Abstract
Purpose
Purposes of this study were to: identify subgroups of patients with distinct trajectories for morning and evening fatigue; evaluate for differences in demographic and clinical characteristics among these subgroups; and compare and contrast the predictors of subgroup membership for morning and evening fatigue.
Methods
Outpatients with breast, gastrointestinal, gynecological, or lung cancer (n=582) completed questionnaires a total of six times over two cycles of CTX. Morning and evening fatigue severity were evaluated using the Lee Fatigue Scale. Latent profile analysis (LPA) was used to identify distinct subgroups.
Results
Three latent classes were identified for morning fatigue (i.e., Low (31.8%), High (51.4%), and Very High (16.8%)) and for evening evening fatigue (i.e., Moderate (20.0%), High (21.8%), and Very High (58.2%)). Most of the disease and treatment characteristics did not distinguish among the morning and evening fatigue classes. Compared to the Low class, patients in the High and Very High morning fatigue class were younger, had a lower functional status and higher level of comorbidity. Compared to the Moderate class, patients in the Very High evening fatigue class were younger, more likely to be female, had child care responsibilities, had a lower functional status, and a higher level of comorbidity.
Conclusion
LPA allows for the identification of risk factors for more severe fatigue. Since an overlap was not observed across the morning and evening fatigue classes and unique predictors for morning and evening fatigue were identified, these findings suggest that morning and evening fatigue may have distinct underlying mechanisms.
Keywords: fatigue, diurnal variations, morning fatigue, evening fatigue, chemotherapy, cancer, latent class analysis, latent profile analysis
Introduction
Fatigue is the most common and disabling symptom associated with cancer and its treatment [1,2]. Fatigue occurs in 14% to 96% of patients undergoing chemotherapy (CTX) [3,4]. Severe fatigue has an impact on patients’ ability to tolerate CTX and may result in discontinuation of therapy [5].
While the majority of research on fatigue has reported mean changes in fatigue severity, work from our research team [6–8] and others [9–11] suggest that the severity of fatigue varies over the course of a day and varies substantially among individuals. In a previous study, we identified distinct subgroups of patients and family caregivers (FCs) with different morning and evening fatigue trajectories [12]. Common (e.g., age) and unique (e.g., Karnofsky Performance Status (KPS) score, gender, children at home) predictors were found among the morning and evening fatigue groups. These findings provide support for the hypothesis that morning and evening fatigue are distinct but related symptoms.
Our previous work referenced above, using growth mixture modeling (GMM), evaluated for diurnal variation in fatigue severity in patients who underwent radiation therapy (RT) and their FCs. To date, only two studies were identified that evaluated diurnal variations in fatigue severity in patients undergoing CTX [9,10]. Although these studies suggest that morning and evening fatigue are distinct symptoms, neither study evaluated for predictors of diurnal variations in fatigue severity associated with CTX. Therefore, the purposes of this study, in a sample of patients who underwent two cycles of CTX (n=582), were to: identify subgroups of patients with distinct morning and evening fatigue trajectories using latent profile analysis (LPA); evaluate for differences in demographic and clinical characteristics among the subgroups; and compare and contrast the common and unique predictors associated with membership in the morning and evening fatigue latent classes.
Methods
Patients and Settings
This study is part of a longitudinal study of the symptom experience of oncology outpatients receiving CTX. Eligible patients were ≥18 years of age; had a diagnosis of breast, gastrointestinal (GI), gynecological (GYN), or lung cancer; had received CTX within the preceding 4 weeks; were scheduled to receive at least 2 additional cycles of CTX; were able to read, write, and understand English; and provided written informed consent. Patients were recruited from 2 Comprehensive Cancer Centers, 1 Veterans Affairs hospital, and 4 community-based oncology programs. A total of 969 patients were approached and 582 consented to participate (60.1% response rate). The major reason for refusal was being overwhelmed with their cancer treatment.
Instruments
A demographic questionnaire obtained information regarding age, sex, ethnicity, marital status, living arrangements, education, employment status, child and elder care responsibilities, exercise regularity, and income. In addition, patients completed the KPS scale that ranged from 30 (I feel severely disabled and need to be hospitalized) to 100 (I feel normal; I have no complaints or symptoms) [13,14].
The Self-Administered Comorbidity Questionnaire (SCQ) was used to assess comorbidity. The questionnaire consists of 13 common medical conditions that were simplified into language that could be understood without any prior medical knowledge. Patients were asked to indicate if they had the condition; if they received treatment for it; and did it limit their activities. For each condition, a patient can receive a maximum of 3 points. Total scores can range from 0 to 39. The SCQ has well-established validity and reliability and has been used in studies of patients with a variety of chronic conditions [15,16].
The Lee Fatigue Scale (LFS) consists of 18 items designed to assess physical fatigue and energy [17]. Each item was rated on a 0 to 10 numeric rating scale (NRS). Total fatigue and energy scores were calculated as the mean of the 13 fatigue items and the 5 energy items, with higher scores indicating greater fatigue severity and higher levels of energy. Patients were asked to rate each item based on how they felt “right now,” within 30 minutes of awakening (i.e., morning fatigue, morning energy) and prior to going to bed (i.e., evening fatigue, evening energy). Cutoff scores of ≥3.2 and ≥5.6 indicate high levels of morning and evening fatigue, respectively [18]. The LFS has well established validity and reliability [8, 17, 19–22]. In this study, Cronbach’s alphas for morning and evening fatigue scales at enrollment were 0.96 and 0.95, respectively.
Study Procedures
The Committee on Human Research at the University of California, San Francisco and the Institutional Review Board at each of the study sites approved the study. Patients were approached by a research staff member in the infusion unit to discuss participation in the study. Written informed consent was obtained from all patients. Depending on the length of their CTX cycles, patients completed questionnaires in their homes, a total of six times over two cycles of CTX (i.e., prior to CTX administration (i.e., recovery from previous CTX cycle), approximately 1 week after CTX administration (i.e., acute symptoms), approximately 2 weeks after CTX administration (i.e., potential nadir)). Medical records were reviewed for disease and treatment information.
Data Analysis
Data were analyzed using SPSS version 22 (IBM, Armonk, NY). Descriptive statistics and frequency distributions were calculated for the demographic and clinical characteristics. Differences among the latent classes were evaluated using analyses of variance (ANOVA) or Chi Square analyses. Post hoc contrasts were done using the Bonferroni procedure. A p-value of <.05 was considered statistically significant.
Latent profile analysis (LPA) of morning and evening fatigue
Unconditional LPA was used to identify the profiles of morning and evening fatigue means that characterized unobserved subgroups (i.e., latent classes) of patients over the six assessments. Typically, GMM or latent class growth modeling of change trajectories would be used to identify latent classes of individuals who change differently over time. However, the data in this study demonstrated a complex pattern of change because a pre-treatment assessment, an immediate post-treatment assessment, and a second post-treatment assessment were done over two cycles of CTX. We expected that the trajectory of change for symptoms, measured six times, over two treatment cycles would have a twin peak pattern that looks like “Λ_Λ”. Therefore, we identified latent classes of patients based on their profiles of means, where the means were estimated from the same symptoms measured on six occasions. In order to incorporate the expected correlations among the repeated measures, we included covariance among measures that were one or two occasions apart (i.e., a covariance structure with a lag of two). In this way, we retained the within person correlation among the measures, at the same time that we focused on the patterns of means that distinguished the latent classes. We limited the covariance structure to a lag of two to accommodate the expected reduction in correlation that would be introduced by two treatments within each set of three measurement occasions, and to reduce model complexity.
Estimation was carried out with full information maximum likelihood with standard errors and a Chi-squared test that are robust to non-normality and non-independence of observations (“estimator=MLR”). Model fit was evaluated to identify the solution that characterized the observed latent class structure with the Bayesian Information Criterion (BIC), entropy, and latent class percentages that were large enough to be reliable (i.e., likely to replicate in new samples; 15% or about 85 patients) [23,24]. Missing data were accommodated with the use of the Expectation-Maximization (EM) algorithm [25].
Mixture models, like LPA, are known to produce solutions at local maxima. Therefore, our models were fit with from 1,000 to 2,400 random starts. This approach ensured that the estimated model was replicated many times and was not due to a local maximum. Estimation was done with Mplus Version 7.2 [23].
Results
Latent Classes for Morning Fatigue
A three-class solution was selected (Table 1) because one of the classes in the four-class solution was too small to be reliable and because the profile of means for two of the classes in the four-class solution did not differ in a meaningful way (i.e., either by profile or mean levels). In addition, the three-class solution fit better than the two-class solution and the profiles of means were clinically meaningfully different. Because one does not expect to experience fatigue in the morning, the clinically meaningful LFS cutoff score for morning fatigue is ≥3.2. In naming the morning fatigue classes for this study, we considered the morning fatigue scores for the three latent classes (i.e., Very Low, Low and High) identified in our previous RT study [12]. Therefore, for this study, the morning fatigue classes were named as Low, High, and Very High.
Table 1.
Latent Profile Analysis Solutions and Fit Indices for Two-Through Four-Classes for Morning and Evening Fatigue Over Six Assessments
| Model | LL | AIC | BIC | Entropy |
|---|---|---|---|---|
| Morning Fatigue | ||||
| 2 Class | −5789.71 | 11645.41 | 11789.51 | .83 |
| 3 Classa | −5614.08 | 11318.16 | 11514.65 | .84 |
| 4 Class | −5544.81 | 11203.62 | 11452.50 | .82 |
| Evening Fatigue | ||||
| 2 Class | −5591.29 | 11248.58 | 11392.67 | .73 |
| 3 Classb | −5502.23 | 11094.45 | 11290.94 | .68 |
| 4 Class | −5430.75 | 10975.50 | 11224.39 | .71 |
The three class solution was selected because one of the classes in the four-class solution was too small to be reliable, and because the profile of means for two of the classes in the four-class solution did not differ in a meaningful way (either by profile or mean levels). In addition, the three class solution fit better than the two-class solution, and the profiles of means were meaningfully different.
The three class solution was selected because one of the classes in the four-class solution was too small to be reliable, and because the profile of means for two of the classes in the four-class solution did not differ in a meaningful way (either by profile or mean levels). In addition, the three class solution fit better than the two-class solution, and the profiles of means were meaningfully different.
Note. AIC = Akaike’s Information Criterion; BIC = Bayesian Information Criterion; LL = log-likelihood.
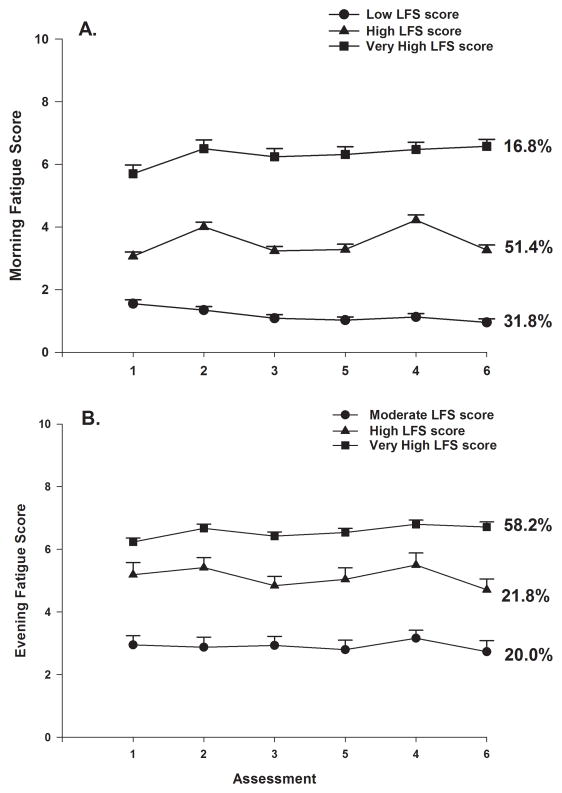
As shown in Figure 1A, the trajectories for morning fatigue differed among the latent classes. For both the Low Morning Fatigue (31.8%) and the Very High Morning Fatigue (16.8%) classes, fatigue scores remained relatively constant across the six assessments. In contrast, for the High Morning Fatigue class (51.4%), fatigue scores oscillated over the two cycles of CTX, with slightly higher scores reported at assessments 2 and 4 (i.e., the week following the administration of CTX).
Figure 1.
Trajectories of morning and evening fatigue in the latent classes
Differences in Demographic and Clinical Characteristics Among Morning Fatigue Classes
Compared to patients in the Low Morning Fatigue class, patients in the High and Very High classes were significantly younger and had a lower KPS score and a higher SCQ score (Table 2). In addition, compared to the Low Morning Fatigue class, patients in the Very High Morning Fatigue class were more likely to be female, less likely to be married or partnered, less likely to exercise on a regular basis, and reported a lower annual household income. In addition, compared to the Low and High Morning Fatigue classes, patients in the Very High Morning Fatigue class had a higher BMI. The majority of the other clinical characteristics did not differ among the morning fatigue classes.
Table 2.
Differences in Demographic and Clinical Characteristics Among the Morning Fatigue Latent Classes (n=582)
| Characteristic | Low LFS score (0) N=185 (31.8%) |
High LFS score (1) N=299 (51.4%) |
Very High LFS score (2) N=98 (16.8%) |
Statistics |
|---|---|---|---|---|
|
| ||||
| Mean (SD) | Mean (SD) | Mean (SD) | ||
|
| ||||
| Age (years) | 58.3 (11.2) | 57.5 (12.4) | 54.2 (11.0) | F=4.07, p=.018 0>1 and 2 |
|
| ||||
| Education (years) | 16.5 (3.0) | 16.4 (3.0) | 16.0 (3.0) | F=0.69, p=.503 |
|
| ||||
| Body mass index (kg/m2) | 25.4 (4.9) | 26.4 (5.7) | 28.3 (7.3) | F=8.14, p<.0001 0 and 1<2 |
|
| ||||
| Karnofsky Performance Status score | 86.2 (9.6) | 79.3 (11.8) | 73.6 (11.6) | F=40.43, p<.0001 0>1>2 |
|
| ||||
| Self-administered Comorbidity Questionnaire score | 4.6 (2.6) | 5.7 (2.9) | 7.0 (3.4) | F=22.41, p<.0001 0<1<2 |
|
| ||||
| Time since diagnosis (years) | 2.3 (4.5) | 2.5 (4.5) | 2.6 (4.0) | F=0.16, p=.858 |
|
| ||||
| Number of prior cancer treatments | 1.8 (1.7) | 1.9 (1.6) | 2.0 (1.6) | F=0.49, p=.611 |
|
| ||||
| Number of metastatic sites including lymph node involvement a | 1.3 (1.2) | 1.4 (1.4) | 1.4 (1.4) | F=0.12, p=.890 |
|
| ||||
| Number of metastatic sites excluding lymph node involvement | 0.8 (1.1) | 0.9 (1.2) | 1.0 (1.2) | F=0.34, p=.709 |
|
| ||||
| Hemoglobin | 11.8 (1.4) | 11.7 (1.4) | 11.7 (1.3) | F=0.73, p=.482 |
|
| ||||
| Hemocrit | 35.2 (4.1) | 34.7 (4.1) | 34.9 (3.8) | F=0.56, p=.569 |
|
| ||||
| % | % | % | ||
|
| ||||
| Gender (% female) | 74.6 | 80.9 | 87.8 | Χ2=7.24, p=.027 0<2 |
|
| ||||
| Self-reported ethnicity | ||||
| White | 77.7 | 69.2 | 74 | Χ2=4.11, p=.128 |
| Non-white | 22.3 | 30.8 | 26 | |
|
| ||||
| Married or partnered (% yes) | 74.3 | 67.2 | 55.7 | Χ2=10.08, p=.006 0>2 |
|
| ||||
| Lives alone (% yes) | 17.6 | 19 | 27.6 | Χ2=4.35, p=.114 |
|
| ||||
| Currently employed (% yes) | 40.4 | 33.2 | 28.6 | Χ2=4.57, p=.102 |
|
| ||||
| Annual household income | KW, p=.003 0>2 |
|||
| Less than $30,000 | 11.1 | 19.9 | 28 | |
| $30,000 to $70,000 | 19.8 | 19.2 | 20.4 | |
| $70,000 to $100,000 | 16 | 18 | 16.1 | |
| Greater than $100,000 | 53.1 | 42.9 | 35.5 | |
|
| ||||
| Child care responsibilities (% yes) | 18.7 | 24.8 | 28 | Χ2=3.68, p=.159 |
|
| ||||
| Elder care responsibilities (% yes) | 5.3 | 10.9 | 9.2 | Χ2=4.20, p=.123 |
|
| ||||
| Exercise on a regular basis (% yes) | 77.7 | 71.6 | 53.1 | Χ2=18.70, p<.0001 0 and 1>2 |
|
| ||||
| Cancer diagnosis | ||||
| Breast cancer | 41.6 | 42.1 | 48 | Χ2=6.05, p=.417 |
| Gastrointestinal cancer | 31.4 | 25.8 | 20.4 | |
| Gynecological cancer | 16.8 | 21.7 | 23.5 | |
| Lung cancer | 10.3 | 10.4 | 8.2 | |
|
| ||||
| Prior cancer treatment | ||||
| No prior treatment | 22.1 | 16.8 | 11.3 | Χ2=7.56 p=.272 |
| Only surgery, CTX, or RT | 37.6 | 41.4 | 48.5 | |
| Surgery and CTX, or surgery and RT, or CTX and RT | 24.9 | 23.2 | 20.6 | |
| Surgery and CTX and RT | 15.5 | 18.9 | 19.6 | |
|
| ||||
| Metastatic sites | ||||
| No metastasis | 28.4 | 34.6 | 31.6 | Χ2=4.55, p=.602 |
| Only lymph node metastasis | 25.1 | 18.8 | 18.4 | |
| Only metastatic disease in other sites | 23 | 20.8 | 23.5 | |
| Metastatic disease in lymph nodes and other sites | 23.5 | 25.8 | 26.5 | |
|
| ||||
| Cycle length | ||||
| 14 day cycle | 37.3 | 35.5 | 33.7 | Χ2=3.58, p=.734 |
| 21 day cycle | 54.6 | 54.8 | 59.2 | |
| 28 day cycle | 7.6 | 9.7 | 7.1 | |
| >28 day cycle | 0.5 | 0 | 0 | |
Total number of metastatic sites evaluated was 9.
Abbreviations: CTX = chemotherapy, kg = kilograms, KW = Kruskal Wallis, m2 = meters squared, RT = radiation therapy, SD = standard deviation
Latent Classes for Evening Fatigue
A three-class solution for evening fatigue was selected (Table 1) based on the same rationales as for morning fatigue. Using the clinically meaningful LFS cutoff score of ≥5.6 for evening fatigue, and the findings from our previous RT study that identified three evening fatigue classes (i.e., Low, Moderate, High) [12], the evening fatigue classes in the current study were named as Moderate, High, and Very High. As shown in Figure 1B, the trajectories of evening fatigue differed among the latent classes. For both the Moderate (20.0%) and Very High (58.2%) Evening Fatigue classes, fatigue scores remained relatively constant across the six assessments. For the High Evening Fatigue class (21.8%), fatigue scores varied over the two cycles of CTX, with slightly higher scores reported at assessments 2 and 4.
Differences in Demographic and Clinical Characteristics Among Evening Fatigue Classes
Compared to the Moderate Evening Fatigue class, patients in the Very High Evening Fatigue class were more likely to be younger, female, reported having child care responsibilities, reported a lower KPS score and a higher SCQ score, and had a diagnosis of breast cancer (Table 3). In addition, compared to the Moderate Evening Fatigue class, patients in the High Evening Fatigue class reported a lower KPS score, a higher SCQ score, and were more likely not to have received prior cancer treatment. Compared to the other two classes, a higher percentage of patients in the Moderate Fatigue class had a diagnosis of GI cancer. The majority of the clinical characteristics did not differ among the latent classes.
Table 3.
Differences in Demographic and Clinical Characteristics Among the Evening Fatigue Latent Classes (n=582)
| Characteristic | Moderate LFS score (0) N=116 (20.0%) |
High LFS score (1) N=127 (21.8%) |
Very High LFS score (2) N=339 (58.2%) |
Statistics |
|---|---|---|---|---|
|
| ||||
| Mean (SD) | Mean (SD) | Mean (SD) | ||
|
| ||||
| Age (years) | 59.7 (11.7) | 57.2 (12.2) | 56.3 (11.7) | F=3.35, p=.036 0 > 2 |
|
| ||||
| Education (years) | 16.3 (3.3) | 15.9 (2.7) | 16.6 (3.0) | F=2.66, p=.071 |
|
| ||||
| Body mass index (kg/m2) | 25.5 (5.1) | 26.6 (6.0) | 26.6 (6.0) | F=1.55, p=.212 |
|
| ||||
| Karnofsky Performance Status score | 85.3 (10.9) | 80.7 (12.1) | 79.1 (11.8) | F=11.06, p<.0001 0 >1 and 2 |
|
| ||||
| Self-administered Comorbidity Questionnaire score | 4.8 (2.8) | 5.8 (3.0) | 5.8 (3.1) | F=5.18, p<.006 0 < 1 and 2 |
|
| ||||
| Time since diagnosis (years) | 2.0 (4.2) | 2.7 (5.4) | 2.5 (4.1) | F=0.90, p=.405 |
|
| ||||
| Number of prior cancer treatments | 1.8 (1.5) | 1.8 (1.6) | 2.0 (1.6) | F=1.02, p=.360 |
|
| ||||
| Number of metastatic sites including lymph node involvement a | 1.4 (1.4) | 1.4 (1.3) | 1.3 (1.3) | F=0.59, p=.553 |
|
| ||||
| Number of metastatic sites excluding lymph node involvement | 1.0 (1.2) | 0.9 (1.2) | 0.9 (1.1) | F=0.42, p=.660 |
|
| ||||
| Hemoglobin | 11.9 (1.4) | 11.6 (1.2) | 11.7 (1.4) | F=1.03, p=.357 |
|
| ||||
| Hematocrit | 35.4 (4.1) | 34.7 (3.6) | 34.8 (4.2) | F=0.96, p=.382 |
|
| ||||
| % | % | % | ||
|
| ||||
| Gender (% female) | 70.7 | 78.7 | 83.8 | Χ2=9.45, p=.009 0<2 |
|
| ||||
| Self-reported ethnicity | ||||
| White | 69.6 | 62.3 | 77.5 | Χ2=11.00, p=.004 |
| Non-white | 30.4 | 37.7 | 22.5 | 1<2 |
|
| ||||
| Married or partnered (% yes) | 70.2 | 65.9 | 67.3 | Χ2=0.53, p=.766 |
|
| ||||
| Lives alone (% yes) | 17.5 | 19.8 | 20.9 | Χ2=0.60, p=.741 |
|
| ||||
| Currently employed (% yes) | 33 | 33.3 | 35.8 | Χ2=0.42 p=.809 |
|
| ||||
| Annual household income | ||||
| Less than $30,000 | 20.6 | 18.6 | 18 | KW, p=.140 |
| $30,000 to $70,000 | 25.5 | 22.1 | 16.7 | |
| $70,000 to $100,000 | 17.6 | 15 | 17.6 | |
| Greater than $100,000 | 36.3 | 44.2 | 47.7 | |
|
| ||||
| Child care responsibilities (% yes) | 14.9 | 20.8 | 27.3 | Χ2=7.82, p=.020 0<2 |
|
| ||||
| Elder care responsibilities (% yes) | 8.4 | 9.2 | 8.8 | Χ2=0.05, p=.976 |
|
| ||||
| Exercise on a regular basis (% yes) | 72.4 | 72.2 | 69.1 | Χ2=0.68, p=.712 |
|
| ||||
| Cancer diagnosis | Χ2=28.90, p<.0001 | |||
| Breast cancer | 31 | 39.4 | 48.4 | 0<2 |
| Gastrointestinal cancer | 41.4 | 22 | 23.3 | 0>1 and 2 |
| Gynecological cancer | 17.2 | 21.3 | 21.2 | n/s |
| Lung cancer | 10.3 | 17.3 | 7.1 | 1>2 |
|
| ||||
| Prior cancer treatment | Χ2=17.57, p=.007 | |||
| No prior treatment | 20.9 | 25 | 13.7 | 1>2 |
| Only surgery, CTX, or RT | 33 | 37.9 | 45.2 | n/s |
| Surgery and CTX, or surgery and RT, or CTX and RT | 32.2 | 20.2 | 21.4 | n/s |
| Surgery and CTX and RT | 13.9 | 16.9 | 19.6 | n/s |
|
| ||||
| Metastatic sites | ||||
| No metastasis | 28.4 | 26.2 | 35.6 | Χ2=6.32, p=.388 |
| Only lymph node metastasis | 21.6 | 23 | 19.6 | |
| Only metastatic disease in other sites | 23.3 | 27 | 19.6 | |
| Metastatic disease in lymph nodes and other sites | 26.7 | 23.8 | 25.2 | |
|
| ||||
| Cycle length | ||||
| 14 day cycle | 47.4 | 30.7 | 33.6 | Χ2=11.15, p=.084 |
| 21 day cycle | 44 | 58.3 | 58.4 | |
| 28 day cycle | 8.6 | 11 | 7.7 | |
| >28 day cycle | 0 | 0 | 0.3 | |
Total number of metastatic sites evaluated was 9.
Abbreviations: CTX = chemotherapy, kg = kilograms, KW = Kruskal Wallis, m2 = meters squared, RT = radiation therapy, SD = standard deviation
Overlap in membership among the fatigue classes
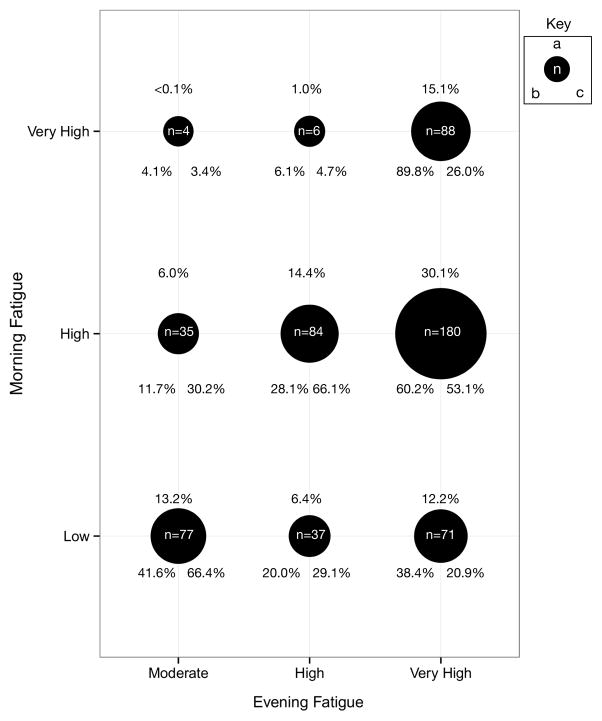
Figure 2 illustrates the overlap in membership between the morning and evening fatigue latent classes. Of the 582 patients enrolled in this study, 15.1% (n=88) were classified in both the Very High Morning and Very High Evening fatigue classes. Of those patients in the Very High Morning Fatigue class (n=98), 89.8% (n=88) were in Very High Evening Fatigue class. In contrast, of those patients in the Very High Evening Fatigue class (n=339), only 26.0% (n=88) of them were in the Very High Morning Fatigue class.
Figure 2.
Overlap in membership among the morning and evening classes. Black circles represent the number (n) and percentage of all patients (a) in the intersecting Morning and Evening Fatigue classes. Percentages of patients found in the Morning Fatigue classes (row totals) and the three Evening Fatigue classes are given by (b). Percentages of patients found in the Evening Fatigue classes (column totals) by the three Morning Fatigue classes are given by (c). Of the 582 patients enrolled in this study, 15.1% (n=88) were classified in both the Very High Morning and Very High Evening fatigue classes. Of those patients in the Very High Morning Fatigue class (n=98), 89.8% (n=88) were also in Very High Evening Fatigue class. In contrast, of those patients in the Very High Evening Fatigue class (n=339), only 26.0% (n=88) of them were in the Very High Morning Fatigue class. Eighty-four patients (14.4%) were classified in both the High Morning and High Evening Fatigue latent classes. Of those patients in the High Morning Fatigue class (n=297), 28.1% (n=84) of them were in the High Evening Fatigue class. In contrast, of those patients in the High Evening Fatigue class (n=127), 66.1% (n=84) of them were in the High Morning Fatigue class. Of those patients in the Low Morning Fatigue class (n=185), 41.6% were in the Moderate Evening Fatigue class (n=77), 20% were in the High Evening class, and 38.4% were in the Very High Evening Fatigue class.
Discussion
This study is the first to use unconditional LPA to identify subgroups of patients with distinct morning and evening fatigue trajectories over two cycles of CTX. As shown in Figures 1A and B, and consistent with our previous study of patients who underwent RT and their family caregivers (FCs) [12], three distinct latent classes were identified for both symptoms. While direct comparisons between this study and the RT study [12] are not possible due to differences in the timing of the assessments (i.e., two cycles of CTX over approximately 2 months versus from prior to the initiation of RT to 4 months after the completion of RT) and sample characteristics (i.e., only patients versus patients and their FCs), several observations warrant consideration. First, in contrast to 61% of participants in the RT study, 80% of the patients in this study experienced clinically meaningful levels of evening fatigue. In addition, while 47% of the participants in the RT study reported clinically meaningful levels of morning fatigue, 67% of the patients in this study had this level of morning fatigue. Second, while in our previous and other RT studies [12,26,27], fatigue severity increased over the course of treatment, in this and previous CTX studies [28,29], fatigue severity scores persisted over the two cycles of CTX. Third, in contrast to our RT study [12], two new classes of patients with “Very High” levels of evening and morning fatigue were identified using LPA. This finding may reflect a shift in the overall distribution of fatigue severity scores rather than a broadening of the range and distribution of these scores. These differences in morning and evening fatigue severity may be partially explained by the systemic effects associated with CTX compared to the more localized effects associated with RT. Taken together, these findings suggest that subgroups of patients are at higher risk for clinically meaningful levels of persistent morning and evening fatigue. In addition, the mechanisms that contribute to differences in the severity and trajectories of morning and evening fatigue associated with CTX versus RT warrant investigation in future studies.
Common predictors of morning and evening fatigue
In order to identify patients at higher risk for clinically meaningful levels of morning and evening fatigue, as well as to evaluate whether morning and evening fatigue are distinct but related symptoms, we compared common and unique predictors of these two symptoms (see Table 4). Age and gender were the only two demographic characteristics that were associated with both morning and evening fatigue latent class membership. Consistent with our RT study [12] and previous reports [30,31], younger age was associated with membership in both of the highest morning (i.e., Very High) and evening (i.e., Very High) fatigue classes. Potential explanations for these associations include: lower doses of CTX being given to older patients [32,33]; age-related changes in the hypothalamic-adrenal-pituitary axis [34]; or a “response shift” in the perception of symptoms in older patients [35].
Table 4.
Common and Unique Predictors of Morning and Evening Fatigue Latent Class Membership
| Common predictors of morning and evening fatigue class membership | Unique predictors of morning fatigue class membership | Unique predictors of evening fatigue class membership |
|---|---|---|
| Age | Marital status | Child care responsibilities |
| Gender | Annual household income | Ethnicity |
| Functional status | Performance of regular exercise | Cancer diagnosis |
| Comorbidity profile | Receipt of prior cancer treatment |
Female gender was associated with membership in both of the highest morning (i.e., Very High) and evening (i.e., Very High) fatigue classes. In our RT study [12], this association was found only for evening fatigue. Findings regarding an association between gender and symptom severity are inconsistent [36–38]. However, as was found in this study, and in our RT study [12], being female and caring for children at home were both associated with higher evening fatigue scores. This finding suggests that the added responsibilities associated with the provision of child care increase the severity and duration of evening fatigue [12].
Consistent with previous reports, patients in both the higher morning and evening fatigue classes reported a poorer functional status [12,39] and a more severe comorbidity profile [40]. Compared to the Moderate Evening Fatigue class, decrements in functional status reported by patients in the High and Very High Evening Fatigue classes represent not only statistically significant but clinically meaningful differences in KPS scores (d=0.39, d=0.52, respectively) [41,42]. Similarly, albeit the effect sizes were larger, compared to the Low Morning Fatigue class, patients in the High and Very High Morning Fatigue classes had clinically meaningful decrements in functional status scores (d=0.58, d=1.06, respectively) [41,42]. While a higher level of comorbidity was noted to contribute to higher mean fatigue severity scores in patients receiving CTX [40], future studies need to examine the specific comorbidities and associated treatments that increase the severity of both morning and evening fatigue.
Unique predictors of morning and evening fatigue
Other than child care responsibilities (discussed above), self-reported ethnicity was the only demographic characteristic that was associated with higher levels of evening, but not morning fatigue. Specifically, compared to the High Evening Fatigue class, a higher percentage of White patients belonged to the Very High Evening Fatigue class. Findings regarding ethnic differences in the occurrence and severity of symptoms in oncology patients are inconsistent [43,44] and warrant additional investigation.
In terms of clinical characteristics, cancer diagnosis and types of prior cancer treatment distinguished among the evening fatigue latent classes. Specifically, patients with a diagnosis of GI cancer were more likely to be classified in the Moderate Evening Fatigue class compared to the other two classes. In addition, patients with lung cancer were more likely to be classified into the High Evening Fatigue class compared to the Very High Evening Fatigue class. Finally, compared to the Moderate Evening Fatigue class, a higher proportion of patients with breast cancer were classified in the Very High Evening Fatigue class. To our knowledge, this study is the first to identify differences in fatigue severity among patients with four different diagnoses. Some of these associations may be related not only to the cancer diagnosis and its associated treatment regimens, but to a variety of demographic (e.g., gender) and clinical (e.g., functional status) characteristics. The specific characteristics associated with increased fatigue severity within each cancer diagnosis warrant investigation in future studies.
In terms of receipt of prior cancer treatment, the only statistically significant post hoc contrast was that a higher percentage of patients who were receiving CTX as their first cancer treatment were more likely to be classified in the High Evening Fatigue class compared to the Very High Evening Fatigue class. Similar to diagnosis, the specific predictors associated with higher fatigue severity in the context of previous cancer treatment(s) warrant additional research.
Marital status, annual household income, BMI, and performance of regular exercise were the four unique characteristics associated with morning fatigue. The finding that, compared to the Low Morning Fatigue class, patients in the Very High Morning Fatigue class were less likely to be married suggests that social support and assistance from a partner may alleviate some of the fatigue associated with CTX. In addition, patients with an annual income of <$30,000 were more likely to be classified in the Very High Evening Fatigue class. The exact reasons for this difference are not known. Future studies need to evaluate the impact of employment status, housing arrangements, insurance status, as well as clinical characteristics that influence fatigue severity in socioeconomically disadvantaged patients.
Consistent with previous reports in patients with breast cancer [45,46], a higher BMI (i.e., 28.3 ± 7.3) which would be classified as overweight [47], was associated with membership in the Very High Morning Fatigue class. The reason why a higher BMI was associated with a higher level of morning fatigue is not readily apparent. However, it is known that obstructive sleep apnea (OSA) can occur in patients with a higher BMI and that OSA is associated with morning fatigue [48]. This hypothesis warrants investigation in future studies. Finally, compared to the other two morning fatigue classes, a significantly lower percentage of patients in the Very High Morning Fatigue class reported that they exercised on a regular basis similar to previous findings in breast cancer patients [45,46]. This finding is not surprising because exercise is the only evidenced-based intervention that can be used to reduce fatigue in oncology patients [49,50]. Future studies need to evaluate for interactions among gender, cancer diagnosis, BMI, and use of regular exercise and the severity of fatigue during and following cancer treatment.
It should be noted that consistent with previous reports [6,8], the majority of disease and treatment characteristics did not predict membership in any of the evening and morning fatigue classes. In particular, while previous reports suggested that anemia may be a potential mechanism for fatigue associated with cancer and its treatment [51,52], no differences in hemoglobin and hematocrit levels were found among the evening or the morning fatigue latent classes.
Overlap among morning and evening fatigue class membership
To evaluate the hypothesis that morning and evening fatigue are distinct but related symptoms, the overlap in membership across the morning and evening fatigue latent classes was evaluated (Figure 2). If these two symptoms had identical underlying mechanisms, the expectation would be that a substantial portion of the patients would be classified in both the higher and the lower fatigue severity classes. In other words, a patient who was classified as being in the Very High Evening Fatigue class would also be classified as being in the Very High Morning Fatigue class, and vice versa. In this study, 89.8% of the patients classified in the Very High Morning Fatigue class were also in the Very High Evening Fatigue class. However, the reverse was not true. Only 26.0% of the patients classified in the Very High Evening Fatigue class were also in the Very High Morning Fatigue class. In other words, a patient who experiences very high evening fatigue is not expected to also experience very high morning fatigue, which suggests that sleep is restorative in these patients. However, those patients who wake with very high levels of morning fatigue are expected to have very high levels of evening fatigue as well. In addition, only 66.4% of the patients who were classified in the Moderate Evening Fatigue class were classified in the Low Morning Fatigue class. Of note, we did not identify a Low Evening Fatigue class in this study. This lack of an overlap across the latent classes, as well as the unique predictors of class membership (see Table 4) support our hypothesis that morning and evening fatigue are distinct but related symptoms. As we reported for our RT study [53], additional work is underway to identify the molecular mechanisms that distinguish between these two symptoms in patients receiving CTX.
Limitations
Several limitations need to be acknowledged. Because patients were not recruited prior to the initiation of CTX, increases in fatigue severity from the initiation of treatment were not evaluated. In addition, since ongoing assessments were not done during the remainder and following the completion of CTX, the reasons for the persistent nature of morning and evening fatigue are not known.
Conclusions
Despite these limitations, the findings from this large, longitudinal study add support to the growing body of evidence that morning and evening fatigue are distinct but related symptoms. In addition, the use of analytic strategies like LPA allows for the identification of demographic and clinical characteristics of patients who are at highest risk for more severe fatigue profiles. Using the risk factors listed in Table 4, clinicians who care for oncology patients receiving CTX can conduct individualized assessments to identify high risk patients, educate patients about morning and evening fatigue, and plan appropriate interventions to decrease morning and evening fatigue.
Future studies are needed to confirm the common and unique risk factors for morning and evening fatigue. In addition, an evaluation of differences in serum biomarkers (e.g., C-reactive protein) among the latent classes warrants investigation. Future studies can evaluate for differences among the latent classes in other symptoms (e.g., depression) and quality of life outcomes. Additional longitudinal studies are warranted that enroll patients prior to the initiation of CTX and follow them to the completion of CTX to confirm the specific latent classes identified in this study and to determine if the severity of fatigue changes across these latent classes.
Acknowledgments
This study was supported by a grant from the National Cancer Institute (NCI, CA134900). Dr Miaskowski is supported by a grant from the American Cancer Society and a K05 award (CA168960) from the NCI.
Footnotes
Financial disclosures: Nothing to declare.
References
- 1.Lawrence DP, Kupelnick B, Miller K, Devine D, Lau J. Evidence report on the occurrence, assessment, and treatment of fatigue in cancer patients. J Natl Cancer Inst Monogr. 2004;(32):40–50. doi: 10.1093/jncimonographs/lgh027. [DOI] [PubMed] [Google Scholar]
- 2.Butt Z, Rosenbloom SK, Abernethy AP, Beaumont JL, Paul D, Hampton D, Jacobsen PB, Syrjala KL, Von Roenn JH, Cella D. Fatigue is the most important symptom for advanced cancer patients who have had chemotherapy. J Natl Compr Canc Netw. 2008;6:448–455. doi: 10.6004/jnccn.2008.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prue G, Rankin J, Allen J, Gracey J, Cramp F. Cancer-related fatigue: A critical appraisal. Eur J Cancer. 2006;42:846–863. doi: 10.1016/j.ejca.2005.11.026. S0959-8049(05)01134-2. [DOI] [PubMed] [Google Scholar]
- 4.Bower JE, Ganz PA, Desmond KA, Bernaards C, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in long-term breast carcinoma survivors: a longitudinal investigation. Cancer. 2006;106:751–758. doi: 10.1002/cncr.21671. [DOI] [PubMed] [Google Scholar]
- 5.Winningham ML, Nail LM, Burke MB, Brophy L, Cimprich B, Jones LS, Pickard-Holley S, Rhodes V, St Pierre B, Beck S, et al. Fatigue and the cancer experience: the state of the knowledge. Oncol Nurs Forum. 1994;21:23–36. [PubMed] [Google Scholar]
- 6.Dhruva A, Dodd M, Paul SM, Cooper BA, Lee K, West C, Aouizerat BE, Swift PS, Wara W, Miaskowski C. Trajectories of fatigue in patients with breast cancer before, during, and after radiation therapy. Cancer Nurs. 2010;33:201–212. doi: 10.1097/NCC.0b013e3181c75f2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fletcher BA, Schumacher KL, Dodd M, Paul SM, Cooper BA, Lee K, West C, Aouizerat BE, Swift PS, Wara W, Miaskowski C. Trajectories of fatigue in family caregivers of patients undergoing radiation therapy for prostate cancer. Res Nurs Health. 2009;32:125–139. doi: 10.1002/nur.20312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miaskowski C, Paul SM, Cooper BA, Lee K, Dodd M, West C, Aouizerat BE, Swift PS, Wara W. Trajectories of fatigue in men with prostate cancer before, during, and after radiation therapy. J Pain Symptom Manage. 2008;35:632–643. doi: 10.1016/j.jpainsymman.2007.07.007. S0885-3924(08)00051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dimsdale JE, Ancoli-Israel S, Ayalon L, Elsmore TF, Gruen W. Taking fatigue seriously, II: variability in fatigue levels in cancer patients. Psychosomatics. 2007;48:247–252. doi: 10.1176/appi.psy.48.3.247. 48/3/247. [DOI] [PubMed] [Google Scholar]
- 10.Jim HS, Small B, Faul LA, Franzen J, Apte S, Jacobsen PB. Fatigue, depression, sleep, and activity during chemotherapy: daily and intraday variation and relationships among symptom changes. Ann Behav Med. 2011;42:321–333. doi: 10.1007/s12160-011-9294-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Molassiotis A, Chan CW. Fatigue patterns in Chinese patients receiving radiotherapy. Eur J Oncol Nurs. 2004;8:334–340. doi: 10.1016/j.ejon.2003.12.009. S146238890300111X. [DOI] [PubMed] [Google Scholar]
- 12.Dhruva A, Aouizerat BE, Cooper B, Paul SM, Dodd M, West C, Wara W, KL, Dunn LB, Langford DJ, Merriman JD, Baggott C, Cataldo J, Ritchie C, Kober K, Leutwyler H, Miaskowski C. Differences in morning and evening fatigue in oncology patients and their family caregivers. Eur J Oncol Nurs. 2013;17:841–848. doi: 10.1016/j.ejon.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karnofsky D. Factors that influence the therapeutic response in cancer: a comprehensive treatise. Plenum Press; New York: 1977. Performance scale. [Google Scholar]
- 14.Karnofsky D, Abelmann WH, Craver LV, Burchenal JH. The use of nitrogen mustards in the palliative treatment of carcinoma. Cancer. 1948;1:634–656. [Google Scholar]
- 15.Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The Self-Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003;49:156–163. doi: 10.1002/art.10993. [DOI] [PubMed] [Google Scholar]
- 16.Brunner F, Bachmann LM, Weber U, Kessels AG, Perez RS, Marinus J, Kissling R. Complex regional pain syndrome 1--the Swiss cohort study. BMC Musculoskelet Disord. 2008;9:92. doi: 10.1186/1471-2474-9-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee KA, Hicks G, Nino-Murcia G. Validity and reliability of a scale to assess fatigue. Psychiatry Res. 1991;36(3):291–298. doi: 10.1016/0165-1781(91)90027-m. [DOI] [PubMed] [Google Scholar]
- 18.Fletcher BS, Paul SM, Dodd MJ, Schumacher K, West C, Cooper B, Lee K, Aouizerat B, Swift P, Wara W, Miaskowski CA. Prevalence, severity, and impact of symptoms on female family caregivers of patients at the initiation of radiation therapy for prostate cancer. J Clin Oncol. 2008;26:599–605. doi: 10.1200/JCO.2007.12.2838. [DOI] [PubMed] [Google Scholar]
- 19.Gay CL, Lee KA, Lee SY. Sleep patterns and fatigue in new mothers and fathers. Biol Res Nurs. 2004;5:311–318. doi: 10.1177/1099800403262142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee KA, Portillo CJ, Miramontes H. The fatigue experience for women with human immunodeficiency virus. J Obstet Gynecol Neonatal Nurs. 1999;28:193–200. doi: 10.1111/j.1552-6909.1999.tb01984.x. [DOI] [PubMed] [Google Scholar]
- 21.Miaskowski C, Lee KA. Pain, fatigue, and sleep disturbances in oncology outpatients receiving radiation therapy for bone metastasis: a pilot study. J Pain Symptom Manage. 1999;17:320–332. doi: 10.1016/s0885-3924(99)00008-1. S0885-3924(99)00008-1. [DOI] [PubMed] [Google Scholar]
- 22.Miaskowski C, Cooper BA, Paul SM, Dodd M, Lee K, Aouizerat BE, West C, Cho M, Bank A. Subgroups of patients with cancer with different symptom experiences and quality-of-life outcomes: a cluster analysis. Oncol Nurs Forum. 2006;33:E79–89. doi: 10.1188/06.ONF.E79-E89. [DOI] [PubMed] [Google Scholar]
- 23.Muthen LK, Muthen BO. Mplus User’s Guide. 7. Muthen & Muthen; Los Angeles, CA: 1998–2012. [Google Scholar]
- 24.Nylund KL, Asparouhov T, Muthen BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. Struct Equ Modeling. 2007;14:535–569. [Google Scholar]
- 25.Muthen B, Shedden K. Finite mixture modeling with mixture outcomes using the EM algorithm. Biometrics. 1999;55:463–469. doi: 10.1111/j.0006-341x.1999.00463.x. [DOI] [PubMed] [Google Scholar]
- 26.Danjoux C, Gardner S, Fitch M. Prospective evaluation of fatigue during a course of curative radiotherapy for localised prostate cancer. Support Care Cancer. 2007;15:1169–1176. doi: 10.1007/s00520-007-0229-8. [DOI] [PubMed] [Google Scholar]
- 27.Greenberg DB, Gray JL, Mannix CM, Eisenthal S, Carey M. Treatment-related fatigue and serum interleukin-1 levels in patients during external beam irradiation for prostate cancer. J Pain Symptom Manage. 1993;8:196–200. doi: 10.1016/0885-3924(93)90127-h. [DOI] [PubMed] [Google Scholar]
- 28.Ancoli-Israel S, Liu L, Rissling M, Natarajan L, Neikrug AB, Palmer BW, Mills PJ, Parker BA, Sadler GR, Maglione J. Sleep, fatigue, depression, and circadian activity rhythms in women with breast cancer before and after treatment: a 1-year longitudinal study. Support Care Cancer. 2014;22:2535–2545. doi: 10.1007/s00520-014-2204-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berger AM. Patterns of fatigue and activity and rest during adjuvant breast cancer chemotherapy. Oncol Nurs Forum. 1998;25:51–62. [PubMed] [Google Scholar]
- 30.Janz NK, Mujahid M, Chung LK, Lantz PM, Hawley ST, Morrow M, Schwartz K, Katz SJ. Symptom experience and quality of life of women following breast cancer treatment. J Womens Health. 2007;16:1348–1361. doi: 10.1089/jwh.2006.0255. [DOI] [PubMed] [Google Scholar]
- 31.Jereczek-Fossa BA, Santoro L, Alterio D, Franchi B, Fiore MR, Fossati P, Kowalczyk A, Canino P, Ansarin M, Orecchia R. Fatigue during head-and-neck radiotherapy: prospective study on 117 consecutive patients. Int J Radiat Oncol Biol Phys. 2007;68:403–415. doi: 10.1016/j.ijrobp.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 32.Townsley C, Pond GR, Peloza B, Kok J, Naidoo K, Dale D, Herbert C, Holowaty E, Straus S, Siu LL. Analysis of treatment practices for elderly cancer patients in Ontario, Canada. J Clin Oncol. 2005;23:3802–3810. doi: 10.1200/JCO.2005.06.742. 23/16/3802. [DOI] [PubMed] [Google Scholar]
- 33.Kumar A, Soares HP, Balducci L, Djulbegovic B National Cancer I. Treatment tolerance and efficacy in geriatric oncology: a systematic review of phase III randomized trials conducted by five National Cancer Institute-sponsored cooperative groups. J Clin Oncol. 2007;25:1272–1276. doi: 10.1200/JCO.2006.09.2759. 25/10/1272. [DOI] [PubMed] [Google Scholar]
- 34.Bower JE, Low CA, Moskowitz JT, Sepah S, Epel E. Benefit finding and physical health: positive psychological changes and enhanced allostasis. Soc Personal Psychol Compass. 2008;2:223–244. doi: 10.1111/j.1751-9004.2007.00038.x. [DOI] [Google Scholar]
- 35.Schwartz CE, Sprangers MA. Methodological approaches for assessing response shift in longitudinal health-related quality-of-life research. Social Science & Medicine. 1999;48:1531–1548. doi: 10.1016/s0277-9536(99)00047-7. [DOI] [PubMed] [Google Scholar]
- 36.Baldwin CM, Grant M, Wendel C, Hornbrook MC, Herrinton LJ, McMullen C, Krouse RS. Gender differences in sleep disruption and fatigue on quality of life among persons with ostomies. J Clin Sleep Med. 2009;5:335–343. [PMC free article] [PubMed] [Google Scholar]
- 37.Cheung WY, Le LW, Gagliese L, Zimmermann C. Age and gender differences in symptom intensity and symptom clusters among patients with metastatic cancer. Support Care Cancer. 2011;19:417–423. doi: 10.1007/s00520-010-0865-2. [DOI] [PubMed] [Google Scholar]
- 38.Hickok JT, Roscoe JA, Morrow GR, Mustian K, Okunieff P, Bole CW. Frequency, severity, clinical course, and correlates of fatigue in 372 patients during 5 weeks of radiotherapy for cancer. Cancer. 2005;104:1772–1778. doi: 10.1002/cncr.21364. [DOI] [PubMed] [Google Scholar]
- 39.Hofso K, Miaskowski C, Bjordal K, Cooper BA, Rustoen T. Previous chemotherapy influences the symptom experience and quality of life of women with breast cancer prior to radiation therapy. Cancer Nurs. 2012;35:167–177. doi: 10.1097/NCC.0b013e31821f5eb5. [DOI] [PubMed] [Google Scholar]
- 40.Berger AM, Gerber LH, Mayer DK. Cancer-related fatigue: implications for breast cancer survivors. Cancer. 2012;118:2261–2269. doi: 10.1002/cncr.27475. [DOI] [PubMed] [Google Scholar]
- 41.Guyatt GH, Osoba D, Wu AW, Wyrwich KW, Norman GR Clinical Significance Consensus Meeting G. Methods to explain the clinical significance of health status measures. Mayo Clin Proc. 2002;77:371–383. doi: 10.1016/S0025-6196(11)61793-X. [DOI] [PubMed] [Google Scholar]
- 42.Osoba D. Interpreting the meaningfulness of changes in health-related quality of life scores: lessons from studies in adults. Int J Cancer Suppl. 1999;12:132–137. doi: 10.1002/(SICI)1097-0215(1999)83:12+<132::AID-IJC23>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 43.Fu OS, Crew KD, Jacobson JS, Greenlee H, Yu G, Campbell J, Ortiz Y, Hershman DL. Ethnicity and persistent symptom burden in breast cancer survivors. J Cancer Surviv. 2009;3:241–250. doi: 10.1007/s11764-009-0100-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luckett T, Goldstein D, Butow PN, Gebski V, Aldridge LJ, McGrane J, Ng W, King MT. Psychological morbidity and quality of life of ethnic minority patients with cancer: a systematic review and meta-analysis. Lancet Oncol. 2011;12:1240–1248. doi: 10.1016/S1470-2045(11)70212-1. [DOI] [PubMed] [Google Scholar]
- 45.Donovan KA, Small BJ, Andrykowski MA, Munster P, Jacobsen PB. Utility of a cognitive-behavioral model to predict fatigue following breast cancer treatment. Health Psychol. 2007;26(4):464–472. doi: 10.1037/0278-6133.26.4.464. 2007-09406-010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Andrykowski MA, Donovan KA, Laronga C, Jacobsen PB. Prevalence, predictors, and characteristics of off-treatment fatigue in breast cancer survivors. Cancer. 2010;116:5740–5748. doi: 10.1002/cncr.25294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.World Health Organization., Food and Agriculture Organization of the United Nations. World Health Organization technical report series. World Health Organization; Geneva: [Google Scholar]
- 48.Haensel A, Norman D, Natarajan L, Bardwell WA, Ancoli-Israel S, Dimsdale JE. Effect of a 2 week CPAP treatment on mood states in patients with obstructive sleep apnea: a double-blind trial. Sleep Breath. 2007;11:239–244. doi: 10.1007/s11325-007-0115-0. [DOI] [PubMed] [Google Scholar]
- 49.Cramp F, Byron-Daniel J. Exercise for the management of cancer-related fatigue in adults. The Cochrane database of systematic reviews. 2012;11:CD006145. doi: 10.1002/14651858.CD006145.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bower JE. Cancer-related fatigue--mechanisms, risk factors, and treatments. Nat Rev Clin Oncology. 2014;11:597–609. doi: 10.1038/nrclinonc.2014.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mock V, Atkinson A, Barsevick A, Cella D, Cimprich B, Cleeland C, Donnelly J, Eisenberger MA, Escalante C, Hinds P, Jacobsen PB, Kaldor P, Knight SJ, Peterman A, Piper BF, Rugo H, Sabbatini P, Stahl C National Comprehensive Cancer N. NCCN Practice Guidelines for Cancer-Related Fatigue. Oncology. 2000;14:151–161. [PubMed] [Google Scholar]
- 52.Ryan JL, Carroll JK, Ryan EP, Mustian KM, Fiscella K, Morrow GR. Mechanisms of cancer-related fatigue. Oncologist. 2007;12:22–34. doi: 10.1634/theoncologist.12-S1-22. 12/suppl_1/22. [DOI] [PubMed] [Google Scholar]
- 53.Dhruva A, Aouizerat B, Cooper B, Paul SM, Dodd M, West C, Wara W, Lee K, Dunn LB, Langford DJ, Merriman J, Baggott C, Cataldo J, Ritchie C, Kober KM, Leutwyler H, Miaskowski C. Cytokine gene associations with self-report ratings of morning and evening fatigue in oncology patients and their family caregivers. Biol Res Nur. 2015;17:175–184. doi: 10.1177/1099800414534313. [DOI] [PMC free article] [PubMed] [Google Scholar]