m2) | ||||
| None | 19,066 (90.7) | 5,332 (98.9) | 7,613 (91.4) | 6,121 (85.7) |
| 1-400 | 807 (3.3) | 28 (0.5) | 343 (3.8) | 436 (4.3) |
| 401-750 | 711 (2.9) | 18 (0.3) | 325 (3.6) | 368 (3.7) |
| >750 | 765 (3.1) | 12 (0.2) | 117 (1.3) | 636 (6.3) |
| History of splenectomy, No. (%) | ||||
| Yes | 1,378 (5.5) | 761 (14.1) | 550 (6.0) | 67 (0.7) |
| No | 20,229 (94.5) | 4,648 (85.9) | 7,944 (94.0) | 7,637 (99.3) |
| Number of survivors with subsequent neoplasms | 1639 | 870 | 180 | 589 |
| Number of subsequent neoplasm, (%) | ||||
| Subsequent neoplasms | 3,115 | 2,018 | 902 | 195 |
| Subsequent malignant neoplasm | 1,026 (34.0) | 523 (25.9) | 364 (41.6) | 139 (72.0) |
| Benign Meningioma | 233 (7.5) | 146 (7.2) | 68 (7.8) | 19 (9.2) |
| Non-melanoma skin cancer | 1,856 (58.5) | 1,349 (66.8) | 470 (50.6) | 37 (18.8) |
| # of person years | 374,638 | 139,489 | 150,506 | 84,643 |
| Mean years of follow up from primary cancer diagnosis, years (SD) | 20.5 (7.5) | 27.6 (7.7) | 21.1 (5.3) | 15.7 (4.1) |
| Number of deaths in the analysis cohort | 1,796 | 732 | 625 | 439 |
| Rate of deathc per 10,000 person years | 63.3 | 74.0 | 57.0 | 53.4 |
Analyses, including reported percentages and means/medians, were weighted to account for undersampling of acute lymphoblastic leukemia (ALL) survivors (1987-1999), with a weight of 1.21 for ALL age 0 or 11-20 years at diagnosis, and a weight of 3.63 for those aged 1-10 years.
577 had data from one or two of the treatments missing. Among the 577, 169 received radiation therapy, 185 received surgery, and 360 received chemotherapy.
Rates are based on the entire eligible cohort, for which NDI has mortality information.
Abbreviations: IQR, interquartile range; SD, standard deviation.
Complete treatment data were available for 83% of the cohort. Between 1970-1999 there were substantial changes in therapies. Radiation therapy decreased from 77% of survivors treated in the 1970s, to 54% in the 1980s and 33% in the 1990s. Median radiation treatment dose decreased from 30 Gy (interquartile range [IQR] 24-44) in the 1970s to 26 Gy (IQR 18-52) in the 1990s. Although the proportion of children treated with alkylating agents and anthracyclines increased over time, median doses decreased. The proportion of children treated with epipodophyllotoxins and platinum agents also increased over the three decades; however, whereas the median cumulative dose of platinum increased with each treatment decade, the median cumulative dose of epipodophyllotoxins increased substantially in the 1980s and then decreased in the 1990s (Table 1).
Cumulative Incidence and Cumulative Burden of Subsequent Neoplasms
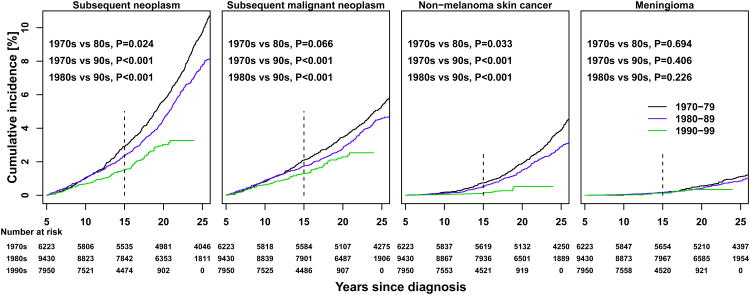
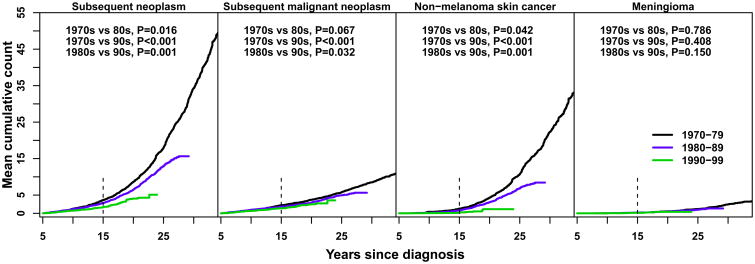
At 15 years from initial diagnosis, the cumulative incidence of subsequent neoplasms was 2.9% (95% CI 2.5-3.3) among individuals diagnosed in the 1970s, 2.4% (95% CI 2.1-2.7) in those diagnosed in the 1980s, and 1.5% (95% CI 1.3-1.8) in survivors from the 1990s (1970s vs. 1980s p=0.024; 1970s vs. 1990s p<0.001; 1980s vs. 1990s p<0.001) (Figure 2A). The cumulative burden of subsequent neoplasms per 100 survivors was 3.6, 2.8 and 1.7 at 15 years in those diagnosed in the 1970s, 1980s and 1990s (Figure 2B), respectively (1970s vs. 1980s p=0.016; 1970s vs. 1990s p<0.001; 1980s vs. 1990s p=0.001). After 20 years from diagnosis, among survivors from the 1970s and 1980s, the steep increase in cumulative burden was secondary to recurrent non-melanoma skin cancer events.
Figure 2. Cumulative incidence (A) and cumulative burden (mean cumulative count per 100 survivors) (B) of subsequent neoplasms, by type and by decade of initial cancer diagnosis.
Black line, 1970-79; blue line, 1980-89; green line, 1990-99. Vertical dashed line at 15 year mark represents the time point of interest. Permutation tests were used to assess differences between curves.
A significantly lower 15-year cumulative incidence of subsequent malignancies was observed in those diagnosed in the 1990s (1.3%, 95% CI 1.1-1.5) compared to the 1980s (1.7%, 95% CI 1.5-2.0, p=0.02) and to the 1970s (2.1%, 95% CI 1.7-2.4, p<0.001) (Figure 2A, eTable 3). A similar decline was seen for non-melanoma skin cancers but not for meningiomas. When assessing incidence by primary cancer diagnosis, declines between decades were seen for Hodgkin lymphoma and Wilms tumor, but only survivors of Hodgkin lymphoma demonstrated a statistically significant decrease in 15-year cumulative incidence of subsequent neoplasms across decades (eFigure 2). Among the most common subsequent malignancies, only soft tissue sarcomas (1970s 0.26%, 95% CI 0.13-0.38 vs. 1990s 0.13%, 95% CI 0.06-0.21, p=0.032) and breast cancers (1970s 0.27%, 95% CI 0.14-0.40 vs. 1990s 0.08% 95% CI 0.02-0.14, p=0.003) had significant decreases in 15-year cumulative incidence from 1970s to 1990s (eFigure 3).
Survivors treated with radiation experienced a higher cumulative incidence of all types of subsequent neoplasms, for all treatment decades (eFigure 4). Cumulative burden for non-melanoma skin cancers compared to cumulative incidence, showed a more pronounced difference based on receipt of radiation therapy, exemplifying the number of radiation-exposed survivors with multiple events. Among irradiated survivors, a significant decrease in the 15-year cumulative incidence of non-melanoma skin cancers was observed for the most recent treatment decade (1970s 1.0%, 95% CI 0.7-1.3; 1980s 0.9%, 95% CI 0.6-1.1; 1990s 0.2%, 95% CI 0.1-0.4; 1970s vs. 1980s, p=0.27; 1970s vs 1990s, p<0.001; 1980s vs. 1990s p<0.001).
Risk of Subsequent Malignant Neoplasms
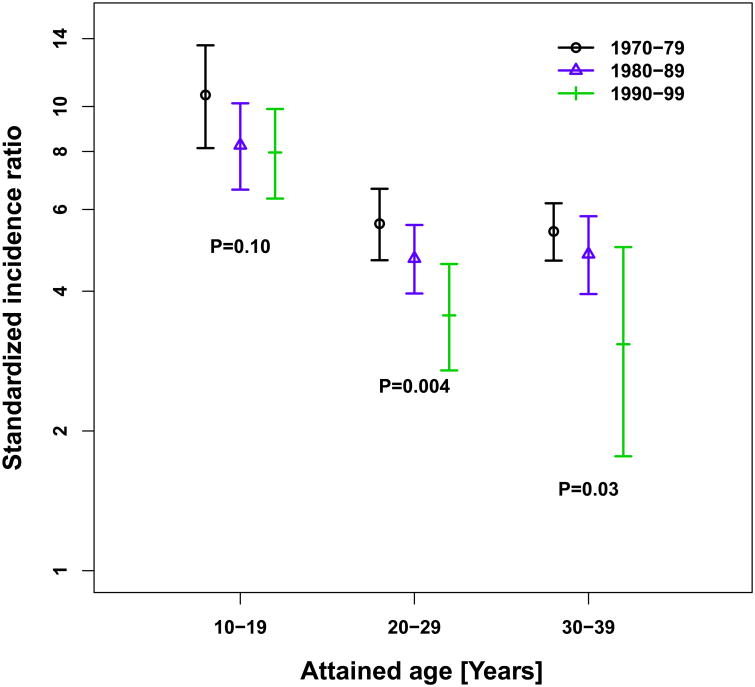
Reference absolute rates per 1,000 person-years was 4.21 (95% CI 3.05-5.81) for subsequent neoplasms, 1.12 (95% CI 0.84-1.57) for subsequent malignancies, 0.16 (95% CI 0.06-0.41) for meningiomas, and 1.71 (95% CI 0.88-3.33) for non-melanoma skin cancers. Lower SIRs were observed by decade of diagnosis for survivors whose attained age was 20-29 years (1970s 5.7, 95% CI 4.7-6.7; 1980s 4.8, 95% CI 4.0-5.6; 1990s 3.6, 95% CI 2.7-4.6; p=0.004) and 30-39 years (1970s 5.6, 95% CI 4.8-6.4; 1980s 4.9, 95% CI 4.1-6.0; 1990s 3.1, 95% CI 1.8-5.0; p=0.03) (Figure 3, eTable 4). Decreases in SIRs across treatment decades within specific subsequent malignancy types were not observed (eFigure 5). Among survivors of Hodgkin lymphoma with attained age ≥20 years, SIRs for subsequent malignancies decreased over time (20-29 years, 1970s 10.7, 95% CI 7.7-14.4; 1980s 6.8, 95% CI 4.4-10.0; 1990s 5.5, 95% CI 3.2-9.0; p=0.016, and 30-39 years, 1970s 10.2, 95% CI 8.2-12.5; 1980s 8.4, 95% CI 6.3-11.0; 1990s 5.2, 95% CI 2.4-9.8; p=0.038) (eTable 5).
Figure 3. Standardized incidence ratios for subsequent malignant neoplasms, by attained age and decade of initial cancer diagnosis.
Black line, 1970-79; blue line, 1980-89; green line, 1990-99. Vertical bars represent 95% confidence intervals. Analyses were weighted to account for undersampling of acute lymphoblastic leukemia (ALL) survivors (1987-1999), with a weight of 1.21 for ALL age 0 or 11-20 years at diagnosis, and a weight of 3.63 for those aged 1-10 years.
The number of individuals from each decade of diagnosis contributing data for each attained age are as follows: 1970s, 10-19 years 5,072; 20-29 years 5,810; 30-39 years 4,809; 1980s, 10-19 years 7,932; 20-29 years 8,283; 30-39 years 3,901; 1990s, 10-19 years 6,622; 20-29 years 5,517; 30-39 years 1,534.
Risk Factors for Subsequent Neoplasms
Multivariable analysis demonstrated that females experienced increased rates of subsequent malignant neoplasms (RR 1.7, 95% CI 1.5-2.0, p<0.001) and meningiomas (RR 1.4, 95% CI 1.0-2.0, p=0.05) compared to males. Treatment with high doses of alkylating agents and platinum agents were also associated with increased rates of subsequent malignancies and therapeutic radiation at all dose increments was associated with increased rates of subsequent malignant neoplasms, meningiomas, and non-melanoma skin cancers (Table 2, crude data reported in eTable 6).
Table 2. Reference absolute rates and relative rates of subsequent neoplasm, overall and by subtypes, according to multivariable analysisa.
| Subsequent neoplasm | Subsequent malignant neoplasm | Meningioma | Non-melanoma skin cancer | |||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Variable | RR (95% CI) | P | RR (95% CI) | P | RR (95% CI) | P | RR (95% CI) | P |
| Reference absolute rate per 1000 person yearsb | 4.21 (3.05-5.81) | 1.12 (0.84-1.57) | 0.16 (0.06-0.41) | 1.71 (0.88-3.33) | ||||
|
| ||||||||
| Sex | ||||||||
| Male | Ref | Ref | Ref | Ref | ||||
| Female | 1.27 (1.05 - 1.52) | 0.01 | 1.73 (1.48 - 2.03) | <0.001 | 1.40 (1.00 - 1.95) | 0.05 | 1.07 (0.80 - 1.44) | 0.66 |
| Age at diagnosis | ||||||||
| 0-4 | Ref | Ref | Ref | Ref | ||||
| 5-9 | 0.68 (0.52 - 0.88) | 0.004 | 1.07 (0.81 - 1.42) | 0.61 | 0.59 (0.38 - 0.92) | 0.02 | 0.56 (0.35 - 0.89) | 0.01 |
| 10-14 | 0.54 (0.40 - 0.73) | <0.001 | 1.09 (0.84 - 1.42) | 0.50 | 0.19 (0.11 - 0.33) | <0.001 | 0.49 (0.30 - 0.81) | 0.005 |
| 15+ | 0.55 (0.41 - 0.74) | <0.001 | 1.12 (0.84 - 1.50) | 0.44 | 0.14 (0.07 - 0.27) | <0.001 | 0.52 (0.32 - 0.85) | 0.009 |
| Year of diagnosis | ||||||||
| Every 5-years | 0.91 (0.84 - 0.98) | 0.01 | 0.93 (0.86 - 1.00) | 0.05 | 0.94 (0.80 - 1.11) | 0.47 | 0.87 (0.76 - 1.00) | 0.05 |
| Maximum radiation treatment dose to any body region (Gy) | ||||||||
| None | Ref | Ref | Ref | Ref | ||||
| 0.1-10 | 4.55 (2.51 - 8.24) | <0.001 | 2.94 (1.48 - 5.86) | 0.002 | 24.39 (4.42 - 134.44) | <0.001 | 6.55 (2.45 - 17.47) | <0.001 |
| 10.1-20 | 3.16 (2.32 - 4.31) | <0.001 | 1.67 (1.23 - 2.27) | 0.001 | 14.77 (5.89 - 37.03) | <0.001 | 5.75 (3.16 - 10.45) | <0.001 |
| 20.1-30 | 3.32 (2.55 - 4.33) | <0.001 | 1.96 (1.49 - 2.58) | <0.001 | 23.44 (9.85 - 55.79) | <0.001 | 4.82 (2.79 - 8.34) | <0.001 |
| 30.1-40 | 4.32 (3.13 - 5.97) | <0.001 | 2.63 (1.98 - 3.50) | <0.001 | 10.91 (3.60 - 33.05) | <0.001 | 6.98 (3.81 - 12.79) | <0.001 |
| 40.1-50 | 5.19 (3.75 - 7.18) | <0.001 | 2.82 (2.15 - 3.71) | <0.001 | 23.80 (9.32 - 60.80) | <0.001 | 8.57 (4.61 - 15.93) | <0.001 |
| 50+ | 3.81 (2.84 - 5.10) | <0.001 | 2.36 (1.73 - 3.21) | <0.001 | 34.93 (14.20 - 85.93) | <0.001 | 4.93 (2.69 - 9.04) | <0.001 |
| History of splenectomy (yes/no) | ||||||||
| Yes | 1.71 (1.34 - 2.17) | <0.001 | 1.46 (1.16 - 1.83) | 0.001 | 0.10 (0.02 - 0.42) | 0.001 | 1.89 (1.36 - 2.63) | <0.001 |
| No | Ref | Ref | Ref | Ref | ||||
| Cyclophosphamide equivalent dose (mg/m2) | ||||||||
| None | Ref | Ref | Ref | Ref | ||||
| 1-3999 | 0.93 (0.67 - 1.28) | 0.65 | 1.23 (0.90 - 1.66) | 0.19 | 0.51 (0.27 - 0.97) | 0.04 | 0.81 (0.47 - 1.41) | 0.46 |
| 4000-7999 | 1.35 (1.00 - 1.83) | 0.05 | 1.46 (1.15 - 1.86) | 0.002 | 1.00 (0.56 - 1.81) | 0.99 | 1.38 (0.85 - 2.23) | 0.19 |
| 8000+ | 1.33 (1.08 - 1.63) | 0.007 | 1.50 (1.25 - 1.81) | <0.001 | 0.54 (0.34 - 0.88) | 0.01 | 1.40 (1.02 - 1.91) | 0.04 |
| Anthracycline (mg/m2) | ||||||||
| None | Ref | Ref | Ref | Ref | ||||
| 0-100 | 1.26 (0.76 - 2.11) | 0.37 | 1.19 (0.71 - 2.00) | 0.51 | 1.10 (0.42 - 2.85) | 0.85 | 1.35 (0.59 - 3.10) | 0.48 |
| 101-300 | 1.20 (0.80 - 1.78) | 0.37 | 1.17 (0.92 - 1.50) | 0.20 | 0.59 (0.32 - 1.10) | 0.10 | 1.35 (0.70 - 2.60) | 0.36 |
| >300 | 0.82 (0.64 - 1.05) | 0.12 | 1.24 (0.99 - 1.55) | 0.07 | 0.58 (0.33 - 1.03) | 0.06 | 0.64 (0.40 - 1.02) | 0.06 |
| Epipodophyllotoxin (mg/m2) | ||||||||
| None | Ref | Ref | Ref | Ref | ||||
| 1-1000 | 1.65 (1.04 - 2.62) | 0.03 | 1.11 (0.68 - 1.81) | 0.68 | 1.88 (0.78 - 4.51) | 0.16 | 2.48 (1.30 - 4.76) | 0.006 |
| 1001-4000 | 0.71 (0.47 - 1.05) | 0.09 | 0.69 (0.43 - 1.11) | 0.13 | 1.15 (0.34 - 3.87) | 0.82 | 0.60 (0.30 - 1.20) | 0.15 |
| >4000 | 0.88 (0.54 - 1.41) | 0.59 | 1.23 (0.72 - 2.09) | 0.45 | 1.73 (0.69 - 4.36) | 0.24 | 0.21 (0.07 - 0.61) | 0.004 |
| Platinum (mg/m2) | ||||||||
| None | Ref | Ref | Ref | Ref | ||||
| 1-400 | 1.37 (0.78 - 2.41) | 0.27 | 1.10 (0.68 - 1.76) | 0.70 | 2.93 (1.37 - 6.27) | 0.006 | 1.24 (0.40 - 3.91) | 0.71 |
| 401-750 | 1.81 (1.21 - 2.69) | 0.004 | 1.90 (1.20 - 3.01) | 0.006 | 2.28 (0.88 - 5.92) | 0.09 | 1.27 (0.57 - 2.86) | 0.56 |
| >750 | 1.86 (1.12 - 3.09) | 0.02 | 1.88 (1.04 - 3.38) | 0.04 | 3.12 (0.92 - 10.59) | 0.07 | 1.18 (0.36 - 3.86) | 0.78 |
In addition to the above variables in the model, attained age was adjusted for in the model for each outcome using cubic splines.
Rate for survivors with attained age at 28 years old (median) and all other variables in the model being the reference group.
Abbreviations: CI, confidence interval; RR, relative rate.
After adjusting for sex, age at diagnosis, and attained age, relative rates declined for every 5-year increment of treatment era for subsequent neoplasms (RR=0.81, 95% CI 0.76-0.86, p<0.001), subsequent malignant neoplasms (RR=0.87, 95% CI 0.82-0.93, p<0.001), meningiomas (RR=0.85, 95% CI 0.75-0.97, p=0.03), and non-melanoma skin cancers (RR=0.75, 95% CI 0.67-0.84, p<0.001; Table 3). Inclusion of all treatment variables in the model attenuated statistically significantly the treatment era-associated decline of subsequent neoplasms (p<0.001), subsequent malignant neoplasms (p<0.001), meningioma (p=0.027) and non-melanoma skin cancer (p<0.001) rates. Further mediation analyses were conducted by modifying the adjustment from all treatment variables to specific components of treatment variables (i.e., maximum radiation dose and all other treatments, including chemotherapy drug doses and splenectomy). These analyses revealed that radiation therapy dose changes were the chief contributor to the era-associated decline of subsequent neoplasm rates and that radiation therapy dose changes were the only component of the treatment variables significantly associated with the decline of subsequent neoplasm rates over time (Table 3).
Table 3. Relative rates of overall and subsequent neoplasm subtypes, per 5-year treatment era, without and with adjustment for treatment variables*.
| Subsequent neoplasm | Subsequent malignant neoplasm | Meningioma | Non-melanoma skin cancer | |||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Treatment era | RR (95% CI) | P | RR (95% CI) | P | RR (95% CI) | P | RR (95% CI) | P |
| Not adjusted for any treatment (a) | 0.81 (0.76-0.86) | <0.001 | 0.87 (0.82-0.93) | <0.001 | 0.85 (0.75-0.97) | 0.034 | 0.75 (0.67-0.84) | <0.001 |
| Adjusted for: | ||||||||
| All treatments except max. radiation dose (b) | 0.84 (0.78-0.90) | <0.001 | 0.87 (0.81-0.94) | <0.001 | 0.80 (0.68-0.92) | 0.003 | 0.81 (0.71-0.92) | 0.001 |
| Max. radiation dose (c) | 0.93 (0.87 - 0.99) | 0.019 | 0.96 (0.90 - 1.02) | 0.195 | 1.01 (0.87 - 1.17) | 0.903 | 0.87 (0.78 - 0.97) | 0.015 |
| All treatments (d) | 0.91 (0.84-0.98) | 0.012 | 0.93 (0.86-1.00) | 0.047 | 0.94 (0.81-1.10) | 0.473 | 0.87 (0.76–1.00) | 0.048 |
| Statistical significance for the coefficient difference | a vs. b | 0.102 | a vs. b | 0.998 | a vs. b | 0.020 | a vs. b | 0.046 |
| a vs. c | <0.001 | a vs. c | <0.001 | a vs. c | <0.001 | a vs. c | <0.001 | |
| a vs. d | <0.001 | a vs. d | <0.001 | a vs. d | <0.001 | a vs. d | 0.027 | |
| b vs. c | <0.001 | b vs. c | <0.001 | b vs. c | 0.024 | b vs. c | <0.001 | |
| b vs. d | <0.001 | b vs. d | <0.001 | b vs. d | <0.001 | b vs. d | <0.001 | |
| c vs. d | 0.242 | c vs. d | 0.046 | c vs. d | 0.900 | c vs. d | 0.104 | |
Separate models were developed for each outcome, adjusting for sex, age at initial cancer diagnosis, attained age as cubic spline. Models adjusting for treatment included maximum radiation dose to the body, splenectomy, cyclophosphamide equivalent dose, anthracycline dose, epipodophyllotoxin dose, and platinum dose.
Abbreviations: CI, cumulative incidence; RR, relative rate.
Discussion
Survival following childhood cancer has improved substantially over the last five decades. As the number of survivors has increased, so has the focus on late outcomes of cancer therapy. Cohort studies, including the CCSS, devoted to understanding the late health consequences of childhood cancer therapies 6 have previously documented the effect of subsequent neoplasms and quantified risk associated with specific therapies, particularly therapeutic radiation 7,26-28. Accordingly, efforts have been directed toward eliminating the use of radiation therapy when possible, or decreasing the volume and/or dose 14,15. An example of this is the near elimination of cranial radiation among children newly diagnosed with ALL 29. As treatment with radiation has decreased, some chemotherapy regimens have intensified 14. This evolution in delivered therapies has reduced late mortality among survivors 4; however, the association with specific outcomes, including subsequent neoplasms, has not been investigated. The current analysis, including more than 23,000 survivors of childhood cancer treated over three decades, demonstrated that the cumulative incidence rates of subsequent neoplasms, subsequent malignant neoplasms, meningiomas and non-melanoma skin cancers were lower among survivors treated in more recent treatment eras and that modifications of primary cancer therapy were associated with these declines.
Hodgkin lymphoma survivors are at particularly high-risk for subsequent malignancies 30-33. Our findings demonstrated a decreased 15-year cumulative incidence of subsequent neoplasms for Hodgkin lymphoma survivors treated in the 1990s compared to earlier decades, with cumulative incidence of subsequent malignant neoplasms at 15 years significantly lower in the 1990s compared to the 1970s. In contrast, a recent report of Dutch long-term survivors of Hodgkin lymphoma demonstrated the cumulative incidence of second cancers did not decrease across treatment decades 34. Within our study the SIR for subsequent malignant neoplasms decreased over time among survivors of Hodgkin lymphoma with attained age ≥20 years. Intensified alkylator dosing has been used in Hodgkin lymphoma since the 1980s to compensate for decreasing therapeutic radiation 14. At high cumulative doses, alkylating agents are associated with increased rates of subsequent malignant neoplasms, which may have attenuated the expected decline in subsequent malignancies among Hodgkin lymphoma survivors, particularly among the Dutch cohort where a decrease in incidence was not observed.
Temporal treatment modifications have also been made using improved risk stratification of children with ALL and Wilms tumor 14,15, among others, which have led to decreased late mortality from subsequent malignant neoplasms among survivors 4. In ALL, cranial irradiation has been replaced with intensive intrathecal therapy in nearly all patients. Meningiomas are among the most frequently observed subsequent neoplasms in survivors of ALL, and, given the latency of >20 years to development of meningiomas 7, it is likely that the full effect of omitting cranial irradiation among more recently treated survivors has not yet been observed. Temporal changes in Wilms tumor therapy include reduction in the dose and even elimination of therapeutic radiation in low risk populations, which has likely contributed to the decreased cumulative incidence of subsequent malignant neoplasms in the 1990s compared to the 1970s.
These data further document the increased cumulative incidence, cumulative burden and elevated risk for subsequent malignant neoplasms, meningiomas and non-melanoma skin cancers in survivors treated with radiation therapy 7. Despite reduced use of therapeutic radiation, radiation continues to be an important component of treatment for many children. As the use of radiation therapy decreases it is possible that other associations may emerge. Specifically, to maintain and improve cure rates, chemotherapy dosing and/or the proportion of patients receiving various agents have been increasing. Although there has been a decrease in the median cumulative doses of alkylating agents and anthracyclines, the proportion of survivors receiving these agents increased. For epipodophyllotoxins and platinum agents, increased median cumulative doses were given in the 1990s compared to the 1970s and the proportion of survivors treated with these agents increased over time. We observed an increased rate of subsequent malignant neoplasms with higher cumulative doses of alkylating agents and platinum agents. These associations may become more prominent as therapeutic radiation use continues to decline. Additionally, the role of genetic susceptibility in the development of subsequent neoplasms may become more evident and interactions between host genetics and chemotherapy doses may emerge as well.
This study represents, to our knowledge, the first comprehensive report of subsequent neoplasms from the CCSS since the expansion of the cohort to include survivors initially diagnosed through 1970-1999. Both the British Childhood Cancer Survivor Study35 and a cohort of childhood cancer survivors from Nordic cancer registries 36 have reported on subsequent neoplasms from patients diagnosed over extended periods of time (British 1940-1991, Nordic 1943-2005); however, this is the first study, to our knowledge, to report changes in subsequent neoplasm incidence and SIRs as a function of treatment era and treatment variables. The mediation analysis 23-25 demonstrated that, in conjunction with (a) reduction in treatment doses and decreased splenectomy frequency across treatment eras (Table 1) and (b) statistically significant associations of treatment variables with subsequent neoplasm rates when controlling for era of treatment and treatment variables (Table 2), that the decline of subsequent neoplasm rates was mediated by treatment variable changes over time. In this analysis the age-specific SIRs and overall cumulative incidence of subsequent malignant neoplasms at 15 years consistently decreased for more recent treatment eras. As observed in previous CCSS reports on subsequent neoplasms 37, SIRs are greatest at younger attained ages because they measure observed counts relative to expected counts in the age- and sex-matched general population. Although significant decreases were observed from the 1970s to 1990s in 15-year cumulative incidence of breast cancer, soft tissue sarcoma and non-melanoma skin cancer, significant decreases were not observed for subsequent leukemia, central nervous system or thyroid malignancies (eFigure 3, eFigure 4). Furthermore, significant decreases in SIRs for specific subsequent malignancies, including breast cancer, soft tissue sarcoma, leukemia, central nervous system and thyroid malignancies were not observed when stratified by decade of diagnosis and attained age (eFigure 5). The lack of significant changes in individual malignancies is in contrast to the overall decrease in subsequent malignancies with more recent treatment eras and the decrease in use of therapeutic radiation; however, the observed numbers of subsequent malignancies are small for each subgroup and the confidence intervals are wide, indicating possible insufficient power to detect significant differences and also suggesting additional mechanisms contributing to subsequent malignancy risk. Ongoing follow-up of survivors from the latest treatment decade is needed to determine changes in risk over time, particularly given the long latency from primary diagnosis to many subsequent malignant neoplasms.
This study has important limitations. Although the CCSS is a large, well-characterized cohort, it is not completely representative of the childhood cancer survivor population and there is potential for selection bias given that 33% of eligible survivors were not included in this analysis. Selected primary diagnoses, including retinoblastoma, germ cell tumor, and hepatoblastoma were not included. Children with heritable retinoblastoma are at significant risk of subsequent neoplasms and their exclusion may have resulted in underestimation of subsequent malignancy risk. Subsequent neoplasms were initially self-reported, which may have led to underreporting of neoplasms, particularly those occurring in the more distant past. Additionally, the design of the CCSS cohort to include only five-year survivors a-priori excludes consideration of cancers occurring prior to five years. Also, therapies for subsequent neoplasms are not completely ascertained, which limits further exploration of the role of treatments among survivors who develop multiple subsequent neoplasms. In addition, interpretation of results should be made within the context of the multiple statistical tests performed to address the hypotheses of interest.
Conclusions
Among childhood cancer survivors, the risk of subsequent malignancies at 15 years after initial cancer diagnosis remained increased for those diagnosed in the 1990s, although the risk was lower compared with those diagnosed in the 1970s. This lower risk was associated with reduction in therapeutic radiation dose.
Supplementary Material
Key Points.
Question
Are treatment-era related changes in chemotherapy or radiation therapy doses associated with changes in the risk of subsequent neoplasms over time among survivors of childhood cancer?
Findings
In this longitudinal cohort study of 23,603 survivors of childhood cancer, reductions in therapeutic radiation doses over time were associated with reduced rates of subsequent neoplasms, including subsequent malignancies, non-melanoma skin cancers and benign meningiomas.
Meaning
Ongoing efforts to reduce long-term therapeutic toxicity were associated with decreasing subsequent neoplasms among five-year survivors of childhood cancer.
Acknowledgments
Drs. Lucie Turcotte and Joseph Neglia had full access to all study data and take responsibility for the integrity of the data and the accuracy of the data analysis. The statistical analysis was performed by Qi Liu, M.S., of the University of Alberta and Yutaka Yasui, Ph.D., of St. Jude Children's Research Hospital. This work was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (UL1TR000114, B.R. Blazar, Principal Investigator) and the National Cancer Institute (CA55727, G.T. Armstrong, Principal Investigator). Support to St. Jude Children's Research Hospital also provided by the Cancer Center Support (CORE) grant (CA21765, C. Roberts, Principal Investigator) and the American Lebanese-Syrian Associated Charities (ALSAC). The funders/sponsors of this study had no role in the: design and conduct of the study, collection, management, analysis, and interpretation of the data, preparation, review, or approval of the manuscript, or decision to submit the manuscript for publication.
Footnotes
The authors have no conflicts of interest to report.
References
- 1.Mertens AC, Liu Q, Neglia JP, et al. Cause-specific late mortality among 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. Journal of the National Cancer Institute. 2008;100(19):1368–1379. doi: 10.1093/jnci/djn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hudson MM, Mertens AC, Yasui Y, et al. Health status of adult long-term survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. JAMA : the journal of the American Medical Association. 2003;290(12):1583–1592. doi: 10.1001/jama.290.12.1583. [DOI] [PubMed] [Google Scholar]
- 3.Kurt BA, Nolan VG, Ness KK, et al. Hospitalization rates among survivors of childhood cancer in the Childhood Cancer Survivor Study cohort. Pediatric blood & cancer. 2012;59(1):126–132. doi: 10.1002/pbc.24017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstrong GT, Chen Y, Yasui Y, et al. Reduction in Late Mortality among 5-Year Survivors of Childhood Cancer. The New England journal of medicine. 2016;374(9):833–842. doi: 10.1056/NEJMoa1510795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27(14):2308–2318. doi: 10.1200/JCO.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatia S, Armenian SH, Armstrong GT, et al. Collaborative Research in Childhood Cancer Survivorship: The Current Landscape. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33(27):3055–3064. doi: 10.1200/JCO.2014.59.8052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedman DL, Whitton J, Leisenring W, et al. Subsequent neoplasms in 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. Journal of the National Cancer Institute. 2010;102(14):1083–1095. doi: 10.1093/jnci/djq238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garwicz S, Anderson H, Olsen JH, et al. Second malignant neoplasms after cancer in childhood and adolescence: a population-based case-control study in the 5 Nordic countries. The Nordic Society for Pediatric Hematology and Oncology. The Association of the Nordic Cancer Registries. Int J Cancer. 2000;88(4):672–678. doi: 10.1002/1097-0215(20001115)88:4<672::aid-ijc24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 9.Jenkinson HC, Hawkins MM, Stiller CA, Winter DL, Marsden HB, Stevens MC. Long-term population-based risks of second malignant neoplasms after childhood cancer in Britain. British journal of cancer. 2004;91(11):1905–1910. doi: 10.1038/sj.bjc.6602226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Armstrong GT, Stovall M, Robison LL. Long-term effects of radiation exposure among adult survivors of childhood cancer: results from the childhood cancer survivor study. Radiation research. 2010;174(6):840–850. doi: 10.1667/RR1903.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borgmann A, Zinn C, Hartmann R, et al. Secondary malignant neoplasms after intensive treatment of relapsed acute lymphoblastic leukaemia in childhood. European journal of cancer (Oxford, England : 1990) 2008;44(2):257–268. doi: 10.1016/j.ejca.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 12.Kim SH, Shin KH, Seok SO, et al. Secondary malignant neoplasms after osteosarcoma: early onset and cumulative alkylating agent dose dependency. Annals of surgical oncology. 2015;22(3):859–865. doi: 10.1245/s10434-014-4070-2. [DOI] [PubMed] [Google Scholar]
- 13.Pole JD, Gu LY, Kirsh V, Greenberg ML, Nathan PC. Subsequent Malignant Neoplasms in a Population-Based Cohort of Pediatric Cancer Patients: A Focus on the First 5 Years. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2015;24(10):1585–1592. doi: 10.1158/1055-9965.EPI-15-0360. [DOI] [PubMed] [Google Scholar]
- 14.Hudson MM, Neglia JP, Woods WG, et al. Lessons from the past: opportunities to improve childhood cancer survivor care through outcomes investigations of historical therapeutic approaches for pediatric hematological malignancies. Pediatric blood & cancer. 2012;58(3):334–343. doi: 10.1002/pbc.23385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green DM, Kun LE, Matthay KK, et al. Relevance of historical therapeutic approaches to the contemporary treatment of pediatric solid tumors. Pediatric blood & cancer. 2013;60(7):1083–1094. doi: 10.1002/pbc.24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leisenring WM, Mertens AC, Armstrong GT, et al. Pediatric cancer survivorship research: experience of the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27(14):2319–2327. doi: 10.1200/JCO.2008.21.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fritz AG. International classification of diseases for oncology: ICD-O. Geneva: World Health Organization; 2000. [Google Scholar]
- 18.Robison LL, Mertens AC, Boice JD, et al. Study design and cohort characteristics of the Childhood Cancer Survivor Study: a multi-institutional collaborative project. Med Pediatr Oncol. 2002;38(4):229–239. doi: 10.1002/mpo.1316. [DOI] [PubMed] [Google Scholar]
- 19.Green DM, Nolan VG, Goodman PJ, et al. The cyclophosphamide equivalent dose as an approach for quantifying alkylating agent exposure: a report from the Childhood Cancer Survivor Study. Pediatric blood & cancer. 2014;61(1):53–67. doi: 10.1002/pbc.24679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dong H, Robison LL, Leisenring WM, Martin LJ, Armstrong GT, Yasui Y. Estimating the burden of recurrent events in the presence of competing risks: the method of mean cumulative count. American journal of epidemiology. 2015;181(7):532–540. doi: 10.1093/aje/kwu289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhakta N, Liu Q, Yeo F, et al. Cumulative burden of cardiovascular morbidity in paediatric, adolescent, and young adult survivors of Hodgkin's lymphoma: an analysis from the St Jude Lifetime Cohort Study. The lancet oncology. 2016 doi: 10.1016/S1470-2045(16)30215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.SEER Cancer Statistics Review (CSR), 1975-2012. http://seer.cancer.gov/csr/1975_2012/
- 23.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. Journal of personality and social psychology. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 24.Hayes AF. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. Guilford Press; 2013. [Google Scholar]
- 25.MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annu Rev Psychol. 2007;58:593–614. doi: 10.1146/annurev.psych.58.110405.085542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armstrong GT, Liu W, Leisenring W, et al. Occurrence of multiple subsequent neoplasms in long-term survivors of childhood cancer: a report from the childhood cancer survivor study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(22):3056–3064. doi: 10.1200/JCO.2011.34.6585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inskip PD, Robison LL, Stovall M, et al. Radiation dose and breast cancer risk in the childhood cancer survivor study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(24):3901–3907. doi: 10.1200/JCO.2008.20.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sigurdson AJ, Ronckers CM, Mertens AC, et al. Primary thyroid cancer after a first tumour in childhood (the Childhood Cancer Survivor Study): a nested case-control study. Lancet. 2005;365(9476):2014–2023. doi: 10.1016/S0140-6736(05)66695-0. [DOI] [PubMed] [Google Scholar]
- 29.Pui CH, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. The New England journal of medicine. 2009;360(26):2730–2741. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Green DM, Hyland A, Barcos MP, et al. Second malignant neoplasms after treatment for Hodgkin's disease in childhood or adolescence. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2000;18(7):1492–1499. doi: 10.1200/JCO.2000.18.7.1492. [DOI] [PubMed] [Google Scholar]
- 31.Omer B, Kadan-Lottick NS, Roberts KB, et al. Patterns of subsequent malignancies after Hodgkin lymphoma in children and adults. British journal of haematology. 2012;158(5):615–625. doi: 10.1111/j.1365-2141.2012.09211.x. [DOI] [PubMed] [Google Scholar]
- 32.Bhatia S, Robison LL, Oberlin O, et al. Breast cancer and other second neoplasms after childhood Hodgkin's disease. The New England journal of medicine. 1996;334(12):745–751. doi: 10.1056/NEJM199603213341201. [DOI] [PubMed] [Google Scholar]
- 33.Bhatia S, Yasui Y, Robison LL, et al. High risk of subsequent neoplasms continues with extended follow-up of childhood Hodgkin's disease: report from the Late Effects Study Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21(23):4386–4394. doi: 10.1200/JCO.2003.11.059. [DOI] [PubMed] [Google Scholar]
- 34.Schaapveld M, Aleman BM, van Eggermond AM, et al. Second Cancer Risk Up to 40 Years after Treatment for Hodgkin's Lymphoma. The New England journal of medicine. 2015;373(26):2499–2511. doi: 10.1056/NEJMoa1505949. [DOI] [PubMed] [Google Scholar]
- 35.Reulen RC, Frobisher C, Winter DL, et al. Long-term risks of subsequent primary neoplasms among survivors of childhood cancer. JAMA : the journal of the American Medical Association. 2011;305(22):2311–2319. doi: 10.1001/jama.2011.747. [DOI] [PubMed] [Google Scholar]
- 36.Olsen JH, Moller T, Anderson H, et al. Lifelong cancer incidence in 47,697 patients treated for childhood cancer in the Nordic countries. Journal of the National Cancer Institute. 2009;101(11):806–813. doi: 10.1093/jnci/djp104. [DOI] [PubMed] [Google Scholar]
- 37.Turcotte LM, Whitton JA, Friedman DL, et al. Risk of Subsequent Neoplasms During the Fifth and Sixth Decades of Life in the Childhood Cancer Survivor Study Cohort. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33(31):3568–3575. doi: 10.1200/JCO.2015.60.9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.